Abstract
Free full text

Pharmacological potential of ginseng and ginsenosides in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis
Abstract
Nonalcoholic fatty liver disease (NAFLD) is a chronic liver disease characterized by hepatic fat accumulation, while nonalcoholic steatohepatitis (NASH) is an advanced form of NAFLD characterized by hepatic inflammation, fibrosis, and liver injury, resulting in liver cirrhosis and hepatocellular carcinoma (HCC). Given the evidence that ginseng and its major bioactive components, ginsenosides, have potent anti-adipogenic, anti-inflammatory, anti-oxidative, and anti-fibrogenic effects, the pharmacological effect of ginseng and ginsenosides on NAFLD and NASH is noteworthy. Furthermore, numerous studies have successfully demonstrated the protective effect of ginseng on these diseases, as well as the underlying mechanisms in animal disease models and cells, such as hepatocytes and macrophages. This review discusses recent studies that explore the pharmacological roles of ginseng and ginsenosides in NAFLD and NASH and highlights their potential as agents to prevent and treat NAFLD, NASH, and liver diseases caused by hepatic steatosis and inflammation.
Graphical abstract
1. Introduction
Nonalcoholic fatty liver disease (NAFLD) is a range of conditions in which fat builds up in the liver and is considered a major cause of chronic liver diseases worldwide. NAFLD ranges from the mild condition of non-alcoholic fatty liver (NAFL) to NASH and sometimes progresses to hepatic fibrosis, cirrhosis, and HCC (Fig. 1) [1]. The majority of NAFLD patients have few or no symptoms, however, some exhibit weight loss, fatigue, hepatomegaly, lipomatosis, acanthosis nigricans, and right upper quadrant discomfort [2]. The prevalence of NAFLD is about 25% worldwide and is rapidly rising in Western countries. The risk of NAFLD increases in patients with metabolic syndromes, including obesity, dyslipidemia, insulin resistance, type 2 diabetes mellitus, and systemic hypertension. NAFLD is classified into two distinct types. The first type is less related to metabolic syndromes, and the primary pathophysiological cause is insulin resistance [3]. The second type of NAFLD is more related to inherited or acquired metabolic syndromes [4] and to infectious pathologies, such as the hepatitis C and human immunodeficiency viruses that can cause liver steatosis [5].
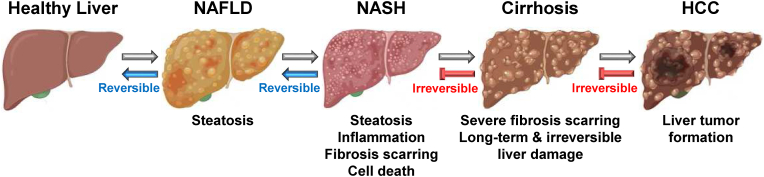
Pathogenesis and progression of NAFLD and NASH. NAFLD develops by fat accumulation (steatosis) and progresses to NASH by hepatic steatosis and inflammation. Fibrosis scarring and hepatocyte death occur in NASH. Severe fibrosis scarring causes cirrhosis, a long-term and irreversible liver damage and injury. In some cirrhosis patients, cirrhosis progresses to HCC. NAFLD and NASH are reversible in a healthy liver, but cirrhosis and HCC are irreversible.
Nonalcoholic steatohepatitis (NASH) is an inflammatory liver disease caused by a buildup of fats in the liver (Fig. 1). Although NASH is a part of NAFLD, NAFLD is a disease characterized by hepatocyte steatosis caused by several factors other than alcohol intake, while NASH is a disease characterized by a fatty liver with histological steatosis associated with inflammation and fibrosis [6,7]. Unlike NAFLD, which has few or no symptoms, NASH shows severe hepatic inflammation and may progress to fibrosis and cirrhosis [6,7]. In some patients, NASH even progresses to end-stage hepatic damage and HCC. Among patients with NAFLD, roughly 20% develop NASH, and the prevalence of NASH is approximately 5% worldwide. The pathogenesis of NASH is complex and has not been completely understood. However, given the evidence of the pathophysiology of NAFLD and NASH with different dietary animal models [8], the development of NASH consists of two steps. The first is a fat-accumulating step in the liver that causes insulin resistance, and the second is the process causing liver damage with cellular and molecular changes that involve fatty acid oxidation, oxidative stress, and inflammation in the liver due to various factors [6,7]. Genetics and changes in metabolism and microbiome may contribute to NASH [9]. Currently, there are no medications approved to treat NAFLD and NASH, and other medications to manage the problems associated with NAFLD and NASH are clinically available, which raises a demand for understanding the pathogenesis and progression of NAFLD and NASH to develop novel therapeutic strategies. Recently, an accumulating number of studies are focusing on identifying and validating novel therapeutics against NAFLD and NASH.
Ginseng is a root plant cultivated in East Asian and North American countries and has long been used as a traditional herbal medicine to treat various physiological and pathological conditions [10]. Ginsengs include different types of bioactive ingredients, such as ginsenosides, polysaccharides, glycosides, phytosterols, and some oils [11]. Among them, ginsenosides, which are steroidal saponins, have been identified as major compounds that modulate numerous biological and physiological conditions in the body. Ginsenosides also have been reported to have pharmacological roles in multiple diseases by regulating various pathological conditions, such as inflammation, oxidative stress, lipogenesis, fibrosis, hypoxia, infection, and cellular apoptosis [[12], [13], [14], [15], [16], [17], [18], [19], [20]]. Recently, an increasing number of studies have demonstrated that ginsengs and ginsenosides play a pharmacological role in the pathogenesis and progression of NAFLD and NASH. Therefore, this review discusses recent studies investigating the pharmacological roles of ginsengs and various ginsenosides in NAFLD and NASH and further highlights the insights into their potential benefits as promising herbal medicines to prevent and treat liver diseases.
2. Pharmacological roles of ginsengs and ginsenosides in NAFLD
2.1. Pharmacological roles of ginsengs in NAFLD
As described earlier, NAFLD is a metabolic disease caused mainly by fat buildup in the liver, and obesity is highly associated with an increased risk of NAFLD. Previous studies have reported the efficacy of ginsengs in obesity and obesity-related metabolic diseases [21,22]. Interestingly, ginseng has been demonstrated to reduce fat buildup in the liver and ameliorate NAFLD by modulating various biological processes and molecules. Hong et al investigated the inhibitory role of Korean Red Ginseng (KRG) in NAFLD. KRG ameliorated NAFLD by reducing the serum triglyceride levels and increasing the serum HDL levels in Otsuka Long-Evans Tokushima Fatty (OLETF) rats [23]. Recent studies demonstrated that NK cells exhibited impaired functionality under obese conditions [24], and Hong et al also showed that KRG increased NK cell activity in OLETF rats [23]. These results suggest that KRG plays an inhibitory role in NAFLD by improving lipid profiles and NK cell activity.
Hong et al also evaluated the inhibitory activity of KRG in patients with NAFLD, focusing on the gut-liver axis. KRG that includes additional ginsenoside Rg1, Rb1, and Rg3 improved aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels in patients with NAFLD [25]. KRG also increased the amounts of Lactobacillus related to improved ALT levels in patients with NAFLD [25]. These results indicate that KRG effectively increases liver enzyme levels by regulating gut microbiota, resulting in the amelioration of NAFLD in patients.
Another study demonstrated the inhibitory effect of Panax notoginseng saponins (PNS) on NAFLD by modulating the gut-liver axis. PNS attenuates hepatic steatosis in high-fat diet (HFD)-treated mice [26]. PNS showed anti-lipogenic and anti-fibrotic activity and, interestingly, reduced the gut-to-liver translocation of microbiota fatty acid production in HFD-induced NAFLD mice [26]. These effects were mediated by the PNS-induced inhibition of TLR4 pathways, which was abolished by the addition of the TLR4 agonist, LPS [26]. These results suggest that PNS has a hepatoprotective role against NAFLD by ameliorating hepatic steatosis and fibrosis, which is mediated mainly by the gut-liver axis in a TLR4-dependent manner and may be used as a potential treatment for NAFLD by modulating the gut-liver axis.
Black ginseng (BG) is processed ginseng that is prepared by steaming and drying white or red ginseng several times. Park et al explored the pharmacological effect of black BG in NAFLD. BG significantly alleviated NAFLD in high-fat/high-fructose (HFHF) diet-fed mice [27]. BG also reduced lipid accumulation, the expression of lipogenic genes, and the production of hepatic steatosis molecules in free fatty acid (FFA)-stimulated hepatocytes and HFHF diet-fed NAFLD mice [27]. This suggests that BG plays an inhibitory role in NAFLD by suppressing hepatic steatosis and may be a potential pharmacological agent for the prevention and treatment of NAFLD.
Some studies investigated the pharmacological effect of ginseng extract enriched in ginsenosides on NAFLD. Wang et al reported the ameliorative role of red ginseng saponin extract enriched in Rh1 and Rg2 (Sap-Rh1/Rg2) in NAFLD in mice. Sap-Rh1/Rg2 improved hepatic steatosis and steatofibrosis in fast food diet (FFD)-induced NAFLD mice [28]. Sap-Rh1/Rg2 also suppressed hepatic inflammation by inhibiting the NLRP3 inflammasome in FFD-induced NAFLD mice [28]. These results suggest that Rh1, along with Rg2, is a key ginsenoside in red ginseng that prevents NAFLD by mitigating hepatic steatosis, fibrosis, and inflammation. Choi et al also demonstrated the protective role of KRG extract enriched in Rd and Rg3 in NAFLD in mice. The fermented KRG with Cordyceps militaris (CRG) extract enriched in Rd and Rg3 (CRG-Rd/Rg3) suppressed the hepatic lipid accumulation and inflammation and prevented hepatic steatosis in FFD-induced NAFLD mice [29]. CRG-Rd/Rg3 exerted its anti-inflammatory activity by inducing mTORC1-mediated M2 polarization of macrophages (RAW264.7 cells) [29]. This study suggests that CRG-Rd/Rg3 plays an inhibitory role in NAFLD by suppressing the lipid-induced pathologic activation of mTORC1 in hepatocytes and macrophages.
2.2. Pharmacological roles of ginsenosides in NAFLD
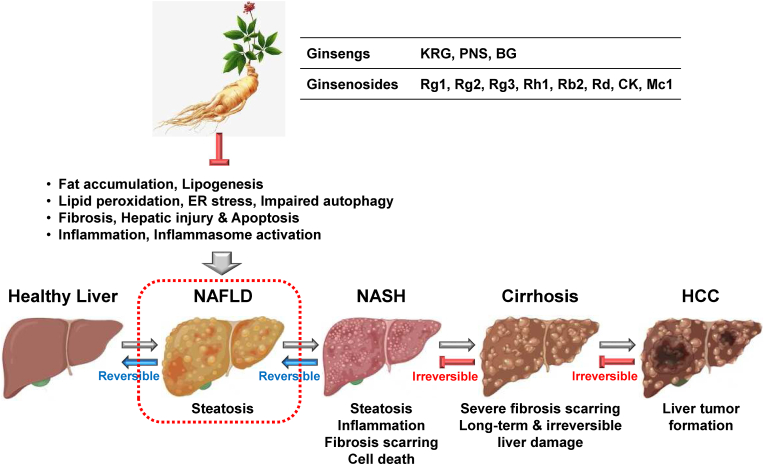
Ginsenosides are the main bioactive saponins in ginsengs, and exhibit a variety of pharmacological activities [12]; numerous studies have demonstrated that ginsenosides are critical agents that have a pharmacological effect on NAFLD. Ginsenoside Rg1 is a triterpene saponin originally found in Panax ginseng and is considered one of the major ginsenosides in ginseng. Several studies have reported the ameliorative role of Rg1 in NAFLD. Li et al demonstrated that Rg1 reduced fat accumulation in medium- and long-chain fat emulsion (MCE)-stimulated hepatocytes and protected the hepatocytes from NAFLD by inhibiting apoptosis via the suppression of the expression of sphingosine-1-phosphate lyase 1 (SGPL1), a key enzyme in the sphingosine signaling pathway [30]. Gu et al evaluated the effect of Rg1 on NAFLD by analyzing the serum proteins and transcriptome in rats. This study showed that Rg1 improved liver function and protected fat accumulation and the liver in HFD-induced NAFLD rats [31]. Moreover, RNA-Seq analysis revealed that Rg1 changed the transcriptome in NAFLD and that Atf3 and Acox2 are key genes for Rg1-mediated efficacy in NAFLD [31]. Qi et al reported the protective role of Rg1 in NAFLD by modulating the FOXO1 transcriptional factor in mice. Rg1 ameliorated NAFLD by protecting the livers from hepatic damage, steatosis, and inflammation in the D-galactose-induced NAFLD mice [32]. Rg1 also suppressed FOXO1 activation, resulting in the reduction of lipid peroxidation in the liver of D-galactose-induced NAFLD mice [32]. Xu et al also reported the protective role of Rg1 in NAFLD in mice. Rg1 mitigated NAFLD by inhibiting lipid peroxidation and endoplasmic reticulum stress in HFD-induced NAFLD mice [33]. Rg1 further inhibited the activation of the NLRP3 inflammasome, resulting in a decrease in the production of pro-inflammatory cytokines, IL-1β, and IL-18 in HFD-induced NAFLD mice [33], suggesting that Rg1 also alleviates NAFLD by inhibiting inflammasome-activated hepatic inflammatory responses. Given the results of these studies, it is clear that Rg1 plays a protective role in NAFLD by modulating various hepatic functions. However, despite these promising results, the protective effect of Rg1 needs to be further evaluated in human patients with NAFLD.
Ginsenoside Rh1 and compound K (CK) are major metabolites in KRG, compared to small amounts in unprocessed ginseng [34], and some studies have demonstrated their pharmacological role in NAFLD. Kim et al demonstrated the anti-steatotic effect of CK in hepatocytes. CK reduced lipid droplet formation and increased the expression of anti-steatotic genes, such as peroxisome proliferator-activated receptor-α (PPARα) and acyl-CoA oxidase (ACOX1) [35] in free FFA-treated hepatocytes, HuH7 cells [36], suggesting that CK has the potential to treat NAFLD. Chen et al further demonstrated the ameliorative role of CK and Rh1 in NAFLD in rats. CK and Rh1 alleviated liver function impairment by lowering hepatic injury and fibrosis in HFD-induced NAFLD rats [37]. Moreover, CK and Rh suppressed the activation of hepatic stellate HSC-T6 cells and reduced the expression of fibrotic factors, such as procollagen (PC)–I, PC-III, and the tissue inhibitor of metalloproteinases 1 (TIMP-1) in hepatic stellate HSC-T6 cells [37], suggesting that CK and Rh1 both play an inhibitory role in NAFLD in the form of hepatoprotective and anti-fibrotic activities.
Ginsenoside Rb2 is a protopanaxadiol (PPD)-type saponin that is one of the major ginsenosides in Panax ginseng [38]. Huang et al investigated the preventive effect of Rb2 on hepatic lipid accumulation by regulating autophagy in obese mice and hepatocytes. Rb2 alleviated hepatic steatosis and restored the impaired hepatic autophagy in db/db mice [39]. Rb2 also induced autophagic flux in hepatocytes [39], suggesting that the inhibitory effect of Rb2 on hepatic steatosis is mediated by a coordinated increase in hepatic autophagy.
Ginsenoside Mc1 is a minor and new deglycosylated ginsenoside that is converted from the ginsenoside Rc [40]. Rho et al examined the protective effect of Mc1 on ER stress-induced hepatic steatosis in obese mice and hepatocytes. Mc1 suppressed palmitate-induced ER stress and apoptosis in hepatocytes [41] and also reduced ER stress and hepatic damage in HFD-induced NAFLD mice [41]. Moreover, Mc1 ameliorated hepatic steatosis in HFD-induced NAFLD mice [41], suggesting that Mc1 plays a protective role in lipogenesis and NAFLD by inhibiting ER stress in hepatocytes.
Given the evidence from the studies above, ginseng and various ginsenosides play a pharmacological role in NAFLD by modulating various cellular functions (Fig. 2) and could be potential complementary and alternative medicines for NAFLD.
3. Pharmacological roles of ginseng and ginsenosides in NASH
3.1. Pharmacological roles of ginsengs in NASH
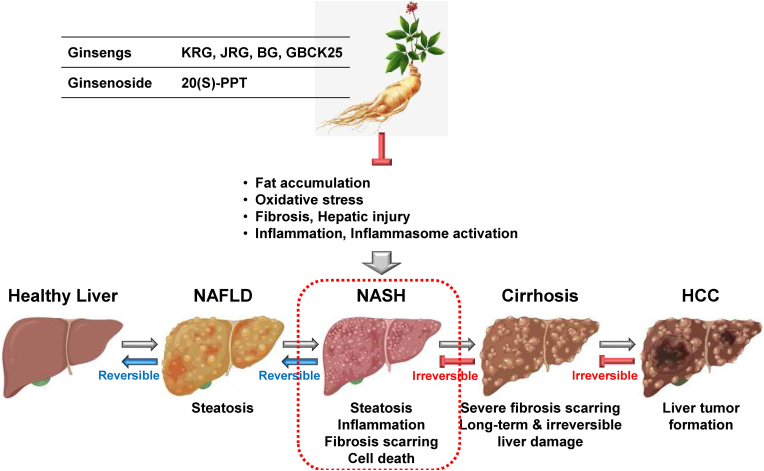
NASH is an inflammatory hepatic disorder in which the pathogenesis is highly associated with NAFLD. Several studies have reported the anti-inflammatory and pharmacological roles of ginsengs in NASH in regulating various cellular molecules and biological functions. Jeong et al investigated the beneficial effect of KRG on NASH progression in mice, hepatocytes, and macrophages. KRG alleviated NASH severity and NASH-mediated hepatic injury in methionine- and choline-deficient high-fat or Western diet-fed NASH mice [42]. KRG also suppressed hepatic inflammation, fibrosis, and steatosis in these NASH mice [42]. Additionally, KRG reduced the expression of FABP4 and pro-inflammatory cytokines in hepatocytes, macrophages, and NASH mice [42], suggesting that KRG plays an inhibitory role in NASH by inhibiting hepatic inflammation and FABP4 expression in the liver. However, the inhibitory role of FABP4 in NASH and its underlying mechanisms require further investigation.
Jinan red ginseng (JRG), another type of KRG produced at Jinan, Jeollabuk-do, was reported to play a pharmacological role in NASH by modulating lipogenesis in mice and hepatocytes. JRG reduced the palmitic acid (PA)-induced lipotoxicity, inflammation, and oxidative stress in hepatocytes and macrophages [43]. JRG also decreased the expression of NASH-associated genes in hepatocytes and macrophages [43]. Moreover, JRG ameliorated hepatic inflammation, injury, lipid metabolism, fibrosis, oxidative stress, and disease severity in Western diet-induced NASH mice [43]. These results indicate that JRG has a potent anti-NASH activity by inhibiting hepatic lipogenesis and inflammation.
The pharmacological role of the processed BG and fermented ginseng was also demonstrated in NASH. Wei et al investigated the protective role of BG against NASH in mice. BG alleviated severe hepatic steatosis and liver damage and reduced the serum levels of pro-inflammatory cytokines and fibrotic-related genes in CCl4-induced NASH mice [44]. Moreover, BG suppressed the inflammatory responses by inhibiting a TLR4/NF-κB pathway in NASH mice [44], suggesting that BG ameliorates NASH by inhibiting hepatic steatosis, inflammation, and fibrosis. BG-mediated anti-inflammatory action in NASH is accomplished by targeting a TLR4/NF-κB pathway. Choi et al also demonstrated the ameliorative role of the fermented ginseng GBCK25 against NASH in mice. GBCK25 reduced hepatic inflammation, oxidative stress, lipid accumulation, and fibrosis in Western diet-induced NASH mice and PA-stimulated hepatocytes and macrophages, resulting in the amelioration of liver injury and NASH in the diseased mice [45]. These results indicate that GBCK25 ameliorated NASH by inhibiting hepatic inflammation, oxidative stress, steatosis, and fibrosis.
3.2. Pharmacological roles of ginsenosides in NASH
The pharmacological role of ginsenosides in NASH was also investigated. Lu et al demonstrated the inhibitory role of ginseng protopanaxatriol (PPT) in NASH and metabolic associated fatty liver disease (MAFLD) by targeting inflammasomes in mice. The ginseng saponin 20(S)-PPT significantly attenuated liver inflammation and fibrosis and restored liver function by inhibiting the NLRP3 inflammasome in the methionine/choline-deficient diet-induced NASH mice and in the primary bone marrow-derived macrophages, Kupffer cells, and hepatocytes [46]. This study suggests that saponin metabolite PPT in ginseng could be a potential therapeutic agent for NASH and MAFLD by targeting NLRP3 inflammasomes in macrophages and hepatocytes.
Given the strong evidence in these studies, ginseng and ginsenosides play a protective and therapeutic role in NASH pathogenesis by inhibiting hepatic inflammation, oxidative stress, steatosis, and fibrosis in hepatocytes and macrophages (Fig. 3).
4. Conclusions and perspectives
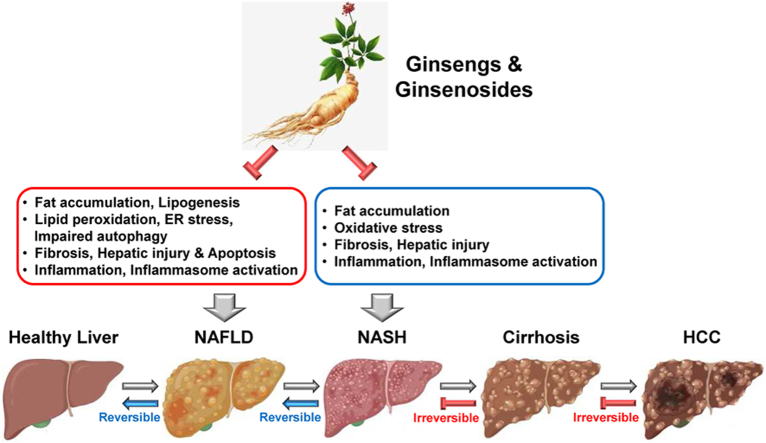
NAFLD is the most common chronic liver disease caused by fat accumulation in the liver. NAFLD ranges from simple hepatic steatosis to NASH characterized by hepatic inflammation, oxidative stress, and fibrosis [1]. Despite the increasing numbers of NAFLD and NASH patients worldwide, the current medications and therapeutic options are very limited. Ginseng is a traditional herbal medicine that has long been used for boosting the body's immunity and alleviating a variety of human diseases [12]. Ginseng includes various bioactive ingredients, such as ginsenosides, glycosides, polysaccharides, peptides, alkaloids, and phenolic compounds [47]. Ginsenosides are saponins and steroid glycosides that play significant pharmacological roles in various human diseases by inhibiting pathogenic conditions, including inflammation, oxidative stress, lipogenesis, and fibrosis [12].
This review comprehensively discusses the studies demonstrating the protective roles of ginseng and ginsenosides in NAFLD and NASH, as well as the underlying mechanisms, as summarized in Table 1, Table 2, respectively. Ginseng and ginsenosides have potent pharmacological activities in NAFLD and NASH by alleviating hepatic steatosis, inflammation, oxidative stress, and fibrosis, resulting in the protection of the liver from NAFLD- and NASH-induced injuries. This improves our understanding of the potential of ginseng and ginsenosides as therapeutic agents against NAFLD and NASH. Despite the evidence, the protective roles of ginseng and ginsenosides in NAFLD and NASH, as well as their underlying molecular and cellular mechanisms, are still largely unknown; therefore, further mechanism studies are required. Furthermore, more than 150 ginsenosides have been identified from ginseng [48], and studies identifying and validating more ginsenosides that have pharmacological roles in NAFLD and NASH are in demand. Moreover, the pharmacological roles of ginseng and ginsenosides and the underlying mechanisms need to be further investigated in other types of liver diseases caused by hepatic pathological conditions, such as liver injury, cirrhosis, and HCC.
Table 1
Pharmacological Roles of Ginsengs and Ginsenosides in NAFLD
| Disease | Ginseng | Activators/inhibitors | Models | Ref. |
|---|---|---|---|---|
| NAFLD | KRG |
| OLETF rats | [23] |
| NAFLD patients | [25] | ||
| PNS |
| HFD-induced NAFLD mice | [26] | |
| BG |
| FFA-treated HepG2 HFHF diet-induced NAFLD mice | [27] | |
| Sap-Rh1/Rg2 |
| FFD-induced NAFLD mice | [28] | |
| KRG-Rd/Rg3 |
| LPS-treated RAW264.7 FFD-induced NAFLD mice | [29] | |
| Rg1 |
| MCE-treated HHL-5 | [30] | |
| HFD-induced NAFLD rats | [31] | ||
| D-galactose-induced NAFLD mice | [32] | ||
| HFD-induced NAFLD mice | [33] | ||
| CK |
| FFA-treated HuH7 | [36] | |
| HSC-T6 HFD-induced NAFLD rats | [37] | ||
| Rh1 |
| HSC-T6 HFD-induced NAFLD rats | [37] | |
| Rb2 |
| HepG2 db/db obese mice | [39] | |
| Mc1 |
| Palmitate-treated HepG2 HFD-induced NAFLD mice | [41] |
Table 2
Pharmacological Roles of Ginsengs and Ginsenosides in NASH
| Disease | Ginseng | Activators/inhibitors | Models | Ref. |
|---|---|---|---|---|
| NASH | KRG |
| MCD or LPS-treated Primary hepatocytes MCD or LPS-treated RAW264.7 MCDHF diet-induced NASH mice Western diet-induced NASH mice | [42] |
| JRG |
| PA-treated AML-12 and RAW264.7 Western diet-induced NASH mice | [43] | |
| BG |
| CCl4-induced NASH mice | [44] | |
| GBCK25 |
| PA-treated AML-12 and RAW264.7 Western diet-induced NASH mice | [45] | |
| 20(S)-PPT |
| MCD diet-induced NASH mice Mouse primary BMDMs, Kupffer cells, hepatocytes | [46] |
In conclusion, accumulating evidence strongly suggests that ginseng and its major bioactive component, ginsenosides, are critical agents exhibiting pharmacological activities in the pathogenesis of NAFLD and NASH. In addition, ginseng and ginsenosides are potential herbal medicines to prevent and treat NAFLD, NASH, and possibly other types of liver diseases caused by hepatic steatosis and inflammation.
Declaration of competing interest
The author declares no conflict of interest.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (RS-2023-00239222).
References
Articles from Journal of Ginseng Research are provided here courtesy of Elsevier
Citations & impact
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/156450468
Article citations
The Regulatory Roles of Inflammation and Inflammasomes in Liver Diseases.
Int J Mol Sci, 25(18):9864, 12 Sep 2024
Cited by: 0 articles | PMID: 39337352 | PMCID: PMC11432471
Research Progress on Effects of Ginsenoside Rg2 and Rh1 on Nervous System and Related Mechanisms.
Molecules, 28(23):7935, 04 Dec 2023
Cited by: 3 articles | PMID: 38067664 | PMCID: PMC10708332
Review Free full text in Europe PMC
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Ginseng Saponin Enriched in Rh1 and Rg2 Ameliorates Nonalcoholic Fatty Liver Disease by Inhibiting Inflammasome Activation.
Nutrients, 13(3):856, 05 Mar 2021
Cited by: 18 articles | PMID: 33807927 | PMCID: PMC7999915
Histopathology of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis.
World J Gastroenterol, 20(42):15539-15548, 01 Nov 2014
Cited by: 207 articles | PMID: 25400438 | PMCID: PMC4229519
Review Free full text in Europe PMC
Potential Therapeutic Application of Estrogen in Gender Disparity of Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis.
Cells, 8(10):E1259, 15 Oct 2019
Cited by: 43 articles | PMID: 31619023 | PMCID: PMC6835656
Review Free full text in Europe PMC
MicroRNA-223 Ameliorates Nonalcoholic Steatohepatitis and Cancer by Targeting Multiple Inflammatory and Oncogenic Genes in Hepatocytes.
Hepatology, 70(4):1150-1167, 05 Jun 2019
Cited by: 67 articles | PMID: 30964207 | PMCID: PMC6783322
Funding
Funders who supported this work.
Ministry of Science, ICT and Future Planning (1)
Grant ID: RS-2023-00239222