Abstract
Free full text

Treatment of patients hospitalized for COVID-19 with remdesivir is associated with lower likelihood of 30-day readmission: a retrospective observational study
Abstract
Aim:
This observational study investigated the association between remdesivir treatment during hospitalization for COVID-19 and 30-day COVID-19-related and all-cause readmission across different variants time periods.
Patients & methods:
Hospitalization records for adult patients discharged from a COVID-19 hospitalization between 1 May 2020 to 30 April 2022 were extracted from the US PINC AI Healthcare Database. Likelihood of 30-day readmission was compared among remdesivir-treated and nonremdesivir-treated patients using multivariable logistic regression models adjusted for age, corticosteroid treatment, Charlson comorbidity index and intensive care unit stay during the COVID-19 hospitalization. Analyses were stratified by maximum supplemental oxygen requirement and variant time period (pre-Delta, Delta and Omicron).
Results:
Of the 440,601 patients discharged alive after a COVID-19 hospitalization, 248,785 (56.5%) patients received remdesivir. Overall, remdesivir patients had a 30-day COVID-19-related readmission rate of 3.0% and all-cause readmission rate of 6.3% compared with 5.4% and 9.1%, respectively, for patients who did not receive remdesivir during their COVID-19 hospitalization. After adjusting for demographics and clinical characteristics, remdesivir treatment was associated with significantly lower odds of 30-day COVID-19-related readmission (odds ratio 0.60 [95% confidence interval: 0.58–0.62]), and all-cause readmission (0.73 [0.72–0.75]). Significantly lower odds of 30-day readmission in remdesivir-treated patients was observed across all variant time periods.
Conclusion:
Treating patients hospitalized for COVID-19 with remdesivir is associated with a statistically significant reduction in 30-day COVID-19-related and all-cause readmission across variant time periods. These findings indicate that the clinical benefit of remdesivir may extend beyond the COVID-19 hospitalization.
Despite the conclusion of the federal COVID-19 Public Health Emergency Declaration in the US, COVID-19 remains a highly consequential disease with short- and long-term health consequences. In 2023 alone, there were 39,514 COVID-19 deaths in the US compared with 3184 influenza deaths up to 10 June 2023 [1]. The US Centers for Disease Control and Prevention have also estimated that one in five people with a prior positive COVID-19 experience post-acute sequelae of SARS-CoV-2 infection, i.e., long COVID-19 [2].
Growing evidence has demonstrated that a significant proportion of discharged COVID-19 patients develop recurrence and are subsequently readmitted. A recent systematic review reported that the prevalence of hospital readmission in the first 30 days following discharge from a COVID-19 hospitalization was 9.0% (95% confidence interval [CI]: 8.9%–11.8%) and post-discharge all-cause mortality was 7.8% (95% CI: 2.8%–13.0%) [3]. Readmission imposes considerable medical and economic burden on both patients and the healthcare system and is an important indicator for hospital quality of patient care. Initiating COVID-19 therapies that are effective in improving both in-hospital and post-discharge outcomes, including readmission risk, remains an essential priority for healthcare systems in managing this evolving disease.
While remdesivir has been shown to reduce time to recovery and improve in-hospital clinical outcomes in COVID-19 patients in randomized controlled trials and observational studies [4–9], the effect of remdesivir on post-discharge outcomes has been examined in limited studies. In the ACTT-1 randomized controlled trial, 5% of patients in the remdesivir group were readmitted to the hospital, as compared with 3% in the placebo group, but this difference was not statistically significant (difference: 2%; 95% CI: 0%–4%) [4]. A previous observational study conducted among patients hospitalized for COVID-19 in Rhode Island between 1 April 2020 and 31 December 2020 demonstrated a significant association between remdesivir and 30-day readmission among patients hospitalized with mild disease (relative risk [RR]: 0.31; 95% CI: 0.13–0.75) but not reaching significance among the overall study cohort (RR: 0.81; 95% CI: 0.59–1.13) [10]. Given the emergence of new variants of concern and improvements in COVID-19 management, it is important to examine the association between remdesivir and readmission across the evolving pandemic in datasets sufficiently large to support the analysis. Understanding the impact of remdesivir specifically across the variants of concern is relevant and important given it is the only antiviral that has remained US FDA approved in the treatment of patients hospitalized for COVID-19 throughout the different variant periods.
The aim of the study was to assess if there is an association between remdesivir treatment during the initial hospitalization for COVID-19 and 30-day COVID-19-related and all-cause readmission across variant time periods including pre-Delta (May 2020–April 2021), Delta (May–November 2021) and Omicron (December 2021–April 2022).
Patients & methods
Study design & data
Hospitalization records for this large, retrospective observational study were extracted from the PINC AI Healthcare Database (PHD, formerly Premier Healthcare Database; www.pinc-ai.com), a large, geographically diverse, all-payer hospital administrative database. This database captures data relating to diagnoses, procedures and administered medications for approximately 25% of all US hospitalizations. Actual dates and times are not available in this database due to privacy reasons, but admission and discharge month and year are available, and all hospitalization information and subsequent readmissions are captured relative to hospital admission day, which allow for extracting of all the information for each day of hospitalization.
Study population
Patients ≥18 years of age hospitalized for COVID-19 and discharged alive between 1 May 2020 and 30 April 2022 were identified in the database as the index COVID-19 hospitalization. COVID-19 hospitalizations were based on the presence of a primary discharge diagnosis code of COVID-19 (International Classification of Diseases, [ICD] 10th revision, clinical modification: U07.1), which was flagged in the database as ‘present on admission’. Patients were excluded if they met any of the following criteria: discharge documented as ‘expired’ or ‘transfer to hospice’, pregnant, erroneous or incomplete records, transferred to or from another hospital, or admitted for elective procedures. Patients were also excluded if they were admitted to hospitals that did not report charges for supplemental oxygen, since some hospitals do not bill supplemental oxygen supply or devices separately and instead include these charges in room charges.
Remdesivir-treated patients received at least a single dose of remdesivir during the index COVID-19 hospitalization, while non-remdesivir patients did not receive remdesivir at any time during the index COVID-19 hospitalization.
Main outcomes & covariates
Thirty-day readmission was defined as readmission to the same hospital within 30 days of being discharged alive from the index COVID-19 hospitalization. Readmission to different hospitals is not captured in the database and as such were not included in the definition of readmission. Primary ICD-10-CM diagnosis code for the readmission was extracted. COVID-19-related (defined as a readmission with a primary or secondary discharge diagnosis of COVID-19) and all-cause readmission within 30 days was examined.
The following covariates were captured for the index COVID-19 hospitalization: demographics (age group, sex, race, ethnicity, primary payor), Charlson comorbidity index (CCI), hospital characteristics (hospital bed size, teaching status, region, urban/rural), maximum supplemental oxygen requirements (no supplemental oxygen charges [NSOc], low-flow oxygen [LFO], high-flow oxygen/non-invasive ventilation [HFO/NIV], invasive mechanical ventilation/extracorporeal membrane oxygenation [IMV/ECMO]), intensive care unit (ICU) stay and use of corticosteroids. These covariates were considered to be of clinical importance for COVID-19-related outcomes as they are indicators of differences in patient demographics, hospital-related variations in care and disease severity. Similar variables have been assessed in other observational studies on COVID-19 using the same database [9,11,12].
Statistical analysis
Analyses were performed overall, by maximal supplemental oxygen requirement during the index COVID-19 hospitalization, and by periods defined by dominant variants including pre-Delta, Delta-predominant and Omicron-predominant.
Descriptive analyses of patient characteristics based on whether or not they were readmitted for any reason within 30 days after discharge, as well as whether or not they had received remdesivir during the index COVID-19 hospitalization were conducted. Multivariable logistic regression models were used to assess the association between remdesivir use and likelihood of 30-day COVID-19-related and all-cause readmission. All models were adjusted for age, corticosteroid use, variant time period (where applicable), CCI, maximum supplemental oxygen requirements (where applicable), and ICU stay during index COVID-19 hospitalization. Multicollinearity between the predictors were tested using variance inflation factor and tolerance. All variance inflation factors were <1.5 and all tolerance values were >0.67, indicating no issues with multicollinearity. In the multivariable model, all covariates were retained in the model irrespective of their p-value. Adjusted odds ratios (aORs) and 95% confidence intervals (CIs) were summarized.
All statistical analyses were conducted using SAS version 9.4 (SAS 9.4, NC, USA, SAS Institute Inc.). All statistical tests were two-sided and the level of significance was assessed at p-value < 0.05.
Results
Overall, 440,601 patients hospitalized with a primary diagnosis of COVID-19 were discharged alive from 852 hospitals (Figure 1) during the study period.
There were 33,217 (7.5%) patients who had an all-cause readmission to the same hospital within 30 days. Compared with non-readmitted patients, readmitted patients were older (median age [interquartile range (IQR)]: 71.0 [60.0; 80.0] vs 63.0 [51.0; 74.0] years), had more comorbidities (CCI ≥4: 36% vs 16%) and were more likely to have received corticosteroid monotherapy during the index COVID-19 hospitalization (38% vs 29%) (Table 1). There was an equal proportion of non-readmitted and readmitted patients requiring HFO/NIV (16%), and IMV/ECMO (3%) during the index hospitalization. The two groups had comparable length of stay (LOS) during the index COVID-19 hospitalization (median [IQR]: 5.0 [3.0; 8.0] vs 5.0 [3.0; 9.0] days) (Table 1). Of the readmissions within 30 days, 30.9% were for a primary discharge diagnosis of COVID-19, 15.0% for sepsis and 2.3% for pneumonia (Table 2).
Table 1.
| Readmitted patients | Non-readmitted patients | Overall | ||
|---|---|---|---|---|
| Patients (n) | 33,217 | 407,384 | 440,601 | |
| Hospitals (n) | 747 | 852 | 852 | |
| Age, years | Median [IQR] | 71.0 [60.0; 80.0] | 63.0 [51.0; 74.0] | 63.0 [51.0; 74.0] |
| Age group, years | 18–49 | 11% | 23% | 22% |
| 50–64 | 24% | 32% | 31% | |
| 65+ | 65% | 45% | 47% | |
| Race | White | 73% | 69% | 70% |
| Black | 18% | 17% | 17% | |
| Asian | 2% | 2% | 2% | |
| Other | 7% | 12% | 11% | |
| Sex | Female | 48% | 49% | 49% |
| Ethnicity | Hispanic | 11% | 17% | 16% |
| Non-Hispanic | 79% | 73% | 73% | |
| Unknown | 10% | 11% | 11% | |
| Primary payor | Commercial | 15% | 31% | 29% |
| Medicare | 70% | 48% | 49% | |
| Medicaid | 10% | 12% | 12% | |
| Other payor | 5% | 10% | 10% | |
| CCI | 0 | 15% | 33% | 32% |
| 1–3 | 49% | 51% | 50% | |
| ≥4 | 36% | 16% | 18% | |
| Hospital setting | Urban | 86% | 86% | 86% |
| Rural | 14% | 14% | 14% | |
| Teaching status | Teaching | 42% | 39% | 40% |
| Census regions | Midwest | 24% | 21% | 21% |
| Northeast | 11% | 10% | 10% | |
| South | 53% | 55% | 55% | |
| West | 12% | 14% | 14% | |
| Bed size | <100 | 6% | 7% | 7% |
| 100–199 | 15% | 17% | 17% | |
| 200–299 | 20% | 20% | 20% | |
| 300–399 | 21% | 19% | 19% | |
| 400–499 | 10% | 9% | 10% | |
| 500+ | 29% | 27% | 27% | |
| Maximum supplemental oxygen requirement (highest level of oxygenation during the index COVID-19 hospitalization) | NSOc | 42% | 39% | 39% |
| LFO | 40% | 42% | 42% | |
| HFO/NIV | 16% | 16% | 16% | |
| IMV/ECMO | 3% | 3% | 3% | |
| LOS | Median [IQR] | 5.0 [3.0; 8.0] | 5.0 [3.0; 9.0] | 5.0 [3.0; 9.0] |
| ICU stay | 19% | 20% | 20% | |
| COVID-19 treatments | Remdesivir | 48% | 57% | 56% |
| Corticosteroids | 86% | 87% | 87% | |
| Remdesivir + corticosteroids | 41% | 48% | 47% | |
| Corticosteroids monotherapy | 38% | 29% | 29% | |
| Remdesivir monotherapy | 1% | 2% | 1% | |
| Remdesivir + corticosteroids + (baricitinib or tocilizumab) | 5% | 8% | 8% | |
| Remdesivir + (corticosteroids or baricitinib or tocilizumab) | 46% | 56% | 55% |
CCI: Charlson comorbidity index; HFO/NIV: High-flow oxygen/non-invasive ventilation; ICU: Intensive care unit; IMV/ECMO: Invasive mechanical ventilation/extracorporeal membrane oxygenation; IQR: Interquartile range; LFO: Low-flow oxygen; LOS: Length of stay; NSOc: No supplementary oxygen charges.
Table 2.
| ICD-10-CM | Description | Readmissions (n) | % |
|---|---|---|---|
| U07.1 | COVID-19, virus identified | 10,257 | 30.9 |
| A41.89 | Other specified sepsis | 2616 | 7.9 |
| A41.9 | Sepsis, unspecified | 2358 | 7.1 |
| J18.9 | Pneumonia, unspecified organism | 752 | 2.3 |
| I13.0 | Hypertensive heart and chronic kidney disease with heart failure and stage 1 through stage 4 chronic kidney disease, or unspecified chronic kidney disease | 721 | 2.2 |
| I26.99 | Other pulmonary embolism without acute cor pulmonale | 634 | 1.9 |
| N17.9 | Acute kidney failure, unspecified | 633 | 1.9 |
| I11.0 | Hypertensive heart disease with heart failure | 562 | 1.7 |
| J96.21 | Acute and chronic respiratory failure with hypoxia | 488 | 1.5 |
| J96.01 | Acute respiratory failure with hypoxia | 418 | 1.3 |
248,785 (56.5%) patients received remdesivir during the index COVID-19 hospitalization (Table 3). Descriptively, compared with non-remdesivir patients, remdesivir-treated patients were younger (median age [IQR]: 62.0 [51.0; 73.0] vs 64.0 [52.0; 76.0]) years) and were more likely to have received some level of supplemental oxygen support (any supplemental oxygen support [1-NSOc] = 70% vs 48%).
Table 3.
| RDV | Non-RDV | ||
|---|---|---|---|
| Patients (n) | 248,785 | 191,816 | |
| Hospitals (n) | 810 | 838 | |
| Age, years | Median [IQR] | 62.0 [51.0; 73.0] | 64.0 [52.0; 76.0] |
| Age group, years | 18–49 | 23% | 21% |
| 50–64 | 33% | 29% | |
| 65+ | 44% | 50% | |
| CCI | 0 | 33% | 29% |
| 1–3 | 52% | 50% | |
| ≥4 | 15% | 21% | |
| Maximum supplemental oxygen requirement (highest level of oxygenation during the index COVID-19 hospitalization) | NSOc | 30% | 52% |
| LFO | 46% | 36% | |
| HFO/NIV | 20% | 10% | |
| IMV/ECMO | 4% | 2% | |
| LOS | Median [IQR] | 5.0 [3.0; 8.0] | 5.0 [3.0; 9.0] |
| ICU stay | 22% | 17% | |
| Variant time period | Pre-Delta | 49% | 57% |
| Delta | 34% | 23% | |
| Omicron | 17% | 20% |
CCI: Charlson comorbidity index; HFO/NIV: High-flow oxygen/non-invasive ventilation; ICU: Intensive care unit; IMV/ECMO: Invasive mechanical ventilation/extracorporeal membrane oxygenation; IQR: Interquartile range; LFO: Low-flow oxygen; LOS: Length of stay; NSOc: No supplementary oxygen charges; RDV: Remdesivir.
The proportion of patients with a COVID-19-related readmission was 3.0% among the remdesivir-treated group compared with 5.4% among the non-remdesivir group (Figure 2). Lower rates of COVID-19-related readmission for the remdesivir-treated group were seen in all three variant time periods including pre-Delta, Delta and Omicron and across all supplemental oxygenation levels. After adjustment for key covariates, the remdesivir-treated group had a 40% lower likelihood of 30-day COVID-19-related readmission compared with the non-remdesivir group (aOR: 0.60; 95% CI: 0.58–0.62; p < 0.0001). This statistically significant reduction in the likelihood of 30-day COVID-19-related readmission was observed consistently across pre-Delta, Delta and Omicron variant time periods and across all supplemental oxygen requirements during the index hospitalization.
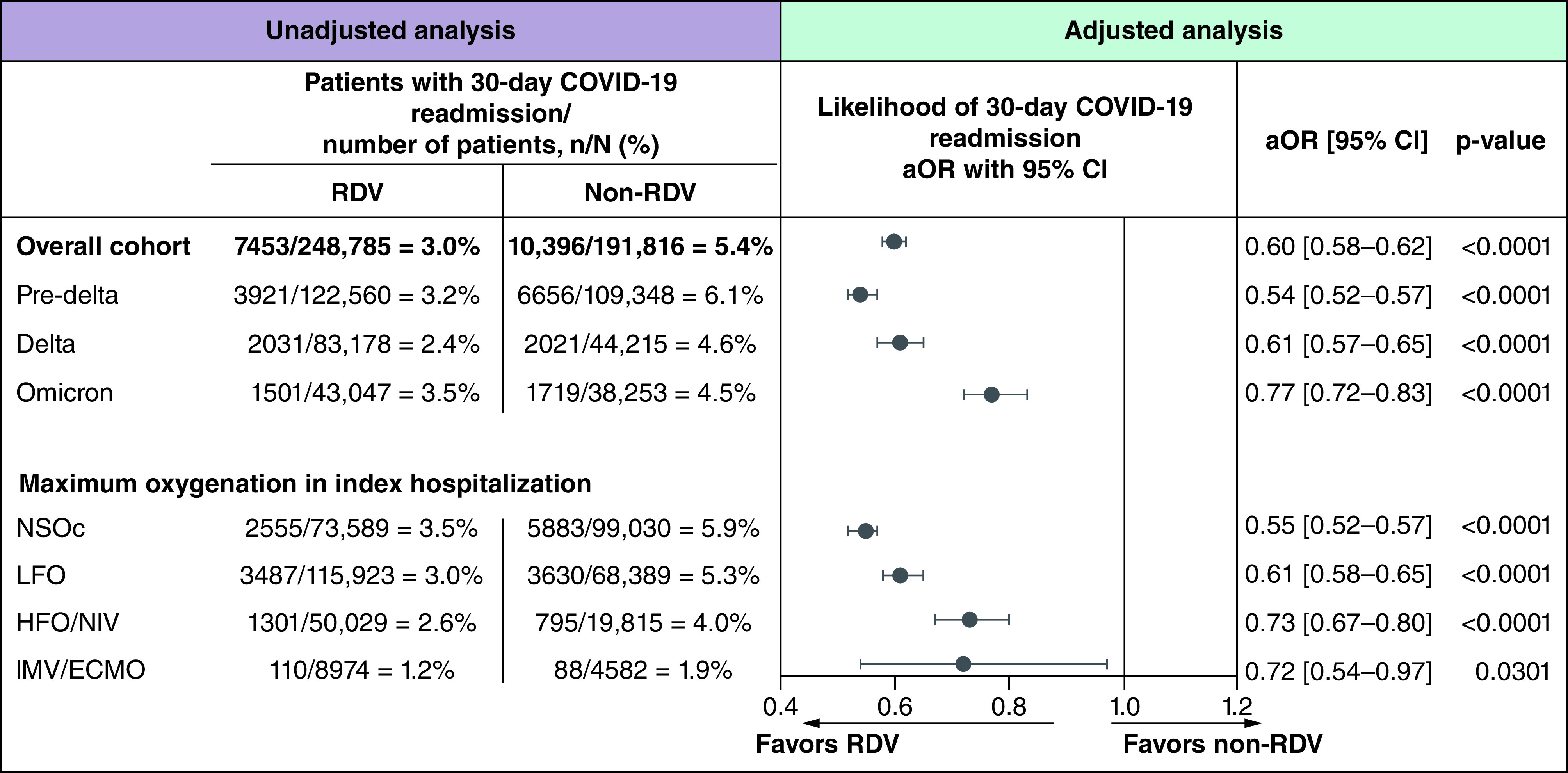
Estimates adjusted for characteristics of index COVID-19 hospitalization: age group, corticosteroids use, variant time period, Charlson comorbidity index, maximum supplemental oxygenation requirements and intensive care unit stay.
95% CI: 95% confidence interval; aOR: Adjusted odds ratio; HFO/NIV: High-flow oxygen/non-invasive ventilation; IMV/ECMO: Invasive mechanical ventilation/extracorporeal membrane oxygenation; LFO: Low-flow oxygen; NSOc: No supplemental oxygen charges; RDV: Remdesivir.
The proportion of patients with an all-cause readmission within 30 days was 6.3% in the remdesivir-treated group and 9.1% in the non-remdesivir group (Figure 3). Lower rates of readmission for the remdesivir-treated group were seen in all three variant time periods including pre-Delta, Delta and Omicron and across all supplemental oxygenation levels. After adjustment for key covariates, remdesivir-treated patients had a 27% lower likelihood of 30-day all-cause readmission compared with non-remdesivir patients (aOR: 0.73; 95% CI: 0.72–0.75; p < 0.0001). Statistically significant lower likelihood of 30-day readmission for the remdesivir-treated group versus non-remdesivir group was observed consistently across pre-Delta, Delta, and Omicron variant time periods and among patients requiring NSOc, LFO or HFO/NIV during index COVID-19 hospitalization (Figure 3).

Estimates adjusted for characteristics of index COVID-19 hospitalization: age group, corticosteroids use, variant time period, Charlson comorbidity index, maximum supplemental oxygenation requirements and intensive care unit stay.
95% CI: 95% confidence interval; aOR: Adjusted odds ratio; HFO/NIV: High-flow oxygen/non-invasive ventilation; IMV/ECMO: Invasive mechanical ventilation/extracorporeal membrane oxygenation; LFO: Low-flow oxygen; NSOc: No supplemental oxygen charge; RDV: Remdesivir.
Discussion
In this large observational study of almost half million patients hospitalized for COVID-19, treatment with remdesivir was associated with a 40% and 27% statistically significant lower likelihood of 30-day COVID-19-related and all-cause readmission, respectively. The lower likelihood of readmission among patients receiving remdesivir was observed across all variant time periods from 1 May 2020 to 30 April 2022 and in patients that did and did not require supplemental oxygen during the index COVID-19 hospitalization, alike. Overall, compared with non-readmitted patients, patients readmitted within 30 days tended to be older, have higher comorbidity burden and did not require supplemental oxygen during their index COVID-19 hospitalization.
This study provides incremental insights to complement and build on existing evidence indicating the important clinical benefit of remdesivir in patients hospitalized for COVID-19. Reductions in hospital readmission results in health benefits and cost savings for both patients and the healthcare systems. Decreasing the stress and burden on healthcare workers and the healthcare system remains an important priority as we recover from the considerable disruption and strain caused by the pandemic and prepare for the evolving endemic phase. This was also imperative amid surge planning, limited bed capacity, rationing of medical supplies and treatments, to utilize effective initial treatment strategies like remdesivir that would avoid downstream readmission mitigating the likelihood these patients would need to consume these finite and constrained resources.
There is extensive evidence supporting the use of remdesivir among patients hospitalized for COVID-19. For example, in the ACTT-1 study, remdesivir use was shown to decrease time to recovery [4]. An individual patient data meta-analysis of eight RCTs demonstrated that remdesivir reduced 28-day mortality in patients hospitalized for COVID-19 not requiring supplemental oxygen but was underpowered to detect an effect among patients requiring supplemental oxygen [13]. Real-world evidence has also demonstrated that remdesivir use is associated with a more rapid clinical improvement in COVID-19 patients [8]. Initiating remdesivir treatment upon admission for COVID-19 has been shown to decrease 30-day mortality in patients who received supplemental oxygen on the day of admission [14], and in complementary analyses for those that received remdesivir in both the general population [6,9] and in immunocompromised individuals [11]. Evidence on post-discharge outcomes are limited. Data from ACTT-1 indicated a higher rate of readmission among patients assigned to remdesivir as compared with placebo (5% vs 2%) but the trial was underpowered to detect significant differences in this outcome [4]. Few observational studies have indicated a potential benefit of remdesivir on readmission rates [10,15–17]. A study using data from the National Veterans Affairs Healthcare System demonstrated significantly reduced risk of 30-day readmission in multiple comparative analyses including propensity-score-quintile adjusted analyses (hazard ratio [HR]: 0.80; 95% CI: 0.72–0.89), propensity-score-matched analyses (HR: 0.83; 95% CI: 0.71–0.97) and inverse probability of treatment weighting analyses (HR: 0.96; 95% CI: 0.78–0.94) [16]. A multi-center study investigating the association between disease-specific factors and post-discharge events observed that treatment with remdesivir was associated with reduced risk of all-cause readmission or mortality within 14 days of discharge (OR: 0.46; 95% CI: 0.36–0.61) in a cohort of 3,508 adult patients discharged alive following hospitalization for COVID-19 [15]. In another US-based multi-center study, remdesivir treatment also led to reduced likelihood of readmission (OR: 0.5; 95% CI: 0.4–0.8) [17]. The findings reported in the present study provide incremental evidence that initiating treatment with remdesivir, a safe and effective antiviral in patients hospitalized for COVID-19 can halt viral replication and improve patient outcomes and reduce hospital readmission.
Future research could build on the findings from this study by examining readmission to any hospital in an appropriate database that allows for longitudinal assessment of care across different hospitals. Given some clear differences in patient characteristics of readmitted and non-readmitted patients, findings from this study may also inform the development of predictive tools to assess risk of readmission and allow for potential preventive measures to be established prior to discharge.
Strengths & limitations
Strengths of this study include the large study population which enabled the conduct of subgroup analyses according to variant time periods and supplemental oxygen requirements during the index COVID-19 hospitalization. As such, it was possible to investigate whether the association between remdesivir and readmission changed with the emergence of new variants of concern. The study focused on patients being hospitalized for COVID-19 to ensure appropriate assessment of readmission outcomes in a well-defined cohort of patients hospitalized for COVID-19.
In terms of study limitations, first, while the analyses adjusted for many potential confounders, there remains the possibility of residual confounding as well as unmeasured confounders. For example, the database does not capture data relating to time of symptom onset or the treatments administered to patients prior to the index COVID-19 hospitalization. In addition, data on vaccination status of the patients were also not available. However, given that we are examining a group of high-risk patients already hospitalized for COVID-19, we do not believe that vaccination is a key aspect for this cohort of hospitalized patients. Second, the absence of billing charges for supplemental oxygen was used to identify the NSOc cohort. Since some hospitals include supplemental oxygen charges within room charges rather than reporting supplemental oxygen charges separately, there is a risk that some patients who received supplemental oxygen could be misclassified as NSOc patients. To minimize the risk of misclassifying the maximum supplemental requirement of the index COVID-19 hospitalizations, those patients who were admitted to hospitals that did not report any LFO charges were excluded. This methodology has been validated previously as successfully identifying a group of patients at lower risk of mortality that patients on LFO [9,11,12]. Last, since the infecting variant of COVID-19 was not available in the dataset, calendar months were grouped according to predominant circulating variants of concern to form a proxy for actual variants of concern. The study period covers the period pre-BA.4/5 and as such future studies can build on these findings for variant time periods after this timeframe.
Conclusion
Hospital readmission is a key marker of quality of care and increases the burden on patients and hospitals. Freedom from rehospitalization is a cardinal marker of recovery of quality-of-life. The clinical benefits of remdesivir have been widely demonstrated in clinical trials and observational studies; and also adopted by clinical guidelines for the treatment of COVID-19. The findings from this study further builds on this evidence by indicating that remdesivir is associated with a reduced likelihood of COVID-19-related readmission and all-cause readmission.
Summary points
Footnotes
Author contributions
All listed authors have contributed to the manuscript substantially and have agreed to the final submitted version.
Financial disclosure
This study was funded by Gilead Sciences, Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Competing interests disclosure
E Mozaffari, C Der-Torossian, T Oppelt and M Berry report employment and being stockholders of Gilead Sciences during the conduct of the study. A Chandak and V Sarda report funding for study, medical writing provided to their Institution (Certara), from Gilead Sciences, during the conduct of the study. RL Gottlieb has served on scientific advisory boards for AbbVie, AstraZeneca, Eli Lilly, Gilead Sciences, GSK, Roche, Johnson & Johnson (COVID-19-related randomized clinical trial, coordinating principal investigator), and Kinevant Sciences (academic steering committee, study investigator; fees to Baylor Scott & White Research Institute), serving as a consultant for Gilead Sciences (honoraria for lectures), Johnson & Johnson, and Kinevant Sciences (through his institution), serving on a speaker bureau for Pfizer unrelated to COVID-19; his institution received a gift-in-kind from Gilead Sciences to facilitate a multicenter clinical trial outside the scope of COVID-19 and reporting a de minimis investment in AbCellera, grants or contracts as a study investigator (fees to Baylor Scott & White Research Institute) from Regeneron, Eli Lilly, Gilead, Pfizer, JNJ, and Roivant Sciences (Kinevant Sciences) and receipt of travel support for original scientific presentations from Gilead Sciences. C Chima-Melton reports payment or honoraria for lectures/speaker from AstraZeneca and participation on advisory board for Gilead Sciences. AC Kalil reports grants from the National Institutes of Health Adaptive COVID-19 Treatment Trial. AN Amin has been a principal investigator or co-investigator of clinical trials sponsored by NIH/NIAID, NeuroRx Pharma, Pulmotect, Blade Therapeutics, Novartis, Takeda, Humanigen, Eli Lilly, PTC Therapeutics, OctaPharma, Fulcrum Therapeutics, Alexion and a speaker and/or consultant for Pfizer, Salix, Alexion, AstraZeneca, Bayer, Ferring, Seres, Spero, Eli Lilly, Nova Nordisk, Gilead, Renibus, GSK, Dexcom, Reprieve, HeartRite, Aseptiscope; these relationships are unrelated to the current work. The authors have no other competing interests or relevant affiliations with any organization/entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Writing disclosure
Medical writing and editing support provided by S Read.
Ethical conduct of research
An ethics approval and informed consent was not required for this study. This analysis of data from the US PINC AI Healthcare Database was conducted under an exemption from Institutional Review Board oversight for US-based studies using de-identified healthcare records, as dictated by Title 45 Code of Federal Regulations (45 CFR 46.101(b)(4)).
Data sharing statement
The data that support the findings of this study are available from Premier, Inc (https://www.premierinc.com/). Restrictions apply to the availability of these data, which were used under license for this study.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit https://creativecommons.org/licenses/by-nc-nd/4.0/
References
Articles from Journal of Comparative Effectiveness Research are provided here courtesy of Becaris Publishing
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Remdesivir Effectiveness in Reducing the Risk of 30-day Readmission in Vulnerable Patients Hospitalized for COVID-19: A Retrospective US Cohort Study Using Propensity Scores.
Clin Infect Dis, ciae511, 15 Oct 2024
Cited by: 0 articles | PMID: 39405450
Association of Treatment with Remdesivir and 30-day Hospital Readmissions in Patients Hospitalized with COVID-19.
Am J Med Sci, 363(5):403-410, 10 Feb 2022
Cited by: 7 articles | PMID: 35151637 | PMCID: PMC8830144
Real-world evaluation of early remdesivir in high-risk COVID-19 outpatients during Omicron including BQ.1/BQ.1.1/XBB.1.5.
BMC Infect Dis, 24(1):802, 08 Aug 2024
Cited by: 0 articles | PMID: 39118052 | PMCID: PMC11312999
Comparison of Time to Clinical Improvement With vs Without Remdesivir Treatment in Hospitalized Patients With COVID-19.
JAMA Netw Open, 4(3):e213071, 01 Mar 2021
Cited by: 86 articles | PMID: 33760094 | PMCID: PMC7991975

 *
10
*
10



