Abstract
Free full text

Essential role of Helicobacter pylori apolipoprotein N-acyltransferase (Lnt) in stomach colonization
ABSTRACT
Bacterial lipoproteins are post-translationally modified with acyl chains, anchoring these proteins to bacterial membranes. In Gram-negative bacteria, three enzymes complete the modifications. Lgt (which adds two acyl chains) and LspA (which removes the signal peptide) are essential. Lnt (which adds a third acyl chain) is not essential in certain bacteria including Francisella tularensis, Neisseria gonorrhoeae, and Acinetobacter baumannii. Deleting lnt results in mild to severe physiologic changes. We previously showed lnt is not essential for Helicobacter pylori growth in vitro. Here, the physiologic consequences of deleting lnt in H. pylori and the role of Lnt in the host response to H. pylori were examined using in vitro and in vivo models. Comparing wild-type, Δlnt, and complemented mutant H. pylori, no changes in growth rates or sensitivity to acid or antibiotics were observed. Since deleting lnt changes the number of acyl chains on lipoproteins and the number of acyl chains on lipoproteins impacts the innate immune response through Toll-like receptor 2 (TLR2) signaling, primary human gastric epithelial cells were treated with a purified lipoprotein from wild-type or lnt mutant H. pylori. Differential gene expression analysis indicated that lipoprotein from the lnt mutant induced a more robust TLR2 response. In a complementary approach, we infected wild-type and Tlr2−/− mice and found that both the wild-type and complemented mutant strains successfully colonized the animals. However, the lnt mutant strain was unable to colonize either mouse strain. These results show that lnt is essential for H. pylori colonization and identifies lipoprotein synthesis as a target for therapeutic intervention.
INTRODUCTION
Helicobacter pylori is a Gram-negative bacterium that persistently colonizes the stomach in about 50% of the human population (i.e., over 4 billion people), eliciting gastric inflammation (1, 2). Without antibiotic therapy, H. pylori can persist in the stomach for decades (3). Over time, H. pylori-induced gastric inflammation can act as an important initiating event leading some H. pylori-infected individuals to develop gastric cancer (2, 4,–7). Gastric cancer is the third leading cause of cancer-related death worldwide, and H. pylori has been classified as a type I carcinogen by the World Health Organization (WHO) (8).
How H. pylori manages to avoid elimination by the host immune response remains incompletely understood. Chronic inflammation develops in response to H. pylori infection, and epithelial cells within the gastric mucosa represent the first line of defense and initiate host innate and inflammatory responses (9,–12). Epithelial cells recognize microbial pathogens via Toll-like receptors (TLRs) that bind a variety of microbial molecular patterns (e.g., lipid, carbohydrate, peptide, lipopeptide, and nucleic acid) and are part of the innate immune response. H. pylori infection activates TLR2, TLR5, TLR9, TLR10, and, to a lesser extent, TLR4 (13,–17) leading to chemokine and cytokine expression that plays an important role in the outcome of infection (18).
Mammalian TLR2 signaling contributes to immune tolerance to H. pylori infection, thereby facilitating long-term bacterial persistence (14, 19). Dendritic cells treated with H. pylori exhibit a TLR2-dependent anti-inflammatory response (14). Additionally, stimulation of dendritic cell:T cell co-cultures with H. pylori promotes development of Foxp3+ Tregs and reduces stimulation of interleukin 17 (IL-17) and interferon gamma (IFNγ)-producing T cells compared to stimulation with a synthetic TLR2 ligand (19). Infection of Tlr2−/− mice resulted in decreased H. pylori density and increased immunopathology in the stomach compared with wild-type (WT) mice, and the H. pylori-specific Th1 response was higher and the Treg and Th17 responses were lower in the Tlr2−/− compared to WT mice (19).
However, consensus has not been reached regarding the H. pylori ligand activating TLR2. Whereas several studies have suggested that H. pylori lipopolysaccharide (LPS) is recognized by TLR2 (20,–22), others have suggested that H. pylori LPS does not activate TLR2 and that conflicting results could arise from contamination of LPS preparations with lipoproteins or nucleic acids (9, 13). TLR2 is widely understood to recognize bacterial lipoproteins (proteins post-translationally modified at their amino-terminal ends by the addition of two or three acyl chains) (23,–25). In a widely held model, triacylated lipoproteins (typically produced by Gram-negative bacteria) are recognized by TLR2/1 heterodimers, and diacylated lipoproteins (typically produced by Gram-positive bacteria) are recognized by TLR2/6 heterodimers (24, 25). Recent reports suggest that signaling via TLR2/1 or TLR2/6 may lead to either immune activation or suppression (26, 27).
Lipoprotein synthesis in Gram-negative bacteria involves three enzymatic steps. Prolipoprotein diacylglyceryl transferase (Lgt) adds a diacylglyceride to the sulfhydryl group of what will become the amino-terminal modified cysteine residue of the lipoprotein. Prolipoprotein signal peptidase (signal peptidase II, LspA) removes the signal peptide, leaving the diacylated cysteine as the amino-terminal residue. Finally, apolipoprotein N-acyltransferase (Lnt) adds a third acyl chain to the terminal amine of the acylated cysteine resulting in the mature, triacylated lipoprotein (28,–30). We previously confirmed the activities of these three enzymes in H. pylori and confirmed that Lgt and LspA were essential for H. pylori growth (31). However, contrary to what is observed in many other bacteria, we showed that Lnt is not essential for H. pylori growth in vitro (31).
A subset of lipoproteins are integral to maintaining the outer membrane in Gram-negative bacteria with essential roles in assembly of LPS, insertion of outer membrane proteins, and sensing envelope stress (32). Thus, deletion of lnt can manifest as disruptions to the outer membrane. For example, lnt mutant strains may exhibit increased sensitivity to antibiotics such as vancomycin or bacitracin that do not normally cross the Gram-negative outer membrane (33, 34). Lnt mutants strains also may exhibit an assortment of other defects including slower growth and altered morphology (34, 35).
In this study, we explore the physiologic consequences of deleting lnt in H. pylori and the role of Lnt in the host response to H. pylori. No differences in growth rates or sensitivities to acid or antibiotic stress were observed when comparing WT, Δlnt mutant, and complemented mutant H. pylori strains. In contrast, incubating TLR reporter cell lines and primary human gastric epithelial cells with a lipoprotein purified from WT or Δlnt mutant H. pylori revealed a heightened response to diacylated lipoprotein isolated from the mutant strain. In a complementary approach, we infected mice with WT H. pylori, a Δlnt mutant, and a complemented mutant strain and found that the mutant strain was unable to colonize mice. These results indicate that lipoprotein acylation plays a crucial role in the ability of H. pylori to colonize the stomach and provides a target for antimicrobial intervention.
RESULTS
Characterization of Δlnt mutant H. pylori in vitro
Lnt (the enzyme responsible for adding a third acyl chain to lipoproteins) is essential in many bacteria including Escherichia coli and Salmonella enterica sv.Typhimurium (33, 35,–37), though it has been shown not to be essential in certain bacteria including Francisella tularensis, Neisseria gonorrhoeae, and Acinetobacter baumannii (33, 34, 38,–40). The physiologic consequences of mutating lnt may be minimal [showing no alterations in growth, (33, 35)] to slower growth and structural defects (34). Mutation of lnt has been characterized by a perturbed outer membrane barrier as evidenced by increased sensitivities to acid and antibiotics that do not readily cross the outer membrane barrier of Gram-negative bacteria (33, 34, 40).
We previously demonstrated that lnt is not essential for H. pylori growth in vitro (31). To analyze H. pylori strains deficient in lnt more thoroughly, we constructed isogenic derivatives of H. pylori strain J166 in which we deleted lnt (strain VM391) and in which we complemented the lnt deletion by introducing a copy of lnt in an alternate locus (strain VM392). The complementing lnt allele was inserted into the hydA-mdaB intergenic region of the chromosome and its expression is driven by a copy of the ureA promoter (31, 41,–43). Analyses of strains J166, VM391, and VM392 by quantitative real-time PCR (qRT-PCR) confirmed loss of lnt expression in strain VM391 and restoration of lnt expression in strain VM392 (Fig. 1A). We then compared the growth of the isogenic strains in broth media and observed that mutation of lnt did not appear to negatively impact the growth rate of the bacteria (Fig. 1B). We next compared the sensitivities of the isogenic strains to various stresses including pH (Fig. 1C) and to antibiotics (e.g., vancomycin and bacitracin, Fig. 1D) but found no differences between strains to these stresses.
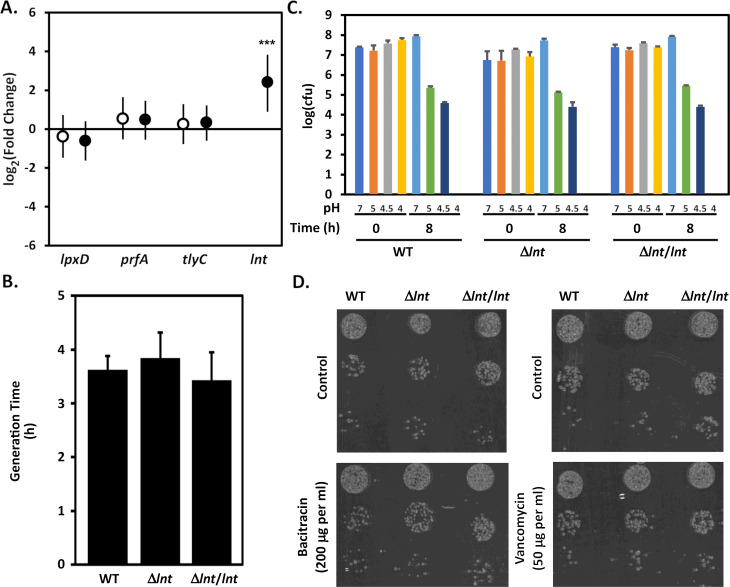
Characterization of WT, lnt mutant, and complemented strains in vitro. (A) Relative abundance of lnt transcript levels were determined as described in Materials and Methods. Relative abundance of lpxD, prfA, and tlyC served as controls. Fold change values compare transcript levels of the indicated genes in Δlnt mutant strain VM391 (open circles) and the complemented mutant strain VM392 (closed circles) compared with J166. The values represent the means and 95% credible limits (error bars) from triplicate cultures analyzed in triplicate. Significance was determined by calculating Bayesian z scores, and a standard z test was performed to derive two-tailed P-values, which were corrected for multiple testing using the Benjamini-Hochberg method (with a false discovery rate of 5%). ***, P = 0.0001. (B) H. pylori strains J166, Δlnt mutant strain VM391, and the complemented mutant strain VM392 were inoculated into bisulfite-free Brucella broth supplemented with cholesterol to an optical density at 600 nm (OD600) of approximately 0.1 and cultures were grown at 37°C in 5% CO2. Growth was monitored by measuring the OD600 at various time points. The generation time was determined during log-phase growth (between 3 and 10 h post inoculation). Results represent the mean and standard deviation from three different experiments, each performed in triplicate, and were not significantly different (analysis of variance). (C) Sensitivity of H. pylori strains to acid stress was determined as described in Materials and Methods. Results represent the mean and standard deviation of triplicate experiments each performed in triplicate. (D) Sensitivity of H. pylori strains to bacitracin and vancomycin stress was determined as described in Materials and Methods. Results are representative of triplicate experiments.
Response of TLR reporter cell lines
Although we did not observe physiologic changes in lnt mutant H. pylori in vitro, the absence of lnt leads to diacylated lipoproteins instead of triacyl lipoproteins (31, 33, 35). This alteration in the number of acyl chains is expected to impact the mammalian TLR2 response (26, 27). As a first step to characterizing the host response to H. pylori lipoproteins, we affinity purified an abundant lipoprotein, Lpp20 from WT and lnt-deficient strains designed to express a human influenza hemagglutinin (HA)-tagged form of the protein (strains VM396 and VM404, Fig. 2A). Lpp20 was chosen as a model lipoprotein based on previous proteomic studies suggesting it is abundantly expressed in H. pylori (44). We next incubated the purified proteins with human embryonic kidney (HEK) reporter cell lines designed to express TLR4, TLR2 and TLR1, or TLR2 and TLR6. As controls, we also added purified Lpp20-HA to control cells including HEK null cells expressing no TLR transgene and HEK cells expressing TLR2 in the absence of TLR1 and TLR6. After 18-h incubation with Lpp20-HA, relative levels of secreted alkaline phosphatase reporter were measured (Fig. 2B). Results showed that diacylated Lpp20-HA (isolated from lnt mutant H. pylori strain VM404) stimulated TLR2-TLR6 expressing cells, with little to no stimulation of TLR2-TLR1 expressing cells. In contrast, triacylated Lpp20-HA isolated from H. pylori strain VM396 stimulated both TLR2-TLR1 expressing cells and TLR2-TLR6 expressing cells. Similar results were obtained using synthetic TLR2 ligands Pam3-CSK4 and Pam2-CSK4 (Fig. 2C). Pam3-CSK4 is a six-amino acid-long peptide (Cys-Ser-Lys-Lys-Lys-Lys) modified by the addition of three palmitic acid chains to mimic a bacterial triacylated lipoprotein (45, 46). Pam2-CSK4 is an analogous peptide containing two palmitic acid chains attached via a diacylglyceride on the Cys sulfhydryl group but does not include a palmitic acid attached to the terminal amine of the Cys and thus mimics a lipopeptide produced in the absence of Lnt (46). No stimulation of TLR4 by either form of Lpp20 was observed whereas purified H. pylori LPS was able to stimulate the TLR4 expressing cells (Fig. 2B and C; Fig. S1). Together, these results show that our purified Lpp20-HA preparations behave similarly to synthetic triacylated or diacylated TLR2 ligands with little detectable contamination by LPS.
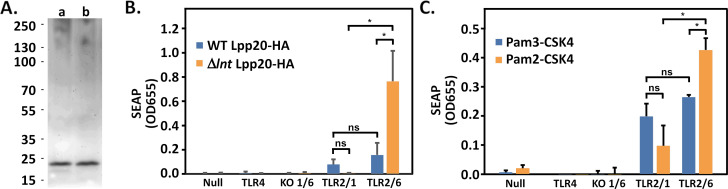
Characterization of purified Lpp20 as model lipoprotein. (A) Lpp20-HA was immunopurified from WT or lnt mutant H. pylori strains VM396 and VM404. A representative Coomassie-stained gel is shown (lane a, WT Lpp20-HA from strain VM396; lane b, Δlnt Lpp20-HA from strain VM404). (B) Purified Lpp20-HA (0.1 µg/mL from either WT or lnt mutant H. pylori) was incubated with secreted embryonic alkaline phosphatase (SEAP) reporter cell lines HEK-Blue Null2 (Null), HEK-Blue hTLR4 (TLR4), HEK-Blue hTLR2 KO-TLR1/6 (KO 1/6), HEK-Blue hTLR2-TLR1 (TLR2/1), and HEK-Blue hTLR2-TLR6 (TLR2/6) from InvivoGen. Relative amounts of SEAP were measured after 16-h treatment. Results show the mean and standard deviation from single Lpp20-HA preparations (from both WT and lnt mutant H. pylori) tested in triplicate and are representative of three independent preparations of each lipoprotein. (C) Synthetic lipopeptides (0.04 µg/mL triacylated Pam3-CSK4 and diacylated Pam2-CSK4) were used to stimulate reporter cell lines as described in B. Results show the mean and standard deviation of triplicate determinations and are representative of three independent experiments. Results in panels B and C were analyzed by analysis of variance followed by Tukey’s post hoc test. Select pair-wise comparisons are indicated as significantly different (*, P < 0.001) or not significantly different (ns).
Response of primary human gastric epithelial cells
To develop a more complete understanding of the host response to H. pylori lipoproteins, we utilized minimally passaged primary human gastric epithelial monolayers. Cells were left untreated or treated with purified triacylated Lpp20-HA, diacylated Lpp20-HA, Pam3-CSK4, or Pam2-CSK4 for 4 h. Following treatment, RNA was isolated from the cells and subjected to RNAseq. Analysis of the untreated control cells showed transcription of KRT18 and TFF1, cell surface mucins MUC1 and MUC16, and secreted mucin MUC5AC as expected for stomach epithelial cells (47, 48). Additionally, transcripts for TLR1, TLR2, TLR3, TLR4, TLR5, TLR6, and TLR9 were detected.
Normalized transcript abundance was determined for each sample and differentially expressed genes (greater than a two-fold change in expression vs control and an adjusted P-value less than 0.05) were identified. We first compared genes differentially expressed between cells treated with triacylated Lpp20-HA vs untreated control cells and between cells treated with triacylated Pam3-CSK4 peptide vs untreated control cells (Fig. 3A, C, and E; Data sets S1 and S2). Results showed a similar (though not identical) pattern of differentially expressed genes; Pam3-CSK4 led to increased expression of a larger number of genes. Analyses identified 40 genes differentially expressed between Lpp20-HA-treated cells and control, and 66 genes differentially expressed between Pam3-CSK4-treated cells and control. Among these genes, 30 were found to be differentially expressed by both Lpp20-HA and Pam3-CSK4, with a strong correlation between the magnitude of fold change between the two groups (Fig. 3E). The similarity between these results suggests that much of the cellular response detected in the RNAseq data is likely due to the lipid anchor of Lpp20 rather than a response to the functional fold of the protein.
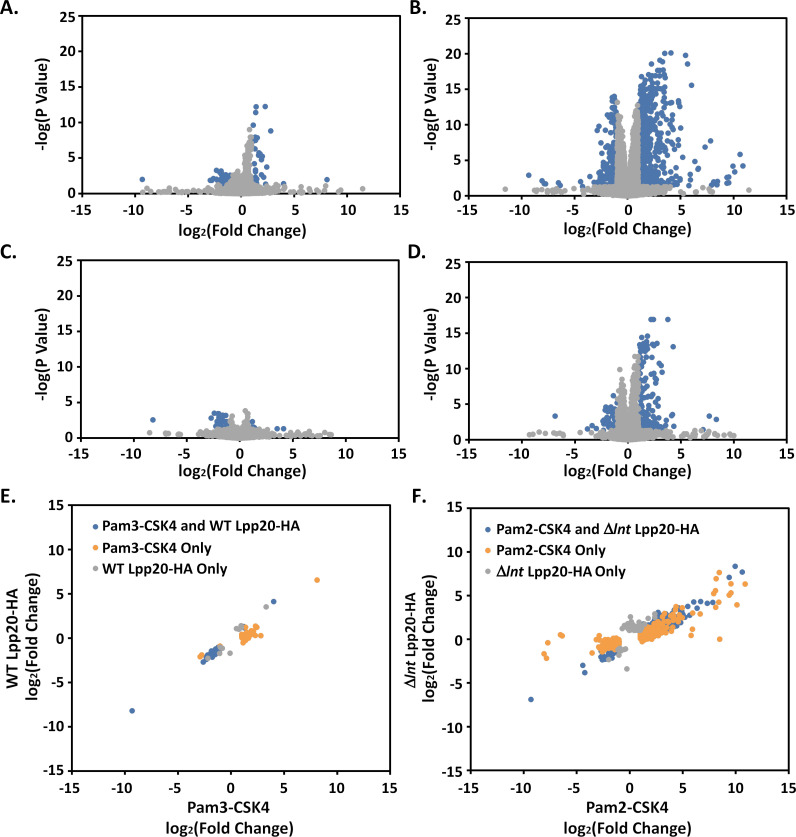
Analysis of RNAseq results. (A–D) RNAseq results were analyzed using edgeR as described in Materials and Methods. Results are summarized by plotting the log2(fold change) compared to untreated control cells vs the negative log of the adjusted P-value (Benjamini-Hochberg) (A, Pam3-CSK4 vs untreated control; B, Pam2-CSK4 vs untreated control; C, WT Lpp20-HA vs untreated control; and D, Δlnt Lpp20-HA vs untreated control). Significant results (greater than a twofold change in expression and an adjusted P-value less than 0.05) are shown in blue. (E and F) To compare results between similar treatment groups, the log2(fold change) for significant differences were plotted against each other (E, cells treated with triacyl lipoproteins Pam3-CSK4 and WT Lpp20-HA; and F, cells treated with diacyl lipoproteins Pam2-CSK4 and Δlnt Lpp20-HA).
Similar analyses were performed comparing genes differentially expressed between cells treated with diacylated Lpp20-HA vs untreated control cells and between cells treated with diacylated Pam2-CSK4 peptide vs untreated control cells (Fig. 3B, D, and F; Data sets S3 and S4). Analyses identified 271 genes differentially expressed between diacylated Lpp20-HA-treated cells and control, and 825 differentially expressed genes between Pam2-CSK4-treated cells and control. Among these genes, 203 were found to be differentially expressed by both diacylated Lpp20-HA and Pam2-CSK4, with a strong correlation between the magnitude of fold change between the two groups (Fig. 3F).
We next performed pathway analysis to provide potential mechanistic insight into the differentially expressed genes. Given the strong correlations between the fold change in gene expression for cells treated with triacylated lipoproteins (Pam3-CSK4 and WT Lpp20-HA, Fig. 3E), pathway analysis was performed by combining the differentially expressed gene lists for Pam3-CSK4- and WT Lpp20-HA-treated cells into a single gene set representing the response to triacylated lipoproteins (49). Similarly, pathway analysis was performed by combining the differentially expressed gene lists for Pam2-CSK4- and Δlnt Lpp20-HA-treated cells into a single gene set representing the response to diacylated lipoproteins. Results are summarized in Tables 1 and 2. The response to triacylated lipoproteins was characterized by increased expression of a number of cytokines and chemokines (CCL2, CCL20, CSF1, CSF2, CSF3, CX3CL1, CXCL1, CXCL2, CXCL3, CXCL6, IL1A, IL1B, IL6, and IL8). The response to diacylated lipoproteins was characterized by the increased expression of a wider array of cytokines and chemokines and an assortment of genes linked to chemokine and cytokine signaling and TLR2 signaling. To help illustrate this, genes belonging to the Kyoto Encyclopedia of Genes and Genomes (KEGG) cytokine-cytokine receptor interaction pathway (hsa04060) that were differentially regulated in one or more treatment groups were identified and the log2(fold change) presented as a heatmap (50) (Fig. 4A). Select genes were analyzed by qRT-PCR to confirm that these are differentially expressed (Fig. 4B). Overall, the response to triacylated lipoproteins was more modest than the response to diacylated lipoproteins. This result was consistent with results observed with the TLR2/1 and TLR2/6 reporter cell lines and with results of previous studies indicating increased inflammatory responses to lipoproteins activating TLR2/6 heterodimers (26).
TABLE 1
Triacylated lipoproteins vs control
| Reactome pathways | Genes in reference lista | Genes in DEG listb | Expected number of genesc | Fold enrichmentd | P-valuee |
|---|---|---|---|---|---|
| Interleukin-10 signaling | 45 | 12 | 0.11 | >100 | 2.41E − 17 |
| Chemokine receptors bind chemokines | 57 | 8 | 0.14 | 55.57 | 9.31E − 09 |
| Interleukin-4 and interleukin-13 signaling | 111 | 6 | 0.28 | 21.4 | 1.07E − 03 |
| Peptide ligand-binding receptors | 196 | 8 | 0.5 | 16.16 | 9.17E − 05 |
| Signaling by interleukins | 448 | 12 | 1.13 | 10.61 | 2.67E − 06 |
| Class A/1 (rhodopsin-like receptors) | 331 | 8 | 0.84 | 9.57 | 4.36E − 03 |
| G alpha (i) signaling events | 315 | 7 | 0.8 | 8.8 | 3.44E − 02 |
| GPCRf ligand binding | 463 | 8 | 1.17 | 6.84 | 4.81E − 02 |
| Cytokine signaling in immune system | 697 | 12 | 1.76 | 6.82 | 3.20E − 04 |
TABLE 2
Diacylated lipoproteins vs control
| Reactome pathways | Genes in reference lista | Genes in DEG listb | Expected number of genesc | Fold enrichmentd | P-valuee |
|---|---|---|---|---|---|
| Interleukin-10 signaling | 45 | 20 | 1.39 | 14.39 | 4.54E − 12 |
| Interferon gamma signaling | 91 | 23 | 2.81 | 8.18 | 7.80E − 10 |
| Interferon alpha/beta signaling | 68 | 17 | 2.1 | 8.09 | 1.10E − 06 |
| Chemokine receptors bind chemokines | 57 | 13 | 1.76 | 7.38 | 3.06E − 04 |
| Interferon signaling | 197 | 34 | 6.09 | 5.59 | 3.62E − 11 |
| Interleukin-4 and interleukin-13 signaling | 111 | 19 | 3.43 | 5.54 | 2.63E − 05 |
| MyD88:MAL(TIRAP) cascade initiated on plasma membrane | 100 | 14 | 3.09 | 4.53 | 1.80E − 02 |
| Toll-like receptor TLR6:TLR2 cascade | 100 | 14 | 3.09 | 4.53 | 1.80E − 02 |
| Toll-like receptor TLR1:TLR2 cascade | 103 | 14 | 3.18 | 4.4 | 2.44E − 02 |
| Toll-like receptor 2 (TLR2) cascade | 103 | 14 | 3.18 | 4.4 | 2.44E − 02 |
| Cytokine signaling in immune system | 697 | 90 | 21.53 | 4.18 | 4.60E − 25 |
| Signaling by interleukins | 448 | 55 | 13.84 | 3.97 | 2.18E − 13 |
| Toll-like receptor 4 (TLR4) cascade | 132 | 16 | 4.08 | 3.92 | 2.19E − 02 |
| Toll-like receptor cascades | 154 | 17 | 4.76 | 3.57 | 3.56E − 02 |
| Peptide ligand-binding receptors | 196 | 20 | 6.05 | 3.3 | 1.91E − 02 |
| Class A/1 (rhodopsin-like receptors) | 331 | 27 | 10.22 | 2.64 | 2.65E − 02 |
| Innate immune system | 1,107 | 62 | 34.2 | 1.81 | 2.81E − 02 |
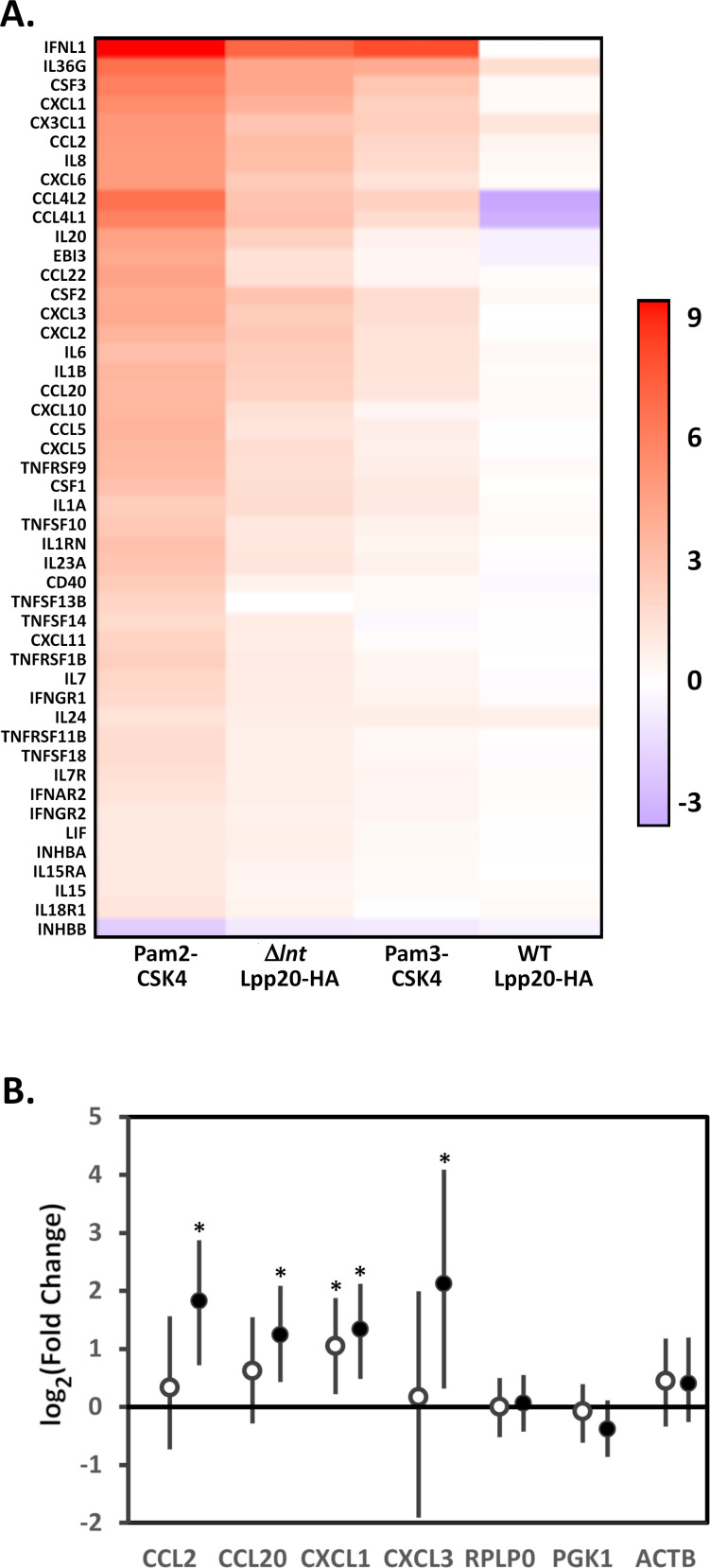
Induction of cytokine and chemokine expression by diacylated lipoproteins. (A) Pathway analysis (Tables 1 and 2) indicated that lipoprotein treatment led to increased expression of numerous chemokines and cytokines. The log2(fold change) for genes within the KEGG cytokine-cytokine receptor interaction pathway (hsa04060) that were differentially regulated in primary gastric epithelial cells by one or more treatment groups (Pam3-CSK4, Pam2-CKS4, WT Lpp20-HA, or Δlnt Lpp20-HA) is presented as a heatmap. (B) Quantitative RT-PCR of select transcripts was performed on the RNA samples from primary human gastric epithelial cells treated with WT Lpp20-HA (open symbols) or Δlnt Lpp20-HA (closed symbols) as described in Materials and Methods; RPLP0, PGK1, and ACTB served as controls. Results represent the mean and 95% credible limits (error bars) from six replicates each. Significance was determined by calculating Bayesian z scores, and a standard z test was performed to derive two-tailed P-values, which were corrected for multiple testing using the Benjamini-Hochberg method (with a false discovery rate of 5%). *, P < 0.03.
Role of lipoprotein acylation in H. pylori infection of mice
We next determined whether expression of diacylated lipoproteins impacted the ability of H. pylori to colonize in a murine model of infection. Eight- to 10-week-old C57Bl/6 mice were infected with H. pylori strain J166, the Δlnt mutant strain VM391, or the complemented mutant strain VM392. Two weeks post infection, animals were sacrificed, and stomach homogenates were cultured to enumerate the number of H. pylori. Both the parental J166 strain and the complemented mutant strain VM392 successfully colonized the stomachs (Fig. 5A). In contrast, the mutant strain VM391 failed to colonize (Fig. 5A). We also tested the ability of strain J166 and the lnt mutant strain VM391 to colonize the stomachs of Tlr2−/− mice. The parental strain J166 successfully colonized three of five animals, whereas the lnt mutant strain VM391 failed to colonize (Fig. 5B). We examined the gastric histology using the modified Sydney scoring system described in Materials and Methods to evaluate possible lnt-dependent changes in the severity of gastric inflammation. Minimal gastric inflammation (histology scores from 0 to 2.5 on a 12-point scale) was observed with no significant difference in inflammation observed between animals infected with wild-type or lnt mutant H. pylori (Fig. S2). These results indicate that lnt is required for H. pylori to colonize or survive within the stomach environment.
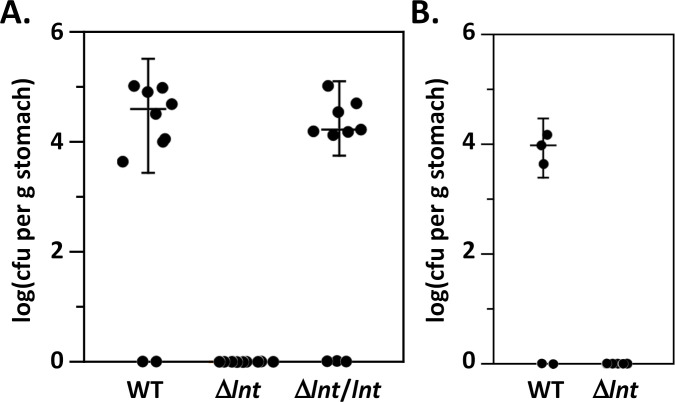
Colonization of mice. (A) Wild-type C57Bl/6 were infected orogastrically with two doses of H. pylori J166 wild type, Δlnt mutant strain VM391, or the complemented mutant strain VM392 (Δlnt/lnt) as indicated. Colony forming units (CFUs) were determined 2 weeks post infection by plating serial dilutions of stomach homogenates. Data are representative of one of three independent experiments. (B) Tlr2−/− mice were infected orogastrically with two doses of H. pylori J166 wild type or Δlnt mutant strain VM391 as indicated. CFU were determined 2 weeks post infection by plating serial dilutions of stomach homogenates.
DISCUSSION
The enzymatic pathway of lipoprotein synthesis is highly conserved among Gram-negative bacteria. For example, we previously showed that the H. pylori Lgt, LspA, and Lnt enzymes can complement their Escherichia coli counterparts despite sharing only 20%–40% amino acid identity (31). Whereas Lgt and LspA are widely believed to be essential, the essential nature of Lnt appears to correlate with differences in the lipoprotein outer membrane localization (Lol) system. Specifically, Lnt appears to be non-essential in bacteria in which LolC and LolE proteins are replaced by a single LolF protein (31, 33, 51).
Previous studies reported a wide range of physiologic consequences to mutating lnt (33,–35, 40). For example, mutation of lnt in Acinetobacter bayli and in A. baumannii leads to significant increases in the generation time, increased sensitivities to an array of antibiotics, and alters cellular morphology (34). Mutation of lnt in Francisella tularensis and Neisseria gonorrhoeae leads to modest changes in antibiotic sensitivities, but changes in generation time were not observed (33). Additionally, F. tularensis lnt mutants retained the ability to survive and replicate within a human monocyte cell line (33). Finally, deletion of lnt in Rhizobium led to acid sensitivity (40). The consequences of deleting lnt are thus difficult to predict. We found that mutation of H. pylori lnt did not alter generation time, antibiotic sensitivity, or acid sensitivity. We previously showed that the H. pylori Cag type IV secretion system (a major virulence determinant) remains active in an lnt mutant despite CagT (an essential component of the system) being a lipoprotein (31). Thus, loss of lnt does not disrupt this important virulence determinant or disrupt proper localization of lipoproteins to the H. pylori outer membrane (31, 33, 35). Even though loss of lnt does not appear to result in profound physiologic changes in H. pylori, lnt is conserved among H. pylori strains. This suggests that H. pylori experiences a selective pressure in vivo to maintain lnt and its addition of a third acyl chain onto H. pylori lipoproteins.
Although multiple studies have indicated an important role for TLR2 in the host response to H. pylori infection, the H. pylori ligands activating TLR2 are poorly understood (9, 13, 14, 18, 19). There have been conflicting studies regarding whether H. pylori LPS is recognized by TLR2 (9, 13, 20,–22). In addition to LPS, previous studies have suggested that H. pylori urease, neutrophil-activating protein (NapA), and heat-shock protein (HSP60) can act as a TLR2 ligands (52,–55). Few studies have explored the role of H. pylori lipoprotein acylation in H. pylori infection despite lipoproteins being widely recognized as TLR2 ligands (24, 25, 56).
In the present study, we found that incubating primary human gastric epithelial cells with triacylated lipoproteins (including Lpp20 as a representative H. pylori lipoprotein) led to increased expression of several chemokines and cytokines including CXCL1, CXCL2, CXCL3, CCL20, CXCL8 (IL8), IL36G, CX3CL1, and IL24. Previous studies incubated primary human gastric epithelial cells with whole H. pylori and found expression of CXCL1, CXCL2, CXCL3, CXCL5, and CXCL8 to be upregulated in a TLR2-dependent manner (48, 57). Together, these results suggest that H. pylori lipoproteins may be important contributors to the immune response to H. pylori. We acknowledge that the synthetic triacylated lipopeptide Pam3-CSK4 induced a wider array of chemokines and cytokines than Lpp20-HA isolated from wild-type H. pylori. Whether these differences are due to differences in the amino acid sequences of Pam3-CSK4 and Lpp20-HA (58), the functional fold of Lpp20, or simply due to differences in the relative concentrations of the lipopeptides is not clear from the present study.
The epithelial response to diacyl lipoproteins (Lpp20-HA from lnt mutant H. pylori and the synthetic Pam2-CSK4) was more robust than the response to triacyl lipoproteins. Both a wider array of chemokines and cytokines was differentially expressed and the magnitude of change was greater than what we observed in response to triacyl lipoproteins. Similar to what we observed with triacyl lipoproteins, we acknowledge that the synthetic diacylated lipopeptide Pam2-CSK4 induced an elevated response compared to Lpp20-HA isolated from lnt mutant H. pylori. Whether these differences are due to differences in the amino acid sequences of Pam2-CSK4 and Lpp20-HA (58), the functional fold of Lpp20, or simply due to differences in the relative concentrations of the lipopeptides is not clear from the present study. It is widely understood that triacylated lipoproteins are recognized by TLR2/1 heterodimers, and diacylated lipoproteins are recognized by TLR2/6 heterodimers (24, 25). Recent reports suggest that signaling via TLR2/1 or TLR2/6 may lead to either immune activation or suppression (26, 27). Thus, differences between the epithelial response to diacylated lipoproteins vs triacylated lipoproteins were not unexpected. The enhanced cellular response to diacylated lipoproteins might contribute to the selective pressure in vivo for H. pylori to maintain lnt and triacylated lipoproteins.
In contrast to the results of our studies, which showed no discernible differences in the physiology between wild-type and lnt mutant H. pylori in vitro; in vivo studies indicated a profound difference between strains. Whereas the wild-type bacteria successfully colonized mice, the lnt mutant strain failed to colonize. We can be confident that this defect is due to loss of lnt as introducing a copy of lnt into an alternate chromosomal locus restored the ability of the mutant strain to colonize the stomach. We saw no difference between wild-type and lnt mutant H. pylori in killing by murine monocytes or in attachment to a human gastric epithelial cell line (data not shown). The mutant strain also failed to colonize the stomachs of Tlr2−/− mice, indicating that the colonization defect does not simply depend on differences in the TLR2-mediated host response to bacteria expressing triacylated lipoproteins vs diacylated lipoproteins. Previous studies have suggested that H. pylori lipoproteins interact with the host in various ways. For example, CagT is required for Cag type IV secretion system effector translocation (31, 59,–61); HpaA contributes to bacterial adhesion to mammalian cells and colonization of the stomach (62); Lpp20 alters cell migration and signaling (63, 64); and ComB7 modulates the activity of the Com system in H. pylori responsible for natural competence which promotes colonization (65,–67). Most suspected H. pylori lipoproteins have unknown functions. We suggest the lnt mutant is unable to colonize due to the dependence of one or more virulence or colonization factors on three acyl chains.
Increasing incidence of clarithromycin resistance has led WHO to declare H. pylori a priority pathogen for development of new antimicrobials (68). The lipoprotein synthesis and localization pathways are unique to bacteria, and several antimicrobial compounds have been identified that target different steps in these pathways (69,–76). This study, demonstrating that lnt is required for colonization, indicates such antimicrobial compounds could be effective alternatives to current H. pylori antibiotic therapies.
MATERIALS AND METHODS
Bacterial strains and culture conditions
Strains and plasmids used in this study are listed in Table 3. H. pylori strains were grown on either trypticase soy agar (TSA) plates containing 5% sheep blood or in serum-free bisulfite-free Brucella broth supplemented with 1× cholesterol lipid concentrate (Life Technologies), at 37°C in room air supplemented with 5% CO2. H. pylori mutant strains were selected using metronidazole (15 µg/mL), kanamycin (12.5 µg/mL), or chloramphenicol (2.5 µg/mL). Plasmids were maintained using E. coli strain DH5α. E. coli strains were grown on Luria-Bertani (LB) agar plates or in LB broth containing ampicillin (100 µg/mL), chloramphenicol (25 µg/mL), or kanamycin (25 µg/mL). To assess acid sensitivity, H. pylori strains were grown in bisulfite-free Brucella broth supplemented with cholesterol and buffered with 50 mM 3-(N-morpholino)propanesulfonic acid (MOPS) (pH 7) or 50 mM citric acid and 100 mM Na2HPO4(pH 5, 4.5, or 4). The number of colony forming units (CFUs) was determined at 0 and 8 h by spotting serial dilutions onto TSA plates containing 5% sheep blood. To assess antibiotic sensitivity, H. pylori strains in bisulfite-free Brucella broth supplemented with cholesterol were serially diluted and spotted onto bisulfite-free Brucella agar supplemented with 10% fetal calf serum with no added antibiotic or with bacitracin (200 µg/mL) or vancomycin (50 µg/mL).
TABLE 3
Bacterial strains and plasmids
| Strain | Genotype/description | Reference |
|---|---|---|
| J166 | (77) | |
| 26695 | (78) | |
| BV199 | 26695 ΔrdxA ΔcagT | (79) |
| VM211 | 26695 Δlnt::aphA3, Kanr | (31) |
| VM391 | J166 Δlnt::aphA3, Kanr | Current study |
| VM392 | VM391 PureA::lnt inserted into hydA-mdaB intergenic region, Kanr, Chlr | Current study |
| VM396 | BV199 ureA::lpp20-HA, Chlr | Current study |
| VM404 | VM396 Δlnt::aphA3, Kanr Chlr | Current study |
| Plasmids | ||
| pAD1 | Vector for introducing recombinant DNA into the H. pylori ureA locus | (80, 81) |
| pMM684 | pGemT-Easy (Promega) containing the H. pylori hydA-mdaB intergenic region | (42, 51) |
| pMM711 | lpp20-HA cloned into pAD1 | Current study |
| pMM712 | lnt cloned into pAD1 | Current study |
| pMM714 | PureA::lnt from pMM712 cloned into pMM684 | Current study |
Construction of recombinant H. pylori strains
Genomic DNA from H. pylori strain VM211 (in which the lnt promoter and open reading frame is replaced by aphA3 and a copy of the lnt promoter is inserted downstream of aphA3 to direct expression of the downstream genes within the operon) was used to transform strain J166 and selection for kanamycin-resistant (Kanr) colonies was used to isolate strain VM391 (Table 3). Strain VM391 was subsequently transformed with plasmid pMM714 which contains H. pylori lnt under the control of the ureA promoter and a cat gene inserted into the hydA-mdaB intergenic region (Table 3) (31, 41,–43). Plasmid pMM714 does not replicate in H. pylori. Selection for chloramphenicol-resistant (Chlr) colonies was used to isolate strain VM392. H. pylori strain BV199 was transformed with plasmid pMM711 which contains an HA-tagged copy of lpp20 and the cat gene in place of ureA (Table 3). Plasmid pMM711 does not replicate in H. pylori and selection for chloramphenicol-resistant colonies was used to isolate VM396. VM396 was subsequently transformed with genomic DNA from strain VM211 and selection for kanamycin-resistant colonies was used to isolate strain VM404 (Table 3).
Purification of Lpp20
H. pylori strains VM394 and VM404 were grown in bisulfite-free Brucella broth supplemented with cholesterol. Bacteria were pelleted (5,000 × g, 10 min), and the pellet was resuspended in 1 M MOPS, pH 7 containing protease inhibitors (Complete Protease Inhibitor) and 1,000 units mutanolysin (Sigma) per gram of bacterial pellet. The bacterial suspensions were incubated with rocking at room temperature for 1 h then passed through a French Press (Glen Mills) at 18000 pounds per square inch. Unlysed bacteria were pelleted (7,500 × g, 10 min) and bacterial membranes pelleted (174,000 × g, 2 h, 4°C). The total membrane pellet was solubilized in 50 mM MOPS, pH 7, 300 mM NaCl, and 1% fos-choline 12. Solubilized membranes were incubated with anti-DYKDDDDK-coated magnetic agarose (Pierce), and the samples were mixed gently at room temperature for 1 h. The beads were collected on a magnet and washed four times in 50 mM MOPS, pH 7, 300 mM NaCl, and 1% fos-choline 12. Affinity-purified proteins were eluted by incubating the beads twice with 50 µL of 2.5 mg/mL DDK peptide in 50 mM MOPS, pH 7, 300 mM NaCl, and 1% fos-choline 12. Eluted proteins were buffer exchanged into 50 mM MOPS, pH 7, 300 mM NaCl using Amicon Ultra ultrafiltration devices (3,000 mw cutoff, Millipore), precipitated with acetone, and dried.
TLR reporter cells and secreted embryonic alkaline phosphatase (SEAP) assays
Reporter cells, derived from HEK293 cells, were purchased from InvivoGen. HEK-Blue Null2 cells express a SEAP reporter gene under the control of the IL-12-p40 minimal promoter fused to five NF-κB and AP-1 binding sites. Similar to HEK-Blue Null2, the HEK-Blue hTLR4 cell line additionally expresses the hTLR4 gene, and the MD-2/CD14 co-receptor gene. The HEK-Blue hTLR2 KO-TLR1/6 cell line carries a double knockout of the TLR1 and TLR6 genes in a SEAP expressing cell line that overexpresses hTLR2 and CD14. HEK-Blue hTLR2-TLR1 were derived by stable transfection of the hTLR1 gene into the HEK-Blue hTLR2 KO-1/6 cell line. HEK-Blue hTLR2-TLR6 were derived by stable transfection of the hTLR6 gene into the HEK-Blue hTLR2 KO-1/6 cell line. Cells were left untreated, treated with Pam3-CSK4 or Pam2-CSK4 (40 ng/mL), or treated with WT Lpp20-HA or Δlnt Lpp20-HA (100 ng/mL) for 16 h. Secreted alkaline phosphatase assays were carried out using HEK-Blue detection media according to manufacturer’s instructions (InvivoGen). Pam3-CSK4 and Pam2-CSK4 were purchased from Tocris Bioscience.
Primary human gastric epithelial cells
Deidentified primary human gastric epithelial cells were purchased from Cell Biologics. Cells at passage 4 were subcultured at 3.4 × 105 cells per well into six-well dishes pretreated with gelatin and cultured in Complete Human Epithelial Cell Medium (Cell Biologics) for 16 h. Cells were left untreated (three wells), treated with Pam3-CSK4 or Pam2-CSK4 (100 ng/mL, three wells each), or treated with WT Lpp20-HA or Δlnt Lpp20-HA (300 ng/mL, six wells each) for 4 h.
RNAseq
For analysis of gene expression in human gastric epithelial cells, cells were lysed in TRIzol and RNA was purified using Direct-zol RNA MiniPrep Plus (Zymo Research) including on-column DNAse treatment. Each RNA sample was eluted in 100 µL of water. Purified RNA (300 ng total RNA per sample) was submitted to the VANTAGE Core who performed RNA quality assessment (2100 Bioanalyzer, Agilent), ribosomal RNA depletion, library preparation, and sequencing on an Illumina NovaSeq 6000 at 150 bp paired-end reads. The number of sequence reads averaged 58 million per sample. Trimmomatic was used for quality trimming and adapter clipping (82). HiSat2 was used to map reads to human b37:hg19 canonical reference genome (83). Alignments were assembled into transcripts using StringTie (84). Differential gene expression was carried out using edgeR using the generalized linear model (85). Fold change values were calculated by comparing the means of normalized pseudocounts for samples from each group (calculated by edgeR). A false discovery rate (FDR), corresponding to a Benjamini-Hochberg adjusted P-value, was calculated using the p.adjust program within edgeR. Differentially expressed genes were defined as those exhibiting an FDR value of <0.05, as well as a >2.0- or <0.5-fold difference in transcript abundance in the above comparisons. Pathway analysis was performed using PANTHER (49).
Mouse infections and tissue harvests
To infect mice, the H. pylori strains (J166, lnt mutant strain VM391 and complemented mutant strain VM392) were grown in liquid culture (bisulfite-free Brucella broth with 10% heat-inactivated FBS and 10 µg/mL vancomycin) under microaerophilic conditions generated by a GasPak EZ Campy Container System (Becton Dickinson, Franklin Lakes, NJ), shaking at 160 rpm for 16–18 h. The orogastric inoculum was prepared after quantification of H. pylori using an optical density at 600 nm (OD600) reading. The cultures for two replicate experiments were serially diluted and plated to verify the accuracy of the inocula (for each strain). For all experiments, mice received two doses of 1 × 109 CFUs of H. pylori in 0.5 mL bisulfite-free, serum-free Brucella broth approximately 48 h apart.
At the time of tissue harvest (2 weeks post infection), the mice were sacrificed, and the stomach was dissected. Gastric tissue containing both antrum and corpus regions were used to determine bacterial burden and inflammation. The H. pylori burden in the mice was quantified by spreading stomach homogenate on tryptic soy agar plates containing sheep blood (5%), nalidixic acid (10 µg/mL), vancomycin (50 µg/mL), amphotericin (2 µg/mL), and bacitracin (100 µg/mL) in microaerophilic conditions at 37°C for 5–7 d.
Inflammation scoring
Inflammation was scored using the updated Sydney System by a single pathologist (M. B. P.) who was unaware of the different strains used for infections. Acute inflammation was scored (0–3) based on increasing density of neutrophils. Chronic inflammation was scored (0–3) based on the density of mononuclear cell infiltration in the lamina propria. Inflammation was scored as absent (grade 0), mild (grade 1), moderate (grade 2), or severe (grade 3) with the antrum and corpus regions of the stomach being scored independently. The total inflammation was calculated as a sum of acute and chronic inflammation in the corpus and the antrum, which allows for quantification of total inflammation on a scale from 0 to 12.
Analysis of gene expression by quantitative RT-PCR
For analysis of gene expression in H. pylori, strains were cultured for 16 h in bisulfite-free Brucella broth supplemented with cholesterol. Bacteria were pelleted, washed three times in fresh media, and used to inoculate new cultures. Following growth for 4 h, RNA was isolated using TRIzol (Life Technologies) according to the manufacturer’s protocol. Purified RNA (1 µg) was treated with Turbo DNA-free (Life Technologies). One-half of each sample was used for first-strand cDNA synthesis using Superscript III (Life Technologies) with random hexamer priming. The other half of each sample was treated in the same manner, except that reverse transcriptase was omitted. qRT-PCR was performed using iQ SYBR Green Supermix (Bio-Rad) and oligonucleotide primers for lgt, and control genes lpxD, prfA, and tlyC. Control genes lpxD, prfA, and tlyC were chosen based on analysis of RNAseq data, indicating stable expression of selected genes in response to in vitro stress (high salt conditions) (86).
Quantitative RT-PCR data were analyzed using generalized linear mixed models based on log-normal Poisson error distribution and fitted using Markov chain Monte Carlo analysis (87). Data were fit using informed models and data from control genes. Amplification efficiencies were determined based on analysis of amplification of targets over a six-log range of template concentrations using purified H. pylori genomic DNA and optimized primer annealing temperatures. The credible intervals were determined by the MCMC.qpcr package in R (41, 51, 87). Based on the Bayesian framework, a credible interval (or credible limit) is an analog of a confidence interval in frequentist statistics. The 95% credible interval indicates there is a 95% probability of the true value falling with this parameter.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (AI163586, MSM and HMSA); the Department of Veterans Affairs (IBX000915A HMSA), the Vanderbilt Digestive Disease Research Center (P30DK058404), and the Vanderbilt-Ingram Cancer Center (P30CA068485).
DATA AVAILABILITY
RNA-seq data were deposited in the GEO database under accession number GSE241938.
ETHICS APPROVAL
The use of animals for this project was approved by the Institutional Animal Care and Use Committee of Vanderbilt University Medical Center (M2100082). Procedures were performed in accordance with the American Association for Accreditation of Laboratory Animal Care guidelines, the American Veterinary Medical Association Guidelines on Euthanasia, National Institutes of Health regulations (Guide for the Care and Use of Laboratory Animals), and the U.S. Animal Welfare Act. All animals were housed at VUMC in an accredited research animal facility that is fully staffed with trained personnel. Eight- to 10-week-old C57Bl/6 mice and Tlr2−/− mice were used for these experiments (#004650, Jackson Laboratory, Bar Harbor, ME).
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/iai.00369-23.
Supplemental figures
iai.00369-23-s0001.pdf:Fig. S1 and S2.
Supplemental data sets
iai.00369-23-s0002.xlsx:Data Sets S1, S2, S3, and S4.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
Articles from Infection and Immunity are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/iai.00369-23
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10715074
Citations & impact
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/156274588
Article citations
Fatty acids of Helicobacter pylori lipoproteins CagT and Lpp20.
Microbiol Spectr, 12(5):e0047024, 19 Mar 2024
Cited by: 0 articles | PMID: 38501821 | PMCID: PMC11064636
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Lipoprotein Processing and Sorting in Helicobacter pylori.
mBio, 11(3):e00911-20, 19 May 2020
Cited by: 15 articles | PMID: 32430470 | PMCID: PMC7240156
Fatty acids of Helicobacter pylori lipoproteins CagT and Lpp20.
Microbiol Spectr, 12(5):e0047024, 19 Mar 2024
Cited by: 0 articles | PMID: 38501821 | PMCID: PMC11064636
TLR2 mediates Helicobacter pylori-induced tolerogenic immune response in mice.
PLoS One, 8(9):e74595, 13 Sep 2013
Cited by: 35 articles | PMID: 24058595 | PMCID: PMC3772856
The role of toll-like receptors in immune tolerance induced by Helicobacter pylori infection.
Helicobacter, 28(6):e13020, 10 Sep 2023
Cited by: 6 articles | PMID: 37691007
Review
Funding
Funders who supported this work.
BLRD VA (1)
Grant ID: I01 BX000915
HHS | NIH | National Institute of Allergy and Infectious Diseases (1)
Grant ID: AI163586
NCI NIH HHS (1)
Grant ID: P30 CA068485
NIAID NIH HHS (1)
Grant ID: R21 AI163586
NIDDK NIH HHS (1)
Grant ID: P30 DK058404
U.S. Department of Veterans Affairs (1)
Grant ID: IBX000915A
 1
,
2
and
1
,
2
and 



