Abstract
Importance
The human gut microbiota, including Bacteroides, is required for the degradation of otherwise undigestible polysaccharides. The gut microbiota uses polysaccharides as an energy source, and fermentation products such as short-chain fatty acids are beneficial to the human host. This use of polysaccharides is dependent on the proper pairing of a TonB protein with polysaccharide-specific TonB-dependent transporters; however, the formation of these protein complexes is poorly understood. In this study, we examine the role of 11 predicted TonB homologs in polysaccharide uptake. We show that two proteins, TonB4 and TonB6, may be functionally redundant. This may allow for the development of drugs targeting Bacteroides species containing only a TonB4 homolog with limited impact on species encoding the redundant TonB6.Free full text

Multiple TonB homologs are important for carbohydrate utilization by Bacteroides thetaiotaomicron
Associated Data
ABSTRACT
The human gut microbiota is able to degrade otherwise undigestible polysaccharides, largely through the activity of Bacteroides. Uptake of polysaccharides into Bacteroides is controlled by TonB-dependent transporters (TBDTs), whose transport is energized by an inner membrane complex composed of the proteins TonB, ExbB, and ExbD. Bacteroides thetaiotaomicron (B. theta) encodes 11 TonB homologs that are predicted to be able to contact TBDTs to facilitate transport. However, it is not clear which TonBs are important for polysaccharide uptake. Using strains in which each of the 11 predicted tonB genes are deleted, we show that TonB4 (BT2059) is important but not essential for proper growth on starch. In the absence of TonB4, we observed an increase in the abundance of TonB6 (BT2762) in the membrane of B. theta, suggesting functional redundancy of these TonB proteins. The growth of the single deletion strains on pectic galactan, chondroitin sulfate, arabinan, and levan suggests a similar functional redundancy of the TonB proteins. A search for highly homologous proteins across other Bacteroides species and recent work in Bacteroides fragilis suggests that TonB4 is widely conserved and may play a common role in polysaccharide uptake. However, proteins similar to TonB6 are found only in B. theta and closely related species, suggesting that the functional redundancy of TonB4 and TonB6 may be limited across Bacteroides. This study extends our understanding of the protein network required for polysaccharide utilization in B. theta and highlights differences in TonB complexes across Bacteroides species.
IMPORTANCE
The human gut microbiota, including Bacteroides, is required for the degradation of otherwise undigestible polysaccharides. The gut microbiota uses polysaccharides as an energy source, and fermentation products such as short-chain fatty acids are beneficial to the human host. This use of polysaccharides is dependent on the proper pairing of a TonB protein with polysaccharide-specific TonB-dependent transporters; however, the formation of these protein complexes is poorly understood. In this study, we examine the role of 11 predicted TonB homologs in polysaccharide uptake. We show that two proteins, TonB4 and TonB6, may be functionally redundant. This may allow for the development of drugs targeting Bacteroides species containing only a TonB4 homolog with limited impact on species encoding the redundant TonB6.
INTRODUCTION
The human gut microbiota performs many important functions that promote human health, including the degradation of complex carbohydrates (fibers or polysaccharides) from our diet (1). Many bacteria in the microbiota ferment polysaccharides, resulting in the release of short-chain fatty acids (SCFAs), such as butyrate, acetate, and propionate (2). These SCFAs then serve as a key energy source for colonocytes and promote intestinal barrier function (3, 4). Understanding the molecular mechanisms of polysaccharide degradation will provide opportunities to develop functional foods as therapeutics or inhibitors of polysaccharide degradation to manipulate microbial metabolism and improve human health.
Gram-negative Bacteroidetes are abundant members of the Western adult gut microbiota and maintain a large capacity to degrade polysaccharides (5, 6). In these bacteria, the genes required for polysaccharide use are organized into polysaccharide utilization loci (PULs) (7). Each PUL is generally transcriptionally activated in response to a distinct polysaccharide substrate (8, 9). Several lipoproteins encoded within each PUL localize to the cell surface to bind, degrade, and import the target substrate (7, 10). The prototypical PUL is the starch utilization system (Sus) from Bacteroides thetaiotaomicron (B. theta) (7, 11, 12). A common feature across all Sus-like systems is at least one pair of proteins homologous to SusC, a putative TonB-dependent transporter (TBDT), and SusD, a starch-binding protein (Fig. 1) (7, 13). Detailed biochemical studies of the additional outer membrane proteins from PULs that target starch, arabinan, levan, chondroitin sulfate, heparin, and several other polysaccharides have helped develop a model of how polysaccharides are initially degraded at the cell surface and elucidated which oligosaccharides are selected and imported into the cell via the SusCD-like complex (14 – 21).
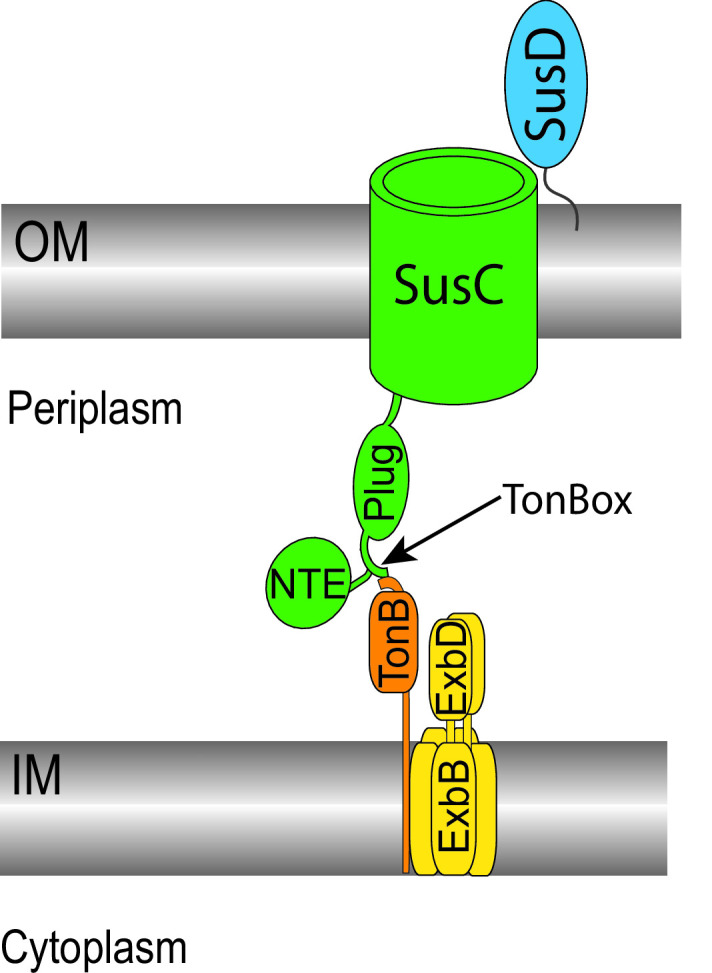
Architecture of the predicted SusC, SusD, TonB, and ExbBD complex. Surface glycan-binding protein SusD is shown in blue, associated with SusC at the outer membrane (OM). TonB-dependent transporter SusC is shown in green, including the plug and N-terminal extension (NTE) domains. The TonBox precedes the plug domain and here is shown pairing with TonB (orange). ExbB and ExbD are shown in yellow in the inner membrane (IM). ExbB is in a pentameric arrangement enclosed around a dimer of ExbD that extends into the periplasm.
SusC is required for starch utilization via transport of maltooligosaccharides across the outer membrane (22). SusC and its thousands of homologs in Bacteroidetes share sequence homology with well-studied TBDTs of Gram-negative organisms. Thus far, one TBDT from Porphyromonas gingivalis and three SusC-like transporters from B. theta have been structurally characterized and show high structural homology with TBDTs, such as the well-studied FhuA and FepA from Escherichia coli (21, 23 – 25).
Key conserved features of these transporters include a 22-β-strand barrel that traverses the outer membrane and houses a plug domain that occludes solute passage until the transporter is activated. The TonB box or TonBox is a sequence of 4–8 conserved residues forming a β-strand that precedes the plug domain and is important for pairing with TonB (Fig. 1) (26). Some B. theta TBDTs are predicted to include two additional domains termed the N-terminal extension (NTE) and the Secretin and TonB N-terminus (STN) domain (25, 27). The precise rearrangement of the plug domain to allow solute passage through the barrel is poorly understood but is facilitated by pairing to an inner membrane complex that harnesses proton motive force. This complex includes the proteins TonB, ExbB, and ExbD (Fig. 1). The structure of the complex with TonB has not been determined, but another characterization of TonB suggests that at least one copy of TonB interacts with the ExbBD complex via the TonB N-terminal membrane-spanning α-helix (28 – 30). This N-terminal α-helix is followed by a linker region that spans the periplasm and a well-ordered C-terminal domain (31). The C-terminal domains of characterized TonB proteins share a common fold of three antiparallel β-sheets with two α-helices (Fig. 2B and C) (32, 33). The final β-strand at the C-terminus directly contacts the TBDT for β-sheet pairing with the TonBox (Fig. 2B and C) (26, 34, 35).
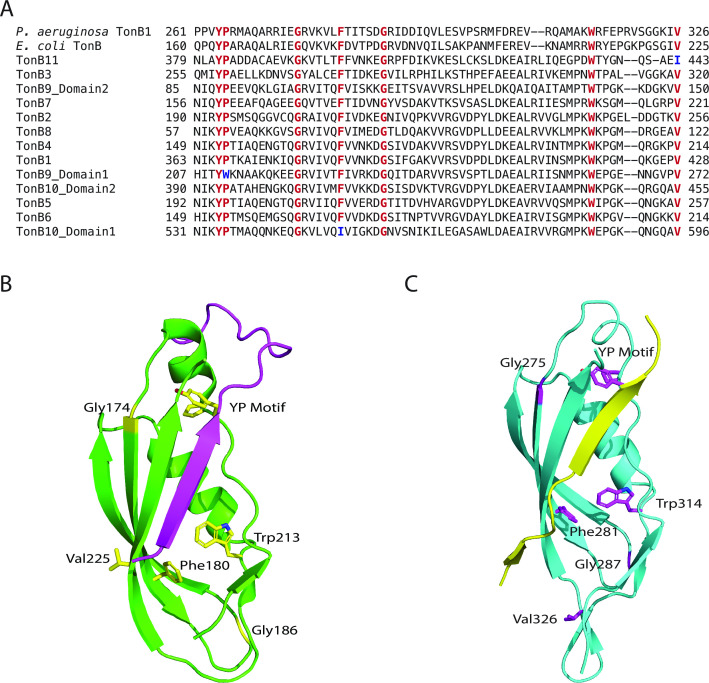
(A) Excerpt of the multiple sequence alignment of the 11 identified TonB proteins from B. thetaiotaomicron with E. coli TonB and Pseudomonas aeruginosa TonB1. Conserved residues are highlighted in red, and deviations from this conservation are highlighted in blue. The full alignment is shown in Fig. S2. (B) Structure of E. coli TonB (green) in complex with the TonBox of BtuB (purple), PDB: 2GSK. Conserved residues from panel A are highlighted in yellow. (C) Structure of Pseudomonas aeruginosa TonB1 (cyan) in complex with the TonBox of FoxA (yellow), PDB: 6I97. Conserved residues from panel A are highlighted in purple.
Unlike E. coli, which expresses only one TonB, B. theta and other Bacteroidetes can encode up to 15 TonB homologs. In some Gram-negative organisms that encode multiple TonB proteins, such as Xanthomonas campestris, there is evidence of TonB-TBDT pairing redundancy such that more than one TonB can energize a discrete transporter (36). Conversely, in Caulobacter crescentus, the deletion of a single TonB completely abrogates the import of maltose (37). TonB-TBDT pairing has been explored in two Bacteroidetes, Riemerella anatipestifer and Bacteroides fragilis. Characterization of the three TonB homologs in R. anatipestifer suggests that each TonB functions differently but that there may be some redundancy in hemin uptake (38). Conversely, in B. fragilis, deletion of a single tonB gene completely eliminated growth on a variety of substrates, including several different polysaccharides, suggesting that a single TonB is responsible for pairing with a variety of transporters under these conditions (39).
The TonB proteins encoded by B. theta differ in both number and sequence from those encoded by B. fragilis, suggesting that TonB pairing may differ even between these closely related species. Through genetic analysis, we identify 11 TonB homologs in B. theta and construct strains containing a gene deletion of each homolog. To better understand Sus as the prototypical PUL, we analyzed the growth of each of these strains on starch and show that deletion of TonB4 leads to less efficient growth on starch. However, in contrast to B. fragilis, this deletion does not completely eliminate growth on starch or other polysaccharides, allowing us to identify a second, redundant TonB important in starch utilization. We then expand our analysis to other B. theta PULs with well-characterized TBDTs showing a similar redundancy in TonB function. This work underscores the roles of TonB homologs during outer membrane transport and expands our understanding of glycan uptake in Bacteroides.
RESULTS
Identification of 11 TonB proteins in B. theta
High sequence diversity has been seen across characterized TonB proteins, making high-confidence annotation of TonB proteins difficult. In fact, varying numbers of genes are annotated as tonB across different analyses of the B. theta genome (13, 39, 40). We compiled a list of 11 potential TonB proteins in B. theta by searching the genome for proteins containing the Gram-negative bacterial TonB protein C-terminal Pfam domain (TonB_C, PF03544) (Table 1, additional information in Fig. S1A). One additional protein (BT3921) showed a match to PF03544 but has not been included in this analysis due to the low confidence of that prediction (E-value = 2.7e-05). This analysis matches the 11 B. theta TonB proteins identified by Parker et al. (39).
TABLE 1
Identified TonB proteins in Bacteroides thetaiotaomicron VPI-5482
| Protein name | Locus tag | Total protein length | Location of PF03544 TonB_C domain | E-value | Additional domain? | Location of additional domain | E-value of additional domain |
|---|---|---|---|---|---|---|---|
| TonB1 | BT0813 | 443 | 363–439 | 1.8e-21 | Peptidase_M56 (PF05569) | 137–266 | 5.7e-16 |
| TonB2 | BT1056 | 269 | 190–268 | 1.4e-14 | No | ||
| TonB3 | BT1668 | 350 | 256–331 | 4.5e-14 | No | ||
| TonB4 | BT2059 | 227 | 149–226 | 2.2e-23 | No | ||
| TonB5 | BT2665 | 270 | 192–269 | 1.6e-22 | No | ||
| TonB6 | BT2762 | 227 | 149–226 | 4.6e-23 | No | ||
| TonB7 | BT3192 | 249 | 156–232 | 1.0e-20 | CarbopepD-reg_2 (PF13715) | 33–112 | 2.5e-11 |
| TonB8 | BT3673 | 283 | 57–134 | 1.1e-20 | DUF4488 (PF14869) | 159–281 | 1.2e-40 |
| TonB9 | BT3896 | 285 | 207–284 a | 1.3e-21 | TonB_C (PF03544) | 85–161 | 4.6e-21 |
| TonB10 | BT3898 | 609 | 531–608 a | 1.1e-22 | TonB_C (PF03544) | 390–467 | 1.8e-18 |
| Peptidase_M56 (PF05569) | 160–261 | 3.2e-13 | |||||
| TonB11 | BT4460 | 449 | 379–440 | 1.6e-07 | CarbopepD-reg_2 (PF13715) | 228–309 | 8.0e-19 |
Sequence similarity between these 11 B. theta TonB proteins and E. coli TonB is low, ranging from 10% to 37% sequence identity (Fig. S1B). This is partially due to the lack of the TonB polyproline region (PF16031) in all candidate B. theta TonB proteins. In E. coli TonB, this region has been shown to be important, though not essential, for properly spanning the periplasm to interact both with the ExbBD complex and TBDTs (31, 41). Characterized TonB proteins with polyproline regions are predominantly from Enterobacteriaceae, so the lack of this region in our identified TonB proteins may suggest that a different structure is needed to properly span the periplasm of other bacteria, including those from the Bacteroidetes phylum (31, 42, 43).
Several B. theta TonB proteins (TonB1, 7, 8, 9, 10, and 11) also contain additional domains appended to the TonB C-terminal domain (Table 1). To our knowledge, TonB proteins with additional domains have not been functionally characterized, so it is difficult to predict if these additional domains confer additional or altered functionality. The predicted peptidase domains (PF05569) in TonB1 and 10 could suggest a role for these proteins in signal transduction. The peptidase M56 family includes the BlaR1 protein that serves as the sensor-transducer for the β-lactam antibiotic resistance pathway in Staphylococci; however, there is no evidence of a similar mechanism for β-lactam resistance in B. theta (44, 45). Previous bioinformatic analysis of TonB proteins identified only nine proteins with this M56 N-terminal extension out of the 263 sequences analyzed (40). These nine TonB proteins included sequences from B. fragilis and X. campestris, suggesting that this domain structure may appear widely across Gram-negative bacteria (40). TonB7 and TonB11 both contain predicted CarboxypepD_reg-like domains (PF13715), which are often also found in TBDTs, including SusC and the levan-targeting BT1763, as the NTE (Fig. 1) (27). The structure of this domain from BT1763 showed an Ig-like fold, and deletion of this domain completely eliminated growth on levan (25). The association with both TBDT and TonB proteins as well as the domain’s importance for BT1763 function may suggest a role in the formation of the TBDT-TonB-ExbBD complex (25, 46). The DUF4488 domain (PF14869) found in TonB8 was structurally characterized in three Bacteroides proteins (47). The function of this domain is unclear, but it appears to be restricted to Bacteroidetes, and the structures revealed an unknown ligand bound to each protein that suggests a role in binding small polar molecules, such as carbohydrates (47). Most DUF4488-containing proteins are made up only of the DUF4488 domain, but 19 of the 146 analyzed sequences contained a TonB C-terminal domain similar to TonB8 (47). TonB9 and 10 contain a second TonB C-terminal domain. This dual TonB domain structure seems to be limited to Bacteroidetes (40). In both TonB9 and 10, the two TonB C-terminal domains share only moderate sequence identity (62.8% and 73.1%, respectively) that is similar or lower than the shared sequence identity of the domains of other B. theta TonB proteins (Fig. S1C, shaded in blue).
Despite these differences in the full-length TonB proteins, the identified B. theta TonB C-terminal domains show moderate sequence similarity to the E. coli TonB C-terminal domain (37.8%–49.4%) and high conservation of amino acids that are important for proper function of E. coli and Pseudomonas aeruginosa TonB (Fig. 2; Fig. S1C and S2). Complete conservation is seen at the YP motif (residues 163–164 in E. coli TonB) and at various points in the downstream region that forms the core of the domain. Notably, none of the conserved residues are in the final β-strand proposed to pair with TBDT TonBox (Fig. 2B and C). The YP motif is the most conserved feature among TonB proteins (40). The tyrosine residue has been shown to interact with E. coli TBDTs BtuB and FecA, and mutation of this residue results in a non-functional P. aeruginosa TonB1 (35, 48 – 50). The proline is not conserved in the second TonB domain of TonB9, but mutation of this residue in other TonB proteins does not disrupt function (50). Complete conservation is seen at residues equivalent to the E. coli TonB Gly174, Gly186, and Trp213. The precise role of these residues is unclear, but mutations in the Gly residues in E. coli TonB reduced E. coli growth on iron and sensitivity to colicins, suggesting that these residues are important for proper function of TonB (51). The equivalent residue to Gly174 in P. aeruginosa TonB1 (Gly275) is also essential for proper function (50). Although multimer formation by TonB is still unclear, Trp213 in E. coli TonB has been suggested to promote dimer formation, as W213C mutants readily form cross-linked dimers (52). Conservation is also seen at E. coli Phe180 in all sequences except Domain 2 of TonB10; however, this residue was not found to be highly conserved in a broader comparison of TonB proteins, and it is unclear what role it plays in E. coli TonB (40). Finally, Val225 of E. coli TonB immediately precedes the region where β-sheet pairing occurs with the TonBox of TBDT, but as side chains do not seem to be important for this interaction, it is unclear why this residue is conserved in all sequences analyzed except TonB11, where it is replaced with an isoleucine (Fig. 2A and B). Additionally, the corresponding valine in P. aeruginosa TonB1 (Val326) is well outside the β-strand that pairs with the TonBox of FoxA, suggesting that this residue may play an additional role in TonB function (Fig. 2C).
To further understand the potential role of these 11 TonB proteins, we analyzed the genomic context of each of the genes (Fig. 3). The tonB genes are dispersed throughout the genome, with most tonB genes being alone without other Ton complex genes. Notable exceptions to this are tonB9 and tonB10, which are found near each other, separated only by one gene predicted to encode a thioredoxin similar to DsbE. Additionally, tonB5 (bt2665) is organized next to and in the same transcriptional orientation as predicted ExbB BT2668 and predicted ExbDs BT2666-2667. The tonb4 (bt2059) gene is also found near predicted ExbB BT2055 and predicted ExbDs BT2052-2053, but the intervening genes include proteins of unknown function (hypothetical proteins), a hydrolase, and isoprenyl synthase that are not predicted to be involved in the formation of the transport complex. Several tonB genes are found near transposases, including tonB5, tonB6, tonB9, and tonB10, although it is not clear if the tonB genes would be transferred by these transposases. Particularly interesting is tonB8, which appears to be found at the end of rhamnogalacturonan-II (RG-II) PUL 3 (20). The bt3673 gene was previously annotated as a hypothetical protein and was not characterized as part of the previous exploration of RG-II degradation, so it is not clear if this gene is important in RG-II degradation. Similarly, tonB1, tonB2, tonB7, and tonB11 are found near predicted transcriptional regulators that may allow for better understanding of the control of expression of these genes.
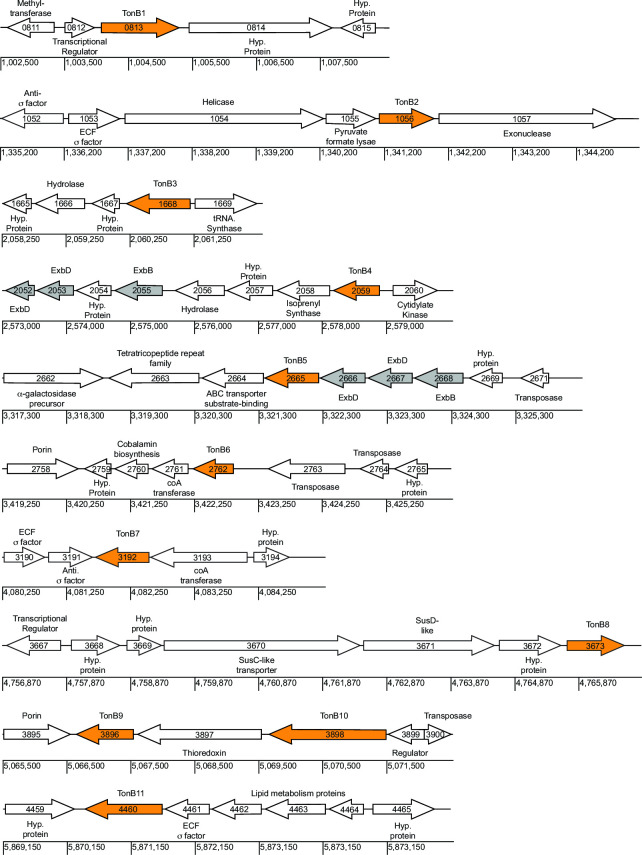
The genomic context of each of the 11 tonB genes. Each locus tag is shown within the arrow depicting the putative open reading frame. Arrow direction depicts the transcription orientation. Scale is shown in base pairs using the reference sequence GCF_000011065.1. The predicted function of the peptide product is depicted outside each gene arrow. Hyp. protein, conserved hypothetical protein.
Taken together, the conservation of key residues and the overall predicted C-terminal domains of the identified B. theta proteins suggest that these proteins are capable of functioning as TonB proteins. However, the addition of unique domains to the overall protein architecture of several of these proteins may allow for the formation of TBDT-TonB-ExbBD complexes that are functionally distinct from characterized complexes, and the lack of genetic organization with ExbBD genes allows for unique assemblies of these complexes. To begin to explore the function of these TonB proteins, we first focused on the formation of a SusC-TonB pair by deleting the TonBox of SusC and constructing in-frame deletions for each of the 11 tonB genes to explore the effect of each deletion on starch utilization.
Deletion of the TonBox of SusC eliminates the growth of B. theta on starch
The canonical E. coli TonBox consensus sequence is acidic-T-hydrophobic-hydrophobic-V-polar-A (26). Conservation of the canonical TonBox sequence is seen across many TBDTs, but some divergence has made it difficult to confidently predict this motif from the sequence alone. To identify the TonBox in SusC, we looked for two features: (i) high conservation across a short region preceding the putative plug of SusC-like transporters in B. theta and (ii) close alignments with the TonBox from characterized TBDTs from other bacteria.
Using an alignment of 100 SusC-like proteins from B. theta, we identified a highly conserved region with the consensus sequence DEVVV(V/T/I) (representative sequences shown in Fig. 4A and Fig. S3). This region also aligns well with the characterized TonBox sequences from FecA (48) and FhuE (53) from E. coli, HasR from Serratia marcescens (54), FoxA (32) and FpvA (55) from P. aeruginosa, and RagA from P. gingivalis (24) (Fig. 4A; Fig. S3). Analysis of BT1763 from B. theta also identified this sequence as the TonBox and showed a significant change in function when the TonBox was mutated or deleted (25). Based on this, we propose that the TonBox sequence in SusC is DEVVVI found at residues 105–110.
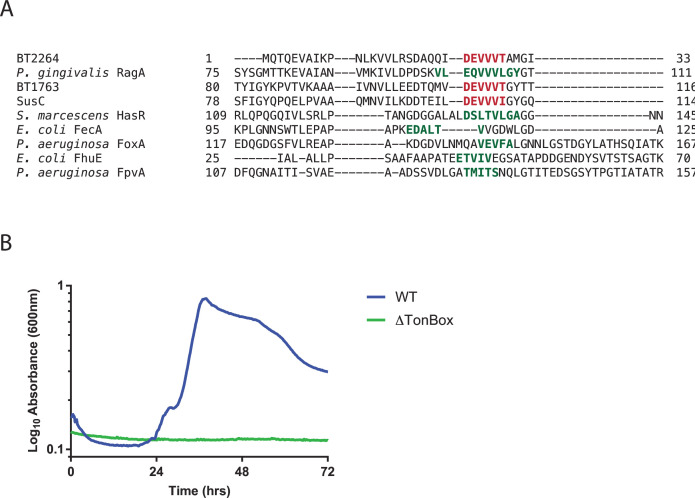
(A) Excerpt of the multiple sequence alignment of SusC from B. thetaiotaomicron with BT1763 and BT2264 for which structures have been determined and characterized TonB-dependent transporters from Porphyromonas gingivalis, Escherichia coli, Serratia marcescens, and Pseudomonas aeruginosa. Identified TonBoxes are shown in green, and our proposed TonBoxes for the B. thetaiotaomicron transporters are shown in red. The full sequence alignment is shown in Fig. S3. (B) Average growth curves of wild-type (WT) and SusC TonBox deletion (ΔTonBox) B. thetaiotaomicron cultured on 2.5 mg mL−1 potato amylopectin. Matched growth experiments in maltose and maltoheptaose are shown in Fig. S4.
We constructed an in-frame deletion of these six residues to create our ΔTonBox strain of B. theta. We chose to delete these residues rather than mutating them, as previous studies have shown that mutations to chemically distinct residues often do not disrupt TBDT function, but deletion of the TonBox disrupts the function of the TBDT likely by eliminating pairing to TonB (26, 54). Our B. theta ΔTonBox strain grows normally on maltose, which does not have to be taken up through SusC (Fig. S4A). However, the ΔTonBox strain cannot grow on amylopectin (Fig. 4B) or other starch substrates, including maltoheptaose (Fig. S4B), that wild-type B. theta can efficiently utilize. This suggests that with the TonBox removed, SusC cannot pair with TonB to import these starch substrates, supporting the role of SusC as a TonB-dependent transporter and the importance of this pairing. Interestingly, a similar TonBox deletion in B. theta levan TBDT BT1763 caused only a lag in growth, while a full deletion of the NTE was needed to eliminate growth on levan (25). These results support the importance of the TonBox but suggest that further characterization of both the TonBox and the NTE may be required to fully understand TBDT-TonB pairing across PULs.
Deletion of TonB4 increases the lag phase of B. theta growth on starch
We explored the role of each TonB 1–11 by assessing the effect of these gene deletions on the function of the prototypical Bacteroides TBDT, SusC, during starch utilization. We began by using glucose or maltose as the sole carbon source, as B. theta does not require TBDTs to import these sugars, and therefore, deletion of TonB proteins should not affect growth. However, deletion of TonB7, but no other TonB genes, resulted in consistently slower growth to both OD = 0.3 and max OD on glucose (representative growth curves shown in Fig. 5A, growth time to OD = 0.3 over four experiments shown in Fig. S5A) and maltose (Fig. S5B). B. theta does require TBDTs to uptake vitamin B12 and heme, which are found in the minimal media used for these growths, so it is possible that TonB7 pairs with at least one of these TBDTs; however, the TonB7 protein was not identified in previous proteomics analysis of B. theta conducted in similar media and well as the proteomics analysis presented here (56, 57). This suggests that the location of this gene in the B. theta genome may play a more important role than the expression of the TonB7 protein, requiring characterization beyond the scope of this work.
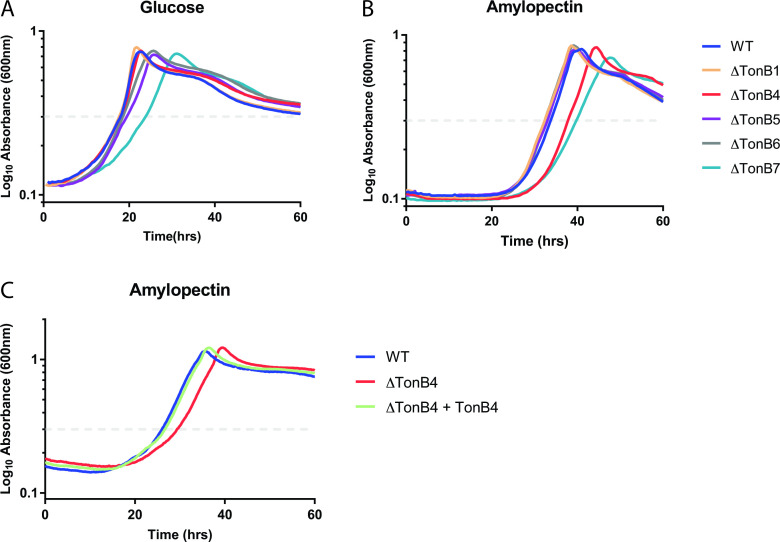
(A and B) Representative average growth curves of wild-type (WT) and select TonB deletion strains of B. thetaiotaomicron cultured on 5 mg mL−1 glucose (A) and 2.5 mg mL−1 potato amylopectin (B). The dashed line indicates OD = 0.3, which is used as a reference point for calculating the lag time. Growth to OD = 0.3 for all TonB deletions (1–11) over four experiments and matched growth experiments in maltose are shown in Fig. S5A through D. (C) Representative average growth curves of WT, TonB4 deletion strain, and the TonB4 deletion strain with the TonB4 gene complemented in another location on the genome cultured on 5 mg mL−1 potato amylopectin. Matched growth experiments in maltoheptaose and maize amylopectin are shown in Fig. S5E and F.
We next assessed the growth of each TonB deletion strain on starch substrates. Deletion of TonB4 resulted in consistently slower growth to both OD = 0.3 and max OD on potato amylopectin (representative growth curves shown in Fig. 5B, growth time to OD = 0.3 over four experiments shown in Fig. S5C and D). The slower growth of TonB7 was consistent with what was seen in glucose and maltose. The slower growth of TonB4 could be rescued by complementing the gene into another location on the chromosome, suggesting that this reduced growth is due to the lack of the TonB4 protein (Fig. 5C; Fig. S5C and D). Similar slow growth of ΔTonB4 and a return to wild-type-like growth in the complementation strain were also seen for other starch substrates, including maltoheptaose (Fig. S5E) and maize amylopectin (Fig. S5F).
TonB6 may compensate for the loss of TonB4
That we observed a lag but not loss of growth for ΔTonB4 led us to question if there is a specific TonB protein that can replace TonB4 in pairing with SusC or if any of the remaining 10 TonB proteins could properly function with SusC. We have previously reported membrane proteomics to quantify the amounts of Sus proteins in cells grown in the presence of maltose to induce Sus expression, and TonB4 was the only TonB protein detected in those samples (56). We chose to revisit membrane proteomics for comparison with the ΔTonB4 strain using a tandem mass tag-based approach for peptide quantification between conditions and strains (Table S1). As expected, we saw a dramatic increase in Sus proteins when both WT and ΔTonB4 were grown on maltose as compared to glucose (SusC shown as an example in Fig. 6, but similar results were seen for SusA-G).
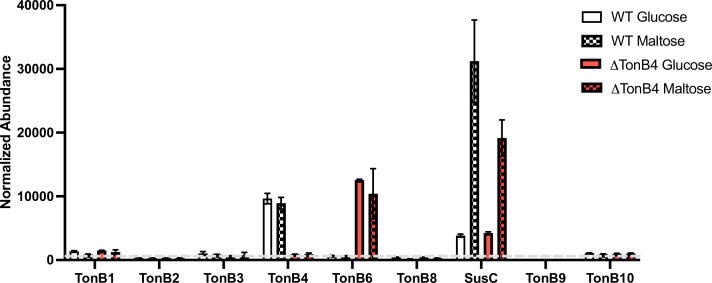
Normalized abundances of TonB and SusC proteins from quantitative membrane proteomics. Mean and standard deviation of two samples are shown. Open bars show data from cells grown on 5 mg mL−1 glucose, while hatched bars are from cells grown on 5 mg mL−1 maltose to induce Sus expression. White bars are samples isolated from wild-type (WT) cells, while red bars are from the TonB4 deletion strain. The dashed line indicates background readings based on TonB4-matched peptides in the TonB4 deletion strain. Full data in Table S1. Quantitation was performed using high-quality MS3 spectra (see Materials and Methods).
Like the previously published data, TonB4 was highly abundant in both glucose- and maltose-grown WT cell membranes (Fig. 6). Unlike the previous data, we also measured low amounts of other TonB proteins but notably did not see expression of TonB7, which also caused a growth defect when deleted. In the ΔTonB4 strain, the abundance of most TonB proteins was unchanged; however, there was an apparent increase in TonB6. Interestingly, TonB6 appeared to be similarly abundant in the ΔTonB4 strain as TonB4 in the WT strain. Therefore, we hypothesize that TonB6 partially complements TonB4 in the ΔTonB4 strain. Furthermore, we have not been able to construct a ΔTonB4/6 double-deletion strain, suggesting that the double mutant is lethal and that these TonB proteins play redundant roles.
TonB4 shows a variable role in growth on other polysaccharides
This evidence supports a model where SusC is normally energized by the TonB4 protein, though it is unclear if this is a specific SusC-TonB4 interaction or if TonB4 is the preferred TonB for all polysaccharide utilization under normal lab conditions. To address this, we assessed the growth of single TonB deletions on various polysaccharide substrates for which the PUL has been defined, and it is known that a single TBDT is responsible for uptake, including arabinan, levan, chondroitin sulfate, and pectic galactan (8, 14, 15, 25, 58). Interestingly, we see a variety of phenotypes across these four polysaccharides, suggesting that the SusC-TonB4 interaction may not be unique, but TonB4 is also not the dominant TonB for all polysaccharide utilization (Fig. 7). The ΔTonB4 strain shows slower growth to both OD = 0.3 and max OD when grown on both pectic galactan and chondroitin sulfate (Fig. 7A and B). This suggests that both TBDTs BT4671 (pectic galactan) and BT3332 (chondroitin sulfate) may primarily pair with TonB4, similar to SusC. Additional work is needed to confirm if TonB6 is the secondary TonB for these transporters. Alternatively, the ΔTonB4 strain shows growth similar to other B. theta strains when grown on arabinan and levan, suggesting that these transporters do not show a preference for pairing with TonB4 and that multiple TonB proteins may be able to facilitate transport of these substrates with similar efficiency (Fig. 7C and D).
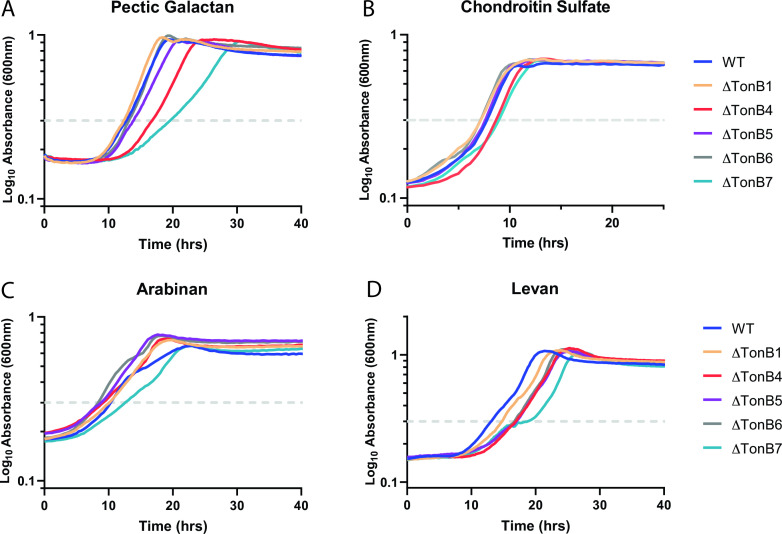
(A to D) Representative average growth curves of wild-type (WT) and select TonB deletion strains of B. thetaiotaomicron cultured on 5 mg mL−1 pectic galactan (A), 5 mg mL−1 chondroitin sulfate (B), 5 mg mL−1 arabinan (C), and 5 mg mL−1 levan (D). The dashed line indicates OD = 0.3, which is used as a reference point for delayed growth.
TonB genes vary across the Bacteroides genus
To understand the conservation of the putative B. theta TonB proteins throughout the genus, we conducted a comparative genomic analysis by searching for homologs of each full-length B. theta TonB protein in a range of fully sequenced Bacteroides species (Fig. 8; Tables S2 and S3). We found that the set of TonB proteins in each species and even varying strains of the same species is highly divergent. Homologs of TonB4, TonB5, and TonB10 were found in almost all of the Bacteroides species we analyzed, suggesting that these TonB proteins may play an essential role in Bacteroides physiology. This also supports that TonB4 may be widely important for polysaccharide uptake, as seen in a recent work analyzing the TonB homologs in B. fragilis, where the TonB4 homolog (B. fragilis TonB3) is essential for growth on a variety of polysaccharides (39). However, sequence similarity of these conserved proteins decreased in species less closely related to B. theta. This is particularly striking for TonB10, where many of the homologs do not consist of the same domain structure as B. theta TonB10, resulting in a similarly low overall sequence. Some TonB proteins, including homologs of TonB7 and TonB9, were found in only a few strains of B. theta (Fig. 8; Table S3). Additional research is needed to understand the unique role these proteins are playing in these strains. Homologs of many TonB proteins, such as TonB6, TonB8, and TonB11, are found only in species closely related to B. theta. This is particularly interesting in the case of TonB6, which is important for supplementing the function of TonB4 in B. theta. The lack of a TonB6 homolog suggests that many of these bacteria may show a higher dependence on proper function of the TonB4 homolog. Indeed, this was recently shown for B. fragilis 638R, where deletion of the TonB4 homolog (B. fragilis TonB3) completely eliminates growth on polysaccharides, and we were not able to identify a TonB6 homolog in this strain (39).
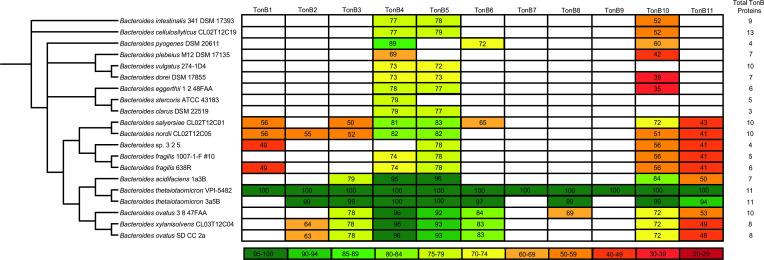
Percent sequence similarities between full-length B. theta TonB proteins (TonB1-11) and proteins in various Bacteroides genomes are shown. The phylogenetic tree shown on the left is based on 16S rRNA sequence similarity. The total TonB proteins identified in each genome are indicated to the right, as many genomes contain predicted TonB proteins that do not show significant sequence similarity to the B. theta TonB proteins.
Many species of Bacteroides have additional TonB proteins that show little homology to the B. theta TonB proteins (Table S4). For example, Bacteroides plebeius not only contains TonB proteins with some homology to TonB4 and TonB10 but also contains five additional predicted TonB proteins (Fig. 8; Table S4). Even in species closely related to B. theta, such as Bacteroides ovatus and Bacteroides acidifaciens, we found predicted TonB proteins with little to no homology to the B. theta TonB proteins. While it is still unclear why Bacteroides maintain such a large number of TonB proteins, the diversity of TonB proteins strongly suggests that they are important, and further characterization of these proteins will allow us to better understand Bacteroides physiology.
DISCUSSION
By deleting either the TonBox portion of the susC gene or the tonB4 gene, we provide data that support that starch is taken up by B. theta through a TonB-dependent mechanism. While deletion of the tonB4 gene causes slower bacterial growth, we show that the levels of TonB6 proteins drastically increase in the absence of TonB4, suggesting that the TonB6 homolog can also energize transport of starch through SusC. The phenomenon does not seem to be restricted only to starch, as growth on pectic galactan and chondroitin sulfate is similarly affected by tonB4 gene deletion. Interestingly, growth on arabinan or levan is not affected by the tonB4 deletion or any other single tonB gene deletion. Taken together, these results suggest that there is specificity of pairing between TBDT and TonB proteins in B. theta but that there is redundancy or overlapping function among some B. theta TonB homologs.
B. theta is often used as a model system to understand Bacteroides, but there are many unique aspects to each Bacteroides species, and the TonB content of each species and even strain is no exception. Our comparative genomic analysis showed that while TonB4 is highly conserved across Bacteroides, individual species typically contain an array of additional tonB genes that are often not highly conserved and may even be specialized for a limited number of strains within a single species. Notably, this analysis identifies TonB4 as the homolog to B. fragilis TonB3, which was shown to be essential for the uptake of heme, vitamin B12, iron, and several complex carbohydrates (39). We were not able to identify a TonB6 homolog in B. fragilis, which likely explains the more drastic effects of the B. fragilis TonB3 deletion as compared to the more moderate effects of the B. theta TonB4 deletion (39).
This importance of TonB4 and the potential redundancy offered by TonB6 provide a useful explanation of previous data exploring the importance of various B. theta genes. In two separate transposon screens of B. theta, no TonB homologs were identified as essential genes (59, 60). However, the strain with a transposon insertion in the tonB4 gene showed a decreased abundance after extended exponential growth and decreased abundance after mono-association in mice as compared to wild-type B. theta (59). This suggests that the growth defect we see as slower growth in the ΔTonB4 strain persists in extended exponential growth and is sufficient to decrease the ability of this strain to colonize mice. However, likely due to the redundancy offered by TonB6, disruption of the tonB4 gene does not eliminate growth or the ability to colonize the mouse intestine (59).
These differential growth outcomes must have a molecular basis in TBDT-TonB pair formation. While we analyzed the role of the SusC TonBox in TBDT-TonB protein pairing, the TonBox is likely not the only region of interaction between these proteins (61). Because the predicted TonBox region is well conserved across Bacteroides TBDTs and we see different specificity for TonB4 across the starch, arabinan, and levan transporters, it is likely that these other interactions are important for determining the specificity of pairing between TBDT and TonB proteins. Sequence variation in the N-terminus of the TBDT is likely important for this specificity, although it is not currently clear if this is limited to the plug domain or if the N-terminal extension and signal transduction domains that are common in Bacteroides TBDTs also play a role (27). It also seems likely that the sequence of the TonB protein is also highly important. Indeed, TonB4 and TonB6 show high sequence similarity, and differences between these proteins may point to important regions for pairing specificity.
The transporter is also not the only protein that TonB must be in contact with to facilitate transport. TonB is also associated with the inner membrane proteins ExbB and ExbD. B theta contains five predicted ExbB homologs and eight predicted ExbD homologs. Previous work in E. coli suggests that TonB interaction with ExbD is essential for TonB to adopt the correct confirmation for interaction with the TBDT (61, 62). Thus, it is likely that only some ExbB and ExbD homologs are capable of properly energizing TonB4 and TonB6 for polysaccharide utilization explored in this paper. Exploration of this inner membrane complex is an essential component that must be explored to fully understand the requirements of TonB-dependent transport in Bacteroides.
A significant open question is the role of the other nine TonB homologs in B. theta. While we have focused on polysaccharide transport here, it seems possible that other TonB homologs may be important for the uptake of B12 and heme that are generally taken up by much smaller TBDTs, although we did not see an abundance of additional TonBs in the membrane proteomics (27). Additionally, it has been shown that for some substrates, TonB-like proteins may play an additional role in transport by directly interacting with the substrate (63). This provides a potential explanation for the unique domain structure of some of the TonB proteins characterized here. This is an important consideration as more TBDT substrates are identified and more TBDTs are characterized in B. theta.
While much focus has been given to carbohydrate-active enzymes and other outer membrane proteins essential for polysaccharide utilization in Bacteroides, this study extends our understanding of the larger protein complex required for polysaccharide utilization in B. theta. TonB-targeting drugs are currently being considered for pathogenic bacteria, and a deeper understanding of this system in B. theta may offer new opportunities for manipulating both the microbiome and pathogenic Bacteroides. The importance and conservation of TonB4 suggests that drugs targeting this protein may offer a way to decrease the growth of all Bacteroides, while the redundancy offered by TonB6 may allow B. theta and related species to survive at low levels while species such as pathogenic B. fragilis are eliminated (39). This work also highlights the many aspects left to understand about TonB-dependent transport. Along with the growing variety of substrates known to be transported through TBDT, the unique domain architectures seen in TonB proteins suggest that the previously characterized structure-function relationships of the TBDT-TonB pair will not be sufficient to fully understand these systems.
MATERIALS AND METHODS
Bacterial strains and culture conditions
The B. thetaiotaomicron VPI-5482 Δtdk strain is the parent strain for all mutations used in this study and is referred to as WT. Mutant strains were generated via allelic exchange as previously described (18, 64). Briefly, the genomic region containing the desired gene deletion was inserted into the counter-selectable allelic exchange vector pExchange_tdk. The primers used in this study were synthesized by IDT DNA Technologies and are described in Table S5. A summary of all plasmids and strains used in this study is provided in Table S6.
All B. theta strains were cultured in a 37°C Coy anaerobic chamber (5% H2/10% CO2/85% N2) from freezer stocks into tryptone-yeast extract-glucose (TYG) medium (65) and grown for 16 h to an OD600 of ~1.0. The cells were then back-diluted 1:100 into Bacteroides minimal media (MM) including 5 mg/mL glucose and grown overnight (16 h).
For kinetic growth experiments in a plate reader, the MM-glucose-grown cells were then washed in minimal media containing no carbon and back-diluted approximately 1:200 into MM with the experimental carbohydrate, glucose, or maltose to a final volume of 200 µL. Thus, both glucose and maltose controls and the experimental carbohydrate-grown cultures were started at the same initial OD600 of 0.1. The carbon sources used for comparison to glucose-grown cultures included 5 mg/mL maltose (Sigma), 2.5 mg/mL (2.17 mM) maltoheptaose (Carbosynth), and 5 mg/mL potato amylopectin (Sigma). Growths were conducted in a flat bottom 96-well plate (Costar) covered with a gas-permeable, optically clear polyurethane membrane (Diversified Biotech, USA). Plates were loaded in a Biostack automated plate-handling device (Biotek Instruments, USA) coupled with a Powerwave HT absorbance reader (Biotek Instruments, USA) inside the anaerobic chamber, and OD600 was recorded every 10–30 min. All plate reader growth experiments were performed in triplicate unless otherwise noted, and the averages are reported in each figure. All biological experiments were repeated at least twice to verify consistent growth phenotypes from day to day.
Gene complementation
The tonB4 (bt2059) gene in a pNBU2 vector containing a constitutively active promoter was introduced into the genome of the ΔTonB4 B. theta strain in a single copy as previously described (16, 66). Briefly, the bt2059 gene was introduced into the pNBU2_erm_us1311 plasmid using restriction enzyme cloning and primers in Table S5. After conjugative transfer of the plasmid into the ΔTonB4 B. theta strain, the plasmid integrated into the genome at the NBU2 att2 site.
Membrane proteomics
Sample preparation
All strains were cultured in TYG and back-diluted into MM containing glucose as described above. The MM-glucose-grown cells were then back-diluted 1:100 into 50 mL of MM containing 5 mg/mL glucose or maltose as indicated. The OD600 was monitored every 30–45 min, and the cells were harvested at mid-log (OD ~0.7–0.8) by centrifugation at 5,000 × g for 5 min. The cell pellet was frozen in liquid nitrogen and stored at −80°C.
To prepare the membrane fraction, cells were thawed and resuspended in 1 mL of 20 mM KH2PO4 pH 7.3. The slurry was gently sonicated on ice. Intact cells were removed by centrifugation at 13,000 × g for 10 min at 4°C. The remaining soluble fraction was ultracentrifuged at 200,000 × g for 2 h at 4°C to pellet total membranes. The supernatant was removed, and the membrane pellet was resuspended in the same buffer, followed by a second round of ultracentrifugation at 200,000 × g. The resulting membrane pellet was resuspended in 20 mM KH2PO4 and 0.1% Tween-20 pH 7.3. Total protein in the final sample was quantified using the BCA Protein Assay Kit (Pierce).
The total membrane samples were submitted to the Mass Spectrometry-Based Proteomics Resource Facility in the Department of Pathology at the University of Michigan (Ann Arbor, MI, USA). Samples were then processed using the TMT 10-plex Mass Tag Labeling Kit (Thermo Scientific) similar to the manufacturer’s protocol and as previously adapted (67). Briefly, upon reduction (5 mM DTT, for 30 min at 45°C) and alkylation of cysteines (15 mM 2-chloroacetamide, for 30 min at room temperature), the proteins were precipitated by adding 6 volumes of ice-cold acetone followed by overnight incubation at −20°C. The precipitate was spun down, and the pellet was allowed to air-dry. The pellet was resuspended in 0.1M TEAB, and overnight (~16 h) digestion with trypsin/Lys-C mix (1:25 protease:protein; Promega) at 37°C was performed with constant mixing using a thermomixer. The TMT 10-plex reagents were dissolved in 41 µL of anhydrous acetonitrile, and labeling was performed by transferring the entire digest to a TMT reagent vial and incubating at room temperature for 1 h. The reaction was quenched by adding 8 µL of 5% hydroxyl amine and further 15-min incubation. Labeled samples were mixed together and dried using a vacufuge. An offline fractionation of the combined sample (~200 µg) into eight fractions was performed using a high pH reversed-phase peptide fractionation kit according to the manufacturer’s protocol (Pierce; Cat #84868). Fractions were dried and reconstituted in 9 µL of 0.1% formic acid/2% acetonitrile in preparation for LC-MS/MS analysis. Details on sample preparation as well as the sample-to-TMT channel are found in Table S1.
Liquid chromatography-mass spectrometry analysis (LC-multinotch MS3)
In order to obtain superior quantitation accuracy, we employed multinotch MS3, which minimizes the reporter ion ratio distortion resulting from fragmentation of co-isolated peptides during MS analysis (68). Orbitrap Fusion (Thermo Fisher Scientific) and RSLC Ultimate 3000 nano-UPLC (Dionex) were used to acquire the data. Two microliters of the sample was resolved on a PepMap RSLC C18 column (75 µm i.d. × 50 cm; Thermo Scientific) at a flow rate of 300 nL/min using a 0.1% formic acid/acetonitrile gradient system (2%–22% acetonitrile in 150 min; 22%–32% acetonitrile in 40 min; 20-min wash at 90% followed by 50-min re-equilibration) and directly sprayed onto the mass spectrometer using an EasySpray source (Thermo Fisher Scientific). The mass spectrometer was set to collect one MS1 scan (Orbitrap; 120K resolution; automatic gain control [AGC] target 2 × 105; max injection time [IT] 100 ms) followed by data-dependent “Top Speed” (3 s) MS2 scans (collision-induced dissociation; ion trap; normalized collison energy (NCE) 35; AGC 5 × 103; max IT 100 ms). For multinotch MS3, the top 10 precursors from each MS2 were fragmented by HCD followed by Orbitrap analysis (NCE 55; 60K resolution; AGC 5 × 104; max IT 120 ms, 100–500 m/z scan range).
Data analysis
Proteome Discoverer (v2.4; Thermo Fisher) was used for data analysis. MS2 spectra were searched against the SwissProt Bacteroides thetaiotaomicron VPI-5482 (ATCC strain 29148) protein database using the following search parameters: MS1 and MS2 tolerance were set to 10 ppm and 0.6 Da, respectively; carbamidomethylation of cysteines (57.02146 Da) and TMT labeling of lysine and N-termini of peptides (229.16293 Da) were considered static modifications; oxidation of methionine (15.9949 Da) and deamidation of asparagine and glutamine (0.98401 Da) were considered variable. Identified proteins and peptides were filtered to retain only those that passed the ≤1% false discovery rate threshold. Quantitation was performed using high-quality MS3 spectra (average signal-to-noise ratio of 10 and <50% isolation interference).
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE (69) partner repository with the data set identifier PXD041518.
Protein sequence analysis
Protein domains, including the TonB protein C-terminal domains, were identified using Pfam version 32.0 (70). To identify the 11 potential TonB proteins, the complete genome of Bacteroides thetaiotaomicron VPI-5482 was searched for sequences that matched PF03544 using the Joint Genome Institute’s Integrated Microbial Genomes & Microbiomes database (71). Each sequence was then searched for additional Pfam domains using the sequence search on the EMBL-EBI Pfam database (72). Predictions of transmembrane helices were made using the TMHMM Server v2.0 (73, 74), and signal peptides were predicted using SignalP-5.0 (75).
Multiple sequence alignment of TonB-dependent transporters and TonB proteins was conducted in Clustal Omega (72). Sequence similarity between TonB proteins was determined using EMBOSS Needle pairwise sequence alignment (72).
Genomic context analysis
The genomic context of each tonB gene was identified by browsing the complete genome of Bacteroides thetaiotaomicron VPI-5482 (GCF_000011065.1). The size, direction, and location of the surrounding genes were annotated and generally included all genes that are encoded on the same strand or all genes that appear to be co-transcribed. Protein function predictions were also analyzed using UniProt Release 2023_02, and the most informative prediction between the two was used (76).
TonB homology analysis
To identify homologs of the 11 TonB proteins found in B. theta, we searched the Integrated Microbial Genomes (IMG) database (current as of May 2018) for all Bacteroides genome sequences and performed BLAST searches of each TonB protein with an E-value cutoff of 1e-50. We chose this stringent cutoff to limit the number of homologs that would match to several of our TonB proteins of interest. These results are shown in Table S2. Despite using this stringent cutoff, we still found that many TonB proteins in other Bacteroides genomes matched several B. theta TonB proteins. For each genome in our data set, we used the E-value and bit score generated through the BLAST search to match each TonB to the single B. theta TonB protein with the highest match. These full results are shown in Table S3, and select genomes are shown in Fig. 8.
Additional TonB proteins in each Bacteroides genome were identified by searching for proteins with regions matching the conserved protein domain family Pfam 03544: Gram-negative bacterial TonB protein C-terminal. The full list of matches to Pfam03544 is shown in Table S4, and the totals for select genomes are shown in Fig. 8. The phylogenetic tree in Fig. 8 was constructed using the 16S rRNA gene from each strain shown.
Protein structure visualization
Structures of E. coli TonB and Pseudomonas aeruginosa TonB1 were visualized in PyMOL (77).
ACKNOWLEDGMENTS
We thank Dr. Venkatesha Basrur for assistance with the membrane proteomics work and all members of the Koropatkin and Martens labs for useful feedback and technical assistance.
This work was supported by a Research Diversity Supplement to grant R01GM118475 awarded to R.M.P. and N.M.K.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/jb.00218-23.
Supplemental figures
jb.00218-23-s0001.pdf:Supplemental Fig. S1 to S5.
Table S1
jb.00218-23-s0002.xlsx:Results of membrane proteomics analysis. Membrane fractions were isolated from wild-type, TonB4 deletion, and TonBox deletion strains’ growth on glucose or maltose and analyzed for abundance of proteins from the Bacteroides thetaiotaomicron VPI-5482 genome.
Table S2
jb.00218-23-s0003.xlsx:BLAST search of B. theta TonB across Bacteroides genomes. Each tab contains the matches for the designated TonB protein.
Table S3
jb.00218-23-s0004.xlsx:Each B. theta TonB protein often has multiple matches within a single Bacteroides species as shown in Table S2. This table identifies only the top match to each B. theta TonB protein within each species.
Table S4
jb.00218-23-s0005.xlsx:Protein matches to Pfam03544 (Gram-negative bacterial TonB protein C-terminal) across Bacteroides species.
Table S5
jb.00218-23-s0006.xlsx:Primers used in this study.
Table S6
jb.00218-23-s0007.xlsx:Strains and plasmids used in this study.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
Articles from Journal of Bacteriology are provided here courtesy of American Society for Microbiology (ASM)
Citations & impact
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/155848463
Article citations
Apple polysaccharide improves age-matched cognitive impairment and intestinal aging through microbiota-gut-brain axis.
Sci Rep, 14(1):16215, 13 Jul 2024
Cited by: 0 articles | PMID: 39003416 | PMCID: PMC11246462
Genome-resolved metatranscriptomics reveals conserved root colonization determinants in a synthetic microbiota.
Nat Commun, 14(1):8274, 13 Dec 2023
Cited by: 6 articles | PMID: 38092730 | PMCID: PMC10719396
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
Pfam (4)
- (3 citations) Pfam - PF13715
- (3 citations) Pfam - PF03544
- (3 citations) Pfam - PF05569
- (2 citations) Pfam - PF14869
Protein structures in PDBe (2)
-
(1 citation)
PDBe - 6I97View structure
-
(1 citation)
PDBe - 2GSKView structure
ProteomeXchange
- (1 citation) ProteomeXchange - PXD041518
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Bacteroides thetaiotaomicron metabolic activity decreases with polysaccharide molecular weight.
mBio, 15(3):e0259923, 20 Feb 2024
Cited by: 0 articles | PMID: 38376161
Multiple Signals Govern Utilization of a Polysaccharide in the Gut Bacterium Bacteroides thetaiotaomicron.
mBio, 7(5):e01342-16, 11 Oct 2016
Cited by: 32 articles | PMID: 27729509 | PMCID: PMC5061871
TonB-dependent transporters in the Bacteroidetes: Unique domain structures and potential functions.
Mol Microbiol, 115(3):490-501, 03 Feb 2021
Cited by: 28 articles | PMID: 33448497
Review
Funding
Funders who supported this work.
HHS | NIH | National Institute of General Medical Sciences (1)
Grant ID: 5R01GM118475-05
NIGMS NIH HHS (1)
Grant ID: R01 GM118475
 1
,
2
,
3
1
,
2
,
3



