Abstract
Free full text

Maintenance of proteostasis by Drosophila Rer1 is essential for competitive cell survival and Myc-driven overgrowth
Abstract
Defects in protein homeostasis can induce proteotoxic stress, affecting cellular fitness and, consequently, overall tissue health. In various growing tissues, cell competition based mechanisms facilitate detection and elimination of these compromised, often referred to as ‘loser’, cells by the healthier neighbors. The precise connection between proteotoxic stress and competitive cell survival remains largely elusive. Here, we reveal the function of an endoplasmic reticulum (ER) and Golgi localized protein Rer1 in the regulation of protein homeostasis in the developing Drosophila wing epithelium. Our results show that loss of Rer1 leads to proteotoxic stress and PERK-mediated phosphorylation of eukaryotic initiation factor 2α. Clonal analysis showed that rer1 mutant cells are identified as losers and eliminated through cell competition. Interestingly, we find that Rer1 levels are upregulated upon Myc-overexpression that causes overgrowth, albeit under high proteotoxic stress. Our results suggest that increased levels of Rer1 provide cytoprotection to Myc-overexpressing cells by alleviating the proteotoxic stress and thereby supporting Myc-driven overgrowth. In summary, these observations demonstrate that Rer1 acts as a novel regulator of proteostasis in Drosophila and reveal its role in competitive cell survival.
Author summary
In developing tissues, cells can stochastically acquire defects that can reduce their fitness. To maintain the overall health of tissues, these unfit cells are identified by the healthier neighboring cells and eliminated via a juxtacrine-acting cellular fitness sensing mechanism called cell competition. An example of such physiological regulation of cellular fitness is the maintenance of proteostasis. Defects in maintaining proteostasis cause proteotoxic stress. Interestingly, proteotoxic stress is observed not only in the unfit loser cells but also in the overgrowing super-competitor cells, for instance, cells with higher levels of Myc. How cell competition is linked to the maintenance of proteostasis is poorly understood. In this study, we have characterized for the first time the function of Drosophila Rer1 protein in development. We demonstrate that Rer1 is essential for maintaining protein homeostasis and loss of Rer1 activates stress-induced unfolded protein responses. Cells lacking Rer1 are identified as unfit cells and become losers when juxtaposed to the normal neighboring cells. Moreover, we show that Myc-overexpressing cells upregulate Rer1 levels, which allows them to maintain a higher demand for stress regulation, caused by increased protein translation. In this work, we propose that Rer1 functions as a stress regulator and that modulating its levels could provide cytoprotection under stress conditions.
Introduction
The development of healthy tissue requires the removal of viable but suboptimal cells. In several growing tissues, this vital culling process is orchestrated through a specific cell-cell interaction called cell competition. In this intricate mechanism, unfit cells, also called “loser”, are eliminated by their surrounding fitter counterparts, the “winner” cells [1,2], thereby maintaining tissue health [3]. The best-known example of cell competition is described in the developing Drosophila epithelium using the heterozygous mutations in a ribosomal protein (Rp) gene (also known as Minute). The Rp+/- flies are viable, however, under mosaic condition the Rp+/- cells are eliminated from the developing epithelium when juxtaposed with the neighboring wild-type (Rp+/+) cells [4–6]. Although the Rp+/- mutation affects cellular physiology autonomously, caspase-dependent apoptosis is observed mostly at the boundary between Rp+/- cells and nearby Rp+/+ cells, which is a hallmark of cell competition [6,7]. The loser fate of the slow growing Rp+/- cells was suggested to be due to reduced protein translation [8–10]. However, recent studies have shown that Rp+/- cells exhibit high proteotoxic stress [11–14] and activate the expression of bZip transcription factor Xrp-1, which plays an essential role in the elimination of the Rp+/- cells [11,15,16]. Interestingly, Xrp-1 appears to be responsible for the manifestation of various defects in Rp+/- cells, including reduced global translation and proteotoxic stress, contributing to the loser status [17].
Moreover, the loser fate is associated with a number of other physiological changes impacting cell fitness. These changes include, 1) reduced metabolic activity due to alteration in the mTOR pathway activity [18,19], 2) loss of apico-basal polarity as a consequence of mutations of the scribble, dlg, and lgl genes [20], 3) defects in endosomal trafficking caused by mutations in the rab5 gene [21], and 4) deregulation of signaling pathways such as Wnt, BMP, and Hippo [22–24]. Cells bearing these perturbations are eliminated through cell competition involving JNK-dependent activation of the proapoptotic factors [6,25].
Interestingly, some perturbations can also provide a competitive advantage to the cells over their wild-type neighbors. For instance, the overexpression of a proto-oncogene Myc, a master regulator of cell proliferation and growth, enhances the relative fitness of the cells. Thus, clonal expression of Myc generates super-competitor cells, which proliferate at the expense of the wild-type neighbors [10,26]. Myc drives cellular growth through its ability to upregulate the expression of a large number of genes and enhance the activity of several crucial metabolic pathways [27–29]. However, Myc-overexpression also leads to proteotoxic stress due to increased protein synthesis [30–32]. Thus, Myc-driven overgrowth is dependent on the activation of the cytoprotective unfolded protein response pathways (UPR) [33]. This includes phosphorylation of eukaryotic initiation factor 2 alpha (eIF2α) via PERK (PKR-like ER kinase) and induction of autophagy to reduce protein translation and clear misfolded proteins, respectively [34,35]. However, a clear understanding of how Myc and UPR cooperate to promote a proliferative cellular environment remains unclear.
Here, we investigated the role of Retention in Endoplasmic Reticulum-1 (Rer1) protein in the competitive cell proliferation in the developing Drosophila wing epithelium. Mutations in the rer1 gene were first described in yeast, where it was identified in a screen as a factor required for proper transport of Sec12p between the endoplasmic reticulum (ER) and Golgi [36]. Later studies have shown that Rer1 is also required for the assembly of multisubunit protein complexes, for example, the tetrameric γ-secretase complex, yeast iron transporter and skeletal muscle nicotinic acetylcholine receptor (nAChR) [37–42]. Rer1 is also known to regulate ER homeostasis, and therefore loss of Rer1 has been shown to induce ER stress in yeast and worms [43]. Despite the fact that Rer1 is evolutionarily conserved from yeast to mammals, its function in the development of organisms remains largely unknown [43–45].
By creating a rer1 loss-of-function mutant, we show that rer1 is an essential gene in Drosophila. Furthermore, we found that loss of Rer1 creates proteotoxic stress in the developing wing epithelium, and when surrounded by wild-type cells, the clonal population of rer1 mutant cells attained the loser fate and were eliminated specifically via the process of cell competition. We have also analyzed the role of Rer1 in Myc-induced overgrowth and Rer1 levels were found to be upregulated upon Myc-overexpression. More importantly, we found that loss of Rer1 is sufficient to suppress Myc-induced overgrowth. In summary, our results demonstrate that Rer1 is an essential protein for proper maintenance of protein homeostasis and competitive cell survival in a developing tissue.
Results
Rer1 is required for Drosophila larval development
We first set out to characterize the role of Rer1 during Drosophila development. To this end, we generated a rer1 knockout mutant by imprecise excision of a p-element insertion in the rer1 locus (see materials and methods). A loss-of-function mutation in rer1 containing a 1560 bp deletion in the coding region was identified (Fig 1A). Quantitative RT-PCR analysis in the homozygous mutant (rer1–/–) animals confirmed a complete loss of rer1 mRNA levels, indicating a complete loss-of-function (S1A Fig). Further analysis showed that the rer1–/–larvae failed to develop into pupae and died during the larval stages (S1B Fig). To rule out the possibility of lethality arising due to a second site mutation in another essential gene, we performed rescue experiments using a genomic-rescue construct expressing GFP-tagged Rer1 via the endogenous promoter (see materials and methods). The expression of GFP-rer1 in homozygous rer1–/–flies led to a complete rescue of lethality, confirming the specificity of the mutant (S1B Fig). These results underscore the indispensability of Rer1 in Drosophila development.
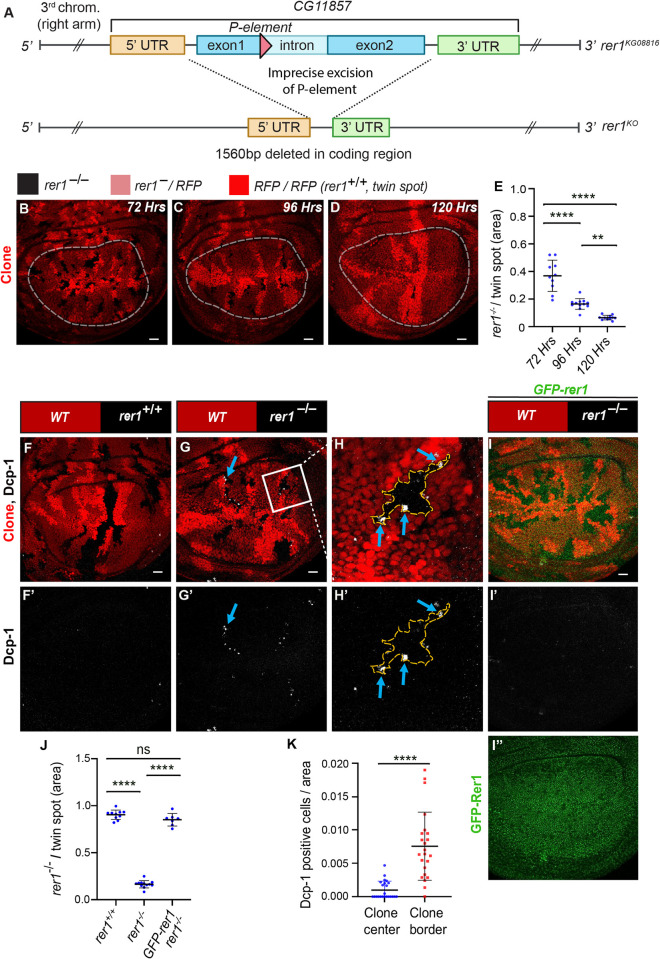
(A) Schematic representation of the rer1KO line. Upon imprecise excision of a p-element inserted in the coding sequence, a 1560bp deletion in the rer1 gene was obtained. (B-D) Wing imaginal disc harboring rer1–/–clones induced by hs-FLP at 72, 96 and 120 hrs prior to dissection of third-instar larvae. RFP-negative (black) represents rer1–/–, lighter red areas represent heterozygous rer1–/RFP, and brighter red areas represent RFP/RFP (rer1+/+; twin spot). (E) The relative size of mutant (RFP-negative) versus twin spots (RFP/RFP) areas at 72 hrs (N = 10 wing discs), 96 hrs (N = 12 wing discs), and 120 hrs (N = 11 wing discs), measured within the white dotted lines. Statistical analysis was performed using the Ordinary one-way ANOVA with Tukey’s multiple comparison test (**** p<0.0001, ** p<0.0036). (F-I) Third-instar larval wing epithelium with hs-FLP-induced (96 hrs AHS) mitotic clones of (F-F’) WT (wild-type; rer1+/+), and (G-G’) rer1–/–genotypes, immuno-stained for the anti-cleaved Dcp-1. (H-H’) A magnified image of the inset (white box) in G. (I-I”) rer1–/–clones in GFP-rer1 background stained with anti-cleaved Dcp-1. I” shows the expression of GFP-Rer1 in the wing imaginal disc. (J) Quantification of the relative size of rer1–/–(RFP-negative) versus twin spots (RFP/RFP) areas in WT control (F, N = 10 wing discs); rer1–/–(G, N = 12 wing discs) and rescue in GFP-Rer1, rer1–/–(I, N = 6 wing discs). Statistical analysis was performed using the Ordinary one-way ANOVA with Tukey’s multiple comparison test (**** p<0.0001). (K) Quantification of cell death at the center and border of rer1–/–clones (two-sided Wilcoxon signed-rank test; N = 23 clones present in 12 wing discs; **** p<0.0001, SB = 20 μm. Also see S1 and S2 Figs.
Cells lacking Rer1 show reduced survival in the developing wing epithelium
We next analyzed the importance of Rer1 at the tissue level using the developing Drosophila wing imaginal discs. We first depleted Rer1 in the posterior compartment of the wing discs by expressing rer1-RNAi using the hedgehog (hh)-Gal4 driver (S1C Fig). To test the efficiency of the knockdown, we expressed rer1-RNAi in the GFP-rer1 genomic-rescue flies. Here, we observed a strong downregulation of the GFP-Rer1 levels (S1D Fig), suggesting that rer1-RNAi effectively downregulated the Rer1 levels. We assessed the impact of Rer1 depletion on cell death by analyzing the levels of cleaved Death caspase-1 (Dcp-1) and Acridine Orange (AO) as apoptosis markers. Rer1 depletion in the posterior compartment led to a strong increase in both Dcp-1 and AO positive cells as compared to the control anterior compartment (S1E–S1H Fig; quantified in S1I and S1J Fig, respectively). Intriguingly, despite the increased cell death, the adult wing of these flies appeared normal (S1K–S1N Fig; quantified in S1O Fig).
To delve further, we generated rer1–/–clones using the heat-shock-inducible Flippase (FLP)-Flp recognition target (FRT)-system (see materials and methods). Clones were induced during early larval stages (48 hrs AEL) and wing discs were dissected at 72 and 96 hrs after heat-shock (AHS). Moreover, some larvae that were delayed and could reach up to 120 hrs were also dissected and analyzed. In these experiments, we observe that the rer1–/–(RFP-negative) clones area reduced over time as compared to the rer1+/+ clones (RFP/RFP; also called twin spot) (Fig 1B–1D; quantified in Fig 1E), indicating progressive removal of rer1–/–cells from the epithelium. Moreover, generation of the rer1–/–clones did not alter the overall wing size (S2A–S2D Fig; quantified in S2E Fig), indicating that the loss of rer1–/–cells was compensated by the neighboring cells.
rer1–/–cells are eliminated through cell competition
To validate these results, we analyzed the Dcp-1 levels in rer1–/–clones. Consistent with the RNAi experiments, rer1–/–clones showed upregulation of Dcp-1 levels (Fig 1F–1H), which was rescued by the expression of GFP-Rer1 (Fig 1I–1I”). Additionally, the rer1–/–clone growth was rescued by the expression of GFP-Rer1 (Fig 1J; quantification of RFP-negative area in Fig 1F, 1G and 1I). Notably, Dcp-1 positive cells were concentrated at the boundary of rer1–/–cells and neighboring control cells (Fig 1H–1H’ see blue-arrows; quantified in Fig 1K; also see S2F–S2G Fig), indicative of elimination via cell competition, a phenomenon observed in Minute/+ cells when competing with normal cells [6,7,19,46–49].
While these results suggest that cell death in rer1–/–clones could arise from competition between two different population of cells, the occurrence of cell death upon depletion of Rer1 in the entire posterior compartment (S1E–S1H Fig), a non-competitive setting, required further analysis. Thus, we revisited the impact of Rer1 depletion on cell death by generating rer1-RNAi expressing MARCM clones (S3A–S3B Fig). Remarkably, these clones exhibit higher cell death at the clone boundary (S3B’–S3B” Fig see blue arrows; quantified in S3C Fig) and reduction in the clone size as compared to control (S3D Fig), consistent with our observation in the rer1 mutant clones, indicating that loss of Rer1 could trigger cell competition.
Next, we sought to further confirm that the elimination of rer1–/–cells occurs via cell competition. A hallmark of cell competition is that the loser or winner fate of the cells depends upon the relative fitness with the neighboring cells [50,51]. Thus, we asked if the fate of rer1–/–cells could be altered by reducing the fitness of their neighbors. To this end, we selected ribosomal protein S3 (RpS3) mutant, which also creates loser cells. However, the homozygous RpS3 mutant (RpS3–/–) cells show autonomous cell lethality, while the heterozygous RpS3 mutant (RpS3+/–) cells are eliminated by surrounding wild-type cells (RpS3+/+) via cell competition [6]. Utilizing this paradigm, we generated rer1–/–clones in RpS3 heterozygous mutant background, causing juxtaposition of rer1–/–cells with the RpS3+/–cells. Growth of wild-type cells in the RpS3 heterozygous background was used as a control. Here, we observed a dramatic increase in the growth of rer1–/–clones, although not to the same extent as control cells versus RpS3+/–(Fig 2A–2D; quantified in Fig 2E). More importantly, the Dcp-1 staining could now be observed in the RpS3+/–cells at the boundary (Fig 2D’–2D” see blue arrows; quantified in Fig 2F), demonstrating that the activation of cell death in rer1–/–could be reversed by reducing the fitness level of the neighbors (Fig 2G–2H). Thus, the boundary cell death in rer1–/–clones observed in otherwise normal background is due to higher fitness of the surrounding wild-type cells. Altogether, these results show that the loss of Rer1 created loser cells which are eliminated via cell competition.
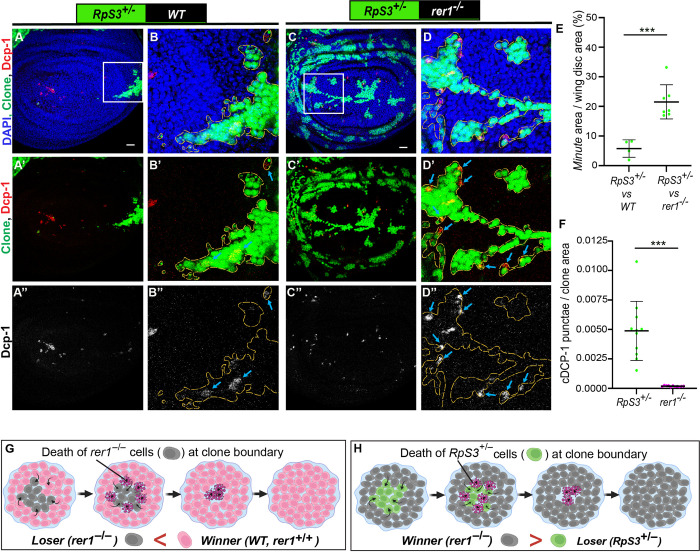
(A-D) Representative images of hs-FLP-induced (96 hrs AHS) mosaic wing imaginal discs containing heterozygous RpS3+/–cells (GFP-positive) juxtaposed to either (A-B) wild-type (WT) cells (GFP-negative) or (C-D) rer1–/–cells (GFP-negative), stained with the anti-cleaved Dcp-1. (E) Quantification shows percentage coverage of the GFP positive Minute area in RpS3+/–vs WT (A, N = 4 wing discs) or RpS3+/–vs rer1–/–(C, N = 7 wing discs). Statistical analysis in E was performed using the two-tailed Welch’s t-test (***p = 0.0002). (F) Quantification of Dcp-1 positive cells in RpS3+/–vs rer1–/–discs shows relatively higher levels of Dcp-1 positive cells in the GFP-positive RpS3+/–tissue as compared to the GFP-negative rer1–/–region (Wilcoxon paired t test; N = 12 wing discs; *** p = 0.0005). (G-H) Schematic diagram illustrating the concept of winner and loser fate in cell competition between WT (rer1+/+) and rer1–/–tissues; and rer1–/–and RpS3+/–tissues, respectively. SB = 20 μm.
Loss of Rer1 creates proteotoxic and oxidative stress
Studies have shown that Rer1 is localized dynamically between the ER and cis-Golgi compartments in Saccharomyces cerevisiae [52,53] and mammalian cells [40,44], where it functions in protein quality control processes [45]. However, its localization and function in Drosophila remains unknown. Utilizing the GFP-rer1 genomic rescue construct, we observed that GFP-Rer1 colocalized with both ER (Calnx) and Golgi (Golgin-245) markers in the wing epithelial cells (S4A–S4B Fig), affirming its conserved localization.
Next, we wondered if the loss of Rer1 also affected protein homeostasis in flies. Thus, we analyzed the level of phosphorylated eIF2α (p-eIF2α), which is a well-established marker of proteotoxic stress [54]. Consistent with the proposed function of Rer1 [43], higher levels of p-eIF2α were observed in both rer1–/–clones (Fig 3A–3C) and RNAi-mediated depletion of Rer1 (S5A–S5F Fig) compared to the control regions, indicating activation of the UPR pathways. The increased level of p-eIF2α in rer1–/–clones was restored upon the expression of GFP-rer1 (Fig 3D–3F).
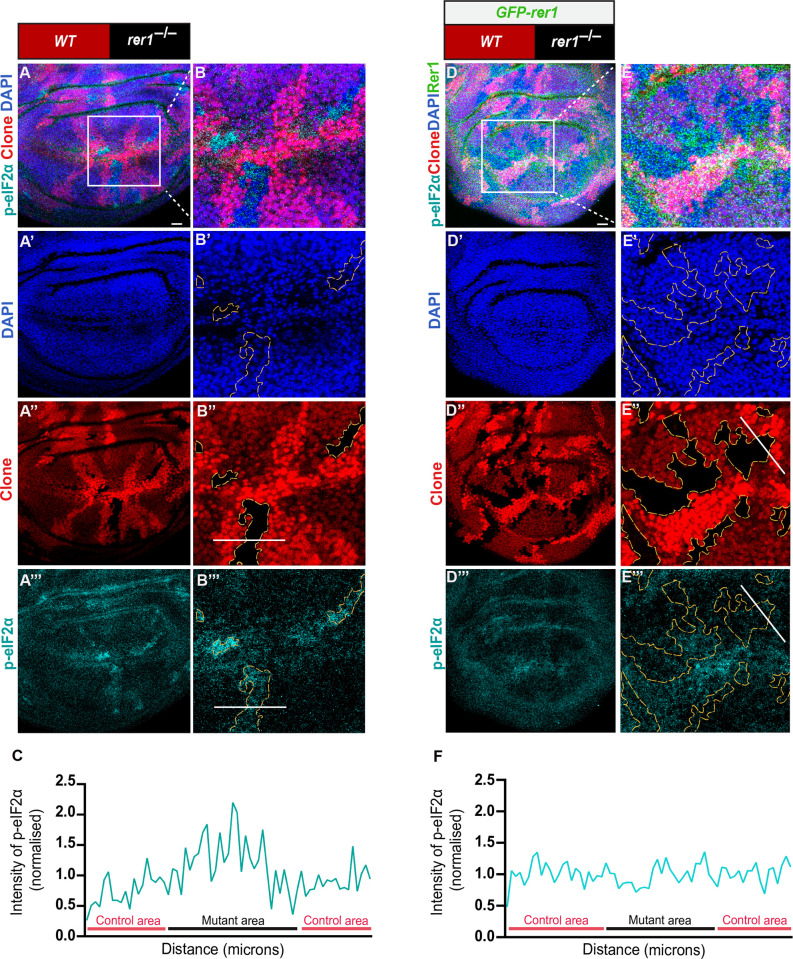
(A) hs-FLP-induced (96 hrs AHS) mitotic clones of rer1–/–tissues in third-instar larval wing epithelium, immuno-stained for the anti-p-eIF2α. (B) A magnified image of the inset (white box) in A. (C) Graph showing the intensity profile of p-eIF2α along the line ROI (white) in B” (N = 12 wing discs). (D) rer1–/–clones in GFP-rer1 background stained with anti-p-eIF2α. (E) A magnified image of the inset (white box) in D. (F) Graph showing the intensity profile of p-eIF2α along the line ROI (white) in E”’ (N = 5 wing discs). SB = 20 μm. Also see S4 and S5 Figs.
We further investigated if the loss of Rer1 also affected redox homeostasis, which has been linked to the activation of cell death response post ER stress [55–57]. Thus, we analyzed the production of reactive oxygen species (ROS) upon loss of Rer1, via Dihydroethidium (DHE) labeling (S1 Text). We observed that the expression of rer1-RNAi in the posterior compartment led to an increase in the levels of DHE as compared to the control anterior compartment, indicating high oxidative stress (S6A–S6D Fig). Altogether, these results suggest that loss of Rer1 increases both proteotoxic and oxidative stress in cells.
PERK-mediated phosphorylation of eIF2α causes elimination of rer1 mutant cells
Four kinases, namely, PERK, GCN2 (general control nonderepressible 2), PKR (protein kinase R) and HRI (heme regulated inhibitor) are known to sense cellular stress and cause phosphorylation of eIF2α, however, in Drosophila only PERK and GCN2 are conserved [58]. Studies suggest that phosphorylation of eIF2α via PERK is due to ER stress [59,60], whereas the phosphorylation via GCN2 is due to amino acid starvation [61,62]. Thus, to further dissect the mechanisms of eIF2α phosphorylation upon loss of Rer1, we depleted PERK or GCN2 in rer1–/–cells, using the MARCM approach (see material and methods), and analyzed p-eIF2α levels. We observed that knockdown of PERK in rer1–/–clones reduced the levels of p-eIF2α, while GCN2 depletion did not show any significant change (Fig 4A–4D, compare Fig 4B”’, 4C”’ and 4D”’; quantified in Fig 4E). Consistent with this, we observed that the expression of PERK-RNAi in the posterior compartment caused reduction in the p-eIF2α, in both control and rer1-RNAi expressing discs (S7A–S7B and S7D–S7E Fig). Whereas coexpression of GCN2-RNAi and rer1-RNAi did not alter p-eIF2α levels (S7F Fig), although expression of GCN2-RNAi alone showed mild reduction (S7C Fig). Altogether, these results show that loss of Rer1 caused ER stress leading to PERK-mediated phosphorylation of e-IF2α.
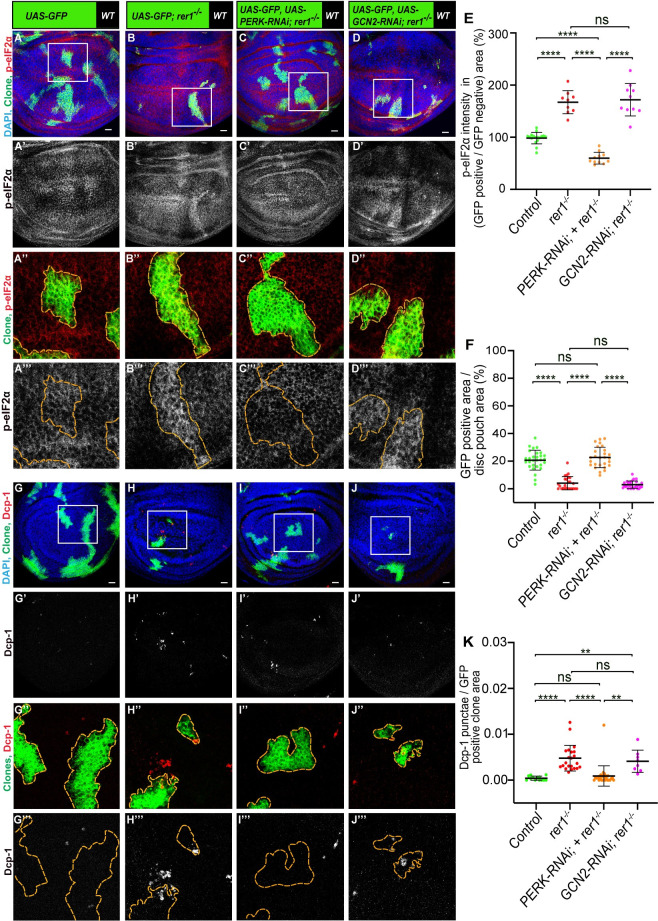
(A-D) Third-instar wing epithelium containing hs-FLP-induced MARCM clones (72 hrs AHS), of following genotypes, (A) UAS-GFP (rer1+/+), (B) UAS-GFP, rer1–/–, (C) UAS-GFP, rer1–/–+ UAS-PERK RNAi and (D) UAS-GFP, rer1–/–+ UAS-GCN2 RNAi, stained with the anti-p-eIF2α antibody. (E) Quantification of the p-eIF2α levels inside the GFP-positive clones with respect to the nearby GFP-negative control tissue in, A (N = 17 wing discs), B (N = 9 wing discs), C (N = 11 wing discs) and D (N = 10 wing discs). Statistical analysis was performed using the Ordinary one-way ANOVA with Tukey’s multiple comparison test (**** p<0.0001). (F) Quantification of the relative size of GFP-labeled clones area in; A (N = 29 wing discs), B, (N = 25 wing discs), C (N = 27 wing discs) and D (N = 29 wing discs). Statistical analysis was performed using the Ordinary one-way ANOVA with Tukey’s multiple comparison test (**** p<0.0001). (G-J) Third-instar wing epithelium containing hs-FLP-induced MARCM clones (72 hrs AHS) of following genotypes, (G) UAS-GFP (rer1+/+), (H) UAS-GFP, rer1–/–, (I) UAS-GFP, rer1–/–+ UAS-PERK RNAi and (J) UAS-GFP, rer1–/–+ UAS-GCN2 RNAi, stained with the anti-Dcp-1 antibody. (K) Quantification of the Dcp-1 in the GFP-labeled clones area in, G (N = 13 clones in 3 wing discs), H (N = 22 clones in 9 wing discs), I (N = 28 clones in 8 wing discs) and J (N = 8 clones in 6 wing discs). Statistical analysis was performed using the Ordinary one-way ANOVA with Tukey’s multiple comparison test (**** p<0.0001, ** p = 0.0029 between G and J, ** p = 0.0041 between I and J). SB = 20 μm. Also see S7 Fig.
We also noticed that depletion of PERK, but not GCN2, significantly increased the size of the rer1–/–clones (quantified in Fig 4F), indicating that higher levels of p-eIF2α may have a negative effect on the survival of rer1–/–cells. To ascertain whether PERK or GCN2 depletion also affected cell death in rer1–/–clones, we analyzed the Dcp-1 levels. We found that PERK depleted rer1–/–clones showed a reduction in Dcp-1, as compared to either control or GCN2-RNAi (Fig 4G–4J; quantified in Fig 4K), suggesting that the activation of PERK is responsible for the elimination of rer1–/–cells.
To further confirm these results, we tested the effect of dephosphorylation of p-eIF2α in rer1–/–cells. Thus, we used the overexpression of growth arrest and DNA damage-inducible 34 protein (GADD34), which provides specificity to protein phosphatase 1 for the dephosphorylation of p-eIF2α [63]. As expected, the overexpression of GADD34 in rer1–/–cells led to a strong reduction in the p-eIF2α levels (Fig 5A–5B; quantified in Fig 5C). Importantly, consistent with the effect of PERK-RNAi, the growth of rer1–/–clones was rescued by the overexpression of GADD34 along with a concomitant decrease in Dcp-1 levels (Fig 5D–5E; quantified in Fig 5F and 5G, respectively). Similarly, coexpression of rer1-RNAi and GADD34 in the posterior compartment showed strong downregulation of p-eIF2α (S8A Fig) and Dcp-1 levels (S8B Fig). These results show that p-eIF2α played a maladaptive role promoting the elimination of rer1–/–loser cells, unlike in the case of Rp mutants, where it was suggested to be adaptive [12] (see discussion).
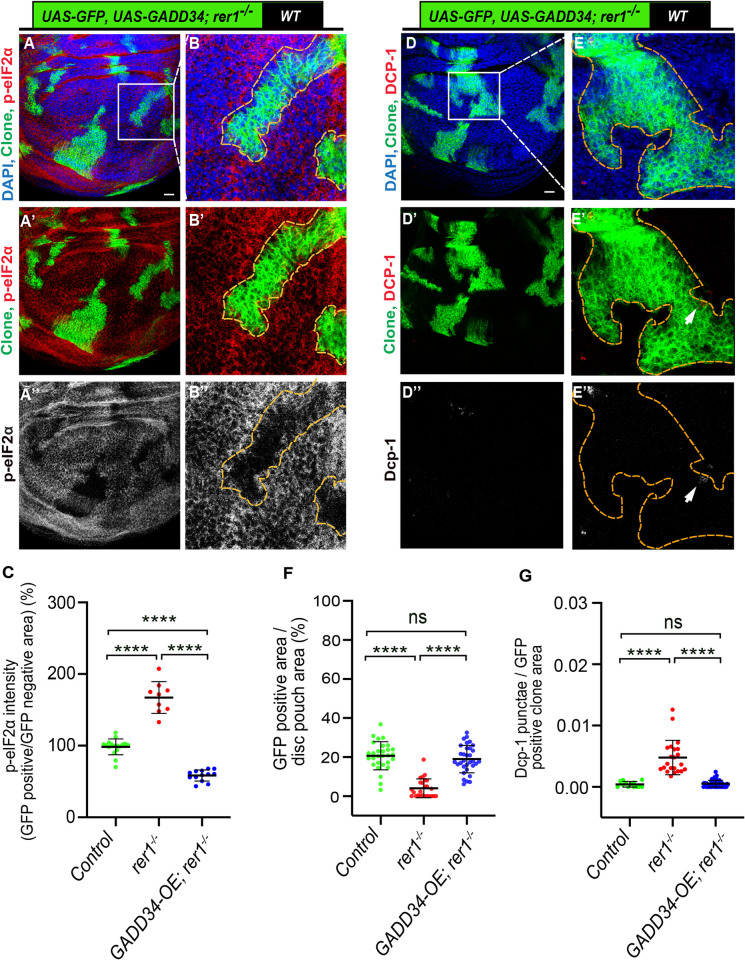
(A-B) Representative images of the third-instar wing epithelium containing hs-FLP-induced MARCM clones (72 hrs AHS) of UAS-GFP, UAS-GADD34; rer1–/–genotype, stained with the anti-p-eIF2α antibody. (B-B”) Magnified images of the insets in A. (C) Quantification of the p-eIF2α levels inside the GFP-positive clones with respect to the nearby GFP-negative tissue in control discs (UAS-GFP, same as in Fig 4A; N = 17 wing discs), rer1–/–(UAS-GFP; rer1–/–, same as in Fig 4B; N = 9 wing discs) and GADD34-OE; rer1–/–(UAS-GFP, UAS-GADD34; rer1–/–; N = 13 wing discs). Statistical analysis was performed using the Ordinary one-way ANOVA with Tukey’s multiple comparison test (**** p<0.0001). (D-E) Representative images of the third-instar wing epithelium containing hs-FLP-induced MARCM clones (72 hrs AHS) of UAS-GFP, UAS-GADD34; rer1–/–genotype, stained with the anti-Dcp-1 antibody. (E-E”) Magnified images of the insets in D. (F) Quantification of GFP-positive clone area in control discs (UAS-GFP, same as in Fig 4A; N = 29 wing discs), rer1–/–(UAS-GFP; rer1–/–same as in Fig 4B; N = 25 wing discs) and GADD34-OE; rer1–/–(UAS-GFP, UAS-GADD34; rer1–/–; N = 32 wing discs). Statistical analysis was performed using the Ordinary one-way ANOVA with Tukey’s multiple comparison test (**** p<0.0001). (G) Quantification of Dcp-1 levels with respect to the GFP-positive clone area in control discs (UAS-GFP, same as in Fig 4G; N = 13 clones in 3 wing discs), rer1–/–(UAS-GFP; rer1–/–, same as in Fig 4H; N = 22 clones in 9 wing discs) and GADD34-OE; rer1–/–(UAS-GFP, UAS-GADD34; rer1–/–; N = 56 clones in 15 wing discs). Statistical analysis was performed using the Ordinary one-way ANOVA with Tukey’s multiple comparison test (**** p<0.0001). SB = 20 μm. Also see S8 Fig.
Being an essential component of the translational machinery, phosphorylation of eIF2α is suggested to cause a reduction in global protein synthesis [54,59,60]. We sought to test whether higher levels of p-eIF2α in rer1–/–also affects protein translation, which perhaps led to their elimination. Thus, we performed an O-propargyl-puromycin (OPP) incorporation assay, where OPP, an analog of puromycin, is incorporated into nascent polypeptide chains directly allowing rapid assessment of global protein synthesis [64]. Surprisingly, in these experiments, rer1–/–cells did not show significant changes in the OPP levels (S9A–S9B Fig; quantified in S9C Fig). While these results are consistent with other reports where phosphorylation of eIF2α did not lead to a reduction in the global protein translation [65–67], it is also conceivable that rer1–/–only have a mild effect on protein translation, that is undetectable with the OPP assay. Furthermore, the loser status of the rer1–/–cells with almost normal translation is consistent with similar observations for other cell competition factors in recent studies [11,16].
Cells lacking Rer1 activates JNK pathway that partially limits their growth
JNK signaling is intricately associated with multiple cellular stress pathways [25] and it is also involved in the elimination of loser cells through cell competition [6,57]. Given that the rer1–/–cells are eliminated due to stress, we aimed to investigate the involvement of the JNK pathway in this process. We first tested if loss of Rer1 led to the activation of JNK signaling. The expression of a well-established JNK reporter, TRE-DsRed [68] was found to be upregulated in rer1–/–clones (Fig 6A–6C). Additionally, the expression of two other known targets of JNK signaling, puckered (puc) and Hid [69–71] were also upregulated upon RNAi-mediated depletion of Rer1 and in rer1–/–clones, respectively (S10A–S10D Fig). Collectively these results show that loss of Rer1 activates JNK signaling.
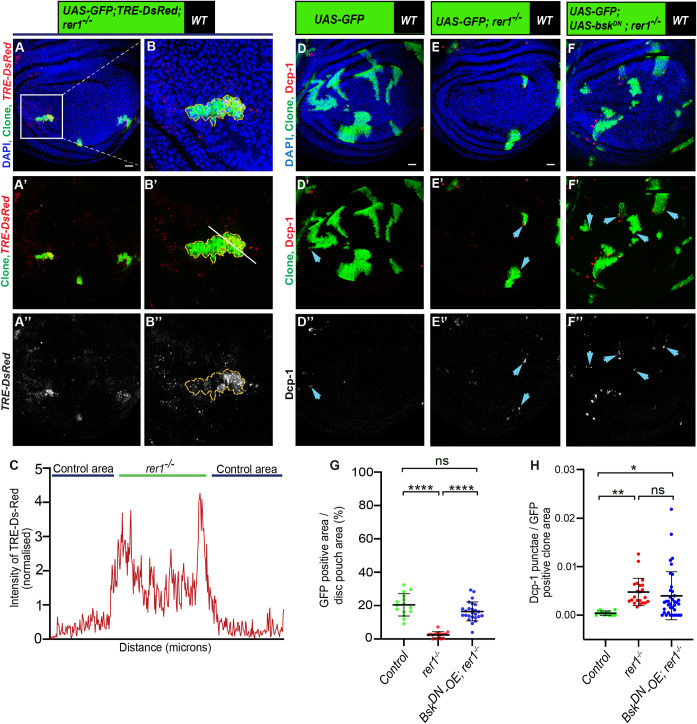
(A-B) Representative images of hs-FLP-induced MARCM clones (72 hrs AHS) of TRE-dsRED, UAS-GFP, rer1–/–genotype (N = 17 wing discs). (C) Graph showing the intensity profile of TRE-dsRED along the line ROI (white) in B’. (D-F) Third-instar wing discs containing hs-FLP-induced MARCM clones (72 hrs AHS) of following genotypes, (D) UAS-GFP (rer1+/+), (E) UAS-GFP, rer1–/–, and (F) UAS-GFP, rer1–/–+ UAS-bskDN, stained with the anti-Dcp-1 antibody. (G) Quantification of GFP-positive clone area in control discs (UAS-GFP; N = 15 wing discs), rer1–/–(UAS-GFP; N = 16 wing discs) and bskDN-OE, rer1–/–(UAS-GFP, UAS-bskDN; rer1–/–; N = 25 wing discs). Statistical analysis was performed using the Ordinary one-way ANOVA with Tukey’s multiple comparison test (**** p<0.0001). (H) Quantification of the Dcp-1 in the GFP-labeled clones area in, D (N = 13 clones in 3 wing discs), E (N = 22 clones in 9 wing discs), and F (N = 37 clones in 12 discs). Statistical analysis was performed using the Ordinary one-way ANOVA with Tukey’s multiple comparison test (** p = 0.0058,* p = 0.0154). SB = 20 μm. Also see S10 Fig.
Subsequently, we explored whether the activation of JNK activity contributed to the elimination of the rer1–/–cell. We observed that the expression of dominant-negative Basket (bskDN), the Drosophila ortholog of JNK [72], modestly improved the growth of rer1–/–cells (Fig 6D–6F; quantified in Fig 6G), however, Dcp-1 levels remained unaffected (Fig 6D–6F; quantified in Fig 6H). This aligns with a previous report on the role of JNK in the survival of Rps3+/- cells, where inhibition of JNK signaling rescued growth without altering cell death [57]. Nevertheless, we tested if the expression of bskDN in rer1–/–clones altered the developmental progression of the larvae, which may have resulted in clone size difference. We find that the pupariation time for the larvae with rer1–/–was similar to the larvae with rer1–/–clones expressing bskDN, indicating that the expression of bskDN did not significantly change developmental timing of larvae harboring rer1–/–clones (S10G Fig). Moreover, p-eIF2α levels remained high in rer1–/–cells expressing bskDN (S10E–S10F Fig), placing JNK activity either downstream or parallel to stress induction. Altogether, these results suggest that activation of JNK signaling contributes towards the maladaptive effects of p-eIF2α in rer1–/–cells, restricting their growth.
Rer1 provides cytoprotection to support Myc-induced overgrowth
Our observation that the loser status of rer1 mutant cells is due to the proteotoxic stress aligns with similar findings in Rp mutants [11–16,56]. However, counter-intuitively, the stress-induced UPR activation (PERK activity) in rer1 mutants appears to be maladaptive (Fig 4), in contrast to the adaptive effect observed in Rp mutants [12]. We next sought to understand this discrepancy further. We reasoned that perhaps Rer1 itself is a component of the adaptive UPR response, providing cytoprotection. To test this, we turned to other UPR-dependent growth paradigms, such as Myc-driven overgrowth, which occurs under high proteotoxic stress and therefore it is highly dependent on the adaptive UPR pathways [33,35]. Thus, we explored the role of Rer1 in Myc-induced growth.
Using the GFP-Rer1 construct, we tested if Rer1 levels adapt to meet the increased proteostasis demand during Myc-overexpression. We found that overexpression of Myc in the posterior compartment via the hh-GAL4 led to an increase in the GFP-Rer1 levels, indicating a potential connection between Rer1 and Myc-induced proteostasis demand (Fig 7A–7B; quantified in Fig 7C). Next, to evaluate the effect of Rer1 loss on proteostasis during Myc overexpression, we employed the ProteoStat aggresome detection assay, which identifies aggregation of misfolded protein. We found that RNAi-mediated depletion of Rer1 in the posterior compartment caused a modest increase in protein aggregation, which was also observed upon Myc overexpression (Fig 7D–7F). However, loss of Rer1 with Myc overexpression resulted in a substantial increase in the aggregation (Fig 7G; quantified in Fig 7H), suggesting that elevated Rer1 levels are required for proper proteostasis during Myc-overexpression.

(A) Images of wing discs with hh-Gal4 driven Myc-overexpression, observed with anti-Myc staining (A”), in GFP-rer1 background (GFP-rer1; hh-Gal4::UAS-RFP-KDEL + UAS-Myc; N = 10 wing discs) show higher levels of GFP-Rer1 in the posterior compartment as compared to the anterior. (B) The increase in GFP-Rer1 is not observed in the control discs (GFP-rer1; hh-Gal4::UAS-RFP-KDEL; N = 9 wing discs). (C) Quantification of GFP-Rer1 intensity in the area marked with white box in A”’, also enlarged in A”“. (D-G) Protein aggregation levels were analysed with ProteoStat assay upon hh-Gal4 mediated control (D; N = 4 wing discs), Rer1 knockdown (E; N = 4 wing discs), Myc overexpression (F; N = 6 wing discs) and overexpression of Myc along with Rer1 depletion (G; N = 9 wing discs). (H) Quantification of protein aggregation punctae in posterior compartment with respect to the anterior compartment in D, E, F and G. Statistical analysis was performed using the Ordinary one-way ANOVA with Tukey’s multiple comparison test (*** p<0.0008,** p = 0.0024,* p = 0.0499 between D and E, * p = 0.0483 between F and G). SB = 20 μm. Anterior and posterior compartments of all wing imaginal discs were placed left and right sides, respectively.
We then asked if Rer1 is required for Myc-induced overgrowth. Thus, we induced MARCM clones overexpressing Myc in either control or rer1–/–clones and analyzed the effect on clone size. Here, we observed that Myc-overexpression in otherwise normal cells increased growth of the clones, which was significantly restricted in the rer1–/–cells (Fig 8A–8D; quantified in Fig 8E). Furthermore, p-eIF2α levels were strongly increased in Myc expressing rer1–/–cell, as compared to either rer1–/–or Myc-overexpression alone (Fig 8A’–8D’ and 8A”’–8D”’; quantified in Fig 8F), indicating that the Rer1 alleviated proteotoxic stress in the Myc-overexpressing cell. However, these experiments did not rule out the possibility of an independent additive effect of Myc-overexpression and loss of Rer1 on p-eIF2α levels and clonal growth.
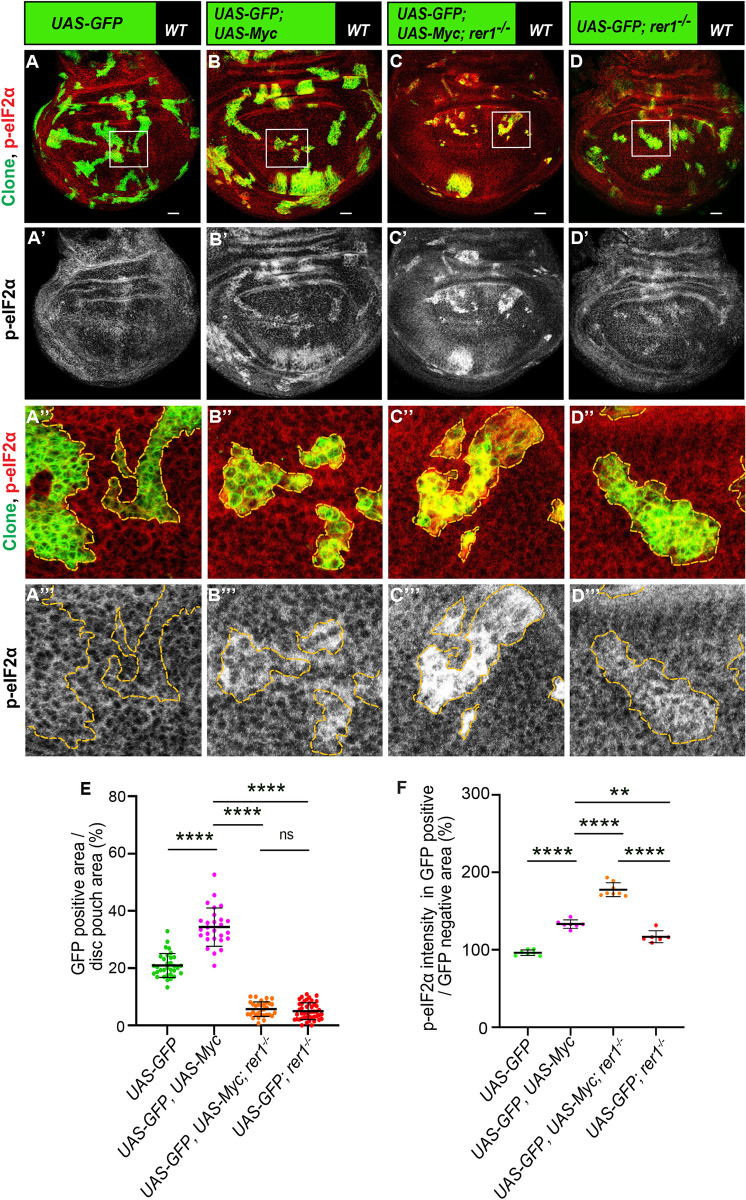
(A-D) Representative images of the third-instar discs containing hs-FLP-induced MARCM clones (96 hrs AHS) of (A) UAS-GFP (rer1+/+), (B) UAS-GFP; UAS-Myc, (C) UAS-GFP, rer1–/–+ UAS-Myc and, (D) UAS-GFP, rer1–/–genotypes, stained with the anti-p-eIF2α antibody. (A”-D”) Magnified image of the insets (white box) in A, B, C and D are shown in A”, B”, C” and D”, respectively. (E) Quantification of the relative size of GFP-labeled clones area in UAS-GFP (rer1+/+) (N = 42 wing discs), UAS-GFP, UAS-Myc, (N = 23 wing discs), UAS-GFP, rer1–/–+ UAS-Myc (N = 31 wing discs) and, UAS-GFP, rer1–/–(N = 46 wing discs). (F) Quantification of the p-eIF2α levels inside the GFP-positive clones with respect to the nearby GFP-negative control tissue in genotypes given in, A (N = 7 wing discs), B (N = 7 wing discs), C (N = 8 wing discs) and D (N = 6 wing discs). Statistical analyses in E and F were performed using the Ordinary one-way ANOVA with Tukey’s multiple comparison test (**** p<0.0001, ** p = 0.0013). SB = 20 μm.
To further strengthen our results, we tested if reducing the overall dosage of Rer1 in the animal will affect Myc-overexpressing cells. To this end, we generated Actin-flip-out-GAL4 (AFG) Myc-overexpressing clones in either wild-type (rer1+/+) or rer1 heterozygous (rer1+/-) background. Consistent with the observation in the MARCM clones, we find that overexpression of Myc in otherwise wild-type background led to an increase in p-eIF2α, which was further enhanced in the rer1+/- background (Fig 9A–9B; compare Fig 9A” and 9B”; quantified in Fig 9C). Moreover, the growth of Myc-expressing cells was reduced in the rer1+/- background as compared to the wild-type (Fig 9A–9B; quantified in Fig 9D). The control clones expressing only GFP did not affect the p-eIF2α levels and showed similar clonal growth in both wild-type and rer1+/- background (S11A–S11D Fig). Altogether, these results show that higher levels of Rer1 provided cytoprotection upon Myc-overexpression thereby supported the overgrowth.
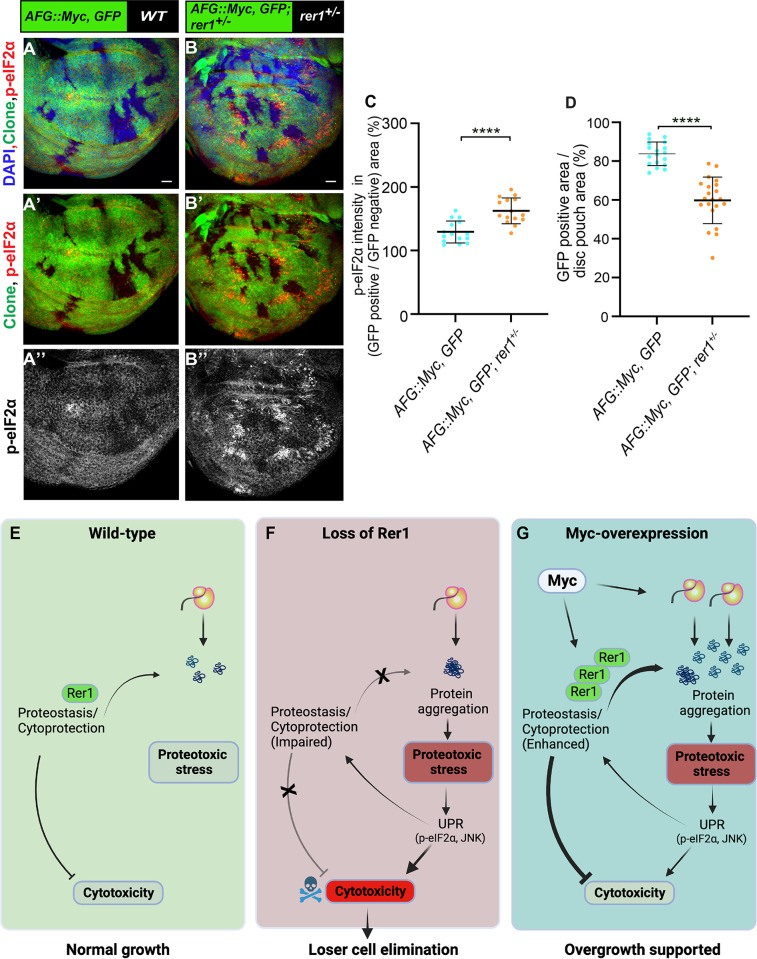
(A-B) Representative images of the wing imaginal discs with hs-FLP induced (at 48 hrs AEL) Actin-FRT-Stop-FRT-Gal4 (AFG)-clones overexpressing GFP and Myc (72 hrs AHS) in either wild-type (A) or rer1+/–background (B). (C) Quantification of p-eIF2α shows higher levels in AFG:: Myc, GFP; rer1+/–(N = 15 wing discs) as compared to AFG:: Myc, GFP in WT background (N = 15 wing discs). (D) Quantification of GFP positive area shows reduction in AFG:: Myc, GFP; rer1+/–(N = 21 wing discs) as compared to AFG:: Myc, GFP in WT background (N = 18 wing discs). Statistical analyses in C and D were performed using the Two-tailed Welch’s unpaired t-test (**** p<0.0001). SB = 20 μm. (E-G) Schematic representation of the involvement of Rer1 in the regulation of proteostasis and its role in supporting Myc-induced overgrowth. (E) Rer1 plays a homeostatic role in the regulation of protein quality providing cytoprotection in the wild-type cells. (F) Cells lacking Rer1 have proteotoxic stress due to improper cytoprotection. This leads to high cytotoxicity downstream of UPR activation, ultimately leading to the competitive elimination of cells identified as losers. (G) Myc-overexpression increases gene expression leading to high proteotoxic stress. Rer1 levels are upregulated upon Myc-overexpression to maintain higher demand of proteostasis, thereby supporting overgrowth. Also see S11 Fig.
Discussion
In this study, we present compelling evidence supporting the role of Rer1 as a regulator of proteostasis in Drosophila. We show that Rer1 is localized to the ER and cis-Golgi compartments and loss of Rer1 activates PERK-mediated phosphorylation of eIF2α, indicating the onset of proteotoxic stress in the ER. These observations are consistent with the proposed function of Rer1 as a regulator of ER proteostasis [73]. Moreover, studies in yeast, worms and mouse cerebral cortex have shown that absence of Rer1 induces ER stress and activates the UPR pathways [43,74], suggesting a well-conserved function of Rer1 across species.
Our results show that proteotoxic stress induced by Rer1 deficiency creates loser cells that are eliminated via cell competition. This is in line with recent studies that have implicated mutations in genes, for example, RNA Helicase Hel25E, E3 ubiquitin ligase Mahjong and Rp mutations, in causing proteotoxic stress and subsequent elimination of mutant cells via cell competition [11,12,15,16,49,56].
Interestingly, our results show that despite both rer1–/–and RpS3+/–cells exhibiting increased p-eIF2α, rer1 mutant cells outperform RpS3+/–cells when juxtaposed. Several potential reasons for the difference in their fitness can be envisaged. First, RpS3+/–cells, that are also rer1+/–, may experience higher stress levels than rer1–/–cells, potentially rescuing later from their loser fate. Second, different downstream pathways may be activated by stress originating in the cytoplasm of RpS3+/–cells as compared to stress in the ER of Rer1-deficient cells. Third, the differential effect of p-eIF2α on translation was observed in RpS3+/–and rer1–/–cells. Our data shows that, despite having higher p-eIF2α, rer1–/–cells do not show reduction in translation. In contrast, RpS3+/–mutants show reduced translation attributed to the transcription factor Xrp1, that is both activated by stress and capable of promoting phosphorylation of eIF2α [15,16,56,75]. While the reduction in translation is probably not sufficient to induce cell competition [17], it may pose a disadvantage to the RpS3+/–cells when competing with the rer1–/–cells. In any case, aligned with other studies, our work shows that proteotoxic stress, even without a reduction in protein translation, is sufficient to drive elimination of the loser rer1–/–cells.
Stress-mediated activation of UPR is associated with both proapoptotic and cytoprotective responses [76,77]. However, whether or not the activation of UPR can provide cytoprotective support to the cell appears to depend on the initial cause of proteotoxic stress. For instance, in RpS mutants, p-eIF2α was shown to be cytoprotective [12], although not sufficiently to rescue their loser fate. In contrast, blocking p-eIF2α by PERK depletion was shown to suppress the competitive elimination of wollknaeuel (wol) mutant clones (wol is involved in the glycosylation of proteins in the ER), Hel25E mutant clones and RpL14+/- cells [15]. Consistent with this, we found that either PERK depletion or dephosphorylation of p-eIF2α by the expression of GADD34 improved the fitness of rer1 mutant cells, indicating that these cells p-eIF2α played a maladaptive role. We propose that this is due to the involvement of Rer1 itself in the cytoprotective processes.
This was evident in our analysis of Rer1 in the regulation of proteostasis in Myc overexpressing cells and Myc-driven overgrowth. Myc-overexpression leads to PERK-mediated phosphorylation of eIF2α [30] and the induction of autophagy, which is required for the Myc-driven overgrowth [33]. This activation of UPR is believed to be an adaptive response to increased gene expression in Myc-overexpressing cells. The increase in p-eIF2α was shown to play a cytoprotective role which is also required for the growth of Myc-expressing tumors in mice [30,35]. We find that Rer1 levels are upregulated upon Myc-overexpression and loss of Rer1 further amplifies the proteotoxic stress and protein aggregation in Myc-overexpressing cells. Importantly, our data shows that the growth of Myc-overexpressing cells was suppressed in rer1+/–background, where cells exhibited higher levels of p-eIF2α. These results suggest that higher levels of Rer1 allowed cells to maintain the increased demand for proteostasis upon Myc-overexpression and thereby mitigating the proteotoxic stress and supporting the overgrowth (Fig 9E–9G).
High Myc expression is associated with various cancers, for instance, pancreatic cancers [78]. However, targeting Myc protein directly as a therapeutic approach has not been successful so far [79]. Interestingly, besides Myc, Rer1 levels are also found to be high in pancreatic cancer cells [80]. Thus, further studies on the effect of Rer1 in Myc-induced overgrowth will be helpful in developing Rer1 as a potential therapeutic target in Myc-driven cancers.
Materials and methods
Drosophila genetics and culturing
The following Drosophila stocks were used: neoFRT82B ry506 rer1KO on 3rd chr. (this paper); GFP-Rer1 genomic rescue line on 2nd chr (this paper), hh-Gal4 on 3rd chr. [81], hs-FLP on 1st chr. and UAS-GFP on 3rd chr. were gifted by A. Teleman (DKFZ, Germany). The following additional stocks were obtained from the Bloomington Drosophila stock center (BDSC); neoFRT82B Ubi-mRFP.nls (BDSC# 30555), neoFRT82B Ubi-GFP.nls (BDSC# 32655), FRT82B Ubi-GFP.nls, RpS3[Plac92] (BDSC# 5627), hs-FLP UAS-GFP tubP-Gal4;; neoFRT82B tubP-Gal80 (BDSC# 86311), UAS-Trip-rer1-RNAi (BDSC# 57435), UAS-GADD34 (BDSC# 76250), UAS-dMyc (BDSC# 9674), hid-LacZ (BDSC# 57435), UAS-bskDN (BDSC# 6409), UAS-RFP.KDEL (BDSC# 30909), TRE-DsRed (BDSC# 59011), UAS-GCN2 RNAi (BDSC# 67215), UAS-PERK RNAi (BDSC# 42499) and Actin5C-FRT-CD2-FRT-Gal4 (BDSC# 4779). The following line was obtained from the Vienna Drosophila RNAi center; UAS-rer1-RNAi (GD ID# 23204).
Standard food composition containing cornmeal, sucrose, yeast, Dextrose and agar was used for growing all fly cultures and crosses. All crosses were maintained at 25°C room temperature unless specifically mentioned. Egg collection, heat shock, dissection (time points), immunostaining, and imaging were kept identical between the control and experiments.
Generation of rer1 mutant and genomic rescue fly-lines
To generate the rer1 knock-out line, the fly line containing a P-element insertion upstream of the rer1 coding sequence (y; ry506 P{SUPor-P}CG11857KG08816/TM3, Sb Ser) was obtained from the Bloomington Drosophila Stock Center Indiana (BDSC# 15137). The P-element imprecise excision was performed, and the excision lines were screened by Polymerase chain reaction (PCR), using primers positioned in the terminal coding sequences of the neighboring genes. The rer1 knock-out line, w;; neoFRTneo82B, ry506, rer1KO/TM6C, Sb, Tb was identified, with 1560bp deletion that covered the whole coding sequence of the rer1 gene (Fig 1A). To generate the GFP-rer1 (CG11857) BAC rescue construct, GFP was recombined to CG11857BAC genomic clone, CH322-101C14 (BACPAC resources) following the P[acman] method [82]. The GFP-CG11857BAC was then inserted into VK18 (2L, 53B2) for genomic rescue.
Antibodies
Larval wing imaginal discs were stained using the following antibodies: Rabbit anti-cleaved Dcp-1 (1:300, Cell Signaling Technology), Rabbit-anti-p-eIF2α (1:300, Cell Signaling Technology), Mouse-anti-dMyc (1:20, a gift from Bruce Edgar lab, University of Utah, USA), Mouse-anti-beta Galactosidase (1:50, 40-1A, DSHB) and Hoechst 33342, H3570 (1:1000, Invitrogen). Fluorescent secondary antibodies used were Alexa-405, Alexa-488, Alexa-594, and Alexa-647 (Invitrogen) at 1:500 dilutions.
ProteoStat assay
For ProteoStat staining, larvae were dissected in 1X PBS and transferred to an Eppendorf tube containing 4% formaldehyde diluted in 1X PAB (ProteoStat assay buffer) for 30 minutes. Following that, the samples were permeabilized 3 times using 0.5% Triton X-100 and 3 mM ethylenediaminetetraacetic acid (pH 8.0) diluted in 1X PAB. Next, it was stained with a ProteoStat detection reagent (Enzo Life Sciences) diluted 1 in 20,000 and Hoechst 33342 at 1 μg ml−1 in PAB incubated for 45 minutes at 4°C and washed thrice by PBS. The samples were then mounted and imaged immediately using a confocal microscope.
Immunostaining
Third instar wandering larvae were used for immunohistochemistry. Larvae were dissected in 1X Phosphate-buffered saline (PBS) and head complexes with wing imaginal discs were fixed for 45 min in 4% paraformaldehyde (PFA) at room temperature. Wing discs were blocked with 0.1% BSA for 1 hour followed by overnight incubation with primary antibody at 4°C and 90 min incubation with fluorophore-conjugated secondary antibody at room temperature. Staining and microscopy conditions for samples used were identical. Wing discs are oriented with the dorsal up and anterior left.
Image acquisition and processing
Images of fixed samples were acquired using the 40x oil objective on the Olympus (FV3000) confocal microscope with each slice (z-stack) equivalent to 1μm. Wing disc images were processed using ImageJ (ImageJ version 1.51j8, NIH) and Photoshop (Adobe Photoshop CS6 extended version 13.0 x64). Figures were made using Illustrator (Adobe Illustrator CS6 Tryout version 16.0.0). All the schematics were created online with BioRender.com.
Statistical analysis
Wing disc pouch regions were taken for all the analysis. Clone size was measured as “total clone area per disc pouch area (%)” by analyzing all of the clones in the pouch area of each genotype using ImageJ (ImageJ version 1.51j8, NIH) software. For cell death analysis, Dcp1 punctae were counted in the tissues (as mentioned in the Figure captions) and normalized with their respective clone area. To analyze the position of cell death in clones, Dcp1 punctae at the clone border and center were counted and divided with the respective common clone area. To calculate the fold change, the average pixel intensities within the area of interest were divided by that of the corresponding wild-type area. All raw data were analyzed using Excel (Microsoft) and graphs were plotted using GraphPad (GraphPad Prism 8 version 8.0.2). Each raw data set is shown as a dot plot, and the horizontal line represents the median.
Statistical analyses were performed by using unpaired two-tailed Welch’s t-test to compare areas in Figs Figs2E,2E, 9C–9D, S3D and S11D, cell death in S1I–S1J Fig, adult wing area in S1O and S2E Figs, OPP intensity in S9C Fig, pupariation time in S10G Fig and p-eIF2α intensity in S11C Fig. Paired two-tailed Wilcoxon signed-rank test was used to compare the cell death between center and border of each clone area in Figs Figs1K,1K, ,2F,2F, and S3C. For multiple comparisons, Ordinary one-way analysis of variance (ANOVA) with Tukey’s test was performed to analyze the data set in Figs 1E, 1J, 4E–4F, ,4K,4K, 5C, 5F–5G, 6G–6H, ,7H,7H, 8E–8F or ANOVA-Dunnett’s test to compare the survivals of rer1–/–and rescued rer1–/–flies with control (rer1+/+) flies in S1B Fig. The significance level was set to p < 0.05. No statistical methods were used to predetermine the sample size. For the quantification of survival and development, first instar larvae were collected, and the numbers of survival larvae in different developmental stages were counted each day. All experiments were independently performed at least three times and were not randomized or blinded. Source data for all the quantitative analyses is included in S1 Data.
Generation of clones
For genetic mosaic analysis, the FLP (Flippase)/FRT (Flippase recognition target) system [83] was used to generate mosaic clones in the wing imaginal disc. Heat shock-inducible FLP was expressed for the mitotic recombination in both mitotic clones and MARCM clones [84,85]. Heat shock was given in a 37°C water bath for 60 minutes at 48 hrs AEL (after egg laying) and larvae were then shifted to 25°C. Larvae were dissected at 72 hrs or 96 hrs or 120 hrs AHS (After heat-shock), as mentioned specifically. Similar heat shock strategy was used for the AFG clones shown in Figs Figs99 and S11, and larvae were dissected 72 hrs AHS kept at 25°C.
Drosophila genotypes
The following genotypes were used in this study:
Fig 1B–1D: hs-FLP/+;; FRT82B, Ubi-RFP.nls/ neoFRT82B, ry506, rer1KO
Fig 1F–1F’: hs-FLP/+;; FRT82B, Ubi-RFP.nls/ FRT82B, Ubi-GFP.nls
Fig 1G–1G’, 1H–1H’: hs-FLP/+;; FRT82B, Ubi-RFP.nls/ neoFRT82B, ry506, rer1KO
Fig 1I–1I”: hs-FLP/+; GFP-rer1/+; FRT82B, Ubi-RFP.nls/ neoFRT82B, ry506, rer1KO
Fig 2A–2A”, 2B–2B”: hs-FLP/+;; FRT82B, Ubi-RFP.nls/ FRT82B, Ubi-GFP.nls, RpS3[Plac92]
Fig 2C–2C”, 2D–2D”: hs-FLP/+;; FRT82B, Ubi-GFP.nls, RpS3[Plac92]/ neoFRT82B, ry506, rer1KO
Fig 3A–3A”’, 3B–3B”’: hs-FLP/+;; FRT82B, Ubi-RFP.nls/ neoFRT82B, ry506, rer1KO
Fig 3D–3D”’, 3E–3E”’: hs-FLP/+; GFP-rer1/+; FRT82B, Ubi-RFP.nls/ neoFRT82B, ry506, rer1KO
Fig 4A–4A”’, 4G–4G”’: hs-FLP, UAS-GFP/+;; tubP-Gal4, neoFRT82B, tubP-Gal80/ neoFRT82B, Ubi-mRFP.nls
Fig 4B–4B”’, 4H–4H”’: hs-FLP, UAS-GFP/+;; tubP-Gal4, neoFRT82B, tubP-Gal80/neoFRT82B, ry506, rer1KO
Fig 4C–4C”’, 4I–4I”’: hs-FLP, UAS-GFP/+; UAS PERK-RNAi/+; tubP-Gal4, neoFRT82B, tubP-Gal80/ neoFRT82B, ry506, rer1KO
Fig 4D–4D”’, 4J–4J”’: hs-FLP, UAS-GFP/+; UAS GCN2 RNAi /+; tubP-Gal4, neoFRT82B, tubP-Gal80/ neoFRT82B, ry506, rer1KO
Fig 5A–5A”, 5B–5B”, 5D–5D”, 5E–5E”: hs-FLP, UAS-GFP/+; UAS-GADD34/+; tubP-Gal4, neoFRT82B, tubP-Gal80/ neoFRT82B, ry506, rer1KO
Fig 6A–6A”, 6B–6B”: hs-FLP, UAS-GFP/+; TRE-DsRed/+; tubP-Gal4, neoFRT82B, tubP-Gal80/ neoFRT82B, ry506, rer1KO
Fig 6D–6D”: hs-FLP, UAS-GFP/+;; tubP-Gal4, neoFRT82B, tubP-Gal80/ neoFRT82B, Ubi-mRFP.nls
Fig 6E–6E”: hs-FLP, UAS-GFP/+;; tubP-Gal4, neoFRT82B, tubP-Gal80/neoFRT82B, ry506, rer1KO
Fig 6F–6F”: hs-FLP, UAS-GFP/UAS-bskDN; +/+; tubP-Gal4, neoFRT82B, tubP-Gal80/ neoFRT82B, ry506, rer1KO
Fig 7A–7A”“:; GFP-rer1/UAS-Myc; hh-Gal4, UAS-RFP-KDEL/+
Fig 7B–7B”’:; GFP-rer1/+; hh-Gal4, UAS-RFP-KDEL/+
Fig 7D–7D’:;; hh-Gal4/+
Fig 7E–7E’:;; UAS-rer1-RNAi, hh-Gal4/+
Fig 7F–7F’:; UAS Myc/+; hh-Gal4
Fig 7G–7G’:; UAS Myc/+; UAS-rer1-RNAi, hh-Gal4/+
Fig 8A–8A”’: hs-FLP, UAS-GFP/+;; tubP-Gal4, neoFRT82B, tubP-Gal80/ neoFRT82B, Ubi-mRFP.nls
Fig 8B–8B”’: hs-FLP, UAS-GFP/+; UAS-Myc/+; tubP-Gal4, neoFRT82B, tubP-Gal80/ neoFRT82B, Ubi-mRFP.nls
Fig 8C–8C”’: hs-FLP, UAS-GFP/+; UAS-Myc/+; tubP-Gal4, neoFRT82B, tubP-Gal80/ neoFRT82B, ry506, rer1KO
Fig 8D–8D”’: hs-FLP, UAS-GFP/+;; tubP-Gal4, neoFRT82B, tubP-Gal80/ neoFRT82B, ry506, rer1KO
Fig 9A–9A”: AFG/hs-FLP; UAS-Myc/+; UAS-GFP/+
Fig 9B–9B”: AFG/hs-FLP; UAS-Myc/+; UAS-GFP/neoFRT82B, ry506, rer1KO
Supporting information
S1 Fig
(Supporting main Fig 1): Analysis of rer1KO (rer1–/–) and rer1-RNAi in growth and cell survival.(A) rer1 mRNA expression levels were measured by quantitative PCR in rer1KG08816 (control, precise excision) and homozygous rer1KO (rer1–/–) flies. Bars show mean±SEM (N = 3 independent experiments). (B) rer1–/–flies failed to hatch out. Most of them died before the pupae stage. Re-introducing the rer1 genomic fragment (GFP-rer1) rescued the phenotype, underscoring that they are caused by rer1 deficiency. Statistical analyses in B were performed using the Ordinary one-way ANOVA with Dunnett’s multiple comparison test, (**** p<0.0001). (C) Scheme of a wing disc illustrating anterior and posterior compartments in which transgene was expressed with a posterior specific Gal4. (D) hh-Gal4 mediated depletion of Rer1 in GFP-rer1 background [GFP-rer1; hh-Gal4::UAS-rer1-RNAi] shows loss of GFP signal in the posterior compartment. (E–F) Dcp-1 staining on (E-E’) control (hh-Gal4, N = 7 wing discs) and (F-F’) Rer1 depleted (hh-Gal4, rer1-RNAi, N = 21 wing discs) wing discs. (G–H) Acridine Orange (AO) staining on (G-G’) control (hh-Gal4, N = 5 wing discs) and (H-H’) Rer1 depleted (hh-Gal4, rer1-RNAi, N = 14 wing discs) wing discs. (I-J) Quantification of Dcp-1 punctae (I) and AO punctae (J) numbers in the posterior compartments of either control or Rer1 depleted discs, normalized to their respective anterior compartments. Statistical analysis was performed using two-tailed Welch’s t-test (**** p<0.0001). SB = 20 μm. (K-N) Adult wings from control flies (hh-Gal4::2x UAS-GFP); male, K, N = 12 and female, M, N = 13) and flies harboring hh-Gal4 mediated Rer1 knockdown along with overexpression of GFP (hh-Gal4::UAS-rer1-RNAi, UAS-GFP); male, L, N = 11 and female, N, N = 22). (O) Quantification of the adult wing areas measured within the dotted line. Statistical analysis was performed using two-tailed Welch’s t-test. P values for male wing comparison (K-L) was p = 0.0793, for female wing comparison (M-N) was p = 0.6704. SB = 500 μm.
(TIF)
S2 Fig
(Supporting main Fig 1): Wing size is unaffected upon induction of rer1 mutant clones albeit higher cell death.(A-B) Images of adult wings from control flies harboring wild-type clones induced at 48hrs AEL (A, male of genotype hs-FLP/Y;; FRT82B, Ubi-RFP.nls/ FRT82B, Ubi-GFP.nls; N = 39 and B, female of genotype hs-FLP/+;; FRT82B, Ubi-RFP.nls/ neoFRT 82B, ry506, rer1KO; N = 44). (C-D) Images of adult wings from flies with rer1 mutant clones induced at 48hrs AEL (C, males of genotype hs-FLP/ Y;; FRT82B, Ubi-RFP.nls/ FRT82B, Ubi-GFP.nls; N = 20 and D, females of genotype hs-FLP/+;; FRT82B, Ubi-RFP.nls/ neoFRT82B, ry506, rer1KO; N = 25). (E) Quantification of the adult wing area measured within the dotted line (Two-tailed Welch’s t-test). P values for male wings size comparison (A-C) was P = 0.1494, for female wings size comparison (B-D) was P = 0.5407. SB = 500 μm. (F-G) Wing imaginal disc harboring RFP negative rer1–/–clones (72 hrs AHS), stained with anti Dcp-1 antibody to show the cell death at clone boundary. (G) A magnified image of the white inset in F. SB = 20 μm.
(TIF)
S3 Fig
(Supporting main Fig 2): MARCM clones expressing rer1-RNAi show cell death at the boundary.(A-A”) Representative images of the wing discs containing hs-FLP induced rer1-RNAi expressing MARCM clones (72 hrs AHS), stained with anti-Dcp-1 antibody. (B-B”) Magnified images of the white box in A. (C) Quantification of Dcp-1 positive cells at the center and border of rer1-RNAi clones (N = 29 clones in 13 wing discs); two-sided Wilcoxon signed-rank test, **** p<0.0001. (D) Quantification of GFP positive clone area in wing disc harboring wild-type (N = 11 wing discs) and rer1-RNAi (N = 16 wing discs) MARCM clones (Two-tailed Welch’s t-test). SB = 20 μm. *** p = 0.0009.
(TIF)
S4 Fig
(Supporting main Fig 3): Colocalization of GFP-Rer1 with ER and Golgi.(A-A”’) Colocalization of GFP-tagged Rer1 and ER, stained with Calnexin (red). (B-B”’) Colocalization of GFP-tagged Rer1 and Golgi, marked by the Golgin-245 (red). White arrows showed the colocalized punctae. SB = 20 μm.
(TIF)
S5 Fig
(Supporting main Fig 3): Knockdown of Rer1 leads to proteotoxic stress.(A) Control wing disc (hh-Gal4::UAS-GFP, N = 5) showing expression of hh-Gal4 in the posterior compartment, marked by GFP. (B–D) hh-Gal4 mediated depletion of Rer1 (hh-Gal4::UAS-GFP, UAS-rer1-RNAi) in the posterior compartment using three different RNAi lines 23203/GD (B; N = 6 wing discs); 23204/GD (C; N = 8 wing discs) and 57435/Trip (D; N = 6 wing discs), shows an increase in the p-eIF2α level compare to the anterior compartment. (E-F) Images of the wing discs containing hs-FLP induced rer1-RNAi (Trip) expressing MARCM clones (72 hrs AHS; N = 3 wing discs), stained with anti-p-eIF2α antibody. (F-F”) Magnified images of the boxed area in E. SB = 20 μm.
(TIF)
S6 Fig
(Supporting main Fig 3): Knockdown of Rer1 results in the accumulation of ROS.(A-D) DHE uptake assay, indicative of ROS levels, performed on the control and Rer1 depleted wing imaginal discs. (A) Depletion of Rer1 in the posterior compartment [hh-Gal4::UAS-rer1-RNAi] showed higher levels of DHE as compared to the anterior compartment (N = 12 wing discs). (B) Magnified images of the inset in A. (C) control disc [hh-Gal4] showed similar DHE levels between anterior and posterior compartments (N = 8 wing discs). (D) Magnified images of the inset in C. Yellow dotted lines mark the anterior-posterior boundary; Nuclei are stained with DAPI. SB = 20 μm.
(TIF)
S7 Fig
(Supporting main Fig 4): Loss of Rer1 triggers PERK-mediated phosphorylation of eIF2α.(A-A’) Control third-instar wing disc (hh-Gal4::2X UAS-GFP) stained with anti-p-eIF2α antibody (N = 10 wing discs). (B-D) Third-instar discs with hh-Gal4 mediated coexpression of GFP with PERK-RNAi (B-B’; N = 12 wing discs), GCN2-RNAi (C-C’; N = 5) and rer1-RNAi (D-D’; N = 10 wing discs), stained with anti-p-eIF2α antibody. (E-E’) Disc with coexpression of rer1-RNAi and PERK-RNAi, stained with anti-p-eIF2α antibody (N = 12). (F-F’) Disc with coexpression of rer1-RNAi and GCN2-RNAi, stained with anti-p-eIF2α antibody (N = 13). Nuclei are stained with DAPI. SB = 20 μm.
(TIF)
S8 Fig
(Supporting main Fig 5): Overexpression of GADD34 rescued cell death following Rer1 depletion.(A-B) Third instar disc with hh-Gal4 mediated overexpression of GADD34 and expression of rer1-RNAi, stained with either anti-p-eIF2α (A; N = 10 wing discs) and anti-cleaved Dcp-1 antibodies (B; N = 15 wing discs). SB = 20 μm.
(TIF)
S9 Fig
Protein translation is unaffected upon loss of Rer1.(A-B) OPP assay on third-instar discs containing hs-FLP-induced MARCM clones (96 hrs AHS) of (A) UAS-GFP (control) and (B) UAS-GFP, rer1–/–genotypes. (C) Quantification of the signal intensity of OPP inside the GFP-positive clones with respect to nearby GFP-negative control tissue in UAS-GFP (A; N = 3 wing discs), UAS-GFP, rer1–/–(B; N = 5 wing discs). Borders between the GFP-positive and GFP-negative areas are marked with yellow dotted lines. Statistical analysis in C was performed using the two-tailed Welch’s t-test (p = 0.9431). SB = 20 μm.
(TIF)
S10 Fig
(Supporting main Fig 6): Loss of Rer1 activates JNK signaling.(A-B) Images representing puc-lacZ promoter activities via beta-galactosidase (red) staining on (A-A’) control discs (puc-lacZ; hh-Gal4/+; N = 9 wing discs), or (B-B’) Rer1 depleted discs puc-lacZ; hh-Gal4, UAS-rer1-RNAi; N = 10 wing discs). (C) Third-instar wing discs containing hs-FLP-induced (96 hrs AHS) MARCM clones of UAS-GFP, rer1–/–genotype (N = 4 wing discs), immuno-stained for the beta-galactosidase (red) to mark the hid-lacZ promoter activity. (D-D’) A magnified image of the inset (white box) in C. The interfaces between the clone areas are marked with yellow dotted lines. (E-F) Representative images of hs-FLP-induced MARCM clones (72 hrs AHS) of UAS-GFP, UAS-bskDN; rer1–/–genotype stained with anti-p-eIF2α antibody (N = 6 wing discs). (G) Quantification of pupariation time for UAS-GFP; rer1–/–(N = 16 technical repeats) and UAS-GFP, UAS-bskDN; rer1–/–(N = 16 technical repeats). Statistical analysis in G was performed using the two-tailed Welch’s t-test (p = 0.1389). SB = 20 μm.
(TIF)
S11 Fig
(Supporting main Fig 9): Analysis of GFP expressing control AFG clones.(A-B) Representative images of the wing imaginal discs with hs-FLP induced (48 hrs AEL) Actin-FRT-Stop-FRT-Gal4 (AFG)-control clones overexpressing GFP in either wild-type (A) or rer1+/–background (B), dissected 72 hrs AHS. (C-D) Quantification of p-eIF2α and GFP positive area in AFG:: GFP in WT background (N = 11 wing discs) and AFG:: GFP in rer1+/–background (N = 5 wing discs). Statistical analyses in C and D were performed using the Two-tailed Welch’s unpaired t-test (p = 0.4193 and p = 0.6089, respectively). SB = 20 μm.
(TIF)
Acknowledgments
We thank IISER Bhopal for the fly facility and the DST-FIST facility for the confocal microscopy. The authors thank Milos Spasic (now at GSK, Belgium) for generating the rer1 mutant flies. We thank Dr. Vimlesh Kumar (IISER Bhopal) for comments on the manuscript. We thank V.C. group members for the discussions on the project and manuscript.
Funding Statement
This work was supported by the Science and Engineering Research Board (SERB), Department of Science & Technology, Government of India (grant number: CRG/2021/004686 to VC). The laboratory of V.C. is also supported by intramural funds from IISER Bhopal and the Department of Biotechnology-EMR (grant number: BT/PR34467/BRB/10/1831/2019 to VC). P.K.P. received fellowship from the Council of Scientific & Industrial Research (09/1020/(0127)/2017-EMR-I). W.A. acknowledges the financial support of the Vlaams Instituut voor Biotechnologie (VIB), KU Leuven (grant number: C14/21/095 and KA.20/085 to WA), the Fonds Wetenschappelijk Onderzoek (FWO) (grant number: I001322N to WA), and the Stichting Alzheimer Onderzoek België (grant number: #2020/0030 to WA). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
References
Decision Letter 0
3 Jul 2023
Dear Dr Chaudhary,
Thank you very much for submitting your Research Article entitled 'Drosophila Rer1 is essential for the maintenance of protein homeostasis and Myc-driven super-competition' to PLOS Genetics.
The manuscript was fully evaluated at the editorial level and by independent peer reviewers. The reviewers appreciated the attention to an important problem, but raised some substantial concerns about the current manuscript. Based on the reviews, we will not be able to accept this version of the manuscript, but we would be willing to review a much-revised version. The reviewers suggest additional experiments to strengthen the manuscript. I agree with their assessment. While I realize these experiments may take a significant amount of time to perform, the additional information they would provide will be essential for a more positive review. We cannot, of course, promise publication at that time.
Should you decide to revise the manuscript for further consideration here, your revisions should address the specific points made by each reviewer. We will also require a detailed list of your responses to the review comments and a description of the changes you have made in the manuscript.
If you decide to revise the manuscript for further consideration at PLOS Genetics, please aim to resubmit within the next 60 days, unless it will take extra time to address the concerns of the reviewers, in which case we would appreciate an expected resubmission date by email to gro.solp@scitenegsolp.
If present, accompanying reviewer attachments are included with this email; please notify the journal office if any appear to be missing. They will also be available for download from the link below. You can use this link to log into the system when you are ready to submit a revised version, having first consulted our Submission Checklist.
To enhance the reproducibility of your results, we recommend that you deposit your laboratory protocols in protocols.io, where a protocol can be assigned its own identifier (DOI) such that it can be cited independently in the future. Additionally, PLOS ONE offers an option to publish peer-reviewed clinical study protocols. Read more information on sharing protocols at https://plos.org/protocols?utm_medium=editorial-email&utm_source=authorletters&utm_campaign=protocols
Please be aware that our data availability policy requires that all numerical data underlying graphs or summary statistics are included with the submission, and you will need to provide this upon resubmission if not already present. In addition, we do not permit the inclusion of phrases such as "data not shown" or "unpublished results" in manuscripts. All points should be backed up by data provided with the submission.
While revising your submission, please upload your figure files to the Preflight Analysis and Conversion Engine (PACE) digital diagnostic tool. PACE helps ensure that figures meet PLOS requirements. To use PACE, you must first register as a user. Then, login and navigate to the UPLOAD tab, where you will find detailed instructions on how to use the tool. If you encounter any issues or have any questions when using PACE, please email us at gro.solp@serugif.
PLOS has incorporated Similarity Check, powered by iThenticate, into its journal-wide submission system in order to screen submitted content for originality before publication. Each PLOS journal undertakes screening on a proportion of submitted articles. You will be contacted if needed following the screening process.
To resubmit, use the link below and 'Revise Submission' in the 'Submissions Needing Revision' folder.
We are sorry that we cannot be more positive about your manuscript at this stage. Please do not hesitate to contact us if you have any concerns or questions.
Yours sincerely,
Ken M. Cadigan
Academic Editor
PLOS Genetics
Gregory P. Copenhaver
Editor-in-Chief
PLOS Genetics
Reviewer's Responses to Questions
Comments to the Authors:
Please note here if the review is uploaded as an attachment.
Reviewer #1: Paul et al present a manuscript on the role of Rer1 role in protein homeostasis and Myc-driven super-competition. Although the manuscript compiles interesting data, the study has limitations that make it difficult to recommend for publication. There are three important results. First, Rer1 is an essential gene in Drosophila. Second, Rer1 mutant cells are eliminated from wing epithelium via cell competition. Lastly, Rer1 is required for Myc-driven cell competition. Currently, an in-depth study of any of the three points is missing. Addressing the below-mentioned points would help authors to improve their manuscript:
1. As per the title of the manuscript, the major claim of the authors is that Rer1 is required for the maintenance of protein homeostasis. The supporting evidence is that Rer1 localizes in Golgi & ER and loss of Rer1 results in increased p-eIf2 α levels. A more detailed study is required here to dissect if Rer1 has a direct role in the regulation of protein homeostasis or if p-eif2α levels increase because of activation of proteotoxic stress signaling in Rer1 mutant, as shown recently in ribosome heterozygous cells or mahjong mutant cells (Langton et al 2021 PLoS genetics). Moreover, does the Rer1 mutant accumulate unfolded proteins? Also, does PERK or GCN2 kinase activity go up or does the dephosphorylation of p-eIf2alpha go down? Perturbation of protein homeostasis also affects autophagic and proteasomal flux, addressing all these points will make the study more comprehensive.
2. Regarding the role of Rer1 in cell competition, the data presented in Fig 2 A-E does not support that Rer1 has a role in cell competition. This data could also be possible because of protein perdurance in somatic clones and mutant cells display cell autonomous cell death.
3. The current model for competitive elimination of RpS3 heterozygous is through Xrp1. Xrp1 regulates proteotoxic stress signaling in RpS3 cells (Langton et al 2021, PLoS Genetics). Thus, data related to the continuous elimination of RpS3/+ cells in the presence of Rer1 mutant, as shown in Fig 2F-I, shows that most likely Xrp1 is still activated in Rp3/+ cells. Interestingly, Xrp1 is known to be active in RpS3/+ cells in a cell-autonomous manner (Lee et al 2018, Dev cell). Therefore, how this data supports the role of Rer1 in cell competition is not clear.
4. The data presented in Fig 1B-E does support the role of Rer1 in cell competition. However, this data need to be validated by knocking down Rer1 through its RNAi and then showing boundary cell death and clone area compared to control clones.
5. The model suggested for Rer1 cell competition would be more complete by studying genetic epistasis between p-eIf2α and JNK signaling in Rer1 mutant cells.
6. p-eIf2α role in cell competition is already known (Naotaka Ochi et al 2021 PLoS Genetics) and it plays a cytoprotective role in different stress conditions. Therefore, how its upregulation results in the elimination of Rer1 is not clear. Moreover, it would be interesting to examine if GADD34 overexpression rescues cell autonomous cell death that occurs upon knockdown of Rer1 in the posterior compartment. This will help to dissect if p-eIf2α has a cell protective role or has a role only in cell competition.
7. The proposed role of Rer1 in Myc cell competition needs further investigation. First, the way this manuscript is written looks like proteotoxic stress activation is associated with Myc super-competition. However, this is not the case. Proteotoxic stress activation is shown to be required for Myc overgrowth phenotype and any association with its super-competition is not known. Therefore, it is important to show that activation of proteotoxic stress activation is required for Myc super-competition.
Additionally. first paper in which the role of Myc was demonstrated in super-competition, it was also shown that differential growth is not sufficient for cell competition (de la Cova, 2004, cell). Therefore, any conclusion on Myc super-competition based on only the growth behavior of cells would lead to the wrong conclusion as shown in Fig 6 of this manuscript.
Moreover, if proteotoxic stress activation has a protective role in Myc overexpressing cells (Nagy et al 2013 PLoS Genetics), then why do these cells with simultaneous loss of Rer1 have smaller clone size? Is it possible that higher p-eIF2alpha levels shown by these cells are because of additive regulation of p-eIF2aplha levels by Myc overexpression and Rer1 loss?
8. What is the significance of Rer-1 upregulation in Myc overexpressing cells? Does Rer1 overexpression result in Myc overexpressing cells grow even better and display lower proteotoxic stress signaling.
Minor points:
1. Abstract “Cell competition is a developmental phenomenon that allows the selection of healthier cells in a developing tissue”
In the above-mentioned line in the abstract, authors have suggested cell competition as a developmental phenomenon. However, this is not the case, and it has much broader significance (Neerven et al 2022, Nature Reviews Molecular Cell Biology).
Reviewer #2: Cell Competition is key mechanism utilised to eliminate potentially dangerous cells for the living organism. In the last decade, the physiological relevance of this phenomenon has been discovered as well as players involved in the elimination of unfit cells. However, the mechanisms which induce unfit cells remain still largely unknown. Paul and Co-authors present in this article a novel Cell Competition regulator Rer1 which appears also to be required for supercompetition. The manuscript is well written and shows convincing evidence that Rer1 regulates cell fitness in wt and supercompetition. Furthermore, I believe that the manuscript will be of interest for the general audience of the journal.
However some points should be clarified or further develop:
Major points:
-Rer1 downregulation in non-competitive scenarios is sufficient to trigger cell death (Figure S1). How authors reconcile this fact with the role Rer1 in cell competition?
-Authors should test the activation of JNK pathway in competitive settings if they want to make statements about the downstream signalling (Fig.3). Given the challenging genetics, authors may want to use in this case Anti-ACTIVE® JNK to detect the activation of JNK pathway in the rer1-/- clones.
-Authors show cell competition phenotypes when downregulating or mutating rer1. It would be very interesting to analyze if Rer1 overexpression in clones is sufficient to induce supercompetitor cells in a wt background. Similarly, to test whether Rer1 overexpression in combination with Myc overexpression in clones, increases the competitive behavior of the Myc overexpressing clones alone.
Minor points:
-Figure 1B does not show the merge of RFP and DCP1, it is just showing the RFP.
-Is Figure 4A showing the experiment 96h AHS? Clones from Figure 1B-D (96h AHS) and Figure 2C (96h AHS) look much bigger.
-Fig. 6: Authors only demonstrate the requirement of Rer1 in supercompetition they do not show higher levels of Rer1 in competitive settings (i.e. Supercompetition).
-Fig. 6J, Distance is not measured in a.u.. Authors may want to say microns/pixels.
-Figure legends for some panels are missing, eg: Fig.1 E”.
-Clarify what authors refer for the sample size (N), number of discs or number of clones.
Reviewer #3: In this work Paul et al generated a rer1 null allele, which presented early larval lethality. They showed that loss of Rer1 leads to increased proteotoxic stress and JNK activation. Additionally they present evidence that Rer1 mutant clones are eliminated through cell competition. They showed that Rer1 levels are upregulated upon Myc overexpression, by using a GFP tagged rer1 genomic rescue construct. The support that Rer1 has a cytoprotective role in Myc induced proteotoxic stress, which is essential for supercompetition. Rer1 acts as a novel regulator of protein homeostasis in Drosophila and reveal its role in competitive cell survival.
Strong points: The author by generating a new Rer1 knockout allele, they manage to show that Rer1 indeed is an essential factor in Drosophila as it is in other organisms and that Rer1 mutant cells presents characteristics of loser fate. This works comes to add another example of proteotoxic driven cell competition.
Weak point: The authors aim to demonstrate that Rer1 has a protective role in Myc induced proteotoxic stress, however, the data does not fully support that the effect of Rer1 is specific to Myc induced mechanism or it is due to its essential function in the cell.
Major Revisions
1. Please provide Dcp1 staining in the experiment with the partial rescue of clonal size of rer1-/- cells by overexpression of bskDN, to show a direct effect on the cell competition hallmark, Dcp1. This is important since there is difference in the size of rer1-/- clone in Figure 2 according to time after heat shock. Therefore, any developmental delay or difference in egg deposition could have an impact in the clone size, not necessarily the competition between the clones and the background cells.
2. Could the authors show reduced Dcp1 for the experiment that they rescue clone size by overexpressing GADD34? To exclude that the clone difference is an secondary effect of developmental timing and not due to competition. (logic similar with the rescue by BskDN).
3. The authors in order to explore if Rer1 plays a role in the growth of Myc-overexpressing cells, they state that “We generated Myc-overexpressing clones in the wing disc, in either wild-type or rer1–/– background”. According to their genotype (Fig 6C : hs-FLP, UAS-GFP/+; UAS-Myc/+; tubP-Gal4, neoFRT82B, tubP-Gal80/ neoFRT 82B, ry506, rer1KO) , the background is rer1+/- heterozygous and only the myc overexpressing clones will be rer1-/-.
4. They showed in Figure 6, that the overgrowth phenotype observed due to the overexpression of Myc was reduced in the rer1–/– cells (Fig 6A – D; quantified in 6E, compare 6B and 6C), underscoring that Rer1 is required for Myc-induced overproliferation. Earlier in the manuscript authors have solid data that Rer1 is an essential protein even in wild type cells. How the authors can exclude the possibility that the absence of rer1 reduces Myc-induced overproliferation, not due to a specific effect on the Myc mechanism, but independently as an essential factor. Therefore, that would mean that Rer1 is required in the cells independently of the Myc induced mechanism.
5. What happens when the authors have the extra copy of the rer1 locus (GFP tagged). Can they see if the supercompetitor status of myc overexpressing cells is increased more with extra Rer1 protein, by checking the clone size for example, to support the cytoprotective role of Rer1 in Myc cells?
6. What happens if they have heterozygosity or homozygosity of rer1 locus in all cells. For example, would it be easy to perform the experiment using this genotype: hs-FLP, UAS-GFP/+; UAS-Myc/+; tubP-Gal4, neoFRT82B, tubP-Gal80, rer1KO / neoFRT 82B, ry506, rer1KO. In that case all the cells will lack rer1, not only the myc overexpressing clones.
7. The above additive effect could also explain the higher levels of p-eIF2a when cells overexpress Myc but lack Rer1 protein. Someone could support that the cells have two different independent stressors that increases p-eIF2a levels and reduces growth of the clones. I think it is important to strengthen this conclusion by other approaches.
Minor Revisions
1. In the abstract where they mention ER add also endoplasmic reticulum (ER)
2. Authors mention that: “Previous studies have suggested that the loser fate of Rp+/- cells is due to a reduction in protein translation [7–9]”. Actually, Lee et al 2018 (citation #7) suggested that reduced translation was likely responsible for the slow growth of Rp+/- cells, but they did not proposed that loser fate of Rp+/- cells is due to a reduction in protein translation.
3. Authors mention that: “However, recently it was shown that it is a result of increased proteotoxic stress due to protein aggregation [10–13].” The word “shown” will be misleading for the audience. I think it is better to use a word not so loud since the existed data do not clearly support that loser fate of Rp+/- cells in Drosophila is due to proteotoxic stress due to protein aggregation. There is still inconsistency in the field
4. In Figure S1 panel I, the Y axis has the “%” symbol. Do the authors mean that the ratio of [Anterior Dcp1]/ [posterior Dcp1] is 32%? Does this mean that posterior compartment has ~3 times more Dcp1 compared to anterior in hhGal4::UAS-rer1-RNAi experiment? In Figure S1F it looks quite more Dcp1 in posterior vs anterior, not just 3 fold. Also, the control hh-Gal4 in panel I should be 100, since the Dcp1 does not differ between anterior and posterior compartment. Therefore the ratio is 1 and if we are transforming this to %, must be 100%. Same comments for panel J.
5. In panel C of Figure 1, the rer1-/- clone that is located in the ventral side of the posterior compartment does not present competitive cell death at the boundaries. Contrary the twin spot (rer1+/+) shows boundary cell death. Have the author noticed any difference in rer1 clones and twin spots between ventral and dorsal compartments? Maybe they could provide more examples of clones. In this experiment (Figure 1C-D) they mention that they have generated clones 96hs after heat shock. Have the authors done Dcp1 staining in 72 hours clones, where the rer1-/- clones are bigger and in the process of elimination (according to their data in Figure 2B)?
6. In panel F of Figure 1 the authors provide the quantification of the RFP negative clones area per disc pouch. Since the clones are generated via heat shock flippase, I think it will be more appropriate to compare the ratio of RFP negative clone area compared to RFP double positive area (twin spot). They have performed this kind of ratio quantification in Figure 2A-E.
7. At what developmental stage the heat shock was done in Figure 2D, in order to have 120hrs clones (5 days). Do the larvae present a developmental delay? Could the authors provide details for the flyfood that was used for these experiments.
8. In panel Figure 2E in the Y axis the symbol (%) is misleading. The twin spot is larger than the clone therefore the percentage of the ratio must be higher than 100%. For example in 120hrs the 20% ratio that is depicted, gives the impression that twin spot is smaller than the clones.
9. For the figure 2F-I, the authors have created rer1-/- clones in RpS3+/- background. They do not present the control experiments to make rer1+/+ clones (wild type) in RpS3+/- background, to investigate if rer1-/- clones perform worsen than rer1+/+ clones when they are next to RpS3+/- cells. I do not find informative the panel 2H. It would be meaningful, if we could compare this with the control situation, where they have wild type cells next to RpS3+/-. In that case they could compare if Dcp1 in RpS3+/- cells is different when the cells next to them have wild type rer1 (rer1+/+) or rer1-/- mutant. This experiment also shows that rer1-/- cells can still outcompete RpS3+/- cells.
10. Have the authors tested if the heterozygosity of the null rer1 allele has any impact in Minutes. In the above experiments, the Minutes have also lost one copy of rer1 gene.
11. In legend 2F they mention”hs-FLP-induced heterozygous RpS3+/– clones (GFP-positive cells) juxtaposed to rer1–/– cells (GFP-negative), and immuno-stained for the anti-cleaved Dcp-1.”. According to their genotype, using the flippase they create rer1-/- clones in RpS3+/- background (the twin spot of these clones were RpS3-/-, rer1+/+, but the cells do not survive since RpS3 is an essential protein). Therefore, the most accurate description is ” hs-FLP-induced rer1–/–clones (GFP-negative) juxtaposed to heterozygous RpS3+/– cells (GFP-positive cells), and immuno-stained for the anti-cleaved Dcp-1.” They use also the term “RpS3+/- clones” in the text. It should be RpS3+/- background
12. Comment for Figure 4A: Could the authors provide another disc with rer1-/- clones, where they will also depict p-eIF2a levels in comparison with DAPI staining (since p-eIF2a levels are cytoplasmic)? For example, in Panel 4B’’ we can see higher levels of p-eIF2a in the top-edge of the Figure where there is no rer1-/- cells. Maybe with the 72 hours AHS mitotic clones, which are bigger in size, the increase in p-eIF2a in rer1-/- mutant clones is more prominent. Indeed, in Figure 5B the increased levels of p-eIF2a in rer1-/- clones is more prominent than in 4A.
13. In Figure S5 could they correlate the DHE staining with the nuclei position. In panel B’’’ there is an area in the anterior compartment with increased DHE, which actually is mainly cytoplasmic, which could be responsible for this. I kind of agree that it seems that DHE is increased in posterior compartment, but I would like to compare different areas of the two compartments with the same nuclei positions.
Thank you.
**********
Have all data underlying the figures and results presented in the manuscript been provided?
Large-scale datasets should be made available via a public repository as described in the PLOS Genetics data availability policy, and numerical data that underlies graphs or summary statistics should be provided in spreadsheet form as supporting information.
Reviewer #1: Yes
Reviewer #2: Yes
Reviewer #3: None
**********
PLOS authors have the option to publish the peer review history of their article (what does this mean?). If published, this will include your full peer review and any attached files.
If you choose “no”, your identity will remain anonymous but your review may still be made public.
Do you want your identity to be public for this peer review? For information about this choice, including consent withdrawal, please see our Privacy Policy.
Reviewer #1: No
Reviewer #2: Yes: Marisa M.Merino
Reviewer #3: Yes: Marianthi Kiparaki
Author response to Decision Letter 0
6 Dec 2023
Attachment
Submitted filename: Paul et al Responses to Reviewers PlosGen.pdf
Decision Letter 1
5 Jan 2024
Dear Dr Chaudhary,
Thank you very much for submitting your Research Article entitled 'Maintenance of proteostasis by Drosophila Rer1 is essential for competitive cell survival and Myc-driven overgrowth' to PLOS Genetics.
The manuscript was fully evaluated at the editorial level and by independent peer reviewers. The reviewers and editors appreciate the effort in providing additional data to address the reviewers' concerns. While these revisions were not sufficient to sway reviewer 1, the editors agree with the viewpoint of reviewers 2 and 3. However, reviewer 3 raises some minor points about Fig. 9 that need to be addressed before the manuscript can be accepted for publication.
We therefore ask you to modify the manuscript according to reviewer 3's recommendations. Your revisions should address the specific points made by reviewer 3.
In addition we ask that you:
1) Provide a detailed list of your responses to the review comments and a description of the changes you have made in the manuscript.
2) Upload a Striking Image with a corresponding caption to accompany your manuscript if one is available (either a new image or an existing one from within your manuscript). If this image is judged to be suitable, it may be featured on our website. Images should ideally be high resolution, eye-catching, single panel square images. For examples, please browse our archive. If your image is from someone other than yourself, please ensure that the artist has read and agreed to the terms and conditions of the Creative Commons Attribution License. Note: we cannot publish copyrighted images.
We hope to receive your revised manuscript within the next 30 days. If you anticipate any delay in its return, we would ask you to let us know the expected resubmission date by email to gro.solp@scitenegsolp.
If present, accompanying reviewer attachments should be included with this email; please notify the journal office if any appear to be missing. They will also be available for download from the link below. You can use this link to log into the system when you are ready to submit a revised version, having first consulted our Submission Checklist.
While revising your submission, please upload your figure files to the Preflight Analysis and Conversion Engine (PACE) digital diagnostic tool. PACE helps ensure that figures meet PLOS requirements. To use PACE, you must first register as a user. Then, login and navigate to the UPLOAD tab, where you will find detailed instructions on how to use the tool. If you encounter any issues or have any questions when using PACE, please email us at gro.solp@serugif.
Please be aware that our data availability policy requires that all numerical data underlying graphs or summary statistics are included with the submission, and you will need to provide this upon resubmission if not already present. In addition, we do not permit the inclusion of phrases such as "data not shown" or "unpublished results" in manuscripts. All points should be backed up by data provided with the submission.
To enhance the reproducibility of your results, we recommend that you deposit your laboratory protocols in protocols.io, where a protocol can be assigned its own identifier (DOI) such that it can be cited independently in the future. Additionally, PLOS ONE offers an option to publish peer-reviewed clinical study protocols. Read more information on sharing protocols at https://plos.org/protocols?utm_medium=editorial-email&utm_source=authorletters&utm_campaign=protocols
Please review your reference list to ensure that it is complete and correct. If you have cited papers that have been retracted, please include the rationale for doing so in the manuscript text, or remove these references and replace them with relevant current references. Any changes to the reference list should be mentioned in the rebuttal letter that accompanies your revised manuscript. If you need to cite a retracted article, indicate the article’s retracted status in the References list and also include a citation and full reference for the retraction notice.
PLOS has incorporated Similarity Check, powered by iThenticate, into its journal-wide submission system in order to screen submitted content for originality before publication. Each PLOS journal undertakes screening on a proportion of submitted articles. You will be contacted if needed following the screening process.
To resubmit, you will need to go to the link below and 'Revise Submission' in the 'Submissions Needing Revision' folder.
Please let us know if you have any questions while making these revisions.
Yours sincerely,
Ken M. Cadigan, PhD
Academic Editor
PLOS Genetics
Gregory P. Copenhaver
Editor-in-Chief
PLOS Genetics
Reviewer's Responses to Questions
Comments to the Authors:
Please note here if the review is uploaded as an attachment.
Reviewer #1: The manuscript looks much better than last time. However, revisiting my previous comments, a major criticism was the absence of an in-depth study. I still feel this aspect has not been adequately addressed. The mechanism governing Rer1 maintenance protein homeostasis remains unclear. As I inquired before: Does Rer1 play a direct or indirect role in activating Perk, and how it contributes to protein homeostasis needs to be thoroughly examined.
Reviewer #2: The authors have now clarified all my concerns and the new version of the manuscript shows further conceptual and experimental insights of this new Cell Competition player.
I recommend the manuscript for publication in PLOS Genetics.
Reviewer #3: Dear Paul, Umarvaish et al,
I find the responses to the reviewer's questions detailed and satisfactory. Thank you.
I have some minor concerns regarding the role of Rer1 in Myc supercompetition.
Minor concerns:
A) Could the authors explain why they were unable to perform the experiment to analyze Myc-driven overgrowth in flies with an extra copy of the rer1 gene (GFP-rer1)? Did the larvae with the appropriate genotype (AFG/hs-FLP; UAS-Myc/GFP-rer1; UAS-GFP/+) die? Were they unable to retrieve clones? I find important for the field to present (or at least mention) the result of Rer1 overexpression.
B) Please provide some details and clarifications of the experiment that is provided in the Figure 9 and gives better support on the role of Rer1 in Myc supercompetition.
More specifically:
1) Could the authors provide the details on the heat shock time and dissection time in the experiment Fig. 9A-B.
2) Is the p-eIF2a ratio in Fig 9C increased in AFG::Myc, GFP, rer1+/- experiment compared to AFG::Myc, GFP due to the decrease of p-eIF2a in rer1+/- background cells? Is the ratio increased because the the Myc, rer1+/- cells have increased p-eIF2a? The images provided in the pdf version are not clear and they give the impression that the p-eIF2a staining is similar in AFG::Myc cells and in AFG::Myc, rer1+/- cells.
3)Have the authors performed control clones in WT and in rer1+/- background, meaning AFG without over-expressing Myc. Do the clones look smaller in rer1+/- background?
4) If the authors have discs that the Myc, GFP cells have not undertaken the whole disc would be useful. Also show discs with similar size (the 9A disc looks bigger than 9B).
C) Please mention that also in Ochi et al, PERK depletion suppressed the elimination of wol clones (wol is involved in the glycosylation of proteins in the ER), of Hel25E clones.
Best wishes,
Marianthi
**********
Have all data underlying the figures and results presented in the manuscript been provided?
Large-scale datasets should be made available via a public repository as described in the PLOS Genetics data availability policy, and numerical data that underlies graphs or summary statistics should be provided in spreadsheet form as supporting information.
Reviewer #1: Yes
Reviewer #2: Yes
Reviewer #3: Yes
**********
PLOS authors have the option to publish the peer review history of their article (what does this mean?). If published, this will include your full peer review and any attached files.
If you choose “no”, your identity will remain anonymous but your review may still be made public.
Do you want your identity to be public for this peer review? For information about this choice, including consent withdrawal, please see our Privacy Policy.
Reviewer #1: No
Reviewer #2: Yes: Marisa M.Merino
Reviewer #3: Yes: Marianthi Kiparaki
Author response to Decision Letter 1
16 Jan 2024
Attachment
Submitted filename: Reviewers Responses PLOS-Genetics minor revision v2.pdf
Decision Letter 2
5 Feb 2024
Dear Dr Chaudhary,
We are pleased to inform you that your manuscript entitled "Maintenance of proteostasis by Drosophila Rer1 is essential for competitive cell survival and Myc-driven overgrowth" has been editorially accepted for publication in PLOS Genetics. Congratulations!
Before your submission can be formally accepted and sent to production you will need to complete our formatting changes, which you will receive in a follow up email. Please be aware that it may take several days for you to receive this email; during this time no action is required by you. Please note: the accept date on your published article will reflect the date of this provisional acceptance, but your manuscript will not be scheduled for publication until the required changes have been made.
Once your paper is formally accepted, an uncorrected proof of your manuscript will be published online ahead of the final version, unless you’ve already opted out via the online submission form. If, for any reason, you do not want an earlier version of your manuscript published online or are unsure if you have already indicated as such, please let the journal staff know immediately at gro.solp@scitenegsolp.
In the meantime, please log into Editorial Manager at https://www.editorialmanager.com/pgenetics/, click the "Update My Information" link at the top of the page, and update your user information to ensure an efficient production and billing process. Note that PLOS requires an ORCID iD for all corresponding authors. Therefore, please ensure that you have an ORCID iD and that it is validated in Editorial Manager. To do this, go to ‘Update my Information’ (in the upper left-hand corner of the main menu), and click on the Fetch/Validate link next to the ORCID field. This will take you to the ORCID site and allow you to create a new iD or authenticate a pre-existing iD in Editorial Manager.
If you have a press-related query, or would like to know about making your underlying data available (as you will be aware, this is required for publication), please see the end of this email. If your institution or institutions have a press office, please notify them about your upcoming article at this point, to enable them to help maximise its impact. Inform journal staff as soon as possible if you are preparing a press release for your article and need a publication date.
Thank you again for supporting open-access publishing; we are looking forward to publishing your work in PLOS Genetics!
Yours sincerely,
Ken M. Cadigan, PhD
Academic Editor
PLOS Genetics
Gregory P. Copenhaver
Editor-in-Chief
PLOS Genetics
Twitter: @PLOSGenetics
----------------------------------------------------
Comments from the reviewers (if applicable):
Reviewer's Responses to Questions
Comments to the Authors:
Please note here if the review is uploaded as an attachment.
Reviewer #3: Dear authors,
Thank you for your revised version.
I am completely satisfied with your replies to my previous comments.
**********
Have all data underlying the figures and results presented in the manuscript been provided?
Large-scale datasets should be made available via a public repository as described in the PLOS Genetics data availability policy, and numerical data that underlies graphs or summary statistics should be provided in spreadsheet form as supporting information.
Reviewer #3: Yes
**********
PLOS authors have the option to publish the peer review history of their article (what does this mean?). If published, this will include your full peer review and any attached files.
If you choose “no”, your identity will remain anonymous but your review may still be made public.
Do you want your identity to be public for this peer review? For information about this choice, including consent withdrawal, please see our Privacy Policy.
Reviewer #3: Yes: Marianthi Kiparaki
----------------------------------------------------
Data Deposition
If you have submitted a Research Article or Front Matter that has associated data that are not suitable for deposition in a subject-specific public repository (such as GenBank or ArrayExpress), one way to make that data available is to deposit it in the Dryad Digital Repository. As you may recall, we ask all authors to agree to make data available; this is one way to achieve that. A full list of recommended repositories can be found on our website.
The following link will take you to the Dryad record for your article, so you won't have to re‐enter its bibliographic information, and can upload your files directly:
http://datadryad.org/submit?journalID=pgenetics&manu=PGENETICS-D-23-00557R2
More information about depositing data in Dryad is available at http://www.datadryad.org/depositing. If you experience any difficulties in submitting your data, please contact gro.dayrdatad@pleh for support.
Additionally, please be aware that our data availability policy requires that all numerical data underlying display items are included with the submission, and you will need to provide this before we can formally accept your manuscript, if not already present.
----------------------------------------------------
Press Queries
If you or your institution will be preparing press materials for this manuscript, or if you need to know your paper's publication date for media purposes, please inform the journal staff as soon as possible so that your submission can be scheduled accordingly. Your manuscript will remain under a strict press embargo until the publication date and time. This means an early version of your manuscript will not be published ahead of your final version. PLOS Genetics may also choose to issue a press release for your article. If there's anything the journal should know or you'd like more information, please get in touch via gro.solp@scitenegsolp.
Acceptance letter
20 Feb 2024
PGENETICS-D-23-00557R2
Maintenance of proteostasis by Drosophila Rer1 is essential for competitive cell survival and Myc-driven overgrowth
Dear Dr Chaudhary,
We are pleased to inform you that your manuscript entitled "Maintenance of proteostasis by Drosophila Rer1 is essential for competitive cell survival and Myc-driven overgrowth" has been formally accepted for publication in PLOS Genetics! Your manuscript is now with our production department and you will be notified of the publication date in due course.
The corresponding author will soon be receiving a typeset proof for review, to ensure errors have not been introduced during production. Please review the PDF proof of your manuscript carefully, as this is the last chance to correct any errors. Please note that major changes, or those which affect the scientific understanding of the work, will likely cause delays to the publication date of your manuscript.
Soon after your final files are uploaded, unless you have opted out or your manuscript is a front-matter piece, the early version of your manuscript will be published online. The date of the early version will be your article's publication date. The final article will be published to the same URL, and all versions of the paper will be accessible to readers.
Thank you again for supporting PLOS Genetics and open-access publishing. We are looking forward to publishing your work!
With kind regards,
Zsofi Zombor
PLOS Genetics
On behalf of:
The PLOS Genetics Team
Carlyle House, Carlyle Road, Cambridge CB4 3DN | United Kingdom
gro.solp@scitenegsolp | +44 (0) 1223-442823
plosgenetics.org | Twitter: @PLOSGenetics
Articles from PLOS Genetics are provided here courtesy of PLOS
Full text links
Read article at publisher's site: https://doi.org/10.1371/journal.pgen.1011171
Read article for free, from open access legal sources, via Unpaywall:
https://journals.plos.org/plosgenetics/article/file?id=10.1371/journal.pgen.1011171&type=printable
Citations & impact
This article has not been cited yet.
Impact metrics
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Membrane protein retrieval from the Golgi apparatus to the endoplasmic reticulum (ER): characterization of the RER1 gene product as a component involved in ER localization of Sec12p.
Mol Biol Cell, 6(11):1459-1477, 01 Nov 1995
Cited by: 64 articles | PMID: 8589449 | PMCID: PMC301304
Human Rer1 is localized to the Golgi apparatus and complements the deletion of the homologous Rer1 protein of Saccharomyces cerevisiae.
Eur J Cell Biol, 74(1):31-40, 01 Sep 1997
Cited by: 32 articles | PMID: 9309388
Bring it back, bring it back, don't take it away from me - the sorting receptor RER1.
J Cell Sci, 133(17):jcs231423, 01 Sep 2020
Cited by: 10 articles | PMID: 32873699
Review
Rer1 and calnexin regulate endoplasmic reticulum retention of a peripheral myelin protein 22 mutant that causes type 1A Charcot-Marie-Tooth disease.
Sci Rep, 4:6992, 11 Nov 2014
Cited by: 23 articles | PMID: 25385046 | PMCID: PMC4227013
Funding
Funders who supported this work.
Council of Scientific & Industrial Research (1)
Grant ID: 09/1020/(0127)/2017-EMR-
Department of Biotechnology, Ministry of Science and Technology, India (1)
Grant ID: BT/PR34467/BRB/10/1831/2019
Department of Science and Technology, Government of India (1)
Grant ID: CRG/2021/004686
Fonds Wetenschappelijk Onderzoek (1)
Grant ID: I001322N
Stichting Alzheimer Onderzoek België (1)
Grant ID: 2020/0030
Vlaams Instituut voor Biotechnologie (1)
Grant ID: C14/21/095 and KA.20/085

 1
,*
1
,*


