Abstract
Free full text

In vitro activity of tedizolid against 43 species of Nocardia species
Abstract
The purpose of the present study was to evaluate the in vitro activity of tedizolid against several clinically significant species of Nocardia by comparing with that of linezolid. A total of 286 isolates of Nocardia species, including 236 clinical isolates recovered from patients in Japan and 50 strains (43 species) purchased from NITE Biological Resource Center, were studied. Antimicrobial susceptibility testing was performed using the broth microdilution method. For the 286 Nocardia isolates, the minimal inhibitory concentration (MIC)50 and MIC90 values of tedizolid were 0.25 and 0.5 μg/ml, and those of linezolid were 2 and 2 μg/ml, respectively. The distribution of the linezolid/tedizolid ratios (MICs of linezolid/MICs of tedizolid) showed that tedizolid had four- to eight-fold higher activity than linezolid in 96.1% (275/286) of Nocardia isolates. Both the tedizolid and linezolid MIC90 values for Nocardia brasiliensis were two-fold higher than those for the other Nocardia species. Both tedizolid and linezolid had low MIC values, 0.25–1 μg/ml and 0.5–4 μg/ml, respectively, even against nine isolates (five species) that were resistant to trimethoprim/sulfamethoxazole. One Nocardia sputorum isolate showed reduced susceptibility to tedizolid (4 μg/ml). Bioinformatics analysis suggests different resistance mechanisms than the oxazolidinone resistance seen in enterococci and staphylococci.
Introduction
Nocardia species are soilborne aerobic actinomycetes that can cause opportunistic infections in humans. Nocardiosis is typically seen in immunocompromised patients with a history of solid organ transplantation, hematopoietic stem cell transplantation, malignancy, or chronic pulmonary diseases; however, it may also occur in immunocompetent individuals1. The mortality rate of disseminated nocardiosis in hematopoietic cell transplantation recipients is high2. A rapid and reliable method for the detection of the causative microorganisms in clinical specimens is essential in order to reduce mortality and expedite appropriate antimicrobial therapy3. The antimicrobial treatment of nocardiosis requires that antibiotics are administered for several months4. Trimethoprim-sulfamethoxazole has long been considered the therapeutic agent of choice, either alone or in combination with amikacin or beta-lactams4. However, some clinically relevant species of Nocardia are multidrug resistant, including trimethoprim/sulfamethoxazole5–7, and there is a paucity of other antimicrobials with sufficient efficacy against these organisms. Thus, new antimicrobials with therapeutic potential are desperately needed.
Linezolid has been used effectively in the therapy of patients with nocardiosis; however, side effects, such as peripheral neuropathy and myelosuppression, have been reported during long-term use in some patients8. On the other hand, new oxazolidinone compounds such as tedizolid have shown excellent activity both in vitro and in vivo against Nocardia brasiliensis9,10. It has also been reported that tedizolid has a low rate of myelosuppression during long-term treatment11 and has activity against linezolid-resistant Staphylococcus aureus and enterococci12–14.
The purpose of the present study was to evaluate the in vitro activity of tedizolid against several clinically significant species of Nocardia compared with those of linezolid.
Materials and methods
Bacterial isolates and identification
A total of 286 strains of Nocardia species, including 236 clinical isolates (15 species/complexes) recovered from patients in 27 microbiology laboratories in Japan and 50 strains (43 species) purchased from the NITE Biological Resource Center (NBRC) (National Institute of Technology and Evaluation, Tokyo, Japan), were studied (Table (Table1).1). Identification of Nocardia species was based on Gram-stain, colonial morphology, and molecular technique. Species identification of all clinical isolates was performed by Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry (microflex LT, Bruker Daltonics)15 and by full-length 16S rRNA gene sequencing16. Nocardia spp. that were difficult to identify via 16S rRNA gene sequencing were classified as a complex17,18. Two strains of new species of N. sputorum (IFM 12275, IFM 12276T), which were previously reported as a novel species by our group19, were included in the N. abscessus complex. These strains were closely related to N. beijingensis NBRC16342T (99.6%) by phylogenetic analysis based on the 16S rRNA sequence19. The GenBank/EMBL/DDBJ accession numbers for the 16S rRNA gene sequence and the complete genome sequence of strains IFM 12276T and IFM 12275 are LC741024 and AP026978, and LC741023 and AP026976 (plasmid: AP026977), respectively.
Table 1
Species distribution of the 286 Nocardia strains subjected in this study.
| Species | No. of isolates | ||
|---|---|---|---|
Clinical isolates (n = = 236) 236) | NBRCa strains (n = = 50) 50) | NBRC No | |
| N. farcinica complexb | 61 | ||
| N. farcinica | 6 | NBRC15532T, NBRC3384, NBRC3423, NBRC3424, NBRC3927, NBRC13510 | |
| N. cyriacigeorgica | 45 | 1 | NBRC100375T |
| N. nova complexc | 37 | ||
| N. nova | 2 | NBRC103080, NBRC15556T | |
| N. elegans | 1 | NBRC108235T | |
| N. aobensis | 1 | NBRC100429T | |
| N. africana | 1 | NBRC100379T | |
| N.kruczakiae | 1 | NBRC101016T | |
| N. veterana | 1 | NBRC100344T | |
| N. vermiculata | 1 | NBRC100427T | |
| N. abscessus complexd | 35 | ||
| N. abscessus | 1 | NBRC100374T | |
| N. asiatica | 1 | NBRC100129T | |
| N. beijingensis | 1 | NBRC16342T | |
| N. arthritidis | 1 | NBRC100137T | |
| N. sputorum | NBRC115476, NBRC115477T | ||
| N. brasiliensis | 17 | 1 | NBRC14402T |
| N. transvalensis complexe | 15 | ||
| N. transvalensis | 1 | NBRC15921T | |
| N. otitidiscaviarum | 12 | 1 | NBRC14405T |
| N. thailandica | 2 | 1 | NBRC100428T |
| N. takedensis | 1 | 2 | NBRC100417T, NBRC100418 |
| N. asteroides | 1 | 1 | NBRC15531T |
| N. vinacea | 1 | 1 | NBRC16497T |
| N. mexicana | 1 | 1 | NBRC108244T |
| N. niigatensis | 1 | 1 | NBRC100131T |
| N. pseudobrasiliensis | 1 | 1 | NBRC108224T |
| N. yamanashiensis | 1 | 1 | NBRC100130T |
| N. araoensis | 1 | NBRC100135T | |
| N. paucivorans | 1 | NBRC100373T | |
| N. brevicatena | 1 | NBRC12119T | |
| N. anaemiae | 1 | NBRC100462T | |
| N. carnea | 1 | NBRC14403T | |
| N. concava | 1 | NBRC100430T | |
| N. exalbida | 1 | NBRC100660T | |
| N. higoensis | 1 | NBRC100133T | |
| N. ignorata | 1 | NBRC108230T | |
| N. inohanensis | 1 | NBRC100128T | |
| N. neocaledoniensis | 1 | NBRC108232T | |
| N. ninae | 1 | NBRC108245T | |
| N. pneumoniae | 1 | NBRC100136T | |
| N. puris | 1 | NBRC108233T | |
| N. shimofusensis | 1 | NBRC100134T | |
| N. sienata | 1 | NBRC100364T | |
| N. terpenica | 1 | NBRC100888T | |
| N. testacea | 1 | NBRC100365T | |
| N. uniformis | 1 | NBRC13702T | |
| Nocardia sp. | 5 | ||
| Total | 236 | 50 | |
aNITE Biological Resource Center (NBRC), bN. farcinica and N. kroppenstedtii were included in the N. farcinica complex, cN. nova, N. elegans, N. aobensis, and N. veterana were included in the N. nova complex, dN. abscessus, N. asiatica, N. beijingensis, N. sputorum, and N. arthritidis were included in the N. abscessus complex, eN. wallacei, N. transvalensis, and N. blacklockiae were included in the N. transvalensis complex.
Antimicrobial susceptibility testing (AST)
Antimicrobial Susceptibility Testing (AST) of 286 Nocardia isolates was performed using the broth microdilution method with frozen panels (Eiken Chemical, Tokyo, Japan), according to the Clinical and Laboratory Standards Institute (CLSI) M24-A3 guidelines20. In brief, a heavy organism suspension was prepared in a small volume of sterile saline with 7–10 3-mm glass beads and was vortexed vigorously. Clumps were allowed to settle for 15 min, and the supernatant was adjusted to a 0.5 McFarland standard using a calibrated nephelometer. For frozen panel inoculation, the adjusted 0.5 McFarland suspension was diluted 30-fold with sterile saline, and 10 µl of the diluted solution was dispensed into each well of the panel. The panels were incubated at 35 °C for 48–72 h and, if needed, daily thereafter for up to 5 days. For trimethoprim/sulfamethoxazole, the MICs were determined as the wells corresponding to 80% inhibition of growth compared to the controls. The MICs were determined for tedizolid, linezolid, trimethoprim/sulfamethoxazole, amikacin, tobramycin, clarithromycin, amoxicillin-clavulanic acid, ciprofloxacin, moxifloxacin, minocycline, imipenem, and ceftriaxone on the same panel, and were interpreted as recommended by CLSI. For this analysis, Staphylococcus aureus ATCC 29213 and Nocardia nova ATCC BAA-2227 were used as the quality control strains.
For determination of trimethoprim/sulfamethoxazole resistance, disk diffusion testing with a 250-μg sulfisoxazole disk (Hardy Diagnostics, CA, USA) was performed according to the CLSI M24-A3 guidelines20. For this analysis, N. nova ATCC BAA-2227 and Escherichia coli ATCC 25922 were used as the quality control strains.
Minimum bactericidal concentration testing
Minimum bactericidal concentration (MBC) testing for tedizolid and linezolid against 23 clinical isolates (eight of Nocardia farcinica complex, four of Nocardia cyriacigeorgica, three of N. brasiliensis, three of N. nova complex, two of Nocardia abscessus complex, two of Nocardia transvalensis complex, and one of Nocardis sp.) was performed according to the Clinical Microbiology Procedures Handbook21. The isolates were selected from species that are frequently isolated from clinical materials. The MBC was defined as the lowest antibiotic concentration able to kill at least 99.9% (reduction of 3 log10) of the initial inoculum.
Analysis of resistance mechanisms in tedizolid-resistant isolate
Mechanisms of tedizolid resistance in IFM 12275, which showed reduced susceptibility to tedizolid (4 μg/ml), were investigated by comparison with those reported in enterococci22–26 and staphylococci12–14. The same analysis was also performed for IFM 12276T, which was susceptible to tedizolid (0.25 μg/ml) as a control strain. Specifically, acquired linezolid resistance genes (cfr, cfr(B), cfr(D), optrA, poxtA25) were analysed using ARG-ANNOT (V6, https://ifr48.timone.univ-mrs.fr/blast/arg-annot_v6.html) and CARD (https://card.mcmaster.ca/) software. Primer-BLAST analysis (https://www.ncbi.nlm.nih.gov/tools/primer-blast/index.cgi?LINK_LOC=BlastHome) using the primer sequences shown in Table S1 was also conducted.
Analysis of nucleotide mutations within the 23SrRNA gene mutations (G2576T, G2505A, U2500A, G2447U, G2534U, G2603U: E. coli J01695 numbering22) in IFM 12275 and IFM 12276T were performed as follows. Briefly, the 23SrRNA gene from N. farcinica strain IFM10152 (https://www.ncbi.nlm.nih.gov/nuccore/NR_076254.1) was used as the reference sequence, and each sequence of the 23SrRNA gene in IFM 12275 and IFM 12276T was mapped to the reference gene by the Nucleotide BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastn&PAGE_TYPE=BlastSearch&LINK_LOC=blasthome). Position numbering, position converted by E. coli J01695 number, was used according to recommendation27.
Analysis of mutations in the 50S ribosomal proteins, L3 (rplC), L4 (rplD), and L22 (rplV)22,24, were performed for comparing homology between their sequences of IFM 12275 and IFM 12276T strains annotated using the annotation pipeline Prokka v.1.14.628. The orthologous gene sets among IFM 12275 and IFM 12276T strains were detected by Panaroo v.1.2.1029.
Ethical statement
The present study was conducted in accordance with the ethical guidelines of the Ministry of Health, Labour and Welfare, Japan. No ethical committee approvals or informed consent were needed for this study.
Conference presentation
A part of this study was presented at the 32nd European Congress of Clinical Microbiology and Infectious Diseases, 23 to 26 April 2022, Lisbon, Portugal [abstr. #00218].)
Results
Antimicrobial Susceptibility Testing
The MIC ranges, MIC50 values, MIC90 values and MIC distributions for tedizolid and linezolid against the 286 isolates of Nocardia species/complexes are shown in Table Table22 and Fig. 1. For the 286 Nocardia isolates, the MIC50 and MIC90 values of tedizolid were 0.25 and 0.5 μg/ml, and those of linezolid were 2 and 2 μg/ml, respectively. Both the tedizolid and linezolid MIC90 values for N. brasiliensis were two-fold higher than those for the other Nocardia species. Both tedizolid and linezolid had low MIC ranges, 0.25–1 (mode =
= 0.5) μg/ml and 0.5–4 (mode
0.5) μg/ml and 0.5–4 (mode =
= 2) μg/ml, respectively, even against the nine isolates that were resistant to trimethoprim/sulfamethoxazole isolates (three of N. otitidiscaviarum, two of N. transvalensis complex, two of N. mexicana, one of N. cyriacigeorgica, and one of N. terpenica). On the other hand, one clinical isolate of N. abscessus complex (N. sputorum IFM 12275) indicated higher MIC of tedizolid (4 μg/ml, measured three times on different days) than the other isolates (up to 1 μg/ml).
2) μg/ml, respectively, even against the nine isolates that were resistant to trimethoprim/sulfamethoxazole isolates (three of N. otitidiscaviarum, two of N. transvalensis complex, two of N. mexicana, one of N. cyriacigeorgica, and one of N. terpenica). On the other hand, one clinical isolate of N. abscessus complex (N. sputorum IFM 12275) indicated higher MIC of tedizolid (4 μg/ml, measured three times on different days) than the other isolates (up to 1 μg/ml).
Table 2
MIC ranges, MIC50 and MIC90 values for tedizolid and linezolid against the 286 isolates of Nocardia species.
| Species (no. of isolates tested) and antimicrobial | MIC (μg/ml) | ||
|---|---|---|---|
| Range | MIC50 | MIC90 | |
| All (286 isolates) | |||
| Tedizolid | 0.06–4 | 0.25 | 0.5 |
| Linezolid | 0.25–4 | 2 | 2 |
| N. farcinica complexa (67 isolates) | |||
| Tedizolid | 0.12–0.5 | 0.5 | 0.5 |
| Linezolid | 0.5–2 | 2 | 2 |
| N. cyriacigeorgica (46 isolates) | |||
| Tedizolid | 0.25–1 | 0.5 | 0.5 |
| Linezolid | 1–4 | 2 | 2 |
| N. nova complexb (45 isolates) | |||
| Tedizolid | 0.12–0.5 | 0.25 | 0.5 |
| Linezolid | 0.5–2 | 1 | 2 |
| N. abscessus complexc (39 isolates) | |||
| Tedizolid | 0.12–4 | 0.25 | 0.25 |
| Linezolid | 0.5–4 | 1 | 2 |
| N. brasiliensis (18 isolates) | |||
| Tedizolid | 0.5–1 | 0.5 | 1 |
| Linezolid | 2–4 | 2 | 4 |
| N. transvalensis complexd (16 isolates) | |||
| Tedizolid | 0.25–0.5 | 0.25 | 0.5 |
| Linezolid | 1–2 | 1 | 2 |
| N. otitidiscaviarum (13 isolates) | |||
| Tedizolid | 0.12–0.5 | 0.5 | 0.5 |
| Linezolid | 0.5–2 | 2 | 2 |
| Other species (42 isolates) | |||
| Tedizolid | 0.06–0.5 | 0.25 | 0.5 |
| Linezolid | 0.5–2 | 1 | 2 |
aN. farcinica and N. kroppenstedtii were included in the N. farcinica complex, bN. africana, N. aobensis, N. elegans, N. kruczakiae, N. nova, N. vermiculata and N. veterana were included in the N. nova complex, cN. abscessus, N. arthritidis, N. asiatica, N. sputorum, and N. beijingensis were included in the N. abscessus complex, dN. blacklockiae, N. transvalensis, and N. wallacei were included in the N. transvalensis complex.
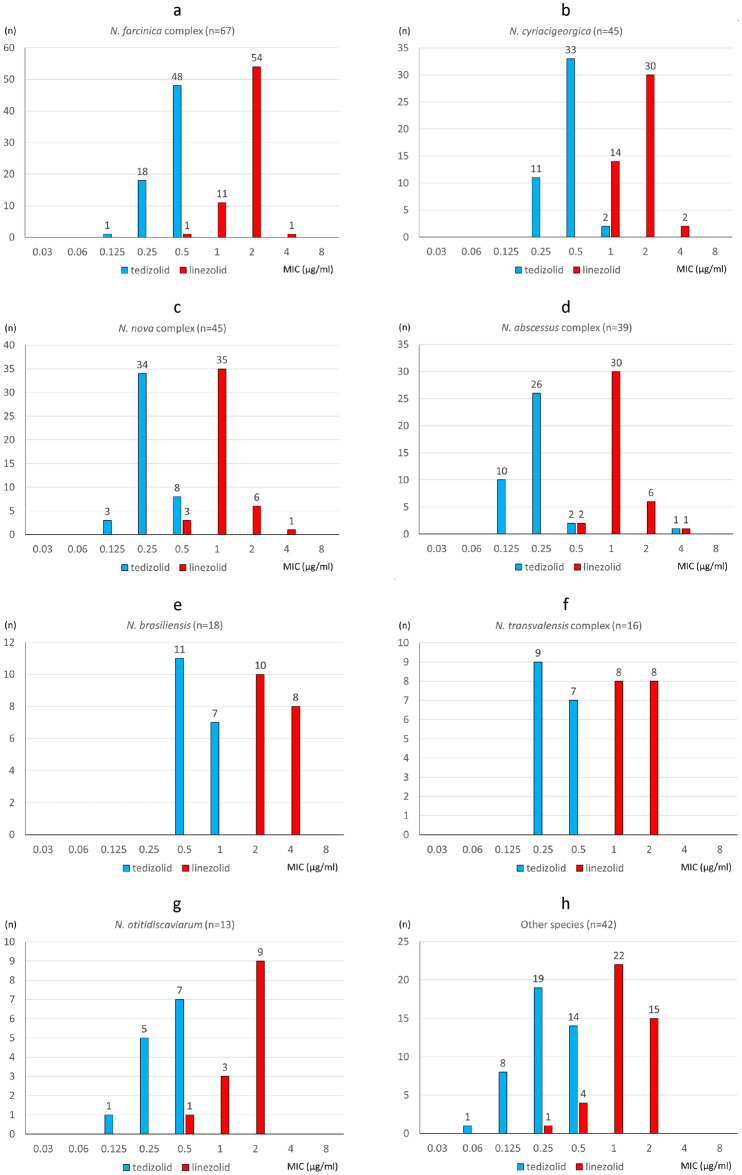
MIC distributions of tedizolid and linezolid against 286 Nocardia isolates. aN. farcinica complex, bN. cyriacigeorgica, cN. nova complex, dN. abscessus complex, eN. brasiliensis, fN. transvalensis complex, gN. otitidiscaviarum, hother species.
The distribution of the linezolid/tedizolid ratios (MICs of linezolid/MICs of tedizolid) against the 286 isolates of Nocardia species are shown in Fig. 2. Tedizolid was four- to eight-fold more active than linezolid in 96.1% (275/286) of Nocardia isolates. One clinical isolate of N. abscessus complex (N. sputorum IFM 12275) showed the same MIC value for linezolid (4 μg/ml) as that for tedizolid.
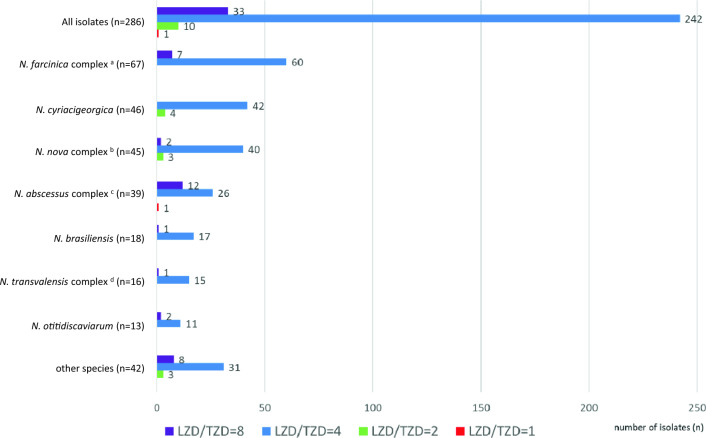
Distribution of the linezolid/tedizolid ratios (MICs of linezolid/MICs of tedizolid) against the 286 isolates of Nocardia species. aN. farcinica and N. kroppenstedtii were included in the N. farcinica complex, bN. africana, N. aobensis, N. elegans, N. kruczakiae, N. nova, N. vermiculata and N. veterana were included in the N. nova complex, cN. abscessus, N. arthritidis, N. asiatica, N. sputorum, and N. beijingensis were included in the N. abscessus complex, dN. blacklockiae, N. transvalensis, and N. wallacei were included in the N. transvalensis complex.
Antimicrobial susceptibility patterns for the different Nocardia species
Antimicrobial susceptibility patterns for the different Nocardia species consisting of 236 clinical isolates and 50 NBRC strains are shown in Table Table3.3. Only three drugs, amikacin, linezolid, and trimethoprim/sulfamethoxazole, showed ≥ 90% susceptibility among the all 286 isolates. The antimicrobial susceptibility patterns varied by species, and multidrug resistance (defined as four or fewer drugs that exhibit a susceptibility rate of 90% or higher) was common among N. farcinica complex, N. nova complex, N. abscessus complex, N. transvalensis complex, N. otitidiscaviarum, N. thailandica, N. mexicana, and N. concava.
90% susceptibility among the all 286 isolates. The antimicrobial susceptibility patterns varied by species, and multidrug resistance (defined as four or fewer drugs that exhibit a susceptibility rate of 90% or higher) was common among N. farcinica complex, N. nova complex, N. abscessus complex, N. transvalensis complex, N. otitidiscaviarum, N. thailandica, N. mexicana, and N. concava.
Table 3
Antimicrobial susceptibility patterns for the different Nocardia species consisting of 236 clinical isolates and 50 NBRC strains.
| Species (no. of isolates tested) | % of susceptible isolates of species or antibiotic susceptible pattern | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Amikacin | Tobramycin | Imipenem | Ceftriaxone | Clarithromycin | Amoxicillin-clavulanic acid | Ciprofloxacin | Moxifloxacin | Minocycline | Linezolid | Trimethoprim-sulfamethoxazole e | |
| All (286) | 94.8 | 51.4 | 74.1 | 44.8 | 23.1 | 48.3 | 16.4 | 34.6 | 21.7 | 100 | 96.8 |
| N. farcinica complexa (67) | 100 | 1.5 | 86.6 | 4.5 | 1.5 | 92.5 | 32.8 | 61.2 | 6 | 100 | 100 |
| N. cyriacigeorgica (46) | 100 | 100 | 100 | 82.6 | 0 | 13 | 0 | 0 | 6.5 | 100 | 97.8 |
| N. nova complexb (45) | 100 | 88.9 | 97.8 | 42.2 | 77.8 | 4.4 | 0 | 2.2 | 0 | 100 | 100 |
| N. abscessus complexc (39) | 100 | 100 | 51.2 | 76.9 | 23.1 | 61.5 | 12.8 | 28.2 | 61.5 | 100 | 100 |
| N. brasiliensis (18) | 100 | 94.4 | 11.1 | 0 | 0 | 94.4 | 0 | 66.7 | 22.2 | 100 | 100 |
| N. transvalensis complexd (16) | 25 | 0 | 75 | 81.3 | 18.8 | 81.3 | 37.5 | 50 | 6.3 | 100 | 87.5 |
| N. otitidiscaviarum (13) | 100 | 69.2 | 0 | 0 | 0 | 0 | 0 | 0 | 84.6 | 100 | 76.9 |
| N. takedensis (3) | 100 | 100 | 100 | 100 | 100 | 66.7 | 0 | 0 | 100 | 100 | 100 |
| N. thailandica (3) | 100 | 66.7 | 66.7 | 0 | 0 | 33.3 | 0 | 0 | 0 | 100 | 100 |
| N. asteroides (2), N. neocaledoniensis (1) | 100 | 100 | 100 | 100 | 0 | 0 | 0 | 0 | 0 | 100 | 100 |
| N. mexicana (2) | 50 | 0 | 50 | 100 | 0 | 0 | 0 | 0 | 0 | 100 | 0 |
| N. niigatensis (2) | 100 | 100 | 0 | 0 | 100 | 0 | 0 | 100 | 0 | 100 | 100 |
| N. pseudobrasiliensis (2) | 50 | 100 | 50 | 0 | 100 | 50 | 100 | 100 | 0 | 100 | 100 |
| N. vinacea (2) | 100 | 100 | 100 | 100 | 50 | 0 | 0 | 100 | 50 | 100 | 100 |
| N. yamanashiensis (2) | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 100 | 0 | 100 | 100 |
| Nocardia sp. (5) | 100 | 100 | 80 | 80 | 20 | 20 | 80 | 100 | 20 | 100 | 100 |
| N. anaemiae (1) | Sf | S | S | S | S | S | |||||
| N. araoensis (1) | S | S | S | S | S | S | S | S | S | ||
| N. brevicatena (1), N. paucivorans (1), N. shimofusensis (1) | S | S | S | S | S | S | S | S | S | S | |
| N. carena (1) | S | S | S | S | S | S | S | S | S | S | S |
| N. concava (1) | S | S | S | S | |||||||
| N. exalbida (1) | S | S | S | S | S | S | S | ||||
| N. higoensis (1) | S | S | S | S | S | S | S | S | |||
| N. ignorata (1) | S | S | S | S | S | S | S | ||||
| N. inohanensis (1) | S | S | S | S | S | S | S | S | S | S | |
| N. ninae (1) | S | S | S | S | S | S | S | S | |||
| N. pneumoniae (1) | S | S | S | S | S | S | |||||
| N. puris (1) | S | S | S | S | S | S | |||||
| N. sienata (1) | S | S | S | S | S | S | S | S | |||
| N. terpenica (1) | S | S | S | S | S | ||||||
| N. testacea (1) | S | S | S | S | S | S | S | ||||
| N. uniformis (1) | S | S | S | S | S | S | S | S | |||
aN. farcinica and N. kroppenstedtii were included in the N. farcinica complex, bN. africana, N. aobensis, N. elegans, N. kruczakiae, N. nova, N. vermiculata and N. veterana were included in the N. nova complex, cN. abscessus, N. arthritidis, N. asiatica, N. sputorum, and N. beijingensis were included in the N. abscessus complex, dN. blacklockiae, N. transvalensis, and N. wallacei were included in the N. transvalensis complex, eResistance to trimethoprim-sulfamethoxazole were determined by disk diffusion testing with a 250-μg sulfisoxazole disks. fS, susceptible (only one isolate), Bold in the table indicates a susceptibility rate of 90% or higher.
Minimum bactericidal concentration testing
The distribution of the MBC/MIC ratios of tedizolid and linezolid against the 23 clinical isolates of the seven Nocardia species showed that the MBC/MIC ratio was greater than or equal to eight for all 23 isolates.
Analysis of resistance mechanisms in tedizolid resistant isolate
The ARGannot analysis on IFM 12275 resulted in hits for ole(C), blab-4, aph(3″), tlr(C), and TlrC, while CARD analysis resulted in hits for vanY, vanW, and rifampin monooxigenase, but acquired linezolid resistance genes known in enterococci, cfr, cfr(B), cfr(D), optrA, and poxtA were not detected by either method. On the other hand, the genes that were hits in the IFM 12275 strain other than TlrC were also detected in the IFM 12276T strain. Of note, the TlrC gene has been reported as a gene associated with resistance for macrolide, lincosamide, and streptogramin group antibiotics30. In addition, in silico analysis by the Primer-blast confirmed that the acquired linezolid resistance gene described above was not detected in IFM 12275.
In the analysis of nucleotide mutations in the 23S rRNA gene (G2576T, G2505A, U2500A, G2447U, G2534U, G2603U), no mutations were observed in either of IFM 12275 and IFM 12276T strains. Furthermore, the 50S ribosomal protein sequences of L3 (rplC), L4 (rplD), and L22 (rplV) were the same between the two strains. Comparing the gene sets in IFM12275 with those in IFM12276T, a total of 6285 orthologous genes were detected, and 500 and 387 unique genes were harboured in IFM12275 and IFM12276T, respectively.
Discussion
Sulfonamides, mainly trimethoprim/sulfamethoxazole, have been the antimicrobials of choice to treat nocardiosis for a long time4. Trimethoprim/sulfamethoxazole still has a good activity against Nocardia species isolated in Japan15. However, adverse reactions to high-dose trimethoprim/sulfamethoxazole therapy, such as myelosuppression and hepatoxicity, are frequent4, and change in antibiotics is often necessary in such cases. Furthermore, the emergence of resistant bacteria due to prophylactic administration of low-dose trimethoprim/sulfamethoxazole has also been regarded as a problem. Averbuch et al. reported that the proportion of trimethoprim/sulfamethoxazole resistant Nocardia spp. was four of 25 (16%) among patients who received trimethoprim/sulfamethoxazole prophylaxis at the time of nocardiosis, compared with two of 36 (6%) among those who did not2.
Tedizolid has bacteriostatic activity against gram-positive cocci by binding to the 23S ribosomal RNA of the 50S subunit of the bacterial ribosome and inhibiting the early steps of bacterial protein synthesis31. Compared to linezolid, tedizolid shows stronger binding at the site of activity due to its unique D-ring substituent31; therefore, tedizolid is four- to 16-fold more potent in vitro than linezolid against many clinically relevant gram-positive cocci, including linezolid-resistant strains26,32. In the present study, we found that both tedizolid and linezolid had bacteriostatic activity (MBC/MIC ratio ≥
≥ 821) against Nocardia spp., and tedizolid was four- to eight-fold more active than linezolid in 96.1% (275/286) of Nocardia isolates. Brown-Elliott et al. reported that tedizolid had higher antibacterial activity in vitro than linezolid against 101 isolates of Nocardia species. They also cited the advantages of tedizolid over linezolid, including fewer serious adverse events, higher oral bioavailability, higher intracellular concentration, and a longer half-life, and speculated on its potential for the treatment of infections caused by Nocardia spp.33. There are some clinical case reports of the successful use of tedizolid for the treatment of disseminated nocardiosis34–37. In the four cases outlined in those case reports, all patients were initially treated with antimicrobial agents including linezolid, but tedizolid was used as an alternative due to myelosuppression caused by linezolid. In all cases, the patients were safely treated with tedizolid on prolonged treatment for two to six months without the development of myelotoxicity34–37. However, some studies have suggested the risk of thrombocytopenia with tedizolid38; further assessment is needed of thrombocytopenia development on prolonged treatment in the clinical setting.
821) against Nocardia spp., and tedizolid was four- to eight-fold more active than linezolid in 96.1% (275/286) of Nocardia isolates. Brown-Elliott et al. reported that tedizolid had higher antibacterial activity in vitro than linezolid against 101 isolates of Nocardia species. They also cited the advantages of tedizolid over linezolid, including fewer serious adverse events, higher oral bioavailability, higher intracellular concentration, and a longer half-life, and speculated on its potential for the treatment of infections caused by Nocardia spp.33. There are some clinical case reports of the successful use of tedizolid for the treatment of disseminated nocardiosis34–37. In the four cases outlined in those case reports, all patients were initially treated with antimicrobial agents including linezolid, but tedizolid was used as an alternative due to myelosuppression caused by linezolid. In all cases, the patients were safely treated with tedizolid on prolonged treatment for two to six months without the development of myelotoxicity34–37. However, some studies have suggested the risk of thrombocytopenia with tedizolid38; further assessment is needed of thrombocytopenia development on prolonged treatment in the clinical setting.
On the other hand, in the present study, we found that one isolate, N. sputorum (IFM12275) showed higher MIC of tedizolid (4 μg/ml) than the other isolates (up to 1 μg/ml). The MIC of tedizolid for N. sputorum (IFM12275) was 16-fold higher than that for the same species, IFM12276T. Interestingly, this isolate had the same MIC value for linezolid (4 μg/ml). In the CLSI method, there is an interpretive breakpoint (BP) of linezolid against Nocardia species at 8 μg/ml, but no BP of tedizolid. Considering the BPs of linezolid and tedizolid are 4 μg/ml and 0.5 μg/ml for S. aureus and 2 μg/ml and 0.5 μg/ml for Enterococcus faecalis, respectively, the Nocardia isolate with MIC of 4 μg/ml in the present study is thought to be resistant to tedizolid. The mechanism of resistance to linezolid and tedizolid is being studied mainly in staphylococci and enterococci12–14,22. In these species, linezolid resistance is mediated through acquisition of resistance genes (cfr, etc.) or through ribosomal mutations in 23S rRNA gene or 50S ribosomal proteins L3, L4, and L2212,22. On the other hand, it has been reported that although tedizolid is active against linezolid-resistant stapylococci possessed cfr gene, it has cross-resistance to linezolid when mutations in chromosomal genes encoding 23S rRNA or ribosomal proteins (L3 and L4) are present13,39. Interestingly, it is also reported that the MICs of linezolid were four- to 16-fold higher than those of tedizolid, even in such cross-resistant strains to linezolid and tedizolid12,14, suggesting that the tedizolid-resistant strain in the present study with the same MIC values as linezolid, IFM 12275, is a very atypical strain.
Valdezate et al. reported two linezolid-resistant N. farcinica strains isolated from patients with cystic fibrosis40. These isolates indicated very high MIC of linezolid with ≥ 256 μg/ml. In their study, Valdezate et al. investigated the presence of genetic resistance determinants by PCR and a complete genome analysis of the strains, but they were unable to identify the resistance mechanism. Neither of the isolates harboured the cfr gene, nor did they show the mutations in 23S RNA (G2576T) allowing linezolid resistance, although the G2608A change was found in one allele of the one strain41. In the present study, we found that the tedizolid-resistant strain did not harbour the acquired linezolid resistance genes, nor did it show the mutations in 23S RNA and the 50S ribosomal protein. These results indicate that oxazolidinone resistance in Nocardia spp. is caused by a different resistance mechanism reported in enterococci and staphylococci. Since 500 genes were uniquely harboured in the tedizolid-resistant strain compared with the tedizolid-susceptible strain, another tedizolid-resistant strain would be helpful in identifying the candidate genes. Unfortunately, in the present study, we were unable to elucidate the mechanism of resistance to tedizolid in Nocardia species. Further research is necessary to elucidate the resistance mechanism and to set interpretive BP for tedizolid against Nocardia species.
256 μg/ml. In their study, Valdezate et al. investigated the presence of genetic resistance determinants by PCR and a complete genome analysis of the strains, but they were unable to identify the resistance mechanism. Neither of the isolates harboured the cfr gene, nor did they show the mutations in 23S RNA (G2576T) allowing linezolid resistance, although the G2608A change was found in one allele of the one strain41. In the present study, we found that the tedizolid-resistant strain did not harbour the acquired linezolid resistance genes, nor did it show the mutations in 23S RNA and the 50S ribosomal protein. These results indicate that oxazolidinone resistance in Nocardia spp. is caused by a different resistance mechanism reported in enterococci and staphylococci. Since 500 genes were uniquely harboured in the tedizolid-resistant strain compared with the tedizolid-susceptible strain, another tedizolid-resistant strain would be helpful in identifying the candidate genes. Unfortunately, in the present study, we were unable to elucidate the mechanism of resistance to tedizolid in Nocardia species. Further research is necessary to elucidate the resistance mechanism and to set interpretive BP for tedizolid against Nocardia species.
A multicentric retrospective cohort study by Takamatsu et al. reported that the most frequently isolated Nocardia species in Japan were N. farcinica (79/317, 24.9%), N. nova complex (61/317, 19.2%), N. abscessus complex (59/317, 18.6%), and N. cyriacigeorgica (44/317, 13.9%)41. Identification of clinical isolates of Nocardia to the species/complex level is important, because Nocardia spp. differ in clinical spectrum and susceptibility patterns42. In the present study, differences in drug susceptibilities and multidrug resistance patterns were also observed depending on the Nocardia species. Recently, matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS)-based identification has been identified as a rapid, easy, and reliable method15,43,44. Accurate identification by MALDI-TOF MS and antimicrobial susceptibility profiles together can help earlier implementation of empirical treatment and improvement of patient prognosis. On the other hand, we found one isolate which showed reduced susceptibility to tedizolid; in addition, linezolid-resistant clinical isolates have already been reported40,45, so performing antimicrobial susceptibility testing against all clinically significant isolates is recommended, especially in disseminated Nocardia infection.
In conclusion, tedizolid may be a useful treatment option for Nocardia infections, such as those in patients with trimethoprim/sulfamethoxazole allergies, and multidrug-resistant Nocardia.
Acknowledgements
The authors thank the microbiology laboratories which provided the clinical isolates.
Author contributions
M.T. designed research; M.T., K.S. and H.S. conducted review and editing; M.T., N.O., M.I., D.T., Y.T., T.Y. and K.O. provided the laboratory tests, data analysis, and resources; M.T. and H.T. conducted bioinformatics analysis and M.T. wrote the paper.
Funding
Supported in part by a research grant from MSD K.K., a subsidiary of Merck Sharp & Dohme LLC, in accordance with the Investigator Initiated Studies Program of Merck Sharp & Dohme LLC. The opinions expressed in this paper are those of the authors and do not necessarily represent those of MSD K.K. nor Merck Sharp & Dohme LLC.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-55916-7.
References
Articles from Scientific Reports are provided here courtesy of Nature Publishing Group
Full text links
Read article at publisher's site: https://doi.org/10.1038/s41598-024-55916-7
Read article for free, from open access legal sources, via Unpaywall:
https://www.nature.com/articles/s41598-024-55916-7.pdf
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Nucleotide Sequences (Showing 6 of 6)
- (1 citation) ENA - AP026976
- (1 citation) ENA - AP026978
- (1 citation) ENA - AP026977
- (1 citation) ENA - J01695
- (1 citation) ENA - LC741024
- (1 citation) ENA - LC741023
Show less
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
In Vitro Susceptibility Testing of Tedizolid against Isolates of Nocardia.
Antimicrob Agents Chemother, 61(12):e01537-17, 22 Nov 2017
Cited by: 9 articles | PMID: 28923878 | PMCID: PMC5700301
In Vitro Susceptibility Testing of Tedizolid against Nontuberculous Mycobacteria.
J Clin Microbiol, 55(6):1747-1754, 22 Mar 2017
Cited by: 39 articles | PMID: 28330892 | PMCID: PMC5442531
In vitro activity of tedizolid (TR-700) against linezolid-resistant staphylococci.
J Antimicrob Chemother, 67(1):167-169, 27 Sep 2011
Cited by: 44 articles | PMID: 21954458
Tedizolid: a novel oxazolidinone with potent activity against multidrug-resistant gram-positive pathogens.
Drugs, 75(3):253-270, 01 Feb 2015
Cited by: 71 articles | PMID: 25673021
Review
Funding
Funders who supported this work.
a research grant from MSD K.K., a subsidiary of Merck Sharp & Dohme LLC (1)
Grant ID: 59705

 1,2,3
1,2,3 


