Abstract
Objectives
To characterize respiratory culture practices for mechanically ventilated patients, and to identify drivers of culture use and potential barriers to changing practices across PICUs.Design
Cross-sectional survey conducted May 2021-January 2022.Setting
Sixteen academic pediatric hospitals across the United States participating in the BrighT STAR Collaborative.Subjects
Pediatric critical care medicine physicians, advanced practice providers, respiratory therapists, and nurses.Interventions
None.Measurements and main results
We summarized the proportion of positive responses for each question within a hospital and calculated the median proportion and IQR across hospitals. We correlated responses with culture rates and compared responses by role. Sixteen invited institutions participated (100%). Five hundred sixty-eight of 1,301 (44%) e-mailed individuals completed the survey (median hospital response rate 60%). Saline lavage was common, but no PICUs had a standardized approach. There was the highest variability in perceived likelihood (median, IQR) to obtain cultures for isolated fever (49%, 38-61%), isolated laboratory changes (49%, 38-57%), fever and laboratory changes without respiratory symptoms (68%, 54-79%), isolated change in secretion characteristics (67%, 54-78%), and isolated increased secretions (55%, 40-65%). Respiratory cultures were likely to be obtained as a "pan culture" (75%, 70-86%). There was a significant correlation between higher culture rates and likelihood to obtain cultures for isolated fever, persistent fever, isolated hypotension, fever, and laboratory changes without respiratory symptoms, and "pan cultures." Respondents across hospitals would find clinical decision support (CDS) helpful (79%) and thought that CDS would help align ICU and/or consulting teams (82%). Anticipated barriers to change included reluctance to change (70%), opinion of consultants (64%), and concern for missing a diagnosis of ventilator-associated infections (62%).Conclusions
Respiratory culture collection and ordering practices were inconsistent, revealing opportunities for diagnostic stewardship. CDS would be generally well received; however, anticipated conceptual and psychologic barriers to change must be considered.Free full text

A Survey of PICU Clinician Practices and Perceptions regarding Respiratory Cultures in the Evaluation of Ventilator-Associated Infections in the BrighT STAR Collaborative
Abstract
OBJECTIVES:
To characterize respiratory culture practices for mechanically ventilated patients, and to identify drivers of culture use and potential barriers to changing practices across PICUs.
DESIGN:
Cross-sectional survey conducted May 2021–January 2022.
SETTING:
Sixteen academic pediatric hospitals across the United States participating in the BrighT STAR Collaborative.
Subjects:
Pediatric critical care medicine physicians, advanced practice providers, respiratory therapists, and nurses.
INTERVENTIONS:
None.
MEASUREMENTS AND MAIN RESULTS:
We summarized the proportion of positive responses for each question within a hospital and calculated the median proportion and IQR across hospitals. We correlated responses with culture rates and compared responses by role. Sixteen invited institutions participated (100%). Five hundred sixty-eight of 1,301 (44%) e-mailed individuals completed the survey (median hospital response rate 60%). Saline lavage was common, but no PICUs had a standardized approach. There was the highest variability in perceived likelihood (median, IQR) to obtain cultures for isolated fever (49%, 38–61%), isolated laboratory changes (49%, 38–57%), fever and laboratory changes without respiratory symptoms (68%, 54–79%), isolated change in secretion characteristics (67%, 54–78%), and isolated increased secretions (55%, 40–65%). Respiratory cultures were likely to be obtained as a “pan culture” (75%, 70–86%). There was a significant correlation between higher culture rates and likelihood to obtain cultures for isolated fever, persistent fever, isolated hypotension, fever, and laboratory changes without respiratory symptoms, and “pan cultures.” Respondents across hospitals would find clinical decision support (CDS) helpful (79%) and thought that CDS would help align ICU and/or consulting teams (82%). Anticipated barriers to change included reluctance to change (70%), opinion of consultants (64%), and concern for missing a diagnosis of ventilator-associated infections (62%).
CONCLUSIONS:
Respiratory culture collection and ordering practices were inconsistent, revealing opportunities for diagnostic stewardship. CDS would be generally well received; however, anticipated conceptual and psychologic barriers to change must be considered.
Ventilator-associated infections (VAIs), including bacterial tracheobronchitis or ventilator-associated pneumonia in patients supported by artificial airways, are clinically significant complications and a common indication for antibiotic treatment (1, 2). Respiratory cultures, (i.e., endotracheal aspirate cultures, sputum cultures, or tracheal aspirate cultures) are often obtained in evaluation of VAI and national guidelines support noninvasive cultures primarily because of lower cost and ease of sampling compared with bronchoalveolar lavage (3). Although these guidelines provide treatment parameters, they do not offer concrete recommendations on when or how to obtain respiratory cultures to optimize diagnostic utility. Interestingly, rate of respiratory cultures varies 10-fold across PICUs in the United States (4), and single-center studies demonstrate heterogeneity of clinical practices within hospitals (5).
The respiratory tract is not sterile, presenting a major diagnostic limitation of respiratory cultures. Positive Gram stain and culture results can reflect bacteria colonizing airways and/or normal flora of the upper respiratory tract and not infection (6–8). Furthermore, differences in specimen collection and laboratory processing impact the culture results and make interpretation of results challenging (6, 9). Clinicians often are unaware of testing limitations and can misinterpret culture data. Studies in adult and pediatric settings show that patients with positive respiratory cultures are more likely to receive antibiotics (10, 11), and hospitals with higher culture rates have higher associated antibiotic utilization (4). The lack of clear indications for respiratory culture use, inconsistent culture collection methods, and associated antibiotic overuse can lead to unintended patient harm from adverse drug effects, increasing antimicrobial resistance and associated complications (12–15).
Some centers have explored local drivers of excessive culturing and have implemented successful diagnostic stewardship initiatives to reduce overuse and standardize respiratory culturing practices (16, 17). Nationally, the variability in respiratory culture practices across hospitals and provider types is unknown. We conducted a multicenter survey of healthcare workers (HCWs) across a geographically diverse group of U.S. PICUs. The objectives of the study were to assess respiratory culture ordering and specimen collection practices, determine HCW perceptions and anticipated barriers to changing practices, and identify patterns associated with higher or lower culture rates to inform diagnostic stewardship efforts.
METHODS
Setting and Participants
Between May 2021 and January 2022, we conducted an anonymous, cross-sectional electronic survey (Qualtrics, Provo, UT) of PICU attending and fellow physicians, advance practice providers (APPs), nurses, and respiratory therapists (RTs) at 16 hospitals participating in the BrighT STAR collaborative; all are academic, tertiary/quaternary centers. BrighT STAR is a quality improvement collaborative of U.S. PICUs including critical care and infectious disease clinicians implementing diagnostic stewardship of cultures. The included PICUs care for critically ill children from birth through early adulthood, typically through age 21 years. In 2021, site leads provided descriptions of their unit and retrospectively obtained respiratory culture rates per 1,000 ventilator-days (18) from January 2019 to December 2020.
Ethical Considerations
Johns Hopkins was the coordinating center. The Johns Hopkins Institutional Review Board (IRB) approved this study as exempt human subjects research with a waiver of informed consent (IRB00235694, January 10, 2020, “Qualitative Study to Identify Barriers of Implementing Respiratory Culture Stewardship to Reduce Antibiotic Use in Critically Ill Children”). Each hospital followed any local IRB protocols for participation. The article follows the Consensus-based checklist for reporting survey studies (19).
Survey Development
The survey questions were developed following the Systems Engineering Initiative for Patient Safety 2.0 model that considers work systems (persons, tasks, internal environment, tools, and organization) involved in the work process that leads to the clinical outcome of obtaining a respiratory culture (20). The survey contained 72 items within five sections (Supplemental Figure 1, http://links.lww.com/PCC/C442): respiratory culture specimen collection, clinical indications for respiratory cultures, perceptions about respiratory cultures in general, perceptions about respiratory cultures practices in the participant’s particular PICU, and anticipated barriers to changing practices. The survey was reviewed by experts in pediatric critical care, infectious diseases, respiratory therapy, quality improvement, and human factors engineering, and then piloted with 32 HCWs from Johns Hopkins All Children’s Hospital PICU, and modified based on feedback.
Survey Administration
Project lead physicians from each hospital were provided a recruitment e-mail and anonymous link to the electronic survey that they distributed directly or via e-mail lists to HCWs in their PICU. To overcome response heterogeneity and capture patterns, we aimed for greater than or equal to 15 responses per site targeting at least five responses from each category of nurses, RTs, or ordering clinicians (i.e., physicians and advanced practice providers [AAPs]). Although Qualtrics cannot distinguish duplicated responses from a different device, there was no incentive and we did not observe duplicative responses.
Analysis
We excluded responses from HCWs not primarily working in the PICU (e.g., consultants) or if data were missing beyond demographic questions. Likert-scale questions were dichotomized into positive responses (i.e., agree and strongly agree) or negative responses (i.e., neutral, disagree and strongly disagree). We aimed to assess pattern differences across hospitals. We summarized responses from each hospital by computing the proportion of respondents selecting a response for each question, and then calculated the median and IQR of the proportion of responses with positive responses to a given question across the hospitals. In the event of missing responses, the denominators for each question reflected the total number of responses per question. Additionally, because ordering clinicians (i.e., medical doctors and AAPs) may have different perspectives from bedside staff contributing to response heterogeneity, we compared responses from clinicians to responses from nurses and RTs using logistic binomial regression assuming an exchangeable correlation for responses within a hospital and robust variance estimate. We considered notable differences if p value less than 0.05 and odds ratios (ORs) were greater than 1.5 or less than 1/1.5.
The association between the log-transformed culture rates and proportion of positive survey responses was quantified using the Pearson correlation coefficient for the 14 units that provided culture rates; p values less than 0.05 were considered significant. Statistical analyses were performed using Stata/IC (version 15; StataCorp, College Station, TX) and R (version R 4.2.2; R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
All 16 (100%) invited institutions participated. After excluding 84 responses (two consultants and 82 insufficient completion), a total of 551 of 1,266 e-mailed HCWs completed the survey (44%, range of 13–107 responses per site). The median site response rate was 60% (range 17–83%). By role, there were 192 physicians (35%), 173 nurses (31%), 134 RTs (24%), and 52 APPs (9%). Respondents had worked in the PICU setting for 0–5 (n = 227, 41%), 6–10 (n = 146, 27%), 11–20 (n = 113, 21%), or greater than 20 years (n = 65, 12%).
PICU characteristics are presented in Table Table11. Across the 16 units, there was a median number of 34 beds (IQR 25–39) with 1,949 annual admissions (1,492–2,046), and 20% of admissions received invasive mechanical ventilation. Thirty-eight percent had cardiac surgery patients, 88% provided extracorporeal membrane oxygenation, and 75% had stem cell transplant and solid organ transplant patients. A few units had previously provided education (n = 3) or guidance (n = 2) for respiratory culture practices. There was variability in laboratory processing practices with 69% having some type of laboratory rejection criteria (specimens consistent with saliva not worked-up [n = 7], specimens repeated within 0–3 days not worked-up [n = 4], or not repeating antibiotic susceptibility testing within 3–7 days [n = 6]).
TABLE 1.
Participating Site Characteristics (n = 16)
| Size of Unit | Median (IQR) |
|---|---|
| No. of ICU beds | 34 (25–39) |
| Annual Admissions 2019 | 1,949 (1,452–2,046) |
| Annual Admissions with ventilation 2019 | 382 (296–614) |
| Antibiotic stewardship/infection control support | No. Sites (%) |
 Conduct Surveillance for VAE or VAP Conduct Surveillance for VAE or VAP | 9 (56)a |
 Antimicrobial stewardship team supports ICU Antimicrobial stewardship team supports ICU | 16 (100) |
 Treatment guidelines for ventilator-associated infections Treatment guidelines for ventilator-associated infections | 4 (25) |
 Prior education for indications of respiratory cultures in unit Prior education for indications of respiratory cultures in unit | 3 (19) |
 Prior algorithm/guidelines for respiratory cultures in unit Prior algorithm/guidelines for respiratory cultures in unit | 2 (13) |
 ICU participates in national quality improvement collaboratives ICU participates in national quality improvement collaboratives | 12 (75) |
 IPSO IPSO | 8 (50) |
 SPS SPS | 10 (63) |
| Clinical staff and training programs | |
 Pediatric residents Pediatric residents | 15 (94) |
 Emergency medicine residents Emergency medicine residents | 14 (88) |
 Pediatric critical care fellows Pediatric critical care fellows | 13 (81) |
 Critical care attendings have overnight in-house call Critical care attendings have overnight in-house call | 16 (100) |
 Advanced practice providers (NPs and PAs) Advanced practice providers (NPs and PAs) | 16 (100) |
 Respiratory therapists specifically assigned to unit Respiratory therapists specifically assigned to unit | 11 (69) |
 Open unit Open unit | 6 (38) |
| Patient populations | |
 Medical patients Medical patients | 16 (100) |
 Surgical patients Surgical patients | 16 (100) |
 Neurosurgical patients Neurosurgical patients | 15 (94) |
 Cardiac surgery patients Cardiac surgery patients | 6 (38) |
 Nonconventional ventilation modes Nonconventional ventilation modes | 15 (94) |
 Extracorporeal membrane oxygenation Extracorporeal membrane oxygenation | 14 (88) |
 Hematopoietic stem cell transplants Hematopoietic stem cell transplants | 12 (75) |
 Solid organ transplants Solid organ transplants | 12 (75) |
 Congenital diaphragmatic hernia Congenital diaphragmatic hernia | 6 (38) |
| Microbiology laboratory restrictions of respiratory cultures | |
 No rejection criteria or restrictions No rejection criteria or restrictions | 5 (31) |
 Laboratory has some type of stewardship criteria Laboratory has some type of stewardship criteria | 11 (69) |
  If specimens are consistent with saliva, no further work-up If specimens are consistent with saliva, no further work-up | 7 (44) |
  Rejects specimens repeated in certain number of days Rejects specimens repeated in certain number of days | 4 (25)b |
  Do not repeat susceptibility testing within certain number of days Do not repeat susceptibility testing within certain number of days | 6 (38)c |
IPSO = improving pediatric sepsis outcomes, NP = nurse practitioner, PA = physician assistant, SPS = solutions for patient safety, VAE = ventilator-associated event, VAP = ventilator-associated pneumonia.
Specimen Collection
Supplemental Table 1 (http://links.lww.com/PCC/C442) has a summary of all survey question responses. Respiratory culture specimens were often collected by RTs (median proportion 99%) or by nurses (51%). For patients with endotracheal tubes (ETTs), specimens often were collected via inline endotracheal aspirate (82%) followed by open suctioning (23%). For patients with tracheostomies, specimens often were collected via inline endotracheal aspirate (71%) followed by open suctioning (40%). In one PICU, respondents often used bronchial brush specimens (77% for ETTs and 49% for tracheostomies). Sites reported using saline lavage when collecting specimens often (39%) or sometimes (41%). However, only 15 total respondents from seven sites (4%) indicated that a standard volume of instilled saline (range 0.5–10 mL) without internal consistency.
mL) without internal consistency.
Ordering Indications
Attendings (86%), fellow physicians (82%), and APPs (69%) often made the decision to order a respiratory culture, whereas resident physicians (43%), nurses (5%), and RTs (5%) less often made the decision. The top most common clinical indications for obtaining respiratory cultures were changes in the description of secretions (33%), new fever (30%), persistent fever (14%), and increased ventilator/oxygen requirement (13%). Figure Figure11 presents the perceived likelihood to obtain cultures for different clinical indications. Respondents indicated cultures were most likely to be obtained for patients with new fever and increased ventilator/oxygen support (90%), new fever and increased secretions without increased ventilatory support (85%), concurrent with blood and urine cultures as a “pan culture” (76%), request from consulting service (76%), and persistent fever without other symptoms (72%). Across hospitals, the scenarios with the most variability in likelihood to obtain cultures were fever with inflammatory laboratory changes (e.g., elevated WBC or C-reactive protein) (median 67%, IQR 54–79%), isolated change in color or thickness of secretions (67%, 54–78%), isolated increase in secretions (53%, 40–62%), laboratory changes without respiratory changes (49%, 38–57%), and isolated new fever (48%, 38–59%).
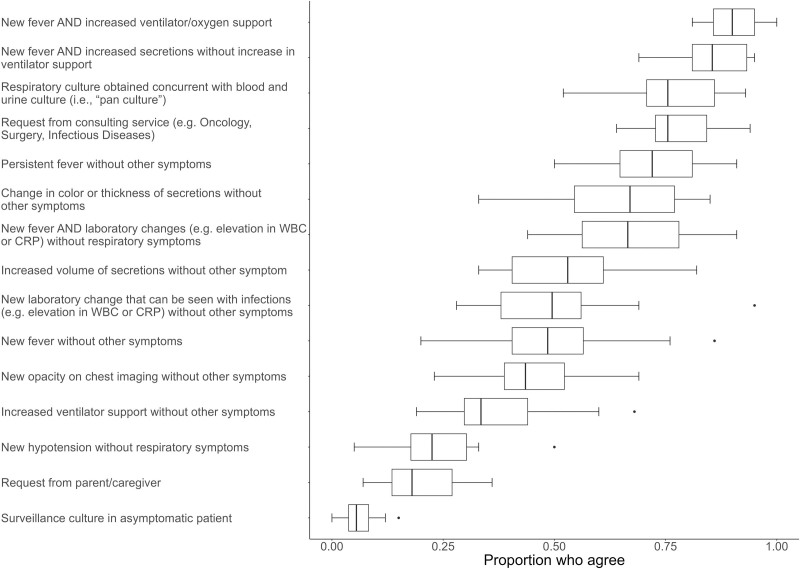
Survey responses: likelihood that respiratory cultures would be obtained for different clinical indications. Box and whiskers plot showing the proportion of respondents across sites who strongly agree or agree that cultures are likely to be obtained in the clinical indications shown on the y-axis. The box indicates the median and IQR, the whiskers indicates the next value within 1.5× IQR, and the points show the outliers.
Perceptions About Respiratory Culture Practices
Figure Figure22 presents the agreement with statements about respiratory cultures. The majority of respondents felt confident about indications to obtain respiratory cultures and that cultures would be helpful to guide antibiotic therapy (80%). Most were aware that medication changes may influence secretion characteristics (84%). Nearly half of respondents did not anticipate that cultures from endotracheal tubes are likely to grow bacteria (42%). Less than one third believed cultures were always indicated for ventilated patients with a new fever (27%) or increased secretions (28%).
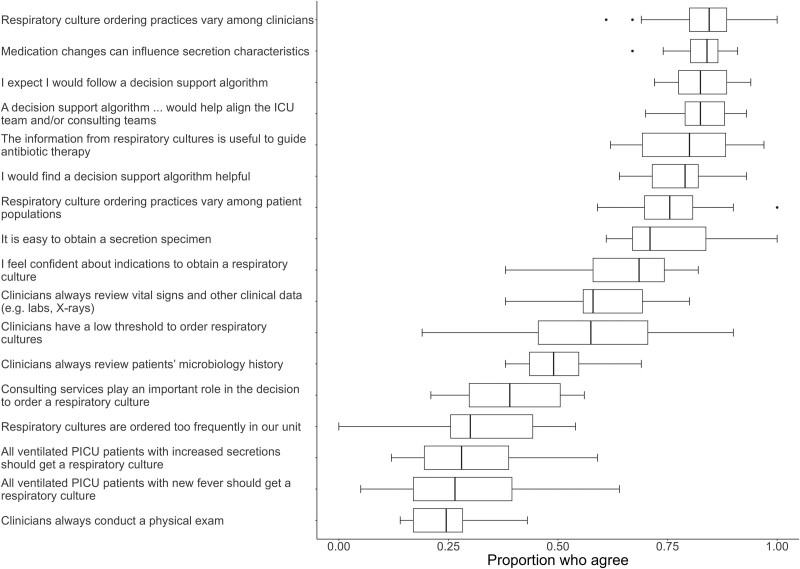
Survey responses: healthcare worker attitudes and perceptions about respiratory culture practices in general and practices in their PICU. Box and whiskers plot showing the proportion of respondents across sites who strongly agree or agree with statements about respiratory cultures in general and respiratory culture practices in their PICU. The box indicates the median and IQR, the whiskers indicates the next value within 1.5× IQR, and the points show the outliers.
Regarding perceptions of their local PICU practices, a median of 24% of respondents perceived clinicians were likely to conduct a physical examination, 49% likely to review the patient’s microbiology history, and 58% likely to review other clinical data prior to ordering a respiratory culture. Only 30% perceived their unit obtained cultures too frequently. Most respondents agreed they would find clinical decision support (CDS) helpful (79%), anticipated they would follow CDS (82%), and that CDS would align the ICU team and consulting teams (82%).
Barriers to Changing Practices
Respondents anticipated the most likely barriers to changing practices would be reluctance to change practices (70%), differing opinions of consulting services (64%), and clinician concern for missing a diagnosis of VAI (62%) (Fig. Fig.33). Less likely barriers included insufficient evidence that respiratory cultures could be reduced safely, and workflow challenges, such as patient workload, conducting physical examinations, or reviewing clinical data.
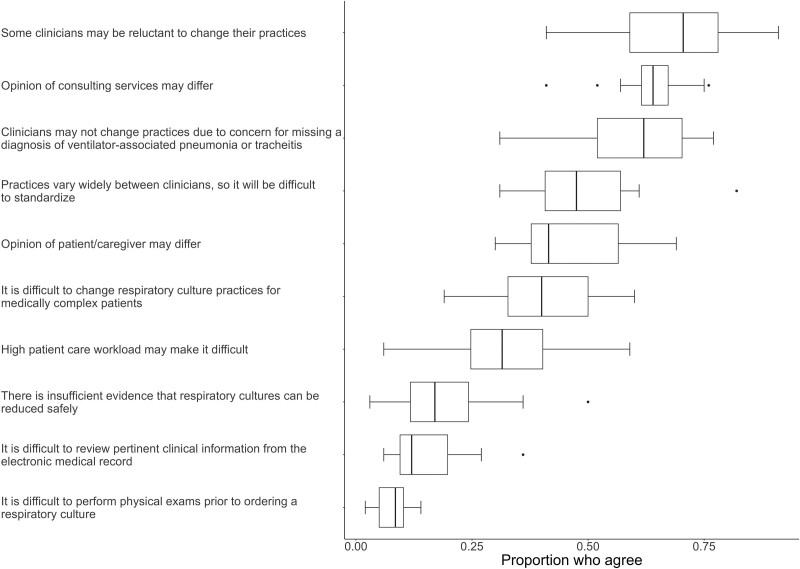
Survey results: anticipated barriers to changing respiratory culture practices. Box and whiskers plot showing the proportion of respondents across sites who strongly agree or agree with potential barriers to changing respiratory culture practices in their PICU. The box indicates the median and IQR, the whiskers indicates the next value within 1.5× IQR, and the points show the outliers.
Differences in Responses by Role
Supplemental Table 3 (http://links.lww.com/PCC/C442) presents evaluation of responses from clinicians compared with nurses and RTs. Clinicians were more likely to report the top indication for cultures as isolated fever (OR 1.78) rather than change in secretions (OR 0.33). Clinicians were less likely to report cultures are obtained for increased volume of secretions (OR 0.47), change in color of secretions (OR 0.29), or new laboratory changes without other symptoms (OR 0.34). Clinicians were less likely to think all ventilated patients with a new fever (OR 0.38) or new increased secretions (OR 0.23) should be cultured or that information from cultures is useful to guide antibiotic therapy (OR 0.42). Clinicians were much more likely to think cultures would grow bacteria (OR 6.81), that respiratory cultures are ordered too frequently (OR 7.29), and that there was a low threshold to obtain cultures in their unit (OR 3.05). Clinicians were more likely to consider that practices vary among clinicians (OR 4.78) and among patient populations (OR 3.60). Overall, clinicians anticipated more barriers to change including reluctance to change (OR 2.72), concern for missing a diagnosis of VAI (OR 3.73), widely varying practices and difficulty to standardize (OR 1.9), insufficient evidence cultures can be reduced safely (OR 4.3), and difficulty changing practices for medically complex patients (OR 3.18). Responses did not differ for the other barriers.
Correlation Between Culture Rates and Perceptions
The median site respiratory culture rate was 9.5 cultures/100 ventilator-days ranging from 1.95 to 16.25 (Supplemental Figure 2, http://links.lww.com/PCC/C442). Statistically significant correlations between proportions of responses and the sites’ culture rates are presented in Table Table22 (complete results in Supplemental Table 2, http://links.lww.com/PCC/C442). Respondents from sites with higher culture rates were more likely to report obtaining cultures for isolated fever, persistent fever, fever with change in secretions, pan cultures, fever with laboratory changes, and isolated hypotension. There was a negative correlation between culture rates and the proportion of respondents who reported that CDS tools are helpful or that CDS would help align the medical team. None of the PICU characteristics correlated with respiratory culture rates.
TABLE 2.
Significant Correlations Between Survey-Reported Perception of Practices and Respiratory Culture Rates per 100 Ventilator-Days
| Variable | Correlation Coefficient | p |
|---|---|---|
| Persistent fever without other symptoms | 0.77 | 0.001 |
| New fever AND increased secretions without increase in ventilatory support | 0.72 | 0.003 |
| New fever without other symptoms | 0.71 | 0.004 |
| New fever AND laboratory changes (e.g., elevation in WBC or C-reactive protein) without respiratory symptoms | 0.63 | 0.02 |
| Respiratory culture obtained concurrent with blood and urine culture (i.e., “pan culture”) | 0.62 | 0.02 |
| New hypotension without respiratory symptoms | 0.61 | 0.02 |
| I would find a decision support algorithm helpful | −0.66 | 0.01 |
| A decision support algorithm... would help align the ICU team and/or consulting teams | −0.57 | 0.04 |
Significant correlations with respiratory culture rates. All other responses did not have a strong or significant correlation with culture rates. The full list of correlation values is presented in Supplemental Table 2, http://links.lww.com/PCC/C442.
DISCUSSION
In this multicenter survey of PICU HCW perceptions about respiratory culture practices, we identified variations in reported specimen collection practices and indications to obtain cultures across hospitals. Importantly, participating hospitals, similar to national patterns, had disparate respiratory culture rates (4). Respiratory cultures were consistently perceived to be obtained for fever and increased ventilatory needs, or for fever and secretion changes. Obtaining cultures for isolated fever and as part of a pan culture concurrent with urine and blood cultures was also common at some institutions. Perceived indications to order cultures that varied the most across hospitals correlated with higher respiratory culture rates, in particular, persistent fever without respiratory changes, fever, and laboratory changes without respiratory symptoms and pan cultures. These correlations strengthen in a sensitivity analysis limited to clinicians’ responses (data not shown). These associations support the concept that culture rates may decline if respiratory cultures were deferred in patients without respiratory tract symptoms.
The method of collecting respiratory secretion specimens impacts culture results and thus interpretation. Saline lavage was a common practice, but no centers reported a specific protocol regarding instilled saline volume. Notably, respiratory therapy guidance does not recommend saline lavage prior to suctioning as it may be harmful to patients without benefit (21–23). The addition of saline also dilutes secretions impacting cell counts, Gram-stain interpretation, and bacterial concentrations in cultures. Inline endotracheal aspirate or open suction from tracheostomies was the most common sampling approach, but this survey did not clarify if HCWs routinely use new vs. existing inline suction catheters. Similar to prior reports, we identified inconsistency in microbiology laboratory sample quality rejection criteria and variation in evaluation of repeat samples submitted within a certain number of days (9). These findings support a need for optimized secretion sampling and processing techniques.
Single-center studies have described within-hospital variability of respiratory culture utilization (5, 17, 24–27). Most survey respondents perceived variability in indications to obtain respiratory cultures between different clinicians and patient populations within their PICU illustrating across and within-site variability in respiratory cultures. Interestingly, clinicians were more likely to perceive variability and overuse compared with nurses and RTs. These survey results suggest there may be tension or an incongruency between clinical knowledge and practice habits as a contributing factor to practice variability. Although many HCWS reported confidence in indications for cultures, many indicated that cultures are likely to be obtained for isolated change in secretions, isolated fever, or pan cultures while reporting that not all ventilated patients with new change in secretions or new fever without associated symptoms should get a respiratory culture. Among PICUs with higher rates of use, there was not a consistent perception that cultures were obtained too frequently. These patterns suggest that PICU clinicians and/or individual units may have developed practice habits that are normalized but not optimized within their setting. Building on prior studies, our survey demonstrates a lack of consistency in respiratory culture practices across hospitals and an opportunity to standardize clinical practice and management of VAI through the development of practice recommendations.
Identification and engagement of key stakeholders are essential components of diagnostic stewardship programs. The survey identified RTs as the most likely to obtain specimens, followed by nurses; however, neither group felt that they were typically involved in the decision to obtain cultures. Nurses and RTs were less likely to think respiratory cultures would have bacterial growth, suggesting opportunities to fill knowledge gaps that respiratory secretions are likely to grow bacteria (6, 8, 28). Importantly, conducting physical examinations and reviewing the clinical data prior to obtaining a new culture was not perceived to be routine, but reassuringly HCWs did not perceive these steps as barriers.
Centers interested in implementing CDS to improve respiratory culture practices may consider the anticipated barriers highlighted in this survey. Encouragingly, clinical data obtainment and workload were not anticipated barriers. Instead, conceptual and psychologic barriers, such as reluctance to change practices and concern for missing VAI, were more likely. Interestingly, HCWs from hospitals with the highest culture rates also viewed CDS less favorably. Single-center studies have identified similar themes of excessive endotracheal aspirate culturing including that clinicians or individual units have developed normalized “default” practices (24), have low thresholds to obtain cultures (29, 30), have high vigilance for sepsis (26), and have fear of missing infection (24–26, 31). These patterns align with psychologic factors relevant for clinical de-implementation, including difficulty breaking habits, and asymmetry of outcomes or loss aversion (e.g., clinician concern for missing a diagnosis feels stronger than the perception of harm from the intervention) (32). The observed patterns also relate to either intrinsic (e.g., regret for errors of omission) and extrinsic (variation in medical practice) drivers of low-value practices in healthcare (33). Future research should consider if additional implementation strategies (e.g., education, provider feedback) are needed in addition to CDS to effectively address these complex clinical decision-making factors.
Limitations of this survey included that the survey asked about perceptions of practices and did not measure actual behaviors. We considered internal validity in several ways: we piloted the survey at another center prior to this study; reduced nonresponse error by aiming for large sample sizes and including HCWs with different roles and perspectives; each site’s responses were reviewed by the site leads. These findings align with single-center studies in which clinicians’ practice behaviors were independently recorded (24) that support that HCWs’ perceptions of practices are representative of actual behaviors. Supporting generalizability, the results demonstrate diversity in respiratory culture rates and perceived practices, the hospitals are geographically dispersed in the United States and most U.S. PICU beds are within academic centers (34). However, this study may not represent all U.S. PICUs, and future studies should consider prospective assessment of clinical practices and include community-based settings.
CONCLUSIONS
This multicenter survey demonstrated variability in perceived respiratory culture practices and attitudes across PICUs and identified clinical patterns that may be associated with higher respiratory culture use. These findings support a need to build consensus and highlight opportunities to optimize respiratory culture practices.
ACKNOWLEDGMENT
We appreciate the engagement and responses from all contributing respondents to make this study possible.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/pccmjournal).
This work was funded by the Donoghue Foundation Greater Value Portfolio, the National Institutes of Health (NIH) grants K23HL161449 to Dr. Sick-Samuels, NIH grant K24AI141580 to Dr. Milstone, and (AHRQ) grant R01HS028634 to Dr. Milstone. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
The authors have disclosed that they do not have any potential conflicts of interest.
The BrighT STAR Authorship group can be found in Appendix 1 (http://links.lww.com/PCC/C441).
REFERENCES
Citations & impact
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/155264643
Article citations
Breaking Boundaries in Pneumonia Diagnostics: Transitioning from Tradition to Molecular Frontiers with Multiplex PCR.
Diagnostics (Basel), 14(7):752, 02 Apr 2024
Cited by: 1 article | PMID: 38611665 | PMCID: PMC11012095
Review Free full text in Europe PMC
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Practices, Perceptions, and Attitudes in the Evaluation of Critically Ill Children for Bacteremia: A National Survey.
Pediatr Crit Care Med, 21(1):e23-e29, 01 Jan 2020
Cited by: 11 articles | PMID: 31702704 | PMCID: PMC6942229
Mixed-methods process evaluation of a respiratory-culture diagnostic stewardship intervention.
Infect Control Hosp Epidemiol, 44(2):191-199, 03 Jan 2023
Cited by: 2 articles | PMID: 36594433
Pediatric Ventilator-Associated Infections: The Ventilator-Associated INfection Study.
Pediatr Crit Care Med, 18(1):e24-e34, 01 Jan 2017
Cited by: 15 articles | PMID: 27828898
Association of Diagnostic Stewardship for Blood Cultures in Critically Ill Children With Culture Rates, Antibiotic Use, and Patient Outcomes: Results of the Bright STAR Collaborative.
JAMA Pediatr, 176(7):690-698, 01 Jul 2022
Cited by: 22 articles | PMID: 35499841 | PMCID: PMC9062771
Funding
Funders who supported this work.
AHRQ HHS (1)
Grant ID: R01 HS028634
NHLBI NIH HHS (1)
Grant ID: K23 HL161449
NIAID NIH HHS (1)
Grant ID: K24 AI141580

 1,2
1,2 

