Abstract
Purpose
Thrombocytopenia (platelet count < 150 × 109/L) is common in intensive care unit (ICU) patients and is likely associated with worse outcomes. In this study we present international contemporary data on thrombocytopenia in ICU patients.Methods
We conducted a prospective cohort study in adult ICU patients in 52 ICUs across 10 countries. We assessed frequencies of thrombocytopenia, use of platelet transfusions and clinical outcomes including mortality. We evaluated pre-selected potential risk factors for the development of thrombocytopenia during ICU stay and associations between thrombocytopenia at ICU admission and 90-day mortality using pre-specified logistic regression analyses.Results
We analysed 1166 ICU patients; the median age was 63 years and 39.5% were female. Overall, 43.2% (95% confidence interval (CI) 40.4-46.1) had thrombocytopenia; 23.4% (20-26) had thrombocytopenia at ICU admission, and 19.8% (17.6-22.2) developed thrombocytopenia during their ICU stay. Absence of acquired immune deficiency syndrome (AIDS), non-cancer-related immune deficiency, liver failure, male sex, septic shock, and bleeding at ICU admission were associated with the development of thrombocytopenia during ICU stay. Among patients with thrombocytopenia, 22.6% received platelet transfusion(s), and 64.3% of in-ICU transfusions were prophylactic. Patients with thrombocytopenia had higher occurrences of bleeding and death, fewer days alive without the use of life-support, and fewer days alive and out of hospital. Thrombocytopenia at ICU admission was associated with 90-day mortality (adjusted odds ratio 1.7; 95% CI 1.19-2.42).Conclusion
Thrombocytopenia occurred in 43% of critically ill patients and was associated with worse outcomes including increased mortality. Platelet transfusions were given to 23% of patients with thrombocytopenia and most were prophylactic.Free full text
Thrombocytopenia and platelet transfusions in ICU patients: an international inception cohort study (PLOT-ICU)
Abstract
Purpose
Thrombocytopenia (platelet count <
< 150
150 ×
× 109/L) is common in intensive care unit (ICU) patients and is likely associated with worse outcomes. In this study we present international contemporary data on thrombocytopenia in ICU patients.
109/L) is common in intensive care unit (ICU) patients and is likely associated with worse outcomes. In this study we present international contemporary data on thrombocytopenia in ICU patients.
Methods
We conducted a prospective cohort study in adult ICU patients in 52 ICUs across 10 countries. We assessed frequencies of thrombocytopenia, use of platelet transfusions and clinical outcomes including mortality. We evaluated pre-selected potential risk factors for the development of thrombocytopenia during ICU stay and associations between thrombocytopenia at ICU admission and 90-day mortality using pre-specified logistic regression analyses.
Results
We analysed 1166 ICU patients; the median age was 63 years and 39.5% were female. Overall, 43.2% (95% confidence interval (CI) 40.4–46.1) had thrombocytopenia; 23.4% (20–26) had thrombocytopenia at ICU admission, and 19.8% (17.6–22.2) developed thrombocytopenia during their ICU stay. Non-AIDS-, non-cancer-related immune deficiency, liver failure, male sex, septic shock, and bleeding at ICU admission were associated with the development of thrombocytopenia during ICU stay. Among patients with thrombocytopenia, 22.6% received platelet transfusion(s), and 64.3% of in-ICU transfusions were prophylactic. Patients with thrombocytopenia had higher occurrences of bleeding and death, fewer days alive without the use of life-support, and fewer days alive and out of hospital. Thrombocytopenia at ICU admission was associated with 90-day mortality (adjusted odds ratio 1.7; 95% CI 1.19–2.42).
Conclusion
Thrombocytopenia occurred in 43% of critically ill patients and was associated with worse outcomes including increased mortality. Platelet transfusions were given to 23% of patients with thrombocytopenia and most were prophylactic.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00134-023-07225-2.
Take-home message
| In this study, two in five acutely admitted adult ICU patients had thrombocytopenia; half of those had thrombocytopenia at ICU admission, which was associated with increased mortality. Platelet transfusions were frequently used in patients with severe and very severe thrombocytopenia, and most transfusions were prophylactic. |
Introduction
Thrombocytopenia, usually defined as a platelet count <
< 150
150 ×
× 109/L, occurs frequently in intensive care unit (ICU) patients [1, 2]. It is often multifactorial with causes including conditions and interventions resulting in reduced platelet production or increased platelet consumption, destruction, or sequestration, and haemodilution [3]. The prevalence and cumulative incidence have been reported in ranges from 8% to 77% and 8% to 56%, respectively, depending on case mix and definitions [4, 5]. Observational studies suggest that thrombocytopenia may impact ICU patients’ short- and long-term outcomes, including increased bleeding, transfusion requirements, use of life-support, length of ICU- and hospital stay, and mortality [4, 5]. However, uncertainty exists as many studies have been single-centred with low external validity [1, 2, 6–9].
109/L, occurs frequently in intensive care unit (ICU) patients [1, 2]. It is often multifactorial with causes including conditions and interventions resulting in reduced platelet production or increased platelet consumption, destruction, or sequestration, and haemodilution [3]. The prevalence and cumulative incidence have been reported in ranges from 8% to 77% and 8% to 56%, respectively, depending on case mix and definitions [4, 5]. Observational studies suggest that thrombocytopenia may impact ICU patients’ short- and long-term outcomes, including increased bleeding, transfusion requirements, use of life-support, length of ICU- and hospital stay, and mortality [4, 5]. However, uncertainty exists as many studies have been single-centred with low external validity [1, 2, 6–9].
Thrombocytopenia may prompt ICU physicians to delay or withhold invasive procedures, reduce thrombosis prophylaxis and consider platelet transfusions [10]. Prophylactic platelet transfusions are frequently used in thrombocytopenic ICU patients to reduce the risk of bleeding [11, 12], but evidence from randomised trials in ICU settings is sparse [13, 14], limited to a recent study evaluating platelet transfusion before central venous catheter (CVC) placement [15].
We conducted an international, prospective, inception cohort study to provide contemporary epidemiological data describing the frequency of thrombocytopenia in ICU patients, its risk factors, current platelet transfusion practice, and clinical outcomes including mortality. We hypothesised that thrombocytopenia would be frequent and associated with worse outcomes and that specific risk factors for thrombocytopenia exist.
Methods
Study design and setting
This was an international, prospective, inception cohort study conducted and analysed in compliance with a published protocol and statistical analysis plan with minor modifications (electronic supplementary material (ESM) 1) [16]. The manuscript was prepared according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement [17] (ESM 2).
The study was performed in 52 ICUs across 10 countries in Europe and North America (ESM 3 and 4) and overseen by a steering committee (ESM 5). Study ICUs were invited to participate through the research network Caring for critically ill immunocompromised patients: Multinational Network (Nine-I).
The study was approved by the Danish Centre for Regional Development (R-21012287) and registered at the Capital Region’s Centre for Data Compliance (P-2021-262). We obtained all necessary approvals in the other participating countries and collected informed consent from patients and/or surrogates if required.
Inception periods of 14 consecutive days were conveniently chosen by participating ICUs from May 2021 to July 2022. Included patients were followed for a maximum of 90 days.
Population
During inception periods, all consecutive adults (≥ 18 years) who were acutely admitted to the ICU were screened for inclusion. Patients transferred from other ICUs were considered eligible if they otherwise met the inclusion criteria. We excluded patients with elective open-heart surgery during current hospitalisation and those who had previously been enrolled or declined informed consent.
18 years) who were acutely admitted to the ICU were screened for inclusion. Patients transferred from other ICUs were considered eligible if they otherwise met the inclusion criteria. We excluded patients with elective open-heart surgery during current hospitalisation and those who had previously been enrolled or declined informed consent.
Data collection and management
We developed an electronic case report form (eCRF) using the Research Electronic Data Capture (REDCap) tool hosted by the Capital Region of Denmark [18, 19]. The eCRF was pre-tested at the coordinating site and local investigators had access to a training version before study initiation.
For each study ICU, we collected data on type of hospital and ICU, number of ICU beds and presence of local guidelines for platelet transfusions (ESM 6).
Patient data were collected from medical records as described in the protocol [16]. Baseline data collected at ICU admission included demographics, comorbidities, selected treatments, Simplified Mortality Score for the Intensive Care Unit (SMS-ICU, an illness severity score ranging from 0 to 42 points with higher scores indicating higher predicted 90-day mortality) (ESM 7) [20, 21], platelet count, and presence of bleeding (ESM 8).
Daily data were collected during ICU stay and resumed upon readmission or transfer to another study ICU for a maximum of 90 days. We collected data on the use of life-support [vasopressor/inotropes, mechanical ventilation, renal replacement therapy, extracorporeal membrane oxygenation (ECMO)], selected treatments, platelet counts, bleeding events, thrombotic events, and transfusions (ESM 9). For platelet transfusions used within ICU departments, we collected data on indications for transfusion (pre-procedural: covering invasive procedures or surgery; prophylactic: reducing the risk of bleeding; therapeutic: treating bleeding) and platelet count before transfusion (ESM 1 and 9). On day 90, we assessed the vital status and number of days in the hospital (ESM 10).
Definitions and classifications
Thrombocytopenia
We defined thrombocytopenia as a platelet count <
< 150
150 ×
× 109/L and classified thrombocytopenia into three categories: ‘baseline thrombocytopenia’ defined as patients with thrombocytopenia at ICU admission, ‘ICU thrombocytopenia’ defined as patients with thrombocytopenia during ICU stay without thrombocytopenia at ICU admission and ‘any thrombocytopenia’ defined as patients who had thrombocytopenia at ICU admission and/or during ICU stay. Severity of thrombocytopenia was classified as mild (100–149
109/L and classified thrombocytopenia into three categories: ‘baseline thrombocytopenia’ defined as patients with thrombocytopenia at ICU admission, ‘ICU thrombocytopenia’ defined as patients with thrombocytopenia during ICU stay without thrombocytopenia at ICU admission and ‘any thrombocytopenia’ defined as patients who had thrombocytopenia at ICU admission and/or during ICU stay. Severity of thrombocytopenia was classified as mild (100–149 ×
× 109/L), moderate (50–99
109/L), moderate (50–99 ×
× 109/L), severe (20–49
109/L), severe (20–49 ×
× 109/L), and very severe (<
109/L), and very severe (< 20
20 ×
× 109/L), based on the platelet count at ICU admission and the nadir platelet count during ICU stay and overall, for baseline-, ICU-, and any thrombocytopenia, respectively [16].
109/L), based on the platelet count at ICU admission and the nadir platelet count during ICU stay and overall, for baseline-, ICU-, and any thrombocytopenia, respectively [16].
Bleeding
We defined and graded bleeding according to a modified World Health Organization (WHO) classification [22–25]. Grade 1 and 2 covers bleedings that do not require red blood cell (RBC) transfusion. Grade 3 covers bleedings requiring up to two RBC transfusions and central nervous system bleedings without symptoms. Grade 4 covers bleedings requiring either (1) more than two RBC transfusions, (2) intubation and mechanical ventilation or (3) surgical intervention, and bleedings from critical sites with symptoms and fatal bleedings (ESM 11) [16].
Outcomes
The primary outcome was the number of patients with any thrombocytopenia. Secondary outcomes were numbers of patients with mild, moderate, severe, and very severe thrombocytopenia; 90-day mortality; days alive without use of life-support; days alive and out of hospital; numbers of patients with at least one bleeding event, at least one thrombotic event, and at least one platelet transfusion during ICU stay; number of platelet transfusions and volumes transfused per patient; and number of transfusions for each indication (ESM 12) [16].
Process variables
Process variables included daily platelet count before in-ICU platelet transfusions for each indication and number of RBC transfusions (ESM 1 and 12).
Statistical methods
Sample size
As we planned to include patients regardless of illness severity and expected length of ICU stay, we made a conservative assumption, that the frequency of any thrombocytopenia would be 20% [4, 5]. We aimed to enroll a minimum of 1,000 patients to determine the frequency with a 95% confidence interval (CI) of 18–23% [16].
Descriptive data
Analyses were performed using R (version 4.2.0) following the statistical analysis plan with minor modifications (ESM 1) [16]. Categorical variables were reported as numbers and percentages and continuous variables as medians with interquartile ranges (IQRs). Primary and secondary outcomes were reported descriptively with 95% CIs calculated using the Clopper–Pearson method for categorical variables and non-parametric percentile-based bootstrapping with 100,000 samples for continuous variables. Results were stratified on the three categories of thrombocytopenia, and for the secondary outcomes, also on the severity of thrombocytopenia [16].
Regression analyses
We used pre-specified logistic regression models [16] to assess a set of potential baseline risk factors for ICU thrombocytopenia (< 150
150 ×
× 109/L and
109/L and <
< 50
50 ×
× 109/L) selected a priori based on literature review [4, 5], clinical knowledge, and the expected number of events. We assessed sex, haematological malignancy, immune deficiency, acute or chronic liver failure, SMS-ICU, bleeding and septic shock in unadjusted models and in a model adjusted for all selected risk factors [16]. We modified the planned definition of ‘immune deficiency’ to, ‘non-acquired immune deficiency syndrome (AIDS)-, non-cancer-related immune deficiency’ to avoid any unintended overlap with cancer patients (ESM 1). Patients with baseline thrombocytopenia
109/L) selected a priori based on literature review [4, 5], clinical knowledge, and the expected number of events. We assessed sex, haematological malignancy, immune deficiency, acute or chronic liver failure, SMS-ICU, bleeding and septic shock in unadjusted models and in a model adjusted for all selected risk factors [16]. We modified the planned definition of ‘immune deficiency’ to, ‘non-acquired immune deficiency syndrome (AIDS)-, non-cancer-related immune deficiency’ to avoid any unintended overlap with cancer patients (ESM 1). Patients with baseline thrombocytopenia <
< 150
150 ×
× 109/L were excluded from these analyses.
109/L were excluded from these analyses.
We assessed associations between baseline thrombocytopenia (< 150
150 ×
× 109/L and
109/L and <
< 50
50 ×
× 109/L) and 90-day mortality in pre-specified logistic regression models adjusted for potential confounders including sex, country, haematological malignancy, SMS-ICU, and septic shock selected based on literature review [4, 5], clinical knowledge and expected number of events.
109/L) and 90-day mortality in pre-specified logistic regression models adjusted for potential confounders including sex, country, haematological malignancy, SMS-ICU, and septic shock selected based on literature review [4, 5], clinical knowledge and expected number of events.
We used Nagelkerke’s R2 [26] and calibration plots [27] to assess model fits (ESM 24). We conducted additional sensitivity analyses including complete case analyses and Cox proportional hazards regressions to assess the impact of missing platelet counts and time-to-event and competing risks, respectively (details in ESM 25 and 27).
Missing data
We handled missing platelet count data, as planned, using logical imputation, and assumed that missing baseline platelet counts were equal to the first platelet count in ICU (ESM 13). Twenty-eight patients were transferred directly from a study ICU to a non-study ICU where daily variables (clinical events, biochemistry, etc.) could not be obtained. We assumed that no events occurred during days spent in non-study ICUs. Following these logical imputations, we performed complete case analysis due to limited remaining missing data (< 5% of patients) [16].
5% of patients) [16].
Results
We included 1168 ICU patients from 52 ICUs across 43 centres in 10 countries. Two patients withdrew consent and were excluded (ESM 14). Most study ICUs were mixed ICUs (76.9%) in university hospitals (94.2%). Few (15.4%) had ICU-specific guidelines for platelet transfusions (ESM 15).
Baseline characteristics
Patients with thrombocytopenia were more often males, admitted from hospital wards, and were more severely ill than those without thrombocytopenia (Table (Table11 and ESM 16). More patients with thrombocytopenia had haematological malignancy, non-AIDS-, non-cancer-related immune deficiencies, acute and chronic liver failure, septic shock, and bleeding at baseline and more received treatment with haematopoietic stem cell transplantation, chemotherapy and platelet transfusions compared to those without thrombocytopenia.
Table 1
Baseline characteristics
| All (n  = = 1166) 1166) | Thrombocytopenia | ||||
|---|---|---|---|---|---|
| None (n  = = 662) 662) | Anya (n  = = 504) 504) | Baselineb (n  = = 273) 273) | ICUc (n  = = 231) 231) | ||
| Age (years) | 63 (49–73) | 64 (47–74) | 63 (51–72) | 61 (50–70) | 65 (53–74) |
| Female sex | 461 (39.5%) | 291 (44%) | 170 (33.7%) | 90 (33%) | 80 (34.6%) |
| Comorbidities | |||||
| Pulmonary disease | 217 (18.6%) | 153 (23.1%) | 64 (12.7%) | 34 (12.5%) | 30 (13%) |
| IHD or HF | 208 (17.8%) | 126 (19%) | 82 (16.3%) | 44 (16.1%) | 38 (16.5%) |
| Chronic renal failure | 100 (8.6%) | 46 (6.9%) | 54 (10.7%) | 29 (10.6%) | 25 (10.8%) |
| Chronic liver failure | 62 (5.3%) | 12 (1.8%) | 50 (9.9%) | 38 (13.9%) | 12 (5.2%) |
| Solid tumour cancer | 162 (13.9%) | 89 (13.4%) | 73 (14.5%) | 45 (16.5%) | 28 (12.1%) |
| Metastatic cancer | 76 (6.5%) | 39 (5.9%) | 37 (7.3%) | 27 (9.9%) | 10 (4.3%) |
| Haematological malignancy | 92 (7.9%) | 17 (2.6%) | 75 (14.9%) | 66 (24.2%) | 9 (3.9%) |
| Non-AIDS-, non-cancer-related immune deficiencyd | 61 (5.2%) | 19 (2.9%) | 42 (8.3%) | 23 (8.4%) | 19 (8.2%) |
| Coagulation disorder | 7 (0.6%) | 4 (0.6%) | 3 (0.6%) | 1 (0.4%) | 2 (0.9%) |
| Previous thrombo-embolism | 150 (12.9%) | 89 (13.4%) | 61 (12.1%) | 35 (12.8%) | 26 (11.3%) |
| Admitted from | |||||
| ED | 571 (49%) | 360 (54.4%) | 211 (41.9%) | 93 (34.1%) | 118 (51.1%) |
| Ward | 340 (29.2%) | 170 (25.7%) | 170 (33.7%) | 120 (44%) | 50 (21.6%) |
| OR | 172 (14.8%) | 95 (14.4%) | 77 (15.3%) | 32 (11.7%) | 45 (19.5%) |
| ICU | 83 (7.1%) | 37 (5.6%) | 46 (9.1%) | 28 (10.3%) | 18 (7.8%) |
| Surgery | |||||
| Elective surgery | 102 (8.7%) | 57 (8.6%) | 45 (8.9%) | 21 (7.7%) | 24 (10.4%) |
| Acute surgery | 219 (18.8%) | 115 (17.4%) | 104 (20.6%) | 47 (17.2%) | 57 (24.7%) |
| Illness severity | |||||
| SMS-ICU predicted 90-day mortality (%)e | 22.8 (14.7–40.1) | 20.5 (13.1–33.8) | 30.8 (16.5–43.4) | 30.8 (16.5–46.7) | 28 (17.4–43.4) |
| Primary reason for ICU admission | |||||
| Neurological | 175 (15%) | 108 (16.3%) | 67 (13.3%) | 33 (12.1%) | 34 (14.7%) |
| Respiratory | 406 (34.8%) | 277 (41.8%) | 129 (25.6%) | 76 (27.8%) | 53 (22.9%) |
| Circulatory | 278 (23.8%) | 125 (18.9%) | 153 (30.4%) | 78 (28.6%) | 75 (32.5%) |
| Renal | 35 (3%) | 13 (2%) | 22 (4.4%) | 13 (4.8%) | 9 (3.9%) |
| Liver | 13 (1.1%) | 1 (0.2%) | 12 (2.4%) | 8 (2.9%) | 4 (1.7%) |
| Metabolic | 55 (4.7%) | 32 (4.8%) | 23 (4.6%) | 13 (4.8%) | 10 (4.3%) |
| Multiple trauma | 29 (2.5%) | 12 (1.8%) | 17 (3.4%) | 4 (1.5%) | 13 (5.6%) |
| TBI | 13 (1.1%) | 8 (1.2%) | 5 (1%) | 3 (1.1%) | 2 (0.9%) |
| Burn | 3 (0.3%) | 2 (0.3%) | 1 (0.2%) | 1 (0.4%) | 0 (0%) |
| Haemorrhage | 48 (4.1%) | 12 (1.8%) | 36 (7.1%) | 19 (7%) | 17 (7.4%) |
| Other | 111 (9.5%) | 72 (10.9%) | 39 (7.7%) | 25 (9.2%) | 14 (6.1%) |
| Acute conditions | |||||
| Sepsis | 161 (13.8%) | 88 (13.3%) | 73 (14.5%) | 52 (19%) | 21 (9.1%) |
| Septic shock | 174 (14.9%) | 58 (8.8%) | 116 (23%) | 62 (22.7%) | 54 (23.4%) |
| Acute liver failure | 42 (3.6%) | 8 (1.2%) | 34 (6.7%) | 18 (6.6%) | 16 (6.9%) |
| COVID-19 | 140 (12%) | 82 (12.4%) | 58 (11.5%) | 40 (14.7%) | 18 (7.8%) |
| Treatments | |||||
| HSCTf | 28 (2.4%) | 0 (0%) | 28 (5.6%) | 27 (9.9%) | 1 (0.4%) |
| Chemotherapyg | 86 (7.4%) | 27 (4.1%) | 59 (11.7%) | 51 (18.7%) | 8 (3.5%) |
| Anticoagulating treatmenth | 399 (34.2%) | 232 (35%) | 167 (33.1%) | 95 (34.8%) | 72 (31.2%) |
| Platelet inhibitorsi | 198 (17%) | 132 (19.9%) | 66 (13.1%) | 31 (11.4%) | 35 (15.2%) |
| Biochemistry | |||||
| Platelet countj | 222 (150.5–300) | 278 (225–341.2) | 146 (93–198) | 97 (50–125) | 204 (175.2–258.8) |
| Bleeding and transfusions 24 h before ICU admission | |||||
| Bleeding | 162 (13.9%) | 66 (10%) | 96 (19%) | 50 (18.3%) | 46 (19.9%) |
| Platelet transfusion | 59 (5.1%) | 9 (1.4%) | 50 (9.9%) | 35 (12.8%) | 15 (6.5%) |
Continuous variables are presented as medians (IQR) and categorical variables as numbers and percentages. Definitions of baseline variables are available in ESM 7 and 8. Additional baseline characteristics are provided in ESM 16
ICU intensive care unit; IHD ischaemic heart disease; HF heart failure; ED emergency department; OR operating room or recovery room; TBI traumatic brain injury; COVID-19 coronavirus disease 2019; HSCT haematopoietic stem cell transplantation; AIDS acquired immune deficiency syndrome; SMS-ICU Simplified Mortality Score for the Intensive Care Unit
aPatients with a platelet count <
< 150
150 ×
× 109/L at ICU admission and/or during ICU stay
109/L at ICU admission and/or during ICU stay
bPatients with a platelet count <
< 150
150 ×
× 109/L at ICU admission
109/L at ICU admission
cPatients with a platelet count <
< 150
150 ×
× 109/L during ICU stay without thrombocytopenia at ICU admission
109/L during ICU stay without thrombocytopenia at ICU admission
dNon-AIDS-, non-cancer-related immune deficiencies including solid organ transplant or conditions requiring long-term (> 30 days) or high-dose (>
30 days) or high-dose (> 1 mg/kg/day) steroids, or any immunosuppressive drug for more than 30 days
1 mg/kg/day) steroids, or any immunosuppressive drug for more than 30 days
ePredicted 90-day mortality based on SMS-ICU (an illness severity score ranging from 0–42 with corresponding predicted 90-day mortality of 3.3–91.0% [20, 21]). The corresponding median (IQR) raw scores were 16.0 (12.0–22.0), 15.0 (11.0–20.0), 19.0 (13.0–23.0), 19.0 (13.0–24.0) and 18.0 (13.5–23.0) in all patients, patients without-, patients with-, patients with baseline- and patients with ICU thrombocytopenia, respectively. Details on SMS-ICU are provided in ESM 7
fHSCT (allogenic or autologous) within one year before ICU admission
gChemotherapy treatment within six weeks before ICU admission
hIncluding treatment with anticoagulating drugs in any prophylaxis and therapeutic dose within 48 h before ICU admission
iTreatment with platelet inhibitors within 48 h before ICU admission
jBaseline platelet counts were unavailable for 195 (16.7%) patients
Common characteristics in patients with baseline thrombocytopenia, were admission from hospital wards, haematological malignancy, and treatment with haematopoietic stem cell transplantation, chemotherapy, and platelet transfusions prior to ICU admission while surgery before ICU admission and admission from emergency settings, operating or recovery rooms were more common in patients with ICU thrombocytopenia.
Primary outcome
The frequency of any thrombocytopenia was 43.2% (95% CI 40.4–46.1); 23.4% (21–26) had baseline thrombocytopenia, and an additional 19.8% (17.6–22.2) developed ICU thrombocytopenia.
Secondary outcomes
ICU thrombocytopenia occurred on median (IQR) day 2 (1–3) with few occurrences after day 5 (10.8%) from ICU admission (ESM 17) and most patients with baseline thrombocytopenia also had thrombocytopenia during ICU stay (95.6%). Among patients with any thrombocytopenia, 42.5% (95% CI 38.1–46.9) had mild-, 31% (26.9–35.2) moderate-, 12.9% (10.1–16.1) severe- and 13.7% (10.8–17) very severe thrombocytopenia (ESM 18) with nadir platelet counts occurring on median (interquartile range, IQR) 3 (1–4) days from ICU admission. Nadir platelet counts for patients with baseline- and ICU thrombocytopenia occurred on median (IQR) days 2 (0–4) and 4 (2–5) from ICU admission, respectively (day 0 representing baseline). The trajectories of thrombocytopenia from baseline through ICU stay are presented in Fig. 1 and ESM 19.
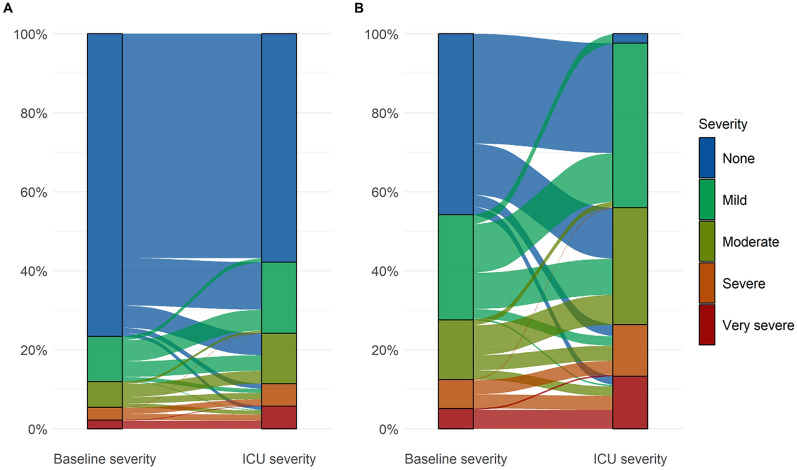
The trajectories of thrombocytopenia. Trajectories of thrombocytopenia in all patients (A) and exclusively in those who had thrombocytopenia (B). The severity of baseline- and ICU thrombocytopenia was based on the platelet count at baseline and the nadir platelet during ICU stay, respectively. The flows are coloured according to the baseline severity. Patients whose thrombocytopenia remained unchanged (i.e., unchanged severity) from baseline throughout their ICU stay are represented by flows moving between identical colours. Patients whose thrombocytopenia got better or worse (i.e., decreased or increased severity) during their ICU stay compared to their starting point at baseline are represented by flows moving between different colours. Only 22 patients had less severe thrombocytopenia during their ICU stay compared to their starting point at baseline (represented by flows mowing from “warmer” to “colder” colour tones)
The overall 90-day mortality was 25.9% (95% CI 23.4–28.5) (ESM 20) and differed between patients with- and without thrombocytopenia at 34.6% and 19.3%, respectively. The highest mortality was observed in patients with severe- and very severe thrombocytopenia (Table (Table22).
Table 2
Secondary clinical outcomes
| Thrombocytopenia | ||||||||
|---|---|---|---|---|---|---|---|---|
| None (n  = = 662) 662) | Anya (n  = = 504) 504) | Mild (n  = = 214) 214) | Moderate (n  = = 156) 156) | Severe (n  = = 65) 65) | Very severe (n  = = 69) 69) | Baselineb (n  = = 273) 273) | ICUc (n  = = 231) 231) | |
| Outcomes | ||||||||
| 90-day mortalityd | 127 (19.3%) | 174 (34.6%) | 56 (26.3%) | 49 (31.4%) | 33 (50.8%) | 36 (52.2%) | 102 (37.4%) | 72 (31.3%) |
| Days alive without life-supporte | 88 (79.5–90) | 82 (0–88) | 86 (0–89) | 83 (0–88) | 0 (0–87) | 0 (0–85) | 81 (0–89) | 83 (0–88) |
| Days alive and out of hospitalf | 71 (20–81) | 44 (0–75) | 63 (0–78) | 47 (0–73) | 0 (0–60) | 0 (0–31) | 30 (0–74) | 50.5 (0–75) |
| Any bleeding in the ICUg | 48 (7.3%) | 139 (27.6%) | 39 (18.2%) | 44 (28.2%) | 24 (36.9%) | 32 (46.4%) | 75 (27.5%) | 64 (27.7%) |
| Worst bleeding in the ICUh | ||||||||
| Grade 1 | 5 (0.8%) | 10 (2%) | 2 (0.9%) | 2 (1.3%) | 3 (4.6%) | 3 (4.3%) | 3 (1.1%) | 7 (3%) |
| Grade 2 | 34 (5.1%) | 49 (9.7%) | 15 (7%) | 17 (10.9%) | 6 (9.2%) | 11 (15.9%) | 29 (10.6%) | 20 (8.7%) |
| Grade 3 | 5 (0.8%) | 37 (7.3%) | 11 (5.1%) | 11 (7.1%) | 6 (9.2%) | 9 (13%) | 20 (7.3%) | 17 (7.4%) |
| Grade 4 | 4 (0.6%) | 43 (8.5%) | 11 (5.1%) | 14 (9%) | 9 (13.8%) | 9 (13%) | 23 (8.4%) | 20 (8.7%) |
| New thrombosis in the ICU | 30 (4.5%) | 38 (7.5%) | 15 (7%) | 16 (10.3%) | 4 (6.2%) | 3 (4.3%) | 19 (7%) | 19 (8.2%) |
| Platelet transfusions | ||||||||
| Transfused with plateletsi | 6 (0.9%) | 114 (22.6%) | 8 (3.7%) | 19 (12.2%) | 29 (44.6%) | 58 (84.1%) | 80 (29.3%) | 34 (14.7%) |
| Number of platelet transfusions per patientj | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–2) | 4 (1–7) | 0 (0–1) | 0 (0–0) |
| Total volume (mL) of platelets transfusions per patientk | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0.–326.2) | 900 (283–1520) | 0 (0–283) | 0 (0–0) |
| Indications for in-ICU transfusionsl | ||||||||
| Prophylaxis | 1 (0.2%) | 73 (14.5%) | 2 (0.9%) | 4 (2.6%) | 16 (24.6%) | 51 (73.9%) | 61 (22.3%) | 12 (5.2%) |
| Pre-procedural | 1 (0.2%) | 29 (5.8%) | 1 (0.5%) | 2 (1.3%) | 10(15.4%) | 16 (23.2%) | 21 (7.7%) | 8 (3.5%) |
| Therapeutic | 1 (0.2%) | 39 (7.7%) | 2 (0.9%) | 9 (5.8%) | 12 (18.5%) | 16 (23.2%) | 22 (8.1%) | 17 (7.4%) |
| RBC transfusions | ||||||||
| Transfused with RBCs | 90 (13.6%) | 217 (43.1%) | 61 (28.5%) | 69 (44.2%) | 41 (63.1%) | 46 (66.7%) | 116 (42.5%) | 101 (43.7%) |
| Number of RBC transfusions per patient | 0 (0–0) | 0 (0–3) | 0 (0–1) | 0 (0–3) | 1 (0–4) | 2 (0–6) | 0 (0–2) | 0 (0–3) |
Continuous variables are presented as medians with IQRs and categorical variables as numbers and percentages. The severity of thrombocytopenia was based on the overall nadir platelet count; mild (100–149 ×
× 109/L), moderate (50–99
109/L), moderate (50–99 ×
× 109/L), severe (20–49
109/L), severe (20–49 ×
× 109/L, and very severe (<
109/L, and very severe (< 20
20 ×
× 109/L). We have presented 95% confidence intervals for the estimates in ESM 20
109/L). We have presented 95% confidence intervals for the estimates in ESM 20
ICU intensive care unit; RBC red blood cell; WHO World Health Organization
aPatients with a platelet count <
< 150
150 ×
× 109/L at ICU admission and/or during the ICU stay and within the specified severity subclasses according to the overall nadir platelet count
109/L at ICU admission and/or during the ICU stay and within the specified severity subclasses according to the overall nadir platelet count
bPatients with a platelet count <
< 150
150 ×
× 109/L at ICU admission
109/L at ICU admission
cPatients with a platelet count <
< 150
150 ×
× 109/L during ICU stay without thrombocytopenia at ICU admission
109/L during ICU stay without thrombocytopenia at ICU admission
dFour patients (0.3%) had missing 90-day mortality data
eDead patients were assigned zero days alive without life-support. Four patients (0.3%) had missing data for this outcome
fDead patients were assigned zero days alive and out of hospital. Four patients (0.3%) had missing data for this outcome
gNumber of patients with at least one WHO bleeding in the ICU. We did not register bleedings occurring during surgery only while admitted to the ICU. Overall, 29 patients with no registered bleeding in the ICU were transfused with 2 or more packed red blood cells during surgery: 11 patients without thrombocytopenia and 18 patients with any thrombocytopenia (6 patients with mild-, 8 patients with moderate-, 3 patients with severe-, 1 patient with very severe thrombocytopenia, respectively). Of those, 8 patients with baseline thrombocytopenia and 10 with ICU thrombocytopenia, respectively
hNumber of patients with at least one WHO bleeding in the ICU graded into 1 to 4 according to the worst graded bleeding episode
iNumber of patients receiving at least one platelet transfusion
jTotal number of transfusions per patient
kTotal volume of platelet transfusions per patient. Two patients (0.2%) had missing data
lNumber of patients that received at least one platelet transfusion that took place in ICU departments for each of the indications. Transfusions administered in operating theatres were excluded as we did not collect data on indications for platelet transfusions administered “outside” the ICU. In total 29 patients received 57 platelet transfusions during surgery: 3 patients without thrombocytopenia received 4 transfusions and 26 patients with thrombocytopenia received 53 transfusions (5 patients with mild thrombocytopenia received 14 transfusions, 7 patients with moderate thrombocytopenia received 12 transfusions, 6 patients with severe thrombocytopenia received 14 transfusions, and 8 patients with very severe thrombocytopenia received 13 transfusions). Of those, 11 patients with baseline thrombocytopenia received 17 transfusions and 15 patients with ICU thrombocytopenia received 36 transfusions
Overall, the median number of days alive without use of life-support was 87 (IQR 0–90) and days alive and out of hospital was 63 (0–79). Patients with thrombocytopenia had fewer days alive without use of life-support and fewer days alive out of hospital than those without thrombocytopenia (Table (Table2).2). More patients with thrombocytopenia received vasopressors (73% vs 45.8%), invasive mechanical ventilation (63.3% vs 44.6%), RRT (25% vs 5.3%) and ECMO (2.8% vs 0.6%) compared with those without thrombocytopenia (ESM 21).
In total, 16% of patients bled in ICU (95% CI 14–18.3) (ESM 20). Among patients without thrombocytopenia, 7.3% bled in ICU, most of whom (81.2%) had bleeding of grade 1 or 2. In contrast, 27.6% of patients with thrombocytopenia bled in ICU, of whom 57.6% had bleeding of grade 3 or 4. Proportionally more patients with severe- and very severe thrombocytopenia had grade 3 or 4 bleeding compared to patients with no- or less severe thrombocytopenia (Table (Table2).2). Thrombotic events occurred in 5.8% (95% CI 4.6–7.3) of all patients (Table (Table22 and ESM 20).
Platelet transfusions were almost exclusively used in patients with thrombocytopenia, of whom 22.6% (95% CI 19–26.5%) were transfused (Table (Table22 and ESM 20). Patients transfused with platelets received median (IQR) 3 (1–5) platelet transfusions. Most platelet transfusions (90.9%) took place within ICU departments (in-ICU) and fewer in operating theatres (9.1%) (EMS 22). The number of in-ICU transfusions increased with higher severity of thrombocytopenia (Fig. 2) and most in-ICU transfusions were prophylactic (64.3%) followed by therapeutic- (27.6%) and pre-procedural transfusions (8.1%) with median platelet counts before transfusion of 15 ×
× 109/L (IQR 9–26), 40
109/L (IQR 9–26), 40 ×
× 109/L (23–76) and 31
109/L (23–76) and 31 ×
× 109/L (20–54), respectively. Platelet counts before transfusion for specific procedures are presented in ESM 23. More patients with thrombocytopenia received RBC transfusion than patients without thrombocytopenia (Table (Table22 and ESM 20).
109/L (20–54), respectively. Platelet counts before transfusion for specific procedures are presented in ESM 23. More patients with thrombocytopenia received RBC transfusion than patients without thrombocytopenia (Table (Table22 and ESM 20).
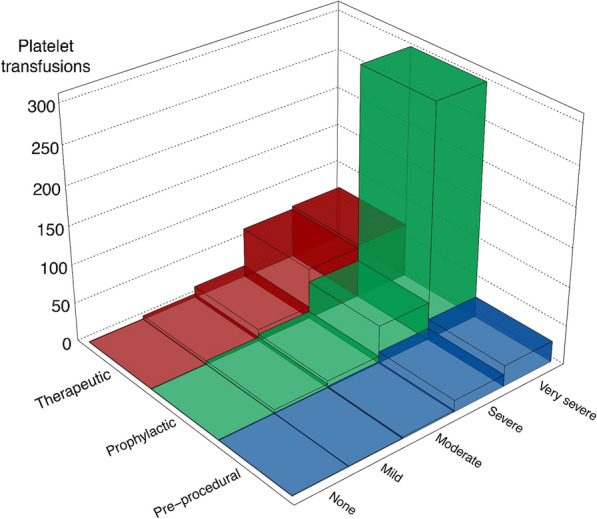
In-ICU platelet transfusions and indications. Number of in-ICU platelet transfusions used in patients with- and without thrombocytopenia. Patients with severe- and very severe thrombocytopenia received 93% of all in-ICU platelet transfusions and in patients with very severe thrombocytopenia, 76% of in-ICU transfusions were prophylactic. We did not collect data on indications for 57 platelet transfusions used during surgery, thus these are excluded (see ESM 22 for details)
Risk factors for ICU thrombocytopenia
In unadjusted and adjusted analyses, male sex, non-AIDS-, non-cancer-related immune deficiency, acute or chronic liver failure, higher illness severity [20, 21], bleeding, and septic shock at baseline were associated with ICU thrombocytopenia <
< 150
150 ×
× 109/L. Meaningful interpretation of risk factors for ICU thrombocytopenia
109/L. Meaningful interpretation of risk factors for ICU thrombocytopenia <
< 50
50 ×
× 109/L were hampered by few events (n
109/L were hampered by few events (n =
= 26) (Table (Table3).3). Results were largely consistent in sensitivity analyses (ESM 25).
26) (Table (Table3).3). Results were largely consistent in sensitivity analyses (ESM 25).
Table 3
Baseline risk factors for ICU thrombocytopenia
ICU thrombocytopenia < < 150 150 × × 109/La 109/LaOR (95% CI) | ICU thrombocytopenia < < 50 50 × × 109/Lb 109/LbOR (95% CI) | |||
|---|---|---|---|---|
| Unadjusted | Adjustedc | Unadjusted | Adjustedc | |
| Male sex | 1.48 (1.08–2.02) | 1.45 (1.04–2.02) | 0.82 (0.38–1.80) | 0.80 (0.35–1.84) |
| SMS-ICUd | 1.07 (1.05–1.10) | 1.06 (1.03–1.09) | 1.10 (1.04–1.17) | 1.06 (0.98–1.13) |
| Bleedinge | 2.25 (1.49–3.39) | 2.27 (1.46–3.52) | 0.91 (0.27–3.07) | 1.12 (0.31–4.03) |
| Haematological malignancy | 1.54 (0.68–3.50) | 1.18 (0.49–2.85) | 2.93 (0.65–13.10) | 3.81 (0.73–19.79) |
| Non-AIDS-, non-cancer-related immune deficiencyf | 3.03 (1.58–5.84) | 4.08 (2.05–8.15) | 6.02 (2.14–16.95) | 7.48 (2.36–23.70) |
| Liver failure | 4.60 (2.44–8.70) | 4.03 (2.06–7.88) | 2.77 (0.80–9.62) | 2.51 (0.66–9.59) |
| Septic shock | 3.18 (2.12–4.77) | 2.47 (1.58–3.85) | 9.15 (4.12–20.36) | 6.90 (2.82–16.89) |
Baseline risk factors for ICU thrombocytopenia in patients without baseline thrombocytopenia (n =
= 893). Few patients (n
893). Few patients (n =
= 26) developed severe thrombocytopenia (<
26) developed severe thrombocytopenia (< 50
50 ×
× 109/L) during ICU stay
109/L) during ICU stay
ICU intensive care unit; CI confidence interval; OR odds ratio; WHO World Health Organization; SMS-ICU Simplified Mortality Score for the Intensive Care Unit; AIDS acquired immune deficiency syndrome
aPatients with a platelet count <
< 150
150 ×
× 109/L during ICU stay without thrombocytopenia at ICU admission
109/L during ICU stay without thrombocytopenia at ICU admission
bPatients with a platelet count <
< 50
50 ×
× 109/L during ICU stay without thrombocytopenia at ICU admission
109/L during ICU stay without thrombocytopenia at ICU admission
cAdjusted for the following baseline variables: male sex, SMS-ICU, bleeding, haematological malignancy, non-AIDS-, non-cancer-related immune deficiency, liver failure (acute or chronic) and septic shock
dOR for a one-point increase in SMS-ICU; an illness severity score ranging from 0–42 points with higher scores indicating higher predicted 90-day mortality
eAny WHO bleeding at baseline
fNon-AIDS-, non-cancer-related immune deficiencies including solid organ transplant or conditions requiring long-term (> 30 days) or high-dose (>
30 days) or high-dose (> 1 mg/kg/day) steroids, or any immunosuppressive drug for more than 30 days
1 mg/kg/day) steroids, or any immunosuppressive drug for more than 30 days
Baseline thrombocytopenia and mortality
When adjusting for country, sex, haematological malignancy, SMS-ICU and septic shock, baseline thrombocytopenia <
< 150
150 ×
× 109/L was associated with increased 90-day mortality (OR 1.7, 95% CI 1.19–2.42), while some uncertainty remained about the effect of baseline thrombocytopenia
109/L was associated with increased 90-day mortality (OR 1.7, 95% CI 1.19–2.42), while some uncertainty remained about the effect of baseline thrombocytopenia <
< 50
50 ×
× 109/L (1.79, 95% CI 0.89–3.61) (ESM 26). Sensitivity analyses yielded largely consistent results (ESM 27).
109/L (1.79, 95% CI 0.89–3.61) (ESM 26). Sensitivity analyses yielded largely consistent results (ESM 27).
Discussion
In this international prospective cohort study of acutely admitted adult ICU patients, we found that 43% had thrombocytopenia; 23% at ICU admission and 20% developed it during ICU stay. Patients with thrombocytopenia had worse outcomes, including 90-day mortality, and several conditions increased the risk of developing thrombocytopenia during ICU stay. Twenty-three percent of patients with thrombocytopenia received platelet transfusion and most in-ICU transfusions were prophylactic.
The observed frequency of thrombocytopenia was similar to findings in smaller cohort studies in general mixed ICU populations [2, 28], and in a larger cohort of ICU patients receiving thromboprophylaxis [29]. However, variation exists, which is likely due to differences in case mix, illness severity, follow-up time and definitions of thrombocytopenia [1, 9, 30, 31].
We found that male sex, non-AIDS-, non-cancer-related immune deficiency, acute or chronic liver failure, higher illness severity, bleeding, and septic shock at baseline increased the risk of developing ICU thrombocytopenia. This aligns well with previously published data [29, 30, 32], although one study using a different definition of thrombocytopenia (< 100
100 ×
× 109/L) found female sex to be associated with the development of ICU thrombocytopenia [30]. Comparing risk factors across studies is challenging as case mix, definitions of thrombocytopenia and analytic methods differ [29, 30, 32].
109/L) found female sex to be associated with the development of ICU thrombocytopenia [30]. Comparing risk factors across studies is challenging as case mix, definitions of thrombocytopenia and analytic methods differ [29, 30, 32].
Consistent with previous studies [4, 5, 29, 30], we observed that patients with thrombocytopenia had worse outcomes, including higher rates of bleeding, transfusion and mortality, fewer days alive without use of life-support and days alive and out of hospital. Associations between thrombocytopenia and bleeding, transfusion, and mortality followed a “dose–response” pattern with worse outcomes accompanying more severe thrombocytopenia. Patients with severe- and very severe thrombocytopenia had markedly higher mortality but were also more likely to experience grade 3 or 4 bleeding and to receive transfusions compared with patients with no- or less severe thrombocytopenia. In this heterogeneous ICU population, it remains unclear whether the observed excess mortality is directly related to thrombocytopenia and its consequences, such as bleeding and transfusions, or whether thrombocytopenia merely represents a marker of illness severity or the pathophysiology of the underlying disease. Importantly, identified risk factors and association with mortality do not represent causal relationships.
Most in-ICU platelet transfusions were prophylactic (64%) with a median platelet count before transfusion of 15 ×
× 109/L. Lower proportions of prophylactic platelet transfusions (40–45%) with higher median platelet counts before transfusion have been reported, which likely reflects differences in case mix and changes in practice over time [11, 12]. The European Society of Intensive Care Medicine (ESICM) recommends against prophylactic platelet transfusion unless the platelet count falls below 10
109/L. Lower proportions of prophylactic platelet transfusions (40–45%) with higher median platelet counts before transfusion have been reported, which likely reflects differences in case mix and changes in practice over time [11, 12]. The European Society of Intensive Care Medicine (ESICM) recommends against prophylactic platelet transfusion unless the platelet count falls below 10 ×
× 109/L, but the recommendation is only conditional due to very low certainty of evidence and randomised trials evaluating this strategy in general ICU patients are warranted [13].
109/L, but the recommendation is only conditional due to very low certainty of evidence and randomised trials evaluating this strategy in general ICU patients are warranted [13].
In our cohort, prophylactic pre-procedural platelet transfusions constituted 8% of in-ICU platelet transfusions and currently, ESICM makes no recommendations regarding this strategy for patients with platelet counts between 10–50 ×
× 109/L [13]. A recent non-inferiority trial enrolling patients in haematology wards and ICUs with platelet counts between 10–50
109/L [13]. A recent non-inferiority trial enrolling patients in haematology wards and ICUs with platelet counts between 10–50 ×
× 109/L showed that withholding versus providing a single prophylactic platelet transfusion before CVC placement was not non-inferior and resulted in more CVC-related bleedings [15]. Interestingly, the occurrence of bleeding was lower in ICU patients compared with haematology ward patients, which might support a no-transfusion strategy with intensive monitoring in ICU patients [15].
109/L showed that withholding versus providing a single prophylactic platelet transfusion before CVC placement was not non-inferior and resulted in more CVC-related bleedings [15]. Interestingly, the occurrence of bleeding was lower in ICU patients compared with haematology ward patients, which might support a no-transfusion strategy with intensive monitoring in ICU patients [15].
This study has some strengths. It was prospective and conducted, analysed and reported in compliance with a published protocol [16]. Further, contrary to many previous studies [1, 2, 6–9], we included a large, unselected, multinational ICU population. This study also provides data on the use of in-ICU platelet transfusions across several countries, which complements previous national studies [11, 12, 33].
Our study also has some limitations. First, 17% of the patients did not have a baseline platelet count and although we handled this as planned, by using the first available platelet count in the ICU measured on median (IQR) day 1 (1–1), some misclassification cannot be ruled out. Second, 7% of patients were transferred directly from other ICUs, with a median stay of 3 days before inclusion. This could underestimate event rates and overestimate baseline thrombocytopenia at the expense of ICU thrombocytopenia. Third, patients with haematological malignancies constituted 8% of the population and 15% of the cases with thrombocytopenia; frequencies of thrombocytopenia may be lower in ICU populations with lower proportions of patients with haematological malignancies. Fourth, 2% of patients were transferred to a non-study ICU without available daily variables; these were analysed under the assumption that no events occurred during days spent in the non-study ICU. Fourth, descriptions of platelet counts before platelet transfusions were added post hoc and assumptions made during computation may have affected results. Fifth, we used pre-specified logistic regression analyses to assess risk factors for ICU thrombocytopenia and associations between baseline thrombocytopenia and 90-day mortality. Although post hoc sensitivity analyses including Cox proportional hazard models accounting for time-to-event and competing risks produced similar results, our regression analyses should be interpreted carefully considering the limited number of included variables and risk of residual confounding (e.g., the effects of baseline thrombocytopenia on 90-day mortality may vary according to the underlying cause, which was not assessed in this study). Lastly, meaningful interpretation of risk factor analyses for ICU thrombocytopenia <
< 50
50 ×
× 109/L was hampered by few events and the analysis of baseline thrombocytopenia
109/L was hampered by few events and the analysis of baseline thrombocytopenia <
< 50
50 ×
× 109/L and 90-day mortality was likely underpowered.
109/L and 90-day mortality was likely underpowered.
Conclusions
In this international, prospective cohort study, 43% of adult acutely admitted ICU patients had thrombocytopenia. Patients with thrombocytopenia had worse outcomes including increased 90-day mortality. Baseline risk factors for the development of thrombocytopenia during ICU stay included male sex, non-AIDS-, non-cancer related immune deficiency, liver failure, higher illness severity, bleeding, and septic shock. Platelet transfusions were given to 23% of patients with thrombocytopenia and most were prophylactic.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This study was performed on behalf of the “Caring for critically ill immunocompromised patients: Multinational Network (Nine-I)” study group. PLOT-ICU Collaborators: Ahmed Khalil, Ahmed Yehia, Haney Salem, Hesham Farahat, Manu Sudevan, Melissa Biggart, Nirmeen Fatima, Mohammed Elkhonezy, Department of Critical Care, King's College Hospital NHS Foundation Trust, London, UK; Anne-Marie Bunzel, Rine M. Siegumfeldt, Stine R. Vestergaard; Department of Anesthesia and Intensive Care, Aalborg University Hospital, Aalborg University, Aalborg, Denmark; Juliette Pelle, Minh-Pierre Lê, Clara Vigneron, Morgane Bertrix, Paul Cirera, Driss Laghlam, Swann Bredin, Nathalie Marin, Médecine Intensive & Réanimation, Hôpital Cochin, Assistance Publique-Hôpitaux de Paris, Université Paris Cité, Paris, France; Maria Toppenberg, Department of Anaesthesiology and Intensive Care, Aarhus University Hospital, Aarhus, Denmark; Brice Benelli, Service de Médecine Intensive Réanimation, Hôpitaux Universitaires Henri-Mondor, Assistance Publique–Hôpitaux de Paris, Paris, France. Amélie Seguin, Charlotte Garret, Florian Guillotin, Gauthier Blonz, Jean-Baptiste Lascarrou, Jérémie Lemarie, Luc Desmedt, Maïté Agbakou, Mathieu Carpentier, Maëlle Martin, Naïla Benkalfate, Olivier Zambon, Paul Decamps, Pauline L. Wilquin, Soraya Benguerfi, Médecine Intensive Réanimation, CHU de Nantes, Université de Nantes, Nantes, France; John Gardner, Critical Care Unit, Queen Elizabeth University Hospital, Glasgow, UK; Natalie Remor, Sheila Carr, Gloria Yang, Critical Care Medicine Service, Department of Anesthesiology & Critical Care Medicine, Memorial Sloan Kettering Cancer Center, New York, NY, USA; Coralie Gernez, Ingrid Thiry, Médecine Intensive Réanimation, Hôpital Louis Mourier, Assistance Publique-Hôpitaux de Paris, DMU ESPRIT, Paris, France; Louai Missri, Service de Médecine Intensive-Réanimation, Hôpital Saint-Antoine, Assistance Publique-Hôpitaux de Paris, Sorbonne Université, Paris, France; Moritz K. G. Denneborg Department of Intensive Care, Holbaek Hospital, Holbaek, Denmark; Katherine Brown, Department of Intensive Care, Glasgow Royal Infirmary, Glasgow, UK; Vanessa Casares, Intensive Care Department, Vall d’Hebron Hospital Universitari, Barcelona, Spain; Mirka Sivula, Elina Lappi, Leena Pettilä, Jonna Heinonen, Minttu Saario, Department of Perioperative and Intensive Care Medicine, University of Helsinki and Helsinki University Hospital, Helsinki, Finland; Manal K. Mecheri, Alezandre Elabbadi, Cyrielle Desnos; Antoine Lafarge, Olfa Mghirbi, Médecine Intensive & Réanimation, Hôpital Saint-Louis, Assistance Publique-Hôpitaux de Paris, Université Paris Cité, Paris, France; Brit Å. Sjøbø, Department of Anaesthesia and Intensive Care, Haukeland University Hospital, Bergen, Norway; Cecilie Christoffersen, Department of Anaesthesia and Intensive Care Medicine, Division of Emergencies and Critical Care, Rikshospitalet, Oslo University Hospital, Oslo, Norway; Frederik H. Bestle, Department of Anaesthesiology and Intensive Care, Copenhagen University Hospital-North Zealand, Hilleroed, Denmark; Claudia Lemos, Department of Intensive Care, Hospital Central do Funchal, Funchal, Portugal;Cristiana V. Gonçalves, Nuno M. B. Jacinto, Monica P. Anselmo, Intensive Care Unit, Hospital Professor Doutor Fernando Fonseca, EPE, Amadora, Portugal; Marius M. Hoeper, Interdisciplinary Intensive Care Unit, Department for Respiratory Diseases and German Centre of Lung Research (DZL), Hannover Medical School, Hannover, Germany; Marja Hoff, Department of Intensive Care B203, Akershus University Hospital, Akershus, Norway; Pedro M. Simões Freire, Department of Intensive Care Medicine, Unit 2, Hospital Egaz Moniz-CHLO, EPE, Lisbon, Portugal.
Author contributions
CTA, FP, AP, EA, KP, AVDL, ABD, SC, PC, PP, LC, VM, MK, TL, TK, JH, MHM and LR conceptualised the study. All authors contributed to the acquisition of the data. CTA and AG performed the statistical analyses. CTA drafted the manuscript with input from the steering committee and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript for publication.
Funding
Open access funding provided by Royal Library, Copenhagen University Library. This study was funded by the Research Council of Rigshospitalet, the Ehrenreich’s Foundation, and the Dagmar Marshalls Foundation. The Memorial Sloan Kettering Cancer Center part of the study was supported by the Core Grant, Grant/Award Number: P30CA008748; Department of Anaesthesiology & Critical Care Medicine, Memorial Sloan Kettering Cancer Center, New York, NY, United States of America. None of the funders had any influence on the design, conduct or reporting of the study.
Data availability
The data that support the findings of this study can be made available from the corresponding author (LR) upon request.
Declarations
The Department of Intensive Care at Rigshospitalet (CTA, AG, MHM, AP, LR) has received funding for other projects from the Novo Nordisk Foundation, Sygeforsikringen ‘danmark’, and Pfizer and has conducted contract research for AM-Pharma. FP has received honoraria for consulting and lectures from Gilead and an institutional grant from Alexion Pharma. AP has received honoraria from Novartis for participation in an advisory board. EA has received research grants from MSD Avenir and Alexion and honoraria for lectures from Alexion, Sanofi and Pfizer. . AVDL has received honoraria from Sanofi for participation in an advisory board. PC has received consulting fees from Sanofi, Gilead, Alexion and Janssen and honoraria for lectures from Merck Sharp & Dohme, Gilead, Alexion and Pfizer. PP has received consulting fees from Sanofi and Gilead and honoraria from Merck Sharp & Dohme, Gilead, Mundipharma and Pfizer for academic and educational work. JH has received consulting fees from Paion and honoraria for lectures from the Finnish Medical Association, Laboratory Medicine and Duodecim. EC received fees for lectures and conference talks and had travel and accommodation expenses related to attending scientific meetings covered by Gilead, Shionogi B.V. and Sanofi-Genzyme. ARH has received honoraria from Pfizer for lectures. MHB has received consulting fees from AM-pharma and Inotrem. Full disclosure forms from all authors are available on the publisher’s website.
Footnotes
PLOT-ICU Collaborators are listed in Acknowledgment section.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
12/11/2023
A Correction to this paper has been published: 10.1007/s00134-023-07291-6
Contributor Information
on behalf of the PLOT-ICU Collaborators and the Nine-I Study Group:
References
Full text links
Read article at publisher's site: https://doi.org/10.1007/s00134-023-07225-2
Read article for free, from open access legal sources, via Unpaywall:
https://link.springer.com/content/pdf/10.1007/s00134-023-07225-2.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/155264896
Article citations
Severe bleeding events among critically ill patients with haematological malignancies.
Ann Intensive Care, 14(1):155, 07 Oct 2024
Cited by: 0 articles | PMID: 39373939 | PMCID: PMC11458868
Platelet count has a nonlinear association with 30-day in-hospital mortality in ICU end-stage kidney disease patients: a multicenter retrospective cohort study.
Sci Rep, 14(1):22535, 28 Sep 2024
Cited by: 0 articles | PMID: 39341971 | PMCID: PMC11439004
Ten things ICU specialists need to know about platelet transfusions.
Intensive Care Med, 50(10):1699-1702, 22 Aug 2024
Cited by: 0 articles | PMID: 39172239
Nonlinear relationship between platelet count and 30-day in-hospital mortality in ICU acute respiratory failure patients: a multicenter retrospective cohort study.
Eur J Med Res, 29(1):312, 08 Jun 2024
Cited by: 1 article | PMID: 38849948
Nonlinear relationship between platelet count and 30-day in-hospital mortality in intensive care unit stroke patients: a multicenter retrospective cohort study.
Front Neurol, 15:1374159, 24 Apr 2024
Cited by: 1 article | PMID: 38721117 | PMCID: PMC11076867
Go to all (10) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Platelet transfusions and thrombocytopenia in intensive care units: Protocol for an international inception cohort study (PLOT-ICU).
Acta Anaesthesiol Scand, 66(9):1146-1155, 23 Aug 2022
Cited by: 2 articles | PMID: 36054145 | PMCID: PMC9542787
Platelet transfusions in adult ICU patients with thrombocytopenia: A sub-study of the PLOT-ICU inception cohort study.
Acta Anaesthesiol Scand, 68(8):1018-1030, 05 Jun 2024
Cited by: 0 articles | PMID: 38840310
Platelet transfusions in adult thrombocytopenic ICU patients: Protocol for a sub-study of the PLOT-ICU cohort.
Acta Anaesthesiol Scand, 68(3):434-440, 19 Dec 2023
Cited by: 0 articles | PMID: 38115558
Thrombocytopenia in the intensive care unit-diagnostic approach and management.
Semin Hematol, 50(3):239-250, 01 Jul 2013
Cited by: 17 articles | PMID: 23953341
Review
Funding
Funders who supported this work.
Dagmar Marshalls Fond
Grosserer Jakob Ehrenreich og Hustru Grete Ehrenreichs Fond
Memorial Sloan-Kettering Cancer Center (1)
Grant ID: P30CA008748
NCI NIH HHS (1)
Grant ID: P30 CA008748

 1,3,4,58 and on behalf of the PLOT-ICU Collaborators and the Nine-I Study Group
1,3,4,58 and on behalf of the PLOT-ICU Collaborators and the Nine-I Study Group


