Abstract
Background
Catheter-related bloodstream infections (CRBSIs) are a major cause of morbidity and mortality among hospitalized patients. Different studies suggest that the use of disinfectant caps (DCs) significantly reduces the rate of CRBSIs. The first purpose of this study is to analyze, through an in-vitro-model, the antiseptic effect of DCs produced by two manufacturers; the second aim is to assess potential differences in terms of effectiveness between the two manufacturers' products.Methods
A know concentration of thirteen different microorganisms was incubated with the sponge drenched in antimicrobial fluid inside DCs and cultured through several assays to investigate the disinfectant effectiveness of some commercially available caps. Disinfectant properties were evaluated under two different conditions: baseline (DCs placed on the needle-free connectors (NFCs) and stress test (DCs directly applied to the catheter hub).Results
Both manufacturers overcame the basal tests (fourteen different assays). Regarding stress tests: the only significant bacterial load was found for Serratia marcescens (104 CFU/mL in ICU Medical™), both at 90 and 180 minutes after incubation; due to the low load, MDR Acinetobacter baumannii was not considered significant (<103 CFU/mL in BD PureHub™).Conclusions
Our results confirm what was reported in BD PureHub™ datasheet and add data not previously shown by ICU Medical™. Moreover, no difference was observed between the two manufacturers products: the use of both DCs on NFCs was able to reclaim the catheter lumen. These findings support the routine use of DCs with NFCs, as part of a structured bundle of interventions, to reduce the incidence of CRBSIs.Free full text

Disinfectant caps in vitro effectiveness
SUMMARY
Background
Catheter-related bloodstream infections (CRBSIs) are a major cause of morbidity and mortality among hospitalized patients. Different studies suggest that the use of disinfectant caps (DCs) significantly reduces the rate of CRBSIs. The first purpose of this study is to analyze, through an in-vitro-model, the antiseptic effect of DCs produced by two manufacturers; the second aim is to assess potential differences in terms of effectiveness between the two manufacturers’ products.
Methods
A know concentration of thirteen different microorganisms was incubated with the sponge drenched in antimicrobial fluid inside DCs and cultured through several assays to investigate the disinfectant effectiveness of some commercially available caps. Disinfectant properties were evaluated under two different conditions: baseline (DCs placed on the needle-free connectors (NFCs) and stress test (DCs directly applied to the catheter hub).
Results
Both manufacturers overcame the basal tests (fourteen different assays). Regarding stress tests: the only significant bacterial load was found for Serratia marcescens (104 CFU/mL in ICU Medical™), both at 90 and 180 minutes after incubation; due to the low load, MDR Acinetobacter baumannii was not considered significant (<103 CFU/mL in BD PureHub™).
Conclusions
Our results confirm what was reported in BD PureHub™ datasheet and add data not previously shown by ICU Medical™. Moreover, no difference was observed between the two manufacturers products: the use of both DCs on NFCs was able to reclaim the catheter lumen. These findings support the routine use of DCs with NFCs, as part of a structured bundle of interventions, to reduce the incidence of CRBSIs.
INTRODUCTION
Catheter-related bloodstream infections (CRBSIs) are clinical conditions characterized by fever and systemic clinical signs. A CRBSI should be diagnosed using paired qualitative (as measured using differential time to positivity-DTP) and/or using paired quantitative (as measured using pour plates) blood cultures from a peripheral vein and from the catheter. For hospitals using DTP, a CRBSI is diagnosed if the same organism is isolated from blood obtained through the catheter hub and from blood obtained from a peripheral vein and the DTP is more than 2 hours (catheter hub culture positive first) [1]. CRBSIs are potentially life-threatening healthcare associated infections, that continue to be one of the most important public health issues throughout the world [2]. Although the reported incidence of CRBSIs is around 0.4 percent, these clinical entities constitute an important cause of hospital-acquired infections associated with elevated morbidity, mortality and increased costs [3, 4].
Researchers have reported that the use of 70% isopropyl alcohol just before needle free connectors (NFCs) is not enough against microbial contamination: most health care organizations use a wipe impregnated with 2% chlorhexidine and 70% isopropyl alcohol, which has been shown to decontaminate NFCs when applied for at least 15 seconds, as recommended by evidence-based guidelines [5–7]. However, manual disinfection of NFCs is significantly affected by expertise and sometimes lack of healthcare workers compliance [8]. Therefore, as recommended by guidelines, NFCs disinfection should be achieved using a disinfectant cap (DC) [5, 8, 9].
The DC (also known as Disinfecting Port Protector) is a plastic device that contains 70% isopropyl alcohol. It is effective within 10 minutes from application and may be used for up to 7 days. Several studies and a recent meta-analysis suggest that the clinical use of DCs significantly reduced the incidence of CRBSIs when compared with manual disinfection [9–15].
To the best of our knowledge, there aren’t independent studies aiming to evaluate DCs effectiveness and to point out potential differences between what the manufacturers claim in their datasheets. The first purpose of this study is to analyze, in an in vitro model, the antiseptic effect of the DCs produced by two manufacturers; we evaluated if, in the event of maximum bacterial contamination, the DCs could still succeed in reclaiming the catheter from various micro-organisms. The second purpose was to analyze potential differences in terms of effectiveness between the two manufacturers products.
MATERIALS AND METHODS
From April 2022 to February 2023, the Microbiology Unit of ASST Fatebenefratelli-Sacco, Milan, Italy, conducted a study to investigate the efficacy of some commercially available DCs, under two different conditions: the baseline, recommended by guidelines (application of DC to NFC) and a stress test (placing the DC directly on the catheter hub) [16]. We then compared the antimicrobial capacity of various Port Protectors in these two conditions. In the first phase of the study (basal test), a known concentration (0.5 McFarland) of different micro-organisms was inoculated in the two NFCs applied on a two-lumen catheter (Arrow®, Teleflex; 20cm length 7 FR dual lumen). After bacterial growth was completed (12 hours after inoculation), we used two disinfectant caps belonging to two different brands (BD PureHub™ and ICU Medical™) as shown in Figure 1. The DCs were placed on NFCs as indicated by guidelines [17]. Ten minutes after application (as stated in DCs datasheets), we removed the sponge drenched in antimicrobial fluid inside the DC and cultured it for 90 and 180 minutes after incubation. To confirm proper contamination, also NFCs were cultured. In sample size calculation, we considered a single proportion with an estimated 3% positivity and a 10% accuracy in terms of confidence level 95%. We calculated a sample size of at least 12 cultures, increased to 14 due to an expected 10% drop out. In the second phase, a known concentration (0.5 MF) of different micro-organisms was inoculated in the two hubs of a two-lumen catheter (Arrow®, Teleflex; 20cm length 7 FR dual lumen) as shown in Figure 1.
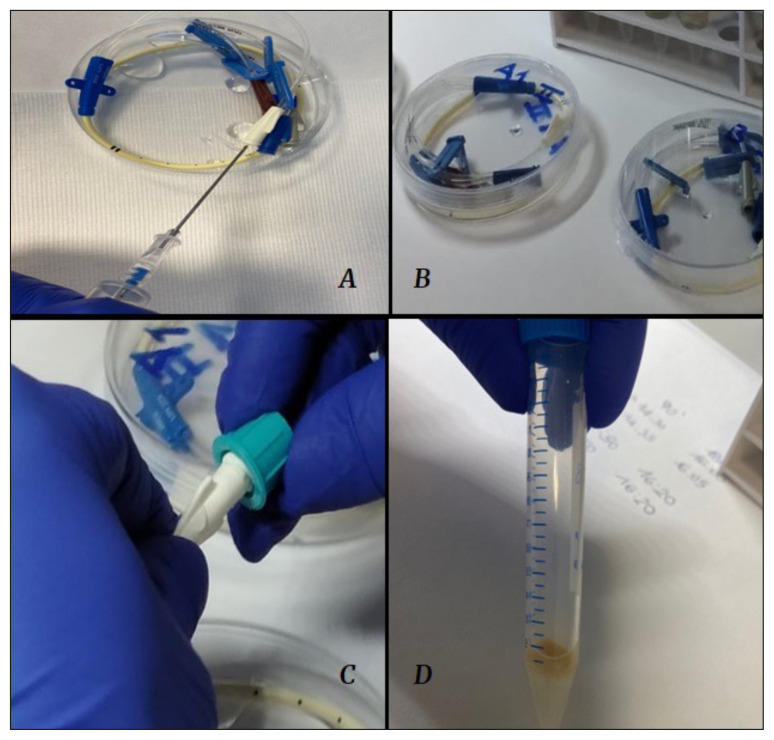
A known concentration (0.5 MF) of different microorganisms was inoculated into a two-lumen catheter (A, B). At the end of bacterial growth, disinfectant caps of two main brands (BD PureHub™ and ICU Medical™) were screwed onto each catheter lumen and retained on it for ten minutes (C). After this time, we removed the filter inside the disinfectant caps (D) and cultured it for 90 and 180 minutes after incubation
After bacterial growth was completed (12 hours after inoculation), we used two disinfectant caps of two different brands (BD PureHub™ and ICU Medical™). The DCs were applied directly on the catheter hubs.
Ten minutes after application (as stated in DCs datasheets), we removed the sponge drenched in antimicrobial fluid inside the DC and cultured it for 90 and 180 minutes after incubation.
We decided to define this as “stress test” because the hollow surface of the catheter hub is usually harder to be disinfected than the smooth surface of the NFC.
To confirm proper contamination, distal catheters hubs were cultured.
The MALDI-TOF MS systems (bioMerieux Coroprate) was used for all bacterial identification in both first and second phase.
In the second phase sample size calculation, we considered a single proportion with an estimated 16% positivity and a 10% accuracy in terms of confidence level 95%. We calculated a sample size of at least 52 culture, increased to 58 due to an expected 10% drop out.
According to the literature and in order to increase the sensitivity value of the test, in both baseline and stress test, each microbial strain was cultured both at 90 and 180 minutes after incubation [18]. Only microbial loads above 104 were considered relevant [18].
Thirteen different strains of the most involved microorganisms in the setting of hospital acquired infections (6 Gram-positive bacteria, 6 Gram-negative bacteria, 1 yeast), were tested.
RESULTS
During the baseline test, fourteen different assays were performed: 3 Gram negative bacteria (carbapenem-resistant Klebsiella pneumoniae, multidrug-resistant Pseudomonas aeruginosa, multidrug-resistant Acinetobacter pittii), one the most involved bacteria in soft tissue infections (methicillin-resistant Staphylococcus aureus) and one fungus (Candida glabrata) were tested once; Escherichia coli was tested twice. As shown in the left section of Table 1, both BD PureHub™ and ICU Medical™ tested negative. All the NFCs cultured were positive to confirm the correct contamination process.
Table 1
Basal Test and Stress Test performed with different pathogens (column 1) on the 2 types (BD PureHub™ and ICU Medical™) of port protectors. NFC means Needle free Connectors. 90 and 180 minutes are times after microbial incubation. N means negative
| BASAL TEST | BD PureHub™ + NFC | ICU Medical™ + NFC | STRESS TEST | BD PureHub™ | ICU Medical™ | ||||
|---|---|---|---|---|---|---|---|---|---|
| 90 | 180 | 90 | 180 | 90 | 180 | 90 | 180 | ||
| Carbapenem-resistant Klebsiella pneumoniae | N | N | N | N | Glycopeptide-resistant Enterococcus faecalis 1 | N | N | N | N |
| Multidrug-resistant Pseudomonas aeruginosa | N | N | N | N | Glycopeptide-resistant Enterococcus faecalis 2 | N | N | N | N |
| Escherichia coli 1 | N | N | N | N | Multidrug-resistant Pseudomonas aeruginosa 1 | N | N | N | N |
| Escherichia coli 2 | N | N | N | N | multidrug-resistant Pseudomonas aeruginosa 2 | N | N | N | N |
| Methicillin-resistant Staphylococcus aureus | N | N | N | N | Multidrug-resistant Pseudomonas aeruginosa 3 | N | N | N | N |
| Multidrug-resistant Acinetobacter pittii | N | N | N | N | Methicillin-resistant Staphylococcus aureus 1 | N | N | N | N |
| Candida glabrata | N | N | N | N | Methicillin-resistant Staphylococcus aureus 2 | N | N | N | N |
| Methicillin-resistant Staphylococcus aureus 3 | N | N | N | N | |||||
| Escherichia coli 1 | N | N | N | N | |||||
| Escherichia coli 2 | N | N | N | N | |||||
| Streptococcus agalactiae 1 | N | N | N | N | |||||
| Streptococcus agalactiae 2 | N | N | N | N | |||||
| Streptococcus agalactiae 3 | N | N | N | N | |||||
| Serratia marcescens 1 | N | N | N | N | |||||
| Serratia marcescens 2 | N | N | N | N | |||||
| Serratia marcescens 3 | N | N | 10^4 | 10^4 | |||||
| Corynebacterium striatum 1 | N | N | N | N | |||||
| Corynebacterium striatum 2 | N | N | N | N | |||||
| Corynebacterium striatum 3 | N | N | N | N | |||||
| Acinetobacter baumannii MDR 1 | 10^3 | 10^3 | N | N | |||||
| Acinetobacter baumannii MDR 2 | N | N | N | N | |||||
| Acinetobacter baumannii MDR 3 | N | N | N | N | |||||
| Candida glabrata | N | N | N | N | |||||
| Staphylococcus capitis 1 | N | N | N | N | |||||
| Staphylococcus capitis 2 | N | N | N | N | |||||
| Staphylococcus capitis 3 | N | N | N | N | |||||
| Staphylococcus epidermidis 1 | N | N | N | N | |||||
| Staphylococcus epidermidis 2 | N | N | N | N | |||||
| Staphylococcus epidermidis 3 | N | N | N | N | |||||
As shown in the right section of Table 1, fifty-eight assays were performed in the stress test: 3 Gram negative (multidrug-resistant P. aeruginosa, Serratia marcescens, MDR Acinetobacter baumannii), and several Gram positive (methicillin-resistant S. aureus, Streptococcus agalactiae, Corynebacterium striatum, Staphylococcus capitis and Staphylococcus epidermidis) were tested three times; glycopeptide-resistant Enterococcus faecalis and E. coli twice; C. glabrata was tested once.
The only significant bacterial load was found for S. marcescens (104 CFU/mL in ICU Medical™), both at 90 and 180 minutes after incubation; due to the low microbial load, MDR Acinetobacter baumannii was not considered significant (<103 CFU/mL in BD PureHub™). All the cultured catheters hub tested positive to confirm the correct contamination process.
DISCUSSION
There are no in vitro and in vivo studies comparing DCs. Moreover, no information about the sponge dimension and the amount of 70% isopropyl alcohol are displayed in the datasheets [19, 20]. Before the beginning of the study, we have asked the manufacturers for the above information and they answered that those data were not public; thus, we remained blind to some important structural differences that could have been useful in DCs comparative evaluation.
To the best of our knowledge, this is the first independent study that evaluates DCs antimicrobial effectiveness and that brings out potential differences between what manufacturers have claimed in their datasheets.
With the aim to compare the results on microbial strains claimed by manufacturers, our study evaluated, in an in vitro model, the ability to suppress bacterial growth of two of the many commercially available DCs: BD PureHub™ and ICU Medical™, despite micro-organisms resistance patterns.
In the in vitro model we chose a high load of bacterial contamination, in order to reproduce the worst clinical settings. The use of femoral catheters, mainly with mid-thigh exit site, is becoming increasingly common in hospitals, particularly in bedridden patients with psychomotor agitation, delirium, and dementia [21–22]. These patients are, among others, those at greater risk of bacterial skin colonization; indeed, many of them live in nursing homes, have had several hospital admissions and undergo bed hygiene [21]. During this cleaning procedure, it is common to get the catheter’s hub dirty and contaminated with a high bacterial load (>0.5 McFarland). The microbiological conditions in this clinical setting could be considered similar to the ones recreated in the present study.
Another clinical setting to be mentioned is the presence of a catheter hub close to a tracheostomy, where respiratory secretions are abundant. During the cleaning of the stoma, even in well positioned venous catheters (with exit site at least 10 cm away from the stoma), a high microbial contamination could happen when aspiration of secretions is performed.
In the baseline test, no significant microbial growth was observed, confirming that the use of NFCs in combination with Port Protectors significantly reduces the risk of CRBSIs [17]. Thus, respecting the aseptic technique of accessing to the system, NFCs are difficult to contaminate.
In the stress test the results obtained in vitro for both DCs were promising; only in one out of fifty-eight trials the bacterial growth was significant. Therefore, our results confirm what has been reported in the BD PureHub™ datasheet and add data not previously shown by ICU Medical™ [19, 20].
Moreover, no difference was shown between the two manufacturers products: the use DCs on NFCs was able to reclaim the catheter lumen in situations. These findings support the routine use of DCs together with NFCs to reduce the incidence of CRBSIs, as part of a structured bundle of interventions.
Finally, the stress test confirms what the producers claim: DCs cannot be used merely as disinfectants of the distal hub, on which the manual scrub-the-hub technique is needed.
A special consideration should be made about the two positive results on S. marcescens and A. baumannii cultures: both are gram-negative, hospital-acquired pathogens responsible for several nosocomial infections, especially CRBSIs [23]. The two bacteria are well known producers of biofilm and that means an advantage on catheters colonization process [24]. Bacterial biofilm is a complex surface-adhered community of viable and dead bacteria, encased within an extracellular matrix composed of polysaccharide, proteins and extracellular DNA. Bacteria in biofilm also exhibit increased resistance to antimicrobial agents for reasons such as resistance to drug penetration and to desiccation, due to metabolic changes such as slower growth rates [25]. Even if biofilm production could be one of the major explanations, other virulence factors cannot be excluded; for this reason, further investigations are needed.
This study has some potential limitations: first of all, we analyzed only one fungus, which was tested once in both basal and stress test; furthermore, this is a monocentric study so it was not possible to highlight any differences in the results among different centers.
As suggested by the recent SHEA guidelines 2022, a single recommendation could be ineffective when used alone: DCs are useful as part of a broad set of strategies to prevent CRBSIs [26].
Further investigations are needed to confirm our data both in vitro and in clinical settings and to compare other manufacturers products.
Footnotes
Conflict of interest: The Authors declare no conflicts of interest.
Funding: All the material used in this in vitro study were provided by our University Hospital ASST Fatebenefratelli-Sacco Milan, Italy, in order to evaluate the best disinfectant cap available on the market.
Avalaibility of data
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
Articles from Le Infezioni in Medicina are provided here courtesy of Edizioni Internazionali s.r.l.
Citations & impact
This article has not been cited yet.
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/157383550
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Impact of universal disinfectant cap implementation on central line-associated bloodstream infections.
Am J Infect Control, 42(12):1274-1277, 25 Nov 2014
Cited by: 20 articles | PMID: 25465256
Preventing central venous catheter-associated bloodstream infections: development of an antiseptic barrier cap for needleless connectors.
Am J Infect Control, 36(10):S174.e1-5, 01 Dec 2008
Cited by: 20 articles | PMID: 19084153
Disinfection of needleless catheter connectors and access ports with alcohol may not prevent microbial entry: the promise of a novel antiseptic-barrier cap.
Infect Control Hosp Epidemiol, 27(1):23-27, 06 Jan 2006
Cited by: 57 articles | PMID: 16418982
Curos™ Disinfection Caps for the Prevention of Infection When Using Needleless Connectors: A NICE Medical Technologies Guidance.
Appl Health Econ Health Policy, 19(2):145-153, 01 Mar 2021
Cited by: 1 article | PMID: 32754850
Review
 2
2

