Abstract
Free full text

Post-COVID-19 Cholangiopathy: Clinical and Radiologic Findings
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2), presents primarily with respiratory manifestations. However, COVID-19 has increasingly been recognized as a multi-systemic disease with extra-pulmonary complications. COVID-19-associated liver injury manifests principally as hepatocellular injury, leading to a mild elevation of liver enzymes during early stages of the disease [1,2]. However, patients may develop severe cholestatic liver injury during the later stages of the disease [1,2]. A subset of these patients who exhibit features of sclerosing cholangitis are diagnosed as having post-COVID-19 cholangiopathy [3,4,5], also termed as COVID-19-associated secondary sclerosing cholangitis. Post-COVID-19 cholangiopathy is a relatively new and under-recognized disease entity. Therefore, raising awareness of its clinical and radiologic features can aid the early diagnosis and management of patients with post-COVID-19 cholangiopathy.
Clinical Manifestations
The main clinical manifestations of post-COVID-19 cholangiopathy are cholestasis and jaundice, characterized by markedly elevated serum alkaline phosphatase, gamma glutamyl transferase, and bilirubin levels. Although definitive criteria have not been established, the generally accepted definition of post-COVID-19 cholangiopathy includes the presence of severe cholestasis, along with bile duct abnormalities on imaging or pathologic examinations, which had not been documented prior to the onset of COVID-19 [3,4,5]. Magnetic resonance cholangiopancreaticography (MRCP) is the most widely used diagnostic examination [4]. Post-COVID-19 cholangiopathy develops almost exclusively in patients with severe disease who receive mechanical ventilation, vasopressor therapy, and extended care in intensive care units [4,6,7,8]. The incidence of post-COVID-19 cholangiopathy among patients hospitalized for COVID-19 is reportedly less than 1% [7]. The clinical manifestations of post-COVID-19 cholangiopathy generally occur during a later stage of the disease, usually after recovery from acute respiratory illness. The average time between the initial infection and the diagnosis of post-COVID-19 cholangiopathy ranges from 90 to 118 days [6,7]. Other consistently reported findings include male predilection, a median age exceeding 50 years [3,4,7,8,9], and comorbidities such as hypertension or diabetes mellitus [4,6,7,10]. Pathologically, post-COVID-19 cholangiopathy presents with portal or periportal inflammation and fibrosis, along with findings of bile duct injury, such as vacuolization, regenerative changes, and apoptosis or necrosis of cholangiocytes [7].
Clinical Course and Prognosis
Although some patients with post-COVID-19 cholangiopathy recover, many ultimately progress to biliary cirrhosis or liver failure, necessitating liver transplantation [3,5]. According to one report, six (50%) of 12 patients with post-COVID-19 cholangiopathy underwent liver transplantation or were waitlisted for transplantation [7]. Since most of the current knowledge regarding post-COVID-19 cholangiopathy is based on anecdotal evidence from case reports/series published in the last three years, its long-term prognosis is unknown. Moreover, the possibility remains that some patients with mild disease might have recovered before undergoing a diagnostic workup and are therefore under-represented in the literature.
Pathophysiology
The pathophysiology underlying post-COVID-19 cholangiopathy is likely multifactorial. Critically ill patients undergoing prolonged management in intensive care units, regardless of the underlying disease, may develop secondary sclerosing cholangitis after recovering from their primary illnesses. This condition, called secondary sclerosing cholangitis in critically ill patients (SSC-CIP) [6,11], has been attributed to ischemic bile duct injury caused by respiratory failure, systemic hypotension, or vasopressor administration. Because of their shared clinicopathologic features, some researchers have proposed that post-COVID-19 cholangiopathy may be a variant of, or at least share pathophysiology with SSC-CIP [5,6,7,11]. Nonetheless, accumulating evidence suggests that SARS-CoV-2 plays a direct role in the development of post-COVID-19 cholangiopathy. Cholangiocytes express angiotensin converting enzyme 2 receptors, which are required for the cellular entry of SARS-CoV-2, suggesting a viral cytopathic effect [12]. A recent case-control study determined that secondary sclerosing cholangitis developed more frequently in patients with chronic liver disease and COVID-19 than it did in a matched group of patients with chronic liver disease and non-COVID-19 pneumonia [1]. This finding indicates that post-COVID-19 cholangiopathy differs from SSC-CIP [6,11], and that SARS-CoV-2 may play a direct role in its development. Other proposed pathophysiologic factors include the release of proinflammatory cytokines as a host response to viral infection [3,10], and toxicity associated with the usage of various drugs. For example, a recent study suggested a potential association between post-COVID-19 cholangiopathy and ketamine [13], a drug often used for patient sedation in intensive care units.
Radiologic Findings
Imaging findings of post-COVID-19 cholangiopathy overlap substantially with those of primary sclerosing cholangitis and other types of secondary sclerosing cholangitis (Figs. 1, ,2).2). Similar to other sclerosing cholangitis types, multifocal intrahepatic bile duct strictures are a predominant and consistent feature in post-COVID-19 cholangiopathy, resulting in a beaded appearance of the intrahepatic bile ducts [4,5,8] (Figs. 1, ,2).2). The intrahepatic bile duct strictures may not be accompanied by obvious dilatation of the upstream ducts. This finding, which may therefore be overlooked on computed tomography, is best evaluated by MRCP (Fig. 1). Bile duct wall thickening with enhancement is frequently observed. Bile stasis and subsequent cholangitis can lead to parenchymal changes, such as inhomogeneous arterial enhancement, edema, or diffusion restriction in peribiliary areas [7,8]. Multifocal clustered cystic lesions, which may indicate saccular dilatation of the intrahepatic bile ducts or bilomas, have also been observed [8] (Fig. 2). Interestingly, ductal changes in post-COVID-19 cholangiopathy primarily manifest in the intrahepatic bile ducts, with the extrahepatic bile ducts remaining unaffected. In a study of 17 patients with post-COVID-19 cholangiopathy, all patients had intrahepatic bile duct involvement, whereas only one (5.9%) exhibited an extrahepatic bile duct stricture [8]. This distinctive pattern may help distinguish post-COVID-19 cholangiopathy from primary sclerosing cholangitis, which frequently affects both intrahepatic and extrahepatic bile ducts [14]. Additional findings include stones or sludges in the gallbladder and bile ducts, as well as hepatic abscesses [9,15]. Biliary casts, which are frequently observed in SSC-CIP, are most likely rarer in post-COVID-19 cholangiopathy, since they were observed in only two (11.8%) of 17 patients [8]. Morphologic changes of the liver, including hepatomegaly and a cirrhotic appearance, have also been reported in approximately 20% of patients with post-COVID-19 cholangiopathy [8].
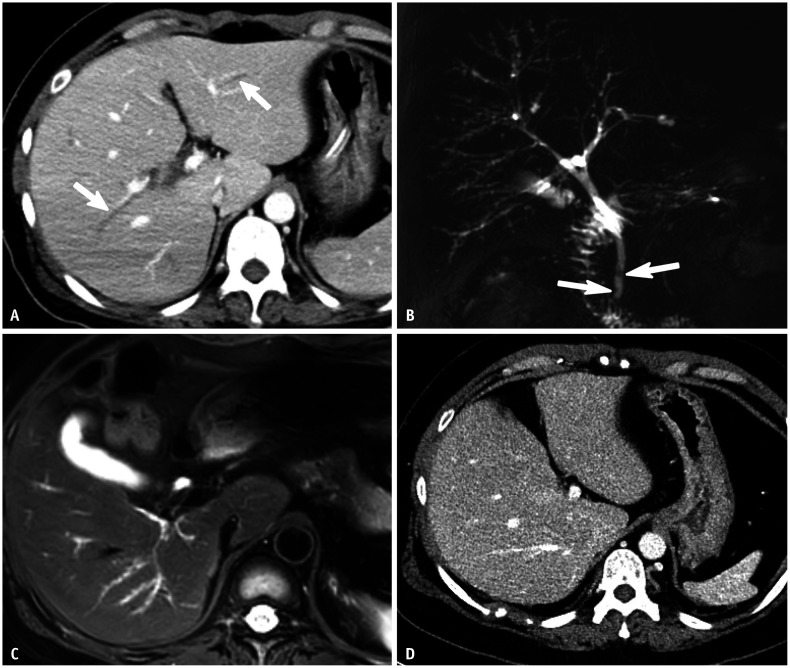

Despite its rarity, post-COVID-19 cholangiopathy is clinically important due to its potential to alter the clinical course of COVID-19. The presence of prolonged cholestasis in patients recovering from severe COVID-19 should raise suspicion of post-COVID-19 cholangiopathy. Such a condition warrants prompt radiologic examination, preferably via MRCP, to ensure an accurate diagnosis of post-COVID-19 cholangiopathy.
Footnotes
Conflicts of Interest: Seung Soo Lee, the editor board member of the Korean Journal of Radiology, was not involved in the editorial evaluation or decision to publish this article. All remaining authors have declared no conflicts of interest.
Author Contributions:
Conceptualization: all authors.
Methodology: all authors.
Resources: all authors.
Visualization: all authors.
Writing—original draft: all authors.
Writing—review & editing: all authors.
Funding Statement: None
References
Articles from Korean Journal of Radiology are provided here courtesy of Korean Society of Radiology
Citations & impact
Impact metrics
Citations of article over time
Article citations
The Impact of Biliary Injury on the Recurrence of Biliary Cancer and Benign Disease after Liver Transplantation: Risk Factors and Mechanisms.
Cancers (Basel), 16(16):2789, 07 Aug 2024
Cited by: 0 articles | PMID: 39199562 | PMCID: PMC11352383
Review Free full text in Europe PMC
Incidence of Secondary Sclerosing Cholangitis in Hospitalized Long COVID-19 Patients: A Retrospective Single Center Study.
Diagnostics (Basel), 14(7):745, 30 Mar 2024
Cited by: 1 article | PMID: 38611659 | PMCID: PMC11011916
Noteworthy Developments in the Korean Journal of Radiology in 2023 and for 2024.
Korean J Radiol, 25(1):1-5, 01 Jan 2024
Cited by: 0 articles | PMID: 38184762 | PMCID: PMC10788598
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Cholangiopathy After Severe COVID-19: Clinical Features and Prognostic Implications.
Am J Gastroenterol, 116(7):1414-1425, 01 Jul 2021
Cited by: 65 articles | PMID: 33993134
Post-COVID cholangiopathy: A narrative review.
Gastroenterol Hepatol, 46(6):474-482, 27 Sep 2022
Cited by: 7 articles | PMID: 36174796 | PMCID: PMC9512521
Review Free full text in Europe PMC
COVID-19 related biliary injury: A review of recent literature.
World J Gastroenterol, 29(14):2127-2133, 01 Apr 2023
Cited by: 1 article | PMID: 37122603 | PMCID: PMC10130971
Review Free full text in Europe PMC
Secondary Sclerosing Cholangitis After SARS-CoV2: ICU Ketamine Use or Virus-Specific Biliary Tropism and Injury in the Context of Biliary Ischemia in Critically Ill Patients?
Hepat Med, 15:93-112, 01 Aug 2023
Cited by: 3 articles | PMID: 37547355 | PMCID: PMC10404108

 1,2
1,2

