Abstract
Objectives
The main purpose of this study was to evaluate whether large granular bovine bone can be as effective as small granular bovine bone in maxillary sinus floor elevation.Methods
A comprehensive online search of eligible articles was conducted using PubMed, EMBASE, Cochrane Library, Scopus, and Web of Science, and a systematic review and meta-analysis was performed from establishment to February, 2023. The outcome indicators were the percentage of connective tissue, the percentage of newly formed bone and the percentage of residual xenograft respectively. The meta-analysis was conducted by using the Stata 15.1 (Stata Conpernarn, USA) and Review Manager software5.4.1.Results
After careful screening and review, a total of 4 studies were included for systematic review and meta-analysis. The data were extracted to compare the histological performance of bovine bones with different particle sizes after maxillary sinus elevation. No significant differences were found in the percentage of connective tissue, the percentage of newly formed bone, and the percentage of residual xenograft.Conclusion
In this study, a systematically review of the previous literature showed that similar histological results were obtained for both large-particle bovine bone and small-particle bovine bone. Therefore, the large granular bovine bone and the small granular bovine bone were equally effective in maxillary sinus elevation. It is difficult to make conclusion from limited evidence from four studies. More clinical evidence was needed.Free full text

The impact of deproteinized bovine bone particle size on histological outcomes in sinus floor elevation: a systematic review and meta-analysis
Abstract
Objectives
The main purpose of this study was to evaluate whether large granular bovine bone can be as effective as small granular bovine bone in maxillary sinus floor elevation.
Methods
A comprehensive online search of eligible articles was conducted using PubMed, EMBASE, Cochrane Library, Scopus, and Web of Science, and a systematic review and meta-analysis was performed from establishment to February, 2023. The outcome indicators were the percentage of connective tissue, the percentage of newly formed bone and the percentage of residual xenograft respectively. The meta-analysis was conducted by using the Stata 15.1 (Stata Conpernarn, USA) and Review Manager software5.4.1.
Results
After careful screening and review, a total of 4 studies were included for systematic review and meta-analysis. The data were extracted to compare the histological performance of bovine bones with different particle sizes after maxillary sinus elevation. No significant differences were found in the percentage of connective tissue, the percentage of newly formed bone, and the percentage of residual xenograft.
Conclusion
In this study, a systematically review of the previous literature showed that similar histological results were obtained for both large-particle bovine bone and small-particle bovine bone. Therefore, the large granular bovine bone and the small granular bovine bone were equally effective in maxillary sinus elevation. It is difficult to make conclusion from limited evidence from four studies. More clinical evidence was needed.
Introduction
Due to maxillary sinus gasification and alveolar bone atrophy, many patients who requires maxillary posterior dental implants faces the problem of insufficient height of residual alveolar bone. In order to obtain sufficient healthy bone mass and good planting results, maxillary sinus floor elevation is a common clinical method to solve such problems [1–3]. For a long time, autologous bone has been considered as the gold standard for maxillary sinus augmentation. Even though, there are a number of disadvantages to the use of autologous bone for maxillary sinus enhancement. For example, there may be a need for hospitalization, the opening of a second surgical site, increased incidence of complications, and the inevitable tendency to absorb a lot [4, 5]. With the development of technology, bone incremental materials from various biological or synthetic origin are also increasingly being used to optimize surgical. Of all the major bone replacement grafts in used (allografts, xenografts, alloplats), the most clinical research are xenografts, such as bovine bone, pork bone, and horse bone. Among them, deproteinized bovine bone mineral has been widely used in sinus augmentation with comparable results and success rates [6, 7].
Bio-Oss® is one of the best documented bone graft materials manufactured in Switzerland for dental implant application [8, 9]. The product is divided into large particles and small particles according to particle size. The large particles are 1–2 mm, and the small particles are 0.25–1 mm. Bovine bone has demonstrated superior clinical presentation and favorable histological results over the years when used alone or in combination with autologous bone or other allografts [10–12]. At the same time, there is no evidence of disease transmission in terms of safety [13–15].
Previous studies have mainly focused on the physical and chemical properties of the graft materials, while relatively few research has been conducted on the effect of the particle size of the material itself on osteogenic. The relative merits of maxillary sinus elevation are unclear, although several studies have compared the clinical and histological outcomes of large and small granular bovine bone.
Based on the facts and background of previous studies, this article conducted a systematic review and meta-analysis to evaluate whether large granular bovine bone can be as effective as small granular bovine bone in maxillary sinus floor elevation. This study aims to provide some reference for future clinical application.
Methods
This article investigated and reported results according to the Cochrane Manual and the Preferred Reporting Project for Systematic Reviews and meta-Analyses (PRISMA) statement. The protocol has been registered in PROSPERO(International Prospective Register of Systematic Reviews) a priori under registration number CRD42022379384.
Search strategies
A comprehensive online search of eligible articles was conducted using PubMed, EMBASE, Cochrane Library, Scopus, and Web of Science. Search strings were created by using Boolean operators specifically combined with the keywords “AND” and “OR”. The search strings were as follows:([“maxillary sinus” OR “sinus”] AND [“floor elevation” OR “lift” OR “floor augmentation” OR “augmentation” OR “floor”]) AND (“xenograft” OR “bovine bone” OR “Bio-Oss” OR “inorganic bovine bone” OR “deproteinized bovine bone matrix”). There were no restrictions on the language of publication or the year of publication. The last search was conducted in February 2023.
Eligibility criteria
Inclusion criteria
All randomized controlled clinical trials conducted in humans were taken into consideration. In addition, there were other aspects need to be considered: Firstly, maxillary sinus lift surgery was required in healthy patients with inadequate bone mass in the maxillary posterior region. Secondly, maxillary sinus lifting was performed using either large (1–2mm) or small (0.25 to 1 mm) Deproteinized Bovine Bone Mineral (DBBM) for bone increment.
Exclusion criteria
At the beginning of this study, all animal trials were excluded. More importantly, this study did not include studies that met several criteria: Firstly, meta-analysis, case reports, proceedings, retrospective and cohort studies, personal communications, and studies without control groups; Secondly, there were no corresponding evaluation index and duplicate studies.
Data extraction
Two authors (XL and SCL) independently completed the literature screening. Eligible references were identified according to inclusion and exclusion criteria. Then data were extracted from the included literature, and the reasons for excluding other literature were recorded. The other authors checked the accuracy of the results.
Quality assessment
Each included study was reviewed separately for risk of bias by XL and SCL authors, and the Cochrane Collaboration tool was used to assess risk of bias in randomized trials. The tool includes seven different domains. All domains and their included issues were evaluated and categorised as low risk, high risk, or representing unclear risk. After determining each domain, an overall estimation of the plausible risk of bias (low, moderate, or high) was performed for each selected study (low risk of bias: all domains were assessed as ‘low risk’; moderate risk of bias: one or more domains were assessed as ‘unclear’; high risk of bias: one or more domains were assessed as ‘high risk’). Differences of opinion between the two authors were resolved by discussion or negotiation with the other authors.The general chart of bias risk was made by Revman 5.4 software.
Outcome measures and data analysis
The primary outcomes of this study were the percentage of connective tissue (CT), the percentage of newly formed bone (NFB), and the percentage of residual xenograft (RX). Authors XL and SCL used the Stata 15.1 (Stata Conpernarn, USA) and Review Manager software 5.4.1 for data synthesis. Weighted mean difference (WMD) and 95% confidence intervals (CI) were calculated to assess the overall efficacy of all included studies. Heterogeneity among studies was quantitatively assessed using the X2-based Q-test and I-squared (I2) statistic. The heterogeneity of the combined study was stronger when P <
< 0.1 and I2
0.1 and I2 >
> 50%, so the random effects model was used, otherwise the fixed-effects model was carried.
50%, so the random effects model was used, otherwise the fixed-effects model was carried.
Results
Search results
This study started with an electronic search through online databases, which produced 149 articles. After removing duplicates, 72 studies were retained by Endnote 20 software. After carefully reading of the full text of the remaining 72 articles and further assessment strictly according to the eligibility criteria, 68 publications were excluded because they did not meet these criteria. The flow chart and reasons for exclusion were shown in Fig. 1. Finally, a total of 4 studies were included in the systematic review.
Study characteristics
The basic characteristics of the four studies included in the meta-analysis were summarized in in Table Table1.1. All the four studies were Randomized Controlled Trials(RCTs). The number of patients in the studies ranged from 20 to 32 (the total number in all studies was 94). The number of implants in the large particle group ranged from 10 to 13 (total =
= 44) and in the small particle group ranged from 10 to 19 (total
44) and in the small particle group ranged from 10 to 19 (total =
= 50).
50).
Table 1
Characteristics of included studies
| Author | Year | Nation | No of patient(T/LP/SP) | No of implants | Patient age(mean) | Sex M/F | RBH (mean  ± ± SD) SD) | Type of surgery | Implant placement | No of biopsies | Follow up period | Main outcomes | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T | LP | SP | M | F | |||||||||||
| Chackartchi | 2011 | Israel | 20 | 10 | 10 | NR | 54.25 (range:46–65) | 6 | 4 | L: 2.45 S: 1.95 | Lateral window | Delayed implantation | 20 | 6–9 month | CT, NFB, RX |
| Tiziano | 2013 | Italy | 22 | 11 | 11 | NR | NR | NR |  < < 5mm 5mm | Lateral window | Delayed implantation | 22 | 6–8 month | CT,NFB,RX | |
| Molon | 2018 | Brazil | 20 | 10 | 10 | 25 | 48.34 ± ± 12.83(range:30–65) 12.83(range:30–65) | 8 | 12 |  < < 5mm 5mm | Lateral window | Delayed implantation | 20 | 8 month | CT,NFB,RX |
| Paksinee | 2022 | Thailand | 32 | 13 | 19 | 32 | L: 56 S: 58.57 | 16 | 16 | L: 3.18 S: 3.33 | Lateral window | Delayed implantation | 32 | 6 month | CT,NFB,RX |
T total, LP large particle DBBM, SP small particle DBBM, RBH residual bone height, NR not report, CT connective tissue(%), NFB newly formed bone(%), RX residual xenograft(%)
Risk of bias
All four articles included in the study were declared to be randomised. All article mentioned allocation except for the study reported by Tiziano (2013). No paper perform blinding except for Chackartchi (2011) and Paksinee (2022). Except for Molon (2018), all included articles contained complete data, and no selective reporting was found. Chackartchi (2011) and Tiziano (2013) were considered to have unclear risk of other bias. Figure 2 presented the methodological quality assessment of the trials included in the review (Table (Table22).
Table 2
Outcome data of included studies
| Author | Year | Percentage of CT | Percentage of NFB | Percentage of RX | |||
|---|---|---|---|---|---|---|---|
| LP | SP | LP | SP | LP | SP | ||
| Chackartchi | 2011 | 39.14 (8.47) | 37.42 (4.15) | 27.14 (3.89) | 28 (6.53) | 33.71 (8.28) | 34.57 (8.08) |
| Tiziano | 2013 | 53.2 (11.5) | 59.6 (9.9) | 26.8 (9.6) | 18.8 ( 4.7) | 20 (9) | 21.7 (10.5) |
| Molon | 2018 | 23.8 (6.16) | 30.4 (8.63) | 36.7 (5.79) | 36.1 (9.6) | 38 (6.92) | 32.4 (8.56) |
| Paksinee | 2022 | 44.36 (26.7) | 66.48 (20.97) | 32.15 (14.04) | 15.99 (14.12) | 23.65 (17.18) | 17.86 (16.42) |
LP large particle DBBM, SP small particle DBBM, CT connective tissue(%), NFB newly formed bone(%), RX residual xenograft(%)
Data are mean(SD)
Quantitative synthesis
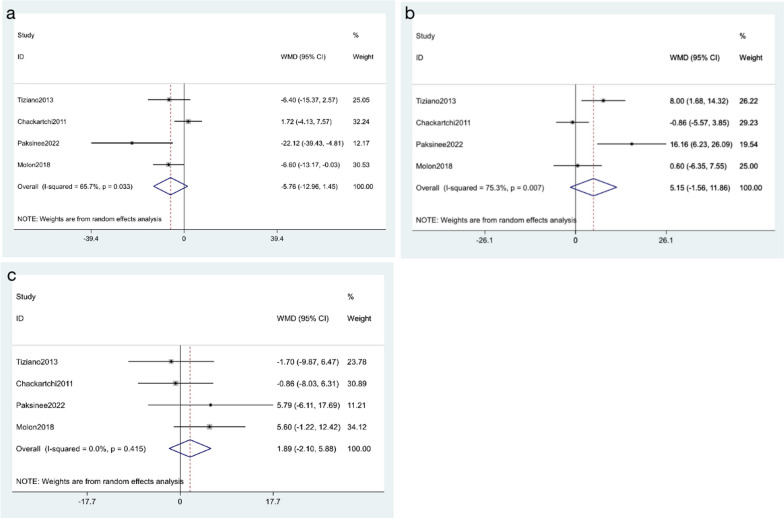
Percentage of connective tissue
All studies reported the percentage of connective tissue after surgery. A total of 94 patients were enrolled, including 44 cases in the small particle size of DBBM group and 50 cases in the large particle size of DBBM group. A random effect model was applied based on the presence of significant heterogeneity (I2 =
= 65.7%, p
65.7%, p =
= 0.033). As shown in Fig. 3a, no significant increase in the percentage of connective tissue was observed among the included findings (WMD
0.033). As shown in Fig. 3a, no significant increase in the percentage of connective tissue was observed among the included findings (WMD =
= − 5.76, 95% CI: − 12.96 to 1.45; p
− 5.76, 95% CI: − 12.96 to 1.45; p =
= 0.117).
0.117).
Percentage of newly formed bone
All studies mentioned the percentage of newly formed bone. There were 94 patients in total, including 44 cases in the small particle size of DBBM group and 50 cases in the large particle size of DBBM group. A random effect model was applied based on a significant heterogeneity (I2 =
= 75.3%, p
75.3%, p =
= 0.007). As shown in Fig. 3b, the results showed that the percentage of newly formed bone was non-significant between the large particle and the small particle (WMD
0.007). As shown in Fig. 3b, the results showed that the percentage of newly formed bone was non-significant between the large particle and the small particle (WMD =
= 5.15,95% CI: − 1.56 to 11.86; p
5.15,95% CI: − 1.56 to 11.86; p =
= 0.132).
0.132).
Percentage of residual xenograft
All studies provided the percentage of residual xenograft. There were 94 patients in total, including 44 cases in the small particle size of DBBM group and 50 cases in the large particle size of DBBM group. A fixed-effects model was applied, due to the I2 =
= 0.0% and p
0.0% and p =
= 0.415. As shown in Fig. 3c, no difference in percentage of residual xenograft was observed between included studie (WMD
0.415. As shown in Fig. 3c, no difference in percentage of residual xenograft was observed between included studie (WMD =
= 1.89, 95% CI: − 2.10 to 5.88; p
1.89, 95% CI: − 2.10 to 5.88; p =
= 0.353).
0.353).
Discussion
This study compared the histological outcomes of DBBM with two different particle sizes during maxillary sinus floor elevation. It was concluded that there was no difference between the two DBBM preparations in histology and maxillary sinus floor lift by searching, screening and analyzing the previous literature. The two groups were similar in terms of percentage of connective tissue, the percentage of newly formed bone, and percentage of residual xenograft.
Multiple bone substitutes can be used in maxillary sinus elevation. According to the source of the materials, they can be divided into four categories: autografts, allografts, xenografts, and synthetic bone substitutes [16].
Autografts itself has osteogenic potential, and can be used as a scaffold for osteogenesis to play a role in osteoconduction and osteoinduction. The latter three types of bone substitutes lack osteoblastic cells, which mainly provide scaffolds for the formation of new bone and play the roles of osteoconduction and osteoinduction.
For maxillary sinus bone grafting with autogenous bone, a second surgical area needs to be opened to obtain sufficient autogenous bone. The opening of the second operative area not only increased the surgical trauma and operation time, but also the discomfort of the patients during and after the operation increased. Many patients have difficulty accepting autogenous bone grafting. Autogenous bone is rarely used in the treatment of maxillary sinus bone grafting [17, 18].
Allografts can be divided into three types: fresh/frozen bone, freeze-dried bone and demineralized freeze-dried bone. Among them, fresh/frozen bone had the greatest osteoinductive and osteoconductive potential. However, due to the risk of disease transmission, it is no longer in clinical application.
Synthetic bone substitutes refer to bioceramics or polymers made from natural materials or synthetic materials. Different synthetic bone substitutes have different physical and chemical properties and can be degraded in vivo or remain stable for a long time. However, as a scaffold material, it has no osteogenesis and osteoinducibility.
Xenogeneic bone refers to the bone graft substitutes derived from different species of biological individuals. The source is generally cattle, pigs, horses and other animals. The currently dominant product in the clinic is DBBM. DBBM is derived from natural calf bone and is a porous carbonate apatite crystal with bone conduction properties. Its physical and chemical properties are very similar to the structure of human bone tissue, and it retains the porous structure and trabecular bone of natural bone. It can provide a scaffold for the expansion of osteoblasts, and ensure the stability of blood clots and the regeneration of blood vessels. In the literature related to maxillary sinus elevation, DBBM as a bone augmentation material has involved the most clinical cases and the most complete data [19–22].
DBBM was a well-documented bone grafting material for maxillary sinus lift [23–26]. The most widely utilized commercial product in clinical practice was Bio-Oss with diameters ranging from 0.25 to 1 mm and 1–2 mm, respectively[27–29]. Bio-Oss was widely used because its characteristics, including its crystallinity and physicochemical properties, were very similar to those of human cancellous bone. DBBM acted as a scaffold and matrix to promote the migration of osteoblasts from the maxillary sinus wall to the graft material, and then increasing the ability of new bone formation [30–32]. There have been a number of studies using DBBM for maxillary sinus elevation and to evaluate the performance of bone healing from a histological perspective, but the application of DBBM with different particle sizes and maxillary sinus elevation has been limited and the results have been confusing. This is because maxillary sinus elevation with different sizes of DBBM results in completely different bone healing in only a few studies [33–36]. It was important to note that only four randomized controlled clinical trials have investigated the use of DBBM and maxillary fundus in different sizes.
A total of four literatures were included in this study. Chackartchi et al. [33] used large and small bovine bones separately in maxillary sinus floor lifting surgery. After a period of 6–9 months, they extracted bone samples from patients and found that both large and small bovine bones showed similar clinical and histological results. When comparing the application of large and small granular bovine bones in maxillary sinus floor elevation, Testori et al. [34] found that the large granular bovine bones produced more new bone than the small granular bovine bones in terms of histomorphometric results at 6–8 months after surgery. In addition, Molon et al. [35] conducted histomorphometric studies to stain the protein expression of osteocalcin, vascular endothelial growth factor and tartrate-resistant acid. The results showed no statistically significant difference between the large and the small, which suggesting that the size of DBBM did not affect the osteogenic effect during maxillary sinus elevation. Through a randomized controlled study, Kamolratanakul et al. [36] found that the application of large particles of DBBM in maxillary sinus floor elevation could induce more angiogenic expression and obtain more new bone. However, the clinical outcomes were similar in both groups. The reasons for their disagreement are multifaceted and the result of various factors, such as sample size, sample selection, population differences, differences in surgical techniques, and the way, location or time of collecting specimens.
In previous studies, bone specimens were obtained in different ways. Testori et al. [34] and Molon et al. [35] took bone specimens from the buccal side of the lateral wall of the maxillary sinus for analysis. On the contrary, Chackartchi et al. [33] and Kamolratanakul et al. [36] used a hollow bone drill to extract bone samples from the alveolar crest (the implant site) for analysis. The presence of partial autogenous cortical or cancellous bone in the bone specimens may affect the histological results, but this article believed that both methods of bone extraction were feasible, and the fact that autogenous bone may be included was unavoidable. Other limitations included the inability to control the size and morphology of the maxillary sinus itself and the size of its bulge. However, as the target of our evaluation was a complex multi-factor biological process, the experimental method and evaluation indicators were more important.
In modern medicine, immunohistochemical analysis can be independent of morphological observation, which can provide a broader overview of biological processes and directions of progress. In a previous report, ABB particles did not affect the expression of genes associated with bone remodeling and inflammation after a 6-month healing period. Histological evidence also suggested that DBBM particles were replaced by new bone formation and did not affect bone healing [37]. Similarly, Pereira et al. performed maxillary floor elevation 6 months after surgery using autogenous bone and DBBM, and they found similar histopathological and immunohistochemical evaluations of RUNX2 and vascular endothelial growth factor (VEGF) [38]. These data suggested that bone remodeling and neovascularization occur at least 6 months after surgery when DBBM was used for external maxillary sinus elevation, which explained why the experimental period of maxillary sinus lift was at least 6 months. Moreover, the use of DBBM did not inhibit the expression of genes related to bone remodeling induction. It is worth noting that the four studies included in this paper also had an observation period of more than 6 months, which revealed the reliability of DBBM in clinical application.
Although both large and small particles used for grafting maxillary sinus lifting after surgery have similar histological results, they have significant advantages and disadvantages from the perspective of clinical application. For large particles, it can safely reduce the amount of biomaterial filling the maxillary sinus without affecting the graft volume, so more space could be obtained for implantation. Another important aspect was that the surgical time can also be shortened due to the reduction in the size of the graft. Conversely, the use of large particles also increased the amount of void space in the whole area, thus in turn increased the risk of infection. For small particles, its application allowed for a better grasp of the space and volume of bone graft, based on the size of the maxillary sinus, the number of implants needed, the anatomy of the maxillary sinus and other factors. On the other hand, the application of DBBM with small particles for suitable patients can not only reduce the use of materials, but also reduce the consumption of patients while achieving the same results as the large particles.
When it comes to implant success rates, even in cases of perfect bone condition, objective factors such as general health must be considered, not to mention the complexities involved in maxillary sinus elevation. The implant stability is regarded as a crucial factor for successful osseointegration and serves as one of the most commonly employed indicators to predict implant stability. There are numerous factors that impact the stability of implants, including but not limited to overall physical health, bone density and quantity, implant surface design, among others. There is little evidence suggests that the utilization of various bone substitutes has impact on implant stability [39–42].
Even if the stability of the implant is not significantly affected by the bone graft material, it does not imply that the bone substitutes are insignificant. No matter which type of bone graft material is used to support the maxillary sinus mucosa, it can effectively maintain the stability of the osteogenic space, particularly when utilizing a small diameter implant tip. Additionally, the bone graft material can disperse pressure on the maxillary sinus mucosa, preventing secondary infections caused by maxillary sinus perforation or collapse of the maxillary sinus mucosa that could reduce osteogenic space.
In conclusion, this study systematically reviewed the previous literature and found that both large-particle DBBM and small-particle DBBM could achieve similar histological results in the following three aspects during maxillary sinus elevation: connective tissue, newly formed bone, and residual xenograft. It can draw a conclusion from the above that the large granular bovine bone and the small granular bovine bone were equally effective in maxillary sinus elevation, which provided a valuable reference for clinical application.It is difficult to make conclusion from limited evidence from four studies. More clinical evidence was needed.
Author contributions
XL and S-cL have equal contributions and share the first author. XL and S-yD designed the study and supervised the overall project; XL and S-cL participated in collecting data; XL, S-cL and S-yD participated in data collecting and analysis; XL, S-cL and S-yD provided the statistical analysis and wrote the manuscript.
Availability of data
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Declarations
This is a systematic review and meta-analysis, ethics approval and consent to participate are not applicable.
Not applicable. This study does not involve human participants.
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xin Li and Shi-chen Lin have contributed equally to this work.
References
Articles from International Journal of Implant Dentistry are provided here courtesy of Springer
Full text links
Read article at publisher's site: https://doi.org/10.1186/s40729-023-00502-1
Read article for free, from open access legal sources, via Unpaywall:
https://journalimplantdent.springeropen.com/counter/pdf/10.1186/s40729-023-00502-1
Citations & impact
Impact metrics
Article citations
Comprehensive sinus contour classification and its characteristics from radiographic examination: a cross-sectional study.
BMC Oral Health, 24(1):1021, 30 Aug 2024
Cited by: 0 articles | PMID: 39215296 | PMCID: PMC11365276
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The impact of deproteinized bovine bone particle size on histological and clinical bone healing outcomes in the augmented sinus: A randomized controlled clinical trial.
Clin Implant Dent Relat Res, 24(3):361-371, 23 Mar 2022
Cited by: 5 articles | PMID: 35320619
Implant stability after sinus floor augmentation with deproteinized bovine bone mineral particles of different sizes: a prospective, randomized and controlled split-mouth clinical trial.
Int J Oral Maxillofac Surg, 45(12):1556-1563, 28 Sep 2016
Cited by: 15 articles | PMID: 27692642
Clinical and Histological Outcomes of Maxillary Sinus Floor Augmentation With Synthetic Bone Substitutes for Dental Implant Treatment: A Meta-Analysis.
J Oral Implantol, 48(2):158-167, 01 Apr 2022
Cited by: 0 articles | PMID: 33465775
Review
Xenograft materials in maxillary sinus floor elevation surgery: a systematic review with network meta-analyses.
Br J Oral Maxillofac Surg, 59(7):742-751, 23 Feb 2021
Cited by: 13 articles | PMID: 34120778
Review