Abstract
Background
While enteric viruses are highly transmissible, household factors associated with transmission are less well documented. We identified individual- and household-level factors associated with viral acute gastroenteritis (AGE) transmission in a large health care network in the United States.Methods
Patients presenting with AGE were enrolled from April 2014 to September 2016. Patients and symptomatic household members were interviewed, and stool specimens were collected and tested for viral pathogens. Within a household, primary cases were those with the earliest symptom onset and a positive viral test result; secondary cases were household contacts (HHCs) with symptom onset 1-7 days from the primary case onset. Transmission households had at least 1 secondary case.Results
Our analysis included 570 primary cases with 1479 HHCs. The overall secondary attack rate was 23%. HHCs were likely to become secondary cases (n = 338) if they were <5 years old (adjusted odds ratio [aOR], 1.8; 95% CI, 1.2-2.6). Secondary transmission was likely to occur if the primary case was aged <5 years (aOR, 2.2; 95% CI, 1.4-3.6) or 5 to 17 years (aOR, 3.3; 95% CI, 1.9-5.7), was norovirus positive (aOR, 2.7; 95% CI, 1.9-3.7), had a diapered contact (aOR: 2.2, 95% CI: 1.6-3.2), or reported symptoms for >4 days (aOR, 1.5; 95% CI, 1.1-2.1). Households with ≥3 members (aOR, 2.1; 95% CI, 1.1-4.5) were more likely to experience transmission.Discussion
Risk of AGE transmission within households increased if the primary case was younger, was norovirus positive, had a longer symptom duration, or had a diapered contact. Targeted prevention messaging around appropriate cleaning, disinfection, and isolation of persons with AGE should be encouraged.Free full text

Household Transmission of Viral Acute Gastroenteritis Among Participants Within an Integrated Health Care Delivery System, 2014–2016
Abstract
Background
While enteric viruses are highly transmissible, household factors associated with transmission are less well documented. We identified individual- and household-level factors associated with viral acute gastroenteritis (AGE) transmission in a large health care network in the United States.
Methods
Patients presenting with AGE were enrolled from April 2014 to September 2016. Patients and symptomatic household members were interviewed, and stool specimens were collected and tested for viral pathogens. Within a household, primary cases were those with the earliest symptom onset and a positive viral test result; secondary cases were household contacts (HHCs) with symptom onset 1-7 days from the primary case onset. Transmission households had at least 1 secondary case.
Results
Our analysis included 570 primary cases with 1479 HHCs. The overall secondary attack rate was 23%. HHCs were likely to become secondary cases (n = 338) if they were <5 years old (adjusted odds ratio [aOR], 1.8; 95% CI, 1.2–2.6). Secondary transmission was likely to occur if the primary case was aged <5 years (aOR, 2.2; 95% CI, 1.4–3.6) or 5 to 17 years (aOR, 3.3; 95% CI, 1.9–5.7), was norovirus positive (aOR, 2.7; 95% CI, 1.9–3.7), had a diapered contact (aOR: 2.2, 95% CI: 1.6-3.2), or reported symptoms for >4 days (aOR, 1.5; 95% CI, 1.1–2.1). Households with ≥3 members (aOR, 2.1; 95% CI, 1.1–4.5) were more likely to experience transmission.
Discussion
Risk of AGE transmission within households increased if the primary case was younger, was norovirus positive, had a longer symptom duration, or had a diapered contact. Targeted prevention messaging around appropriate cleaning, disinfection, and isolation of persons with AGE should be encouraged.
Acute gastroenteritis (AGE) causes >179 million illnesses and 1.6 million deaths annually in the United States [1]. Enteric viruses such as norovirus, rotavirus, sapovirus, and astrovirus are leading causes of AGE and are known to cause outbreaks via person-to-person contact or through contaminated food, water, or fomites [2–7]. A recent study reported the overall incidence of medically attended AGE (MAAGE) as 40.6 episodes per 1000 person-years and found norovirus to be the most important contributor, followed by sapovirus, astrovirus, and rotavirus [6]. Although contact with a person with viral AGE, especially within the same household, is a known risk factor for acquiring AGE, studies exploring factors affecting household transmission of viral AGE are limited [8–14]. Thus, an improved understanding of household transmission dynamics of viral AGE is needed to help identify high-risk groups and target prevention measures to effectively curb the chain of transmission. Additionally, with norovirus vaccine candidates in development, identifying high-risk groups causing household transmission could aid vaccine allocation strategies and further assist in preventing severe disease in other household members.
The MAAGE study was an active surveillance program established within Kaiser Permanente Northwest (KPNW), an integrated health care delivery system serving the northwest Oregon and southwest Washington regions, and was demographically similar to the total population of the underlying catchment area [15]. This platform collected survey data and stool samples from patients with MAAGE. It also allowed for the assessment of transmission within participant households through collection of additional data and stool samples from a subset of their household members. Using this active surveillance platform, we sought to identify individual- and household-level risk factors for viral AGE transmission by comparing households that experienced secondary cases with those that did not.
METHODS
We leveraged data collected from KPNW members enrolled as part of the MAAGE study. A detailed description of the surveillance methods has been published previously [15]. To summarize, from April 2014 to September 2016, we identified KPNW members of all ages with MAAGE, defined as any encounter—including email, telephone, or video visit; ambulatory care clinic, urgent care, or emergency department visit; or hospital admission—within the KPNW health care delivery system for which an AGE-related ICD code (for in-person encounters) or an AGE-related chief complaint (for remote encounters) was documented (Supplementary Table 1). An age-stratified representative sample was selected, and participants were contacted via telephone within 10 days of their MAAGE encounters. Patients who provided consent were asked to complete a baseline survey at recruitment and a follow-up survey 2 weeks following enrollment.
The baseline interview collected information on demographic characteristics, current illness symptoms, symptom onset and end dates, and information on possible exposures. Information on household size, household members' gender and age, and presence of AGE symptoms (including start and end dates) was also collected. A follow-up interview was conducted 2 weeks after enrollment to assess resolution of previously reported symptoms and whether any of the healthy household members identified at the baseline survey subsequently developed AGE symptoms. Household members with AGE symptoms at the time of the baseline or follow-up survey were queried for symptom and exposure history. Household members did not have to be a KPNW member and may or may not have sought medical care.
MAAGE participants and household members with chronic gastrointestinal symptoms (defined as AGE symptoms with duration ≥30 days; 329 MAAGE participants, 1 household member) and those with missing/unclear onset or end dates (2 MAAGE participants, 1 household member) were excluded to focus on acute cases with gastrointestinal symptoms. Households with a size of 1 (n = 373) were also excluded from our analysis.
Primary, Secondary, and Household Contact Definitions
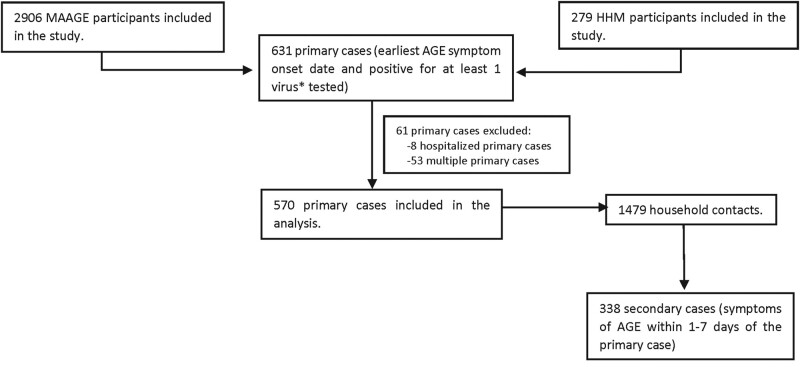
Primary cases were individuals with the earliest symptom onset date in a household who were positive for any of the following enteric viruses tested: norovirus, sapovirus, astrovirus, or rotavirus. All individuals residing at the same address as the primary case were considered household contacts (HHCs). Secondary cases were HHCs with AGE symptom onset between 1 and 7 calendar days from the primary case's symptom onset date. Households with multiple primary cases based on onset date (n = 53) or primary cases requiring hospitalized were excluded from our analysis (n = 8). The attack rate among HHCs was calculated as the number of secondary cases divided by the total number of HHCs (secondary attack rate).
Individual-Level Risk Factors for Transmission
To identify potential risk factors that contributed to secondary transmission of viral AGE among HHCs, we assessed the following primary case characteristics: age, exposures within the last 7 days (ie, travel outside the United States, eating food at restaurants/fast food/vendors, contact with diapered children or adults, eating food prepared by others at a potluck/event, contact with children in day care or nursery school, and contact with persons living in a nursing home), symptom duration, contact with persons with AGE symptoms, and type of virus detected. We also assessed the contribution of household size (<3 and ≥3) and HHC age (<5, 5–17, 18–64, ≥65 years) to individual-level transmission dynamics. We used generalized estimating equation logistic regression with an exchangeable correlation matrix with household as the cluster to assess factors associated with AGE among HHCs. Covariates with P < .2 in univariate analysis were included in the multivariate model by a manual backward elimination method.
Household-Level Risk Factors for Transmission
To evaluate risk factors for household-level transmission, we categorized households into transmission and nontransmission households and compared characteristics. Households with ≥1 secondary case were categorized as transmission households, and those not meeting this definition were categorized as nontransmission households. In addition to the characteristics included in the individual-level analysis, we assessed household income as a potential risk factor. Univariate and multivariate logistic regression was conducted to assess this outcome. Covariates with P < .2 in univariate analysis were included in the multivariate model by a manual backward elimination method.
Collinearity was assessed in the individual- and household-level models, and no variables were collinear. Sensitivity analyses were conducted to assess whether primary cases aged <5 years were driving the transmission and to explore the impact of modifying the secondary case definition on transmission dynamics.
Stool Collection
All recruited study participants (patients with MAAGE and symptomatic household members) were asked to self-collect a stool sample and submit for viral pathogen testing within 7 days of collection. Samples were tested for norovirus, sapovirus, astrovirus, and rotavirus RNA at the Oregon State Public Health Laboratory using TaqMan real-time reverse transcriptase polymerase chain reaction protocols developed by the Centers for Disease Control and Prevention, as described previously [15].
Patient Consent Statement
The study obtained a waiver of signed consent, and all participants provided verbal informed consent at enrollment. This project was reviewed and approved by the KPNW institutional review board (FWA00002344).
RESULTS
Participant Description
From April 2014 to September 2016, 3667 eligible participants with MAAGE and 288 symptomatic household members agreed to participate and provided stool samples for testing. Of these, 3610 (98%) MAAGE participant samples and 284 (99%) household member samples were tested for viral enteric pathogens. A total of 2906 MAAGE participants and 279 household members were subsequently included in our study after exclusion of participants with chronic AGE, a household size of 1, and missing/unclear onset or end dates (Supplementary Figure 1). Of these, 631 participants met the primary case definition. After exclusion of hospitalized primary cases and multiple primary cases within households, 570 primary cases with 1479 HHCs were ultimately included in our analysis. Among all HHCs, 338 symptomatic individuals met the secondary case definition and were classified as secondary cases (Figure 1). Of the 570 households, 209 (37%) were categorized as transmission households.
Primary and Secondary Case Characteristics
Among primary cases, the median age was 7 years, 53% were male, 75% were White, and 15% were of Hispanic ethnicity. The median AGE symptom duration among primary cases was 5 days. Common exposures reported by primary cases included eating at a restaurant/fast food/vendor (71%), having contact with a diapered child or adult (51%), and being exposed to children in day care or nursery school (43%) in the past 7 days (Table 1). Of all primary cases <5 years of age with data available on vaccination status (n = 213), 201 (94%) received rotavirus vaccine.
Table 1.
Characteristics of Primary and Secondary Cases, Kaiser Permanente Northwest, April 2014–September 2016
| Cases, Median (Range) or No. (%) | ||
|---|---|---|
| Characteristic | Primary (n = 570) | Secondary (n = 338)a |
| Age, y | 7 (0–96) | 28 (0–89) |
| Symptom duration, d | 5 (0–30) | 4 (1–32) |
| Age group, y | ||
 0–4 0–4 | 244 (43) | 53 (16) |
 5–17 5–17 | 82 (14) | 61 (18) |
 18–64 18–64 | 151 (26) | 206 (61) |
 ≥65 ≥65 | 93 (16) | 18 (5) |
| Gender: male | 301 (53) | 180 (53) |
| Race | ||
 White White | 427 (75) | 81 (85) |
 Black Black | 14 (2) | 3 (3) |
 Asian Asian | 22 (4) | 4 (4) |
 Multiple races specified Multiple races specified | 53 (9) | 2 (2) |
 Other Other | 37 (7) | 4 (4) |
 Unknown Unknown | 4 (1) | 1 (1) |
 Refused Refused | 2 (0.3) | 0 (0) |
| Hispanic ethnicity | 88 (15) | 7 (8) |
| Education of primary caseb | ||
 Less than high school Less than high school | 7 (3) | 2 (4) |
 High school equivalent High school equivalent | 60 (25) | 7 (14) |
 Some college Some college | 82 (34) | 14 (27) |
 College graduate College graduate | 93 (38) | 28 (55) |
 Don’t know Don’t know | 1 (0.4) | 0 |
 Refused Refused | 1 (0.4) | 0 |
| Symptomc | ||
 Diarrhea Diarrhea | 539 (95) | 81 (85) |
 Vomiting Vomiting | 423 (74) | 74 (78) |
 Fever Fever | 271 (48) | 36 (38) |
 Headache Headache | 249 (44) | 49 (52) |
 Muscle ache Muscle ache | 235 (41) | 58 (61) |
 Abdominal pain Abdominal pain | 425 (75) | 77 (76) |
 Lethargy Lethargy | 472 (83) | 80 (84) |
 Chills Chills | 250 (44) | 38 (40) |
 Nausea Nausea | 450 (79) | 79 (83) |
 Bloody stool Bloody stool | 15 (3) | 4 (4) |
| Exposure characteristicsd | ||
 Travel outside US Travel outside US | 17 (3) | 4 (4) |
 Dine at a restaurant/fast food/vendor Dine at a restaurant/fast food/vendor | 402 (71) | 66 (69) |
 Consume food at potluck/event Consume food at potluck/event | 172 (30) | 24 (25) |
| Contact with | ||
 Diapered children/adult Diapered children/adult | 289 (51) | 76 (81) |
 Children in day care/nursery Children in day care/nursery | 240 (43) | 54 (57) |
 Person at nursing home Person at nursing home | 43 (8) | 2 (2) |
aData available only for 102 secondary cases. Age and gender information available for all secondary cases.
bEducation information available for individuals aged >18 years: primary cases, n = 259; secondary, cases, n = 55.
cSymptoms are not mutually exclusive.
dIn the 7 days prior to symptom onset.
Among secondary cases, the median age was 28 years, 53% were male, 85% were White, and 8% were of Hispanic ethnicity. The median AGE symptom duration among secondary cases was 4 days. The most common exposure reported by secondary cases was having contact with a diapered child or adult (81%), followed by having eaten at a restaurant/fast food/vendor (69%) and having contact with children in day care or nursery (57%) in the past 7 days (Table 1). Secondary cases most frequently cited symptom onset within 2 days (25%) of the primary case's symptom onset date (Supplementary Figure 2).
Virus Distribution
Of the 4 viruses tested, norovirus (53%) was the most prevalent viral pathogen detected among primary cases, followed by sapovirus (24%), astrovirus (14%), and rotavirus (12%). Among norovirus-positive primary cases, GII was the predominant genogroup (89%).
Attack Rates
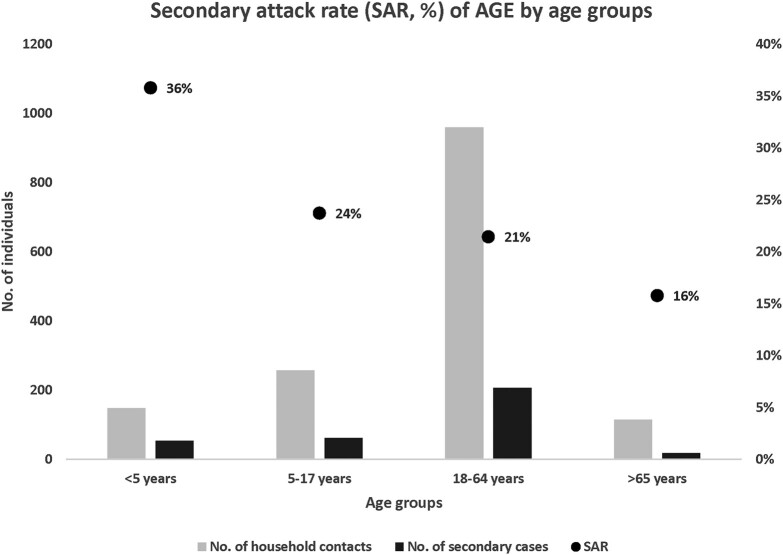
Of 1479 HHCs, 338 met the secondary case definition, leading to an overall secondary attack rate of 23% (338/1479). Children <5 years of age had the highest secondary attack rate (36%); those for HHCs aged 5 to 17 years and 18 to 64 years were similar (24% and 21%, respectively); and seniors aged >65 years had a lower secondary attack rate (16%; Figure 2).
Risk Factors for AGE Among HHCs
HHCs had significantly higher odds of secondary transmission if they were <5 years of age (adjusted odds ratio [aOR], 1.8; 95% CI, 1.2–2.6) or if the primary case in their household was aged <5 years (aOR, 2.2; 95% CI, 1.4–3.6) or between 5 and 17 years (aOR, 3.3; 95% CI, 1.9–5.7). HHCs were also more likely to acquire AGE if the primary case was positive for norovirus (aOR, 2.7; 95% CI, 1.9–3.7), had diapered contact 7 days prior to symptom onset (aOR, 2.2; 95% CI, 1.6–3.1), had symptoms for ≥4 days (aOR, 1.5; 95% CI, 1.1–2.1), or reported vomiting (aOR, 1.6; 95% CI, 1.1–2.4; Table 2).
Table 2.
Univariate and Multivariate Logistic Regression Analysis With Generalized Estimating Equations: Risk Factors for Transmission of Viral Gastroenteritis Among Household Contacts, Kaiser Permanente Northwest, April 2014–September 2016
| Univariate Analysis | Multivariate Analysis | ||||||
|---|---|---|---|---|---|---|---|
| Secondary Cases, No. | Total Household Contacts, No. | Attack Rate, % | OR (95% CI) | P Valuea | aOR (95% CI) | P Value | |
| Household characteristics | |||||||
| Household size ≥3 | |||||||
 No No | 22 | 138 | 16 | 1 [Reference] | |||
 Yes Yes | 316 | 1341 | 24 | 1.7 (1.1–2.7) | .032 | … | |
| Household contact characteristics | |||||||
| Age, y | |||||||
 <5 <5 | 52 | 144 | 36 | 1.9 (1.3–2.7) | .001 | 1.8 (1.2–2.6) | .006 |
 5–17 5–17 | 61 | 257 | 24 | 1.3 (.9–1.7) | .149 | 1.3 (.9–1.8) | .130 |
 18–64 18–64 | 206 | 960 | 21 | 1 [Reference] | |||
 ≥65 ≥65 | 18 | 114 | 16 | 0.7 (.4–1.2) | .296 | 1.1 (.6–2.1) | .757 |
| Primary case characteristics | |||||||
| Duration of symptoms >4 d | |||||||
 No No | 163 | 794 | 21 | 1 [Reference] | |||
 Yes Yes | 175 | 716 | 26 | 1.3 (.9–1.8) | .080 | 1.5 (1.1–2.1) | .024 |
| Diarrhea | |||||||
 No No | 20 | 93 | 22 | 1 [Reference] | |||
 Yes Yes | 318 | 1386 | 23 | 1.01 (.5–1.93) | .967 | … | |
| Vomiting | |||||||
 No No | 56 | 353 | 16 | 1 [Reference] | |||
 Yes Yes | 282 | 1126 | 25 | 1.7 (1.2–2.5) | .007 | 1.6 (1.1–2.4) | .032 |
| Age, y | |||||||
 <5 <5 | 200 | 746 | 27 | 2.4 (1.5–3.7) | <.001 | 2.2 (1.4–3.6) | <.001 |
 5–17 5–17 | 73 | 273 | 27 | 2.4 (1.4–4.1) | <.001 | 3.3 (1.9–5.7) | <.001 |
 18–64 18–64 | 47 | 347 | 14 | 1 [Reference] | |||
 ≥65 ≥65 | 18 | 113 | 16 | 1.2 (.7–2.2) | .528 | 1.6 (.7–3.5) | .223 |
| Exposuresb | |||||||
| Travel outside US | |||||||
 No No | 329 | 1430 | 23 | 1 [Reference] | |||
 Yes Yes | 7 | 38 | 18 | 0.6 (.2–2.0) | .472 | … | |
| Dine at a restaurant/fast food/vendor | |||||||
 No No | 105 | 441 | 24 | 1 [Reference] | |||
 Yes Yes | 239 | 1017 | 23 | 0.9 (.6–1.2) | .621 | … | |
| Contact with diapered children/adult | |||||||
 No No | 105 | 676 | 16 | 1 [Reference] | |||
 Yes Yes | 226 | 772 | 29 | 2.3 (1.6–3.1) | <.001 | 2.2 (1.6–3.2) | <.001 |
| Consume food at potluck/event | |||||||
 No No | 233 | 1002 | 23 | 1 [Reference] | |||
 Yes Yes | 94 | 459 | 20 | 0.9 (.6–1.2) | .386 | … | |
| Contact with person at nursing home | |||||||
 No No | 320 | 1369 | 23 | 1 [Reference] | |||
 Yes Yes | 16 | 93 | 17 | 0.8 (.4–1.5) | .441 | … | |
| Contact with children in day care/nursery | |||||||
 No No | 164 | 814 | 20 | 1 [Reference] | |||
 Yes Yes | 173 | 644 | 27 | 1.6 (1.1–2.2) | .001 | … | |
| Type of viral pathogen detected | |||||||
| Norovirus | |||||||
 Negative Negative | 106 | 601 | 15 | 1 [Reference] | |||
 Positive Positive | 232 | 772 | 30 | 2.3 (1.7–3.2) | <.001 | 2.7 (1.9–3.7) | <.0001 |
| Rotavirus | |||||||
 Negative Negative | 312 | 1301 | 24 | 1 [Reference] | |||
 Positive Positive | 26 | 178 | 15 | 0.6 (.3–.9) | .014 | … | |
| Astrovirus | |||||||
 Negative Negative | 309 | 1250 | 25 | 1 [Reference] | |||
 Positive Positive | 29 | 229 | 13 | 0.5 (.3–.8) | .002 | … | |
| Sapovirus | |||||||
 Negative Negative | 279 | 1136 | 25 | 1 [Reference] | |||
 Positive Positive | 59 | 343 | 17 | 0.7 (.5–.9) | .030 | … | |
Household contacts, n = 1479; secondary cases, n = 338.
Abbreviations: aOR, adjusted odds ratio; OR, odds ratio.
a P < .2 was included in the multivariate analysis.
bIn the 7 days prior to symptom onset.
We found lower odds of secondary transmission among HHCs if the primary case was positive for rotavirus (odds ratio, 0.6; 95% CI, .3–.9); however, this association was not significant in the adjusted multivariate model.
Risk Factors for Transmission vs Nontransmission Household
Households that reported AGE transmission were more likely to have ≥3 members (aOR, 2.1; 95% CI, 1.1–4.5) or have a primary case aged <5 years (aOR, 2.4; 95% CI, 1.4–4.1) or between 5 and 17 years (aOR, 3.5; 95% CI, 1.8–6.8). Higher odds of transmission were also found among households if the primary case was norovirus positive (aOR, 2.0; 95% CI, 1.3–3.0) or had contact with a diapered child/adult (aOR, 1.9; 95% CI, 1.3–2.9) (Table 3).
Table 3.
Univariate and Multivariate Logistic Regression Analysis: Risk Factors Among Transmission vs Nontransmission Households, Kaiser Permanente Northwest, April 2014–September 2016
| Household, No. (%) | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| Transmission (n = 209) | Nontransmission (n = 361) | OR (95% CI) | P Valuea | aOR (95% CI) | P Value | |
| Household characteristics | ||||||
| Household size | ||||||
 Median (range) Median (range) | 4 (2–7) | 3 (2–10) | ||||
 Members ≥3 Members ≥3 | 187 (89) | 245 (68) | 4.0 (2.5–6.6) | <.001 | 2.1 (1.1–4.5) | .043 |
| Household income, $ | ||||||
 ≤75 000 ≤75 000 | 86 (41) | 155 (43) | 1 [Reference] | |||
 >75 000 >75 000 | 93 (45) | 147 (41) | 1.1 (.8–1.7) | .487 | … | |
 Unknown Unknown | 22 (11) | 45 (12) | 0.9 (.5–1.6) | .666 | … | |
 Refused Refused | 8 (4) | 14 (4) | 1.0 (.4–2.6) | .949 | … | |
| Primary case characteristics | ||||||
| Age, y | ||||||
 <5 <5 | 102 (56) | 129 (36) | 3.2 (2.0–5.3) | <.0001 | 2.4 (1.4–4.1) | .002 |
 5–17 5–17 | 35 (19) | 40 (11) | 3.5 (1.9–6.4) | <.0001 | 3.5 (1.8–6.8) | <.001 |
 18–64 18–64 | 29 (16) | 116 (32) | 1 [Reference] | |||
 ≥65 ≥65 | 15 (8) | 74 (21) | 0.8 (.4–1.6) | .550 | 1.2 (.5–2.7) | .693 |
| Exposuresb | ||||||
 Travel outside US Travel outside US | 4 (2) | 13 (4) | 0.5 (.2–1.6) | .244 | … | |
 Dine at a restaurant/fast food/vendor Dine at a restaurant/fast food/vendor | 145 (70) | 257 (72) | 0.9 (.6–1.3) | .611 | … | |
 Contact with a diapered children/adult Contact with a diapered children/adult | 133 (65) | 156 (44) | 2.3 (1.6–3.3) | <.001 | 1.9 (1.3–2.9) | .003 |
 Consume food at potluck/event Consume food at potluck/event | 61 (30) | 141 (31) | 0.9 (.7–1.4) | .775 | … | |
 Contact with person at nursing home Contact with person at nursing home | 14 (7) | 29 (8) | 0.8 (.4–1.6) | .540 | … | |
 Contact with children in day care/nursery Contact with children in day care/nursery | 106 (51) | 134 (38) | 1.7 (1.2–2.4) | .002 | … | |
| Type of viral pathogen detected | ||||||
 Norovirus Norovirus | 127 (61) | 174 (48) | 1.7 (1.2–2.4) | .004 | 2.0 (1.3–3.0) | <.001 |
 Rotavirus Rotavirus | 23 (11) | 46 (12) | 0.9 (.5–1.5) | .604 | … | |
 Astrovirus Astrovirus | 22 (11) | 58 (16) | 0.6 (.4–1.0) | .069 | … | |
 Sapovirus Sapovirus | 43 (21) | 94 (26) | 0.7 (.5–1.1) | .142 | … | |
Abbreviations: aOR, adjusted odds ratio; OR, odds ratio.
a P < .2 was included in the multivariate analysis.
bIn the 7 days prior to symptom onset.
Sensitivity Analyses
Sensitivity analysis to explore transmission among HHCs of primary cases aged <5 years showed higher odds of transmission among HHCs with primary cases who had diarrhea, had a diapered contact, or were positive for norovirus. Modifying the secondary case definition time frame from 1–7 days to 2–7 days from primary case symptom onset did not change the original result.
DISCUSSION
Although enteric viruses are known to be highly transmissible, literature on factors affecting household transmission of viral AGE is limited. We addressed this gap by not only identifying individual-level risk factors that affect transmission of AGE within households but also understanding characteristics of households that experienced transmission vs those that did not. Our individual- and household-level assessment showed increased transmission of viral AGE if primary cases were aged <5 or 5–17 years vs 18–64 years, were exposed to diapered children or adults in the 7 days prior to symptom onset, or were positive for norovirus. Additionally, we found that HHCs were more likely to acquire AGE if the primary case reported vomiting or had a longer duration of symptoms. Larger households (≥3 members) were more likely to have increased transmission of viral AGE.
Children play an important role in household AGE transmission. Younger primary cases (<5 or 5–17 years) were more likely to lead to AGE transmission within a household in both our analyses. Additionally, HHCs <5 years of age showed higher odds of acquiring AGE from a primary case. These findings are similar to those reported in a cross-sectional study conducted in the United Kingdom by de Lusignan et al, which revealed a higher AGE incidence rate among households with preschool children as compared with other age groups [16]. Our results are also consistent with a retrospective household survey conducted in Japan that reported a higher secondary attack risk of AGE among HHCs of index cases who were 0 to 14 years of age vs other age groups [17]. Götz et al analyzed a large foodborne outbreak among children and staff at day care centers in Sweden and determined that children were more likely to cause transmission of norovirus-like AGE within households as compared with adults [13]. Thus, targeting prevention strategies such as isolation, disinfection, and interventions (eg, vaccination) in this population could reduce transmission and have indirect beneficial effects on other children or adults living in the same household [18].
In our individual-level transmission assessment, we found higher secondary transmission among HHCs of primary cases with a duration of symptoms >4 days, indicating that the longer symptoms last, the possibility of spread among HHCs increases. Additionally, HHCs of primary cases with vomiting were more likely to acquire AGE in our study, suggesting higher transmissibility among patients exposed to vomiting. This supports prior evidence indicating a higher risk of secondary transmission on exposure to vomiting in individuals who were virus positive, especially with norovirus [12, 13, 19]. Vomiting can cause significant environmental contamination and effectively result in transmission through fomites and airborne droplets [20]. Prevention strategies—such as cleaning and disinfecting surfaces with bleach-based cleaners, cleaning soiled laundry, and regular hand washing—are simple but important measures that can prevent transmission.
A large proportion of primary cases in our analysis were <5 years (44%) of age, while most secondary cases fell between 18 and 49 years (57%) vs other age categories within each group. Younger children (<5 years) or school-going children (5–17 years) are highly likely to get exposed to AGE in communal settings such as day cares, preschools, or schools, as these are common settings for viral AGE outbreaks [4, 21] and may lead to transmission among caregivers or parents [22]. This is consistent with studies showing a higher risk of viral AGE among children attending day care and is associated with transmission in parents of day care attendees [22, 23]. Individuals in direct contact with diapered children or adults were more likely to transmit viral AGE in both our analyses. Restricting our analyses to primary cases <5 years of age showed increased secondary transmission among HHCs when primary cases had diarrhea. These findings indicate a high exposure risk when handling younger children with AGE and emphasize the need for appropriate hygiene practices among those caring for sick children.
Of the 4 viruses tested, norovirus was the most common pathogen (54%) detected. We found that HHCs of norovirus-positive primary cases were more likely to acquire AGE in our individual-level risk factor analysis for transmission. Additionally, our household-level assessment demonstrated higher odds of transmission among households with norovirus-positive primary cases. This is consistent with a household survey–based cohort study conducted in the Netherlands demonstrating a high burden of norovirus transmission within households [24]. A recent case-control study among US veteran population also identified contact with an individual with AGE within a household to be a risk factor for norovirus-associated AGE [14]. Our findings are similar to those from a household transmission study conducted in Ecuador reporting norovirus transmissibility among one-third of HHCs of symptomatic children [25].
We observed that rotavirus-positive primary cases were less likely to cause secondary transmission among HHCs. Although not statistically significant in multivariate analysis, this may be attributed to the high rotavirus vaccination rates (94%) in the population and underscores the importance of norovirus vaccination in preventing the risk of transmission among close contacts [26].
Our study had limited interview data available for a small number of symptomatic HHCs (n = 102), although we were able to obtain age, gender, and symptom onset and end dates from the MAAGE patient baseline survey for those HHCs who were not interviewed (n = 1448). Additionally, our definition of secondary case included HHCs with symptom onset 1 to 7 calendar days from the primary case onset, which may have misclassified those who were exposed on the same day as the primary case but developed symptoms 1 to 2 days after the primary case’s onset. However, we did not find any changes in the results when the time frame for the secondary case definition was changed to 2 to 7 days from the primary case’s onset. Our stool samples were tested only for viral pathogens, making our analysis generalizable to viral AGE rather than all-cause AGE, and it was unclear what proportion of all-cause AGE may be due to nonviral pathogens or co-detections. The questionnaire did not collect data on behavioral factors such as household cleaning/disinfection or personal hygiene practices, which is important in understanding transmission among those who reside in the same household; thus, we were unable to account for the considerable role that behaviors related to hygiene and disinfection may have contributed.
Our findings suggest that children aged <5 years and school-age children aged 5 to 17 years play a major role in household transmission of viral AGE. To reduce transmission of viral AGE, it is important to target prevention messaging to parents. caregivers, schools and day care facilities and emphasize the importance of measures such as hand hygiene, cleaning and disinfection of surfaces, proper handling of diapers, and isolation of sick contacts. Norovirus was the most frequently detected pathogen among primary viral AGE cases and a risk factor for transmission of AGE across households and HHCs, highlighting the infectiousness and transmissibility of the pathogen among close contacts. With norovirus vaccines in development, our findings support the need to consider young children as a target population to help break the chain of transmission within households and in communities.
Contributor Information
Neha Balachandran, Division of Viral Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, USA. Cherokee Nation Assurance, Arlington, Virginia, USA.
Claire P Mattison, Division of Viral Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, USA. Cherokee Nation Assurance, Arlington, Virginia, USA.
Laura E Calderwood, Division of Viral Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, USA. Cherokee Nation Assurance, Arlington, Virginia, USA.
Rachel M Burke, Division of Viral Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Mark A Schmidt, Center for Health Research, Kaiser Permanente Northwest, Portland, Oregon, USA.
Judy Donald, Center for Health Research, Kaiser Permanente Northwest, Portland, Oregon, USA.
Sara A Mirza, Division of Viral Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank the study site participants for their time and contributions. Additionally, we acknowledge the following individuals for their contributions to the study: Dr Aron Hall, Dr Julia Baker, and Suzanne B. Salas.
Disclaimer. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Centers for Disease Control and Prevention or the US government. Takeda had no role in the study design, data collection and analysis, the decision to publish, or the preparation of the manuscript. The Centers for Disease Control and Prevention received no funding from Takeda.
Financial support. This work was supported by institutional research funding from the CDC Foundation and through investigator-initiated research grants from Takeda Vaccines, Inc (IISR-2015-101015 and IISR-2017-101938), with M. A. S. as the recipient.
References
Articles from Open Forum Infectious Diseases are provided here courtesy of Oxford University Press
Citations & impact
This article has not been cited yet.
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/157422561
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Community burden and transmission of acute gastroenteritis caused by norovirus and rotavirus in the Netherlands (RotaFam): a prospective household-based cohort study.
Lancet Infect Dis, 20(5):598-606, 20 Feb 2020
Cited by: 21 articles | PMID: 32087775
Evidence for Household Transmission of Rotavirus in the United States, 2011-2016.
J Pediatric Infect Dis Soc, 9(2):181-187, 01 Apr 2020
Cited by: 12 articles | PMID: 30753568
Household transmission of SARS-CoV-2 and risk factors for susceptibility and infectivity in Wuhan: a retrospective observational study.
Lancet Infect Dis, 21(5):617-628, 18 Jan 2021
Cited by: 145 articles | PMID: 33476567 | PMCID: PMC7833912