Abstract
Objectives
This study aimed to identify long-term distinct trajectories of multimorbidity with ageing from 50 to 85 years among Chinese older adults and examine the relationship between exposure to early-life adversity (ELA; including specific types of adversity and accumulation of different adversities) and these long-term multimorbidity trajectories.Design
The group-based trajectory models identified long-term multimorbidity trajectories. Multinomial logistic regression models were used to examine the relationship between ELA and the identified multimorbidity trajectories.Setting
This study used data from the China Health and Retirement Longitudinal Study (CHARLS, 2011-2018) and the 2014 Life History Survey.Participants
We used data from 9112 respondents (aged 60 and above) of the 2018 wave of CHARLS.Outcome measures
Each respondent's history of chronic conditions and experiences of ELA were collected from the 2011-2018 waves of CHARLS and the 2014 Life History Survey.Results
Four heterogeneous long-term trajectories of multimorbidity development were identified: 'maintaining-low' (19.1%), 'low onset-rapidly increasing' (23.3%), 'middle onset-moderately increasing' (41.5%) and 'chronically-high' (16.2%). Our findings indicated that the heterogeneity can be explained by ELA experiences. Across various types of different ELA experiences, exposure to food insufficiency (relative risk ratios from 1.372 (95% CI 1.190 to 1.582) to 1.780 (95% CI 1.472 to 2.152)) and parental quarrel/divorce (relative risk ratios from 1.181 (95% CI 1.000 to 1.394) to 1.262 (95% CI 1.038 to 1.536)) had the most prominent associations with health deterioration. The accumulation of more different ELA experiences was associated with a higher relative risk of developing more severe multimorbidity trajectories (relative risk ratio for five to seven ELAs and chronically high trajectory: 7.555, 95% CI 4.993 to 11.431).Conclusions
There are heterogeneous long-term trajectories of multimorbidity in Chinese older adults, and the risk of multimorbidity associated with ELA accumulates over the lifespan. Our findings highlight the role of a supportive early-life family environment in promoting health development across the lifespan, advocating for the integration of life-course approaches to implementing health disparity interventions.Free full text

Original research
Exposure to early-life adversity and long-term trajectories of multimorbidity among older adults in China: analysis of longitudinal data from the China Health and Retirement Longitudinal Study
Abstract
Objectives
This study aimed to identify long-term distinct trajectories of multimorbidity with ageing from 50 to 85 years among Chinese older adults and examine the relationship between exposure to early-life adversity (ELA; including specific types of adversity and accumulation of different adversities) and these long-term multimorbidity trajectories.
Design
The group-based trajectory models identified long-term multimorbidity trajectories. Multinomial logistic regression models were used to examine the relationship between ELA and the identified multimorbidity trajectories.
Setting
This study used data from the China Health and Retirement Longitudinal Study (CHARLS, 2011–2018) and the 2014 Life History Survey.
Participants
We used data from 9112 respondents (aged 60 and above) of the 2018 wave of CHARLS.
Outcome measures
Each respondent’s history of chronic conditions and experiences of ELA were collected from the 2011–2018 waves of CHARLS and the 2014 Life History Survey.
Results
Four heterogeneous long-term trajectories of multimorbidity development were identified: ‘maintaining-low’ (19.1%), ‘low onset-rapidly increasing’ (23.3%), ‘middle onset-moderately increasing’ (41.5%) and ‘chronically-high’ (16.2%). Our findings indicated that the heterogeneity can be explained by ELA experiences. Across various types of different ELA experiences, exposure to food insufficiency (relative risk ratios from 1.372 (95% CI 1.190 to 1.582) to 1.780 (95% CI 1.472 to 2.152)) and parental quarrel/divorce (relative risk ratios from 1.181 (95% CI 1.000 to 1.394) to 1.262 (95% CI 1.038 to 1.536)) had the most prominent associations with health deterioration. The accumulation of more different ELA experiences was associated with a higher relative risk of developing more severe multimorbidity trajectories (relative risk ratio for five to seven ELAs and chronically high trajectory: 7.555, 95% CI 4.993 to 11.431).
Conclusions
There are heterogeneous long-term trajectories of multimorbidity in Chinese older adults, and the risk of multimorbidity associated with ELA accumulates over the lifespan. Our findings highlight the role of a supportive early-life family environment in promoting health development across the lifespan, advocating for the integration of life-course approaches to implementing health disparity interventions.
Introduction
Chronic diseases are among the leading causes of death, placing a huge burden on healthcare systems worldwide.1 Multimorbidity, defined as the coexistence of two or more chronic conditions, has been observed at an extremely high prevalence in old age.2 More than half of Chinese older adults are estimated to have two or more chronic conditions,3 and this number is expected to increase with the continuing ageing of the population and improvements in the survival of people with chronic diseases. Extensive studies have documented a range of adverse outcomes resulting from multimorbidity, including a higher risk of mortality, disability and increased healthcare use.4
Despite the growing interest in studying multimorbidity and its associated factors, previous research has mostly focused on a list of arbitrarily chosen conditions at a single point or period in time and thus does not cover the complete time-varying picture of multimorbidity.5 6 Recent research has indicated that older individuals not only show variations in the timing of disease onset but also develop multimorbidity at different rates over time.7 8 In particular, several studies have successfully identified distinct subgroups of multimorbidity within study populations.9–11 Similar groups emerged across these studies, including a group of older individuals maintaining few conditions, a group characterised by a consistently high number of conditions and a group of individuals reporting a rapidly increasing number of conditions.12 13 To our knowledge, however, no study has examined heterogeneous multimorbidity trajectories by longitudinally capturing the accumulation of chronic conditions throughout the whole period starting from an individual’s first-onset condition to his or her very old age. Identifying such long-term trajectories will not only enhance the understanding of the development of multimorbidity but also help prognostic studies better target people at risk of more severe multimorbidity progression and associated adverse outcomes.
Early-life adversity (ELA) has been documented as a potential risk factor for worse health outcomes, including an increased risk of multimorbidity in old age.14–16 ELA is commonly defined as exposure to traumatic events such as parental death, parental divorce or abuse during childhood.17 18 The life-course perspective provides two important theoretical models explaining the mechanisms underlying the association between ELA and health development.19 The critical period model asserts that exposure to ELA has long-lasting influence on individuals’ developmental trajectories of health. This influence could vary across different types of ELA experiences and operate by shaping the structural aspects of individuals’ brain architecture and behavioural development.20 21 Existing studies supporting this model have demonstrated that exposure to specific types of ELA (eg, domestic violence and food deprivation) could increase the risk of multimorbidity in various older populations through the dysregulation of centrally mediated stress response processes22 23 and the promotion of detrimental behaviours or poor social ties.24 25 Alternatively, the accumulation of risk model suggests that early-life disadvantages increase one’s exposure to later health risks, contributing to the development of the adulthood health ‘chain of risk’, where ELA experiences at different life stages tend to sequentially compound.19 20 Empirical evidence supporting this model has revealed that individuals who experienced four or more accumulated ELAs reported increased risks of multimorbidity and developing specific chronic conditions (eg, dyslipidaemia and psychiatric disease) compared with those who did not experience ELAs.26
In addition to the life-course perspective, the cumulative disadvantage (CDA) framework also provides important viewpoints for understanding the impact of ELA on later-life health disparities, highlighting the mechanism of path dependence. It posits that initial disparities can be linked to later-life health outcomes indirectly through influencing adulthood exposure to environmental risks and health opportunities.27 Empirical studies have revealed that disadvantaged early environment (eg, lower educational attainment) may lead to poorer socioeconomic status in adulthood (eg, placement in the occupational hierarchy), subsequently exerting significant effects on later-life health through the influence of lifestyle choices or access to medical services.28
To our knowledge, no existing studies have incorporated the life-course theory and the CDA framework for understanding the impact of ELA experiences on health development in later life, particularly by linking the two aspects of ELA experiences (types and accumulation) with long-term distinct trajectories of multimorbidity. Using population-based longitudinal data, the present study aimed to identify long-term heterogeneous trajectories of multimorbidity among Chinese older adults starting in late adulthood and followed up to 35 years and to examine the relationship between exposure to ELA (including specific types of adversity and accumulation of different adversities) and these long-term multimorbidity trajectories. Drawing from the existing literature, we expected that exposure to certain types of ELA and the accumulation of more different ELA types would be associated with a higher likelihood of more severe multimorbidity trajectories. Clarifying the impact of ELA experiences on later-life health will provide a basis for healthcare providers to develop comprehensive life-cycle health interventions.
Methods
Data and sampling
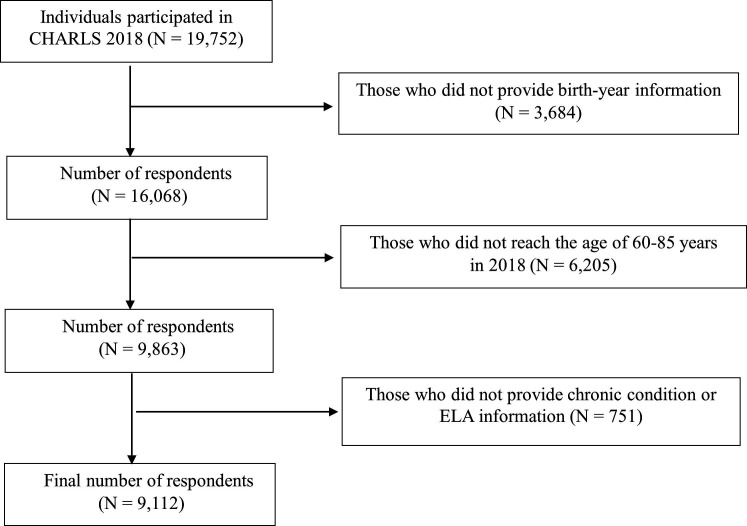
The present study used data from the four waves (2011–2018) of the China Health and Retirement Longitudinal Study (CHARLS) and the 2014 Life History Survey. The 2011 baseline survey collected data on demographic, socioeconomic and health characteristics from 17 706 respondents aged 45 and above. The 2014 Life History Survey interviewed respondents who participated in the 2011 and 2013 surveys and collected information on their early-life experiences, including living environment, family socioeconomic status and health history. For the purpose of the present study, we restricted our analytical sample to respondents who met the following inclusion criteria: (1) participated in the 2018 wave and the 2014 Life History Survey; (2) birth year information available from any of the 2011–2018 waves; (3) provided valid data on the measurement of ELA and history of chronic conditions; and (4) aged ≥60 years in 2018. In addition, we excluded respondents aged over 85 in 2018 because the oldest age group was not well represented in CHARLS (the proportion of respondents aged over 85 in the 2018 CHARLS survey was 1.6%). The final analytical sample included 9112 respondents (see figure 1 for details about the sample selection process). In order to verify that the sample size of this study allowed for subgroup analysis, a power analysis was conducted using Stata V.17. The results showed that the sample size required to detect a significant relationship between the study variables at an α level of 0.05 and a power of 0.8 was approximately 863 respondents.
706 respondents aged 45 and above. The 2014 Life History Survey interviewed respondents who participated in the 2011 and 2013 surveys and collected information on their early-life experiences, including living environment, family socioeconomic status and health history. For the purpose of the present study, we restricted our analytical sample to respondents who met the following inclusion criteria: (1) participated in the 2018 wave and the 2014 Life History Survey; (2) birth year information available from any of the 2011–2018 waves; (3) provided valid data on the measurement of ELA and history of chronic conditions; and (4) aged ≥60 years in 2018. In addition, we excluded respondents aged over 85 in 2018 because the oldest age group was not well represented in CHARLS (the proportion of respondents aged over 85 in the 2018 CHARLS survey was 1.6%). The final analytical sample included 9112 respondents (see figure 1 for details about the sample selection process). In order to verify that the sample size of this study allowed for subgroup analysis, a power analysis was conducted using Stata V.17. The results showed that the sample size required to detect a significant relationship between the study variables at an α level of 0.05 and a power of 0.8 was approximately 863 respondents.
Variables
Development of chronic conditions
We obtained information about each respondent’s history of chronic conditions from the 2011–2018 CHARLS survey. In each survey, respondents were asked if they had been diagnosed by a healthcare provider with any of the following 14 chronic conditions: hypertension, dyslipidaemia, diabetes, cancer or malignant tumour, chronic lung diseases, liver disease, heart disease, stroke, kidney disease, stomach or other digestive diseases, arthritis or rheumatism, asthma, emotional or psychiatric problems, and memory-related disorders.29 For each condition, respondents who answered in the affirmative were then asked to report the year when this condition was first diagnosed. Using the self-reported information on the starting year of each condition, we calculated the age at onset of each chronic condition for each respondent. Given that most studies on chronic conditions used age 50 as a starting point and the high prevalence of chronic conditions in people over 50, we tracked each individual’s chronic conditions from the age of 50.30 31 Then, the time-varying variable ‘development of chronic conditions’ was generated for each respondent, indicating his or her total number of chronic conditions being diagnosed in each year between the age of 50 and the current age when participating in the 2018 CHARLS survey. For more details about the distribution of this time-varying variable, please see online supplemental figure 1. This variable was further used for the identification of distinctive long-term multimorbidity trajectories within this population.
Supplementary data
Early-life adversity
We extracted eight indicators of ELA from the CHARLS 2014 survey, including parental illness, parental death, parental quarrel/divorce, relative poverty, food insufficiency, domestic violence and bullying. The detailed questionnaire items and the definitions of each ELA indicator are available in online supplemental table 1. Responses to each item were dichotomised and summed to generate a cumulative ELA score for each respondent, ranging from 0 to 7. Higher scores indicated more experience with ELA. To investigate the relationship between the accumulation of different ELA experiences and the long-term trajectories of multimorbidity, we further categorised the accumulation of ELA experiences according to ELA scores: none, 1–2, 3–4 and 5–7 types of ELA experience accumulation. The measurement has been accepted and widely used in empirical studies.32
Supplementary data
Covariates
We controlled for a number of confounding factors identified in previous studies that may contribute to the association between ELA and multimorbidity.10 33 34 These factors include (1) demographic characteristics: gender, residence area (urban vs rural) and marital status (married vs other status, including unmarried/divorced/separated/widowed); (2) socioeconomic characteristics: educational attainment (below high school vs high school and above), whether receiving Dibao assistance and types of medical insurance (none, urban employee, urban and rural resident, and others); and (3) health behaviour factors: smoking, drinking and physical activity (no, light-intensity, moderate-intensity and vigorous-intensity physical activity).
Analytical strategy
The group-based trajectory modelling (GBTM) approach was used to model progression in the number of chronic conditions over time (ie, from late adulthood to old age).35–37 The GBTM approach is designed to identify distinctive groups of individuals sharing a similar trajectory using finite mixtures of suitably defined probability distributions.35 Applied to this study, this approach enables the assignment of individuals into distinct trajectories based on the similarity of multimorbidity trajectories as they age. Specifically, each individual was assigned to a single group in which they have the highest posterior probability of membership.6 The fundamental concept of interest is the distribution of outcomes (in our study, the number of chronic conditions) conditional on age. We examined a series of foundational assumptions of GBTM.38 39 First, GBTM assumes that the data follow continuous distribution with finite starting and ending points (in this study, the chronic conditions data are continuous, ranging from 0 to 14). Given the slight skewness in the data, we examined the applicability of the censored normal (cnorm) model and the zero-inflated Poisson model. The results indicated a better fit for the cnorm model. Second, GBTM assumes that heterogeneity between groups arises from differences in trajectory development rather than other variables. We compared the model with random effects, including gender, with the model without random effects and the results did not show significant differences. Third, GBTM posits that all trajectories exhibit steady changes (in this study, the number of chronic conditions in individuals increased over time). Fourth, GBTM assumes that the sample size of each subgroup should not be less than 5% of the total sample size. In this study, the smallest subgroup constituted 16.2% of the total sample (the sample sizes of the four groups were 1743, 2119, 3779 and 1471, respectively), exceeding the model requirements significantly.
Following the standard procedure for applying this approach, we conducted a sequence of GBTM models. A small amount of missingness occurred due to no response or incomplete responses to the chronic conditions and ELA information; these missing data were treated as missing at random. For model selection, we used the following model fit indices and statistical criteria in combination with conceptual considerations of group distinctiveness and interpretability: (1) the Bayesian information criteria (BIC), which is the most widely used criteria for model selection, with a BIC closer to 0 indicating better model fit; (2) relative entropy, which estimates the accuracy (convergence) of classification of individuals into the different latent classes, with entropy values close to 1 indicating lower classification uncertainty; (3) the average posterior probability of group assignment that measures the probability of a group individual belonging to this specific trajectory group was set at greater than 0.7; and (4) according to the posterior probability of the group member, a minimum membership of 5% is required in each trajectory group.40 These criteria were applied iteratively, and the final model was chosen based on a comprehensive evaluation of statistical fit and conceptual coherence, striking a balance between model complexity and interpretability. After identifying the optimal number of trajectory groups, univariate analysis was used to test the differences in trajectory groups from the final model among a range of individual characteristics at baseline. Multivariate logistic models were used to assess the association between ELA and trajectory group memberships after controlling for various confounding factors, including demographic features, socioeconomic factors and health behaviour factors.
We performed a series of sensitivity analyses. (1) We reran the GBTM analyses using 12 chronic conditions (excluding memory-related and emotional/psychiatric problems given their low prevalence in the study population). (2) We also reran the main analyses using a different set of ELA indicators (including two additional indicators that have been suggested to have a potential influence on health in later life) and using different categorisations for the accumulation of ELA. (3) All the analyses were stratified by gender and urban–rural residence.
We followed the Strengthening the Reporting of Observational Studies in Epidemiology checklist for reporting observational studies (see online supplemental table 2).
Supplementary data
Patient and public involvement
None.
Results
Sample characteristics by number of ELAs in older adults
Table 1 shows the characteristics of individuals (measured in the 2018 CHARLS survey) by the accumulation of ELA experiences (none, 1–2, 3–4 and 5–7 types). Among individuals with three or more chronic conditions, the proportion of individuals who reported five to seven types of ELA accumulation (57.2%) was significantly higher than of those who reported fewer types of ELA accumulation (none, 1–2 and 3–4 types). Individuals from the four accumulation groups of ELA experiences significantly differ in their demographic, socioeconomic characteristics and health behaviours.
Table 1
Sample characteristics by number of ELAs in CHARLS 2018 (N=9112)
| Variables (%) | Number of ELAs | P value | |||
| 0 | 1–2 | 3–4 | 5–7 | ||
| Number of chronic conditions | <0.001 | ||||
 0–1 0–1 | 38.2 | 33.7 | 28.2 | 21.8 | |
 2 2 | 19.5 | 22.8 | 21.5 | 21.1 | |
 ≥3 ≥3 | 42.3 | 43.5 | 50.3 | 57.2 | |
| Female | 63.2 | 56.2 | 46.3 | 44.7 | <0.001 |
| Rural | 57.7 | 60.8 | 65.7 | 70.8 | <0.001 |
| Marital status | <0.001 | ||||
 Married Married | 71.7 | 78.1 | 80.3 | 81.3 | |
 Other status Other status | 28.3 | 21.9 | 19.7 | 18.8 | |
| Educational attainment | 0.001 | ||||
 Below high school Below high school | 87.2 | 88.8 | 91.0 | 92.1 | |
 High school and above High school and above | 12.8 | 11.2 | 9.0 | 7.9 | |
| Receiving Dibao assistance | 10.8 | 8.3 | 10.9 | 12.4 | <0.001 |
| Types of medical insurance | 0.001 | ||||
 None None | 3.9 | 2.9 | 2.7 | 2.8 | |
 Urban employee Urban employee | 16.1 | 13.4 | 13.2 | 9.3 | |
 Urban and rural resident Urban and rural resident | 76.6 | 81.1 | 81.9 | 86.3 | |
 Others Others | 3.5 | 2.6 | 2.2 | 1.6 | |
| Smoking | 65.1 | 58.4 | 51.4 | 51.1 | <0.001 |
| Drinking | 72.6 | 67.9 | 63.1 | 61.5 | <0.001 |
| Physical activity | <0.001 | ||||
 None None | 15.7 | 14.2 | 11.8 | 9.5 | |
 Low intensity Low intensity | 40.0 | 35.5 | 31.1 | 26.8 | |
 Moderate intensity Moderate intensity | 29.3 | 27.5 | 26.9 | 26.4 | |
 High intensity High intensity | 15.0 | 22.8 | 30.2 | 37.2 | |
The table shows the results of the χ2 test for the variables.
The information is from the CHARLS 2018. Other status of marriage includes unmarried/divorced/separated/widowed. Urban and rural resident medical insurance integrated urban resident medical insurance and new rural cooperative medical insurance.
CHARLS, China Health and Retirement Longitudinal Study; ELA, early-life adversity.
Long-term trajectories of multimorbidity among older adults
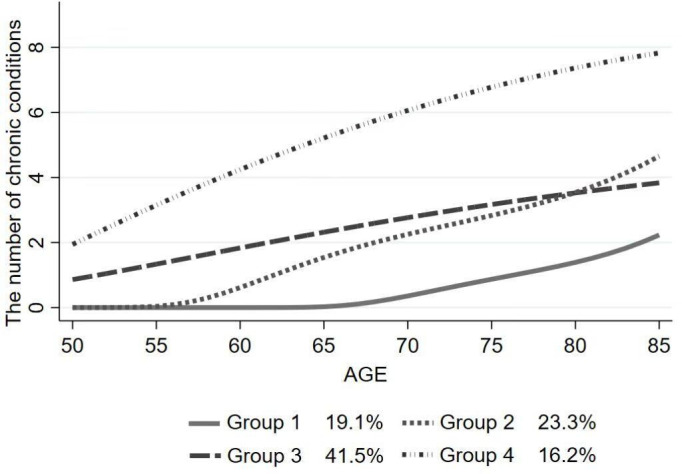
We used the GBTM approach to identify distinct groups of individuals sharing a similar long-term trajectory of multimorbidity from ages 50 to 85. Models were estimated with one to six groups (table 2). Comparatively, the BIC and entropy indicated that groups 4 and 6 were more suitable than the other groups. The optimal BIC was found in group 6 (closest to 0), and group 4 exhibited the highest entropy (closest to 1, at 0.976). Meanwhile, we observed a diminished rate of BIC reduction after group 4, suggesting that further increasing model complexity no longer yields significant benefits.40 Based on considerations of changes in BIC and entropy, a logit model with four trajectory groups was the most suitable fit for the data.
Table 2
Tabulated BIC and 2(ΔBIC)
| Group number | BIC | Entropy | 2(ΔBIC) |
| 1 | −308557.37 | – | – |
| 2 | −253000.24 | 0.967 | 111 114.26 114.26 |
| 3 | −226640.40 | 0.975 | 52 719.68 719.68 |
| 4 | −213163.38 | 0.976 | 26 954.04 954.04 |
| 5 | −201166.33 | 0.973 | 23 994.10 994.10 |
| 6 | −193334.26 | 0.941 | 15 664.14 664.14 |
N=9112. The closer the entropy is to 1, the better the model fits.
BIC, Bayesian information criterion.
Figure 2 illustrates the distribution of older individuals from the four identified long-term trajectories of multimorbidity. The number of chronic conditions in group 1 (‘maintaining-low’) was consistently low, with most remaining free of chronic conditions until age 75. Older individuals in group 2 (‘low onset-rapidly increasing’) had no chronic condition at the starting point, but their number of chronic conditions showed a rapid increase after age 65. Older individuals in group 3 (‘middle onset-moderately increasing’) had one chronic condition at the starting point, and their number of chronic conditions increased steadily with age. Older adults in group 4 (‘chronically-high’) had two chronic conditions at the starting point, with the number of chronic conditions increasing rapidly with age. Please see online supplemental table 3 for more information on the characteristics of the long-term trajectory distribution of multimorbidity.
ELA predicts long-term trajectories of multimorbidity
Model 1 (table 3) presents the results of the multinomial logistic regressions for the relationship between specific types of ELA and multimorbidity trajectories. Exposure to parental illness, parental quarrel/divorce, relative poverty, food insufficiency, domestic violence and bullying was associated with more severe long-term trajectories of multimorbidity. Individuals who had experienced parental quarrel/divorce reported a higher relative risk of being in the ‘low onset-rapidly increasing’ group, the ‘middle onset-moderately increasing’ group and the ‘chronically-high’ group than in the ‘maintaining-low’ group. Similar results have also been found among individuals who reported childhood food insufficiency.
Table 3
Multinomial logistic regressions for ELA experiences and multimorbidity trajectories (model 1)
| Variables | Group 2 ‘Low onset-rapidly increasing’ | Group 3 ‘Middle onset-moderately increasing’ | Group 4 ‘Chronically-high’ |
RRR (95% CI) CI) | RRR (95% CI) CI) | RRR (95% CI) CI) | |
| ELA experiences | |||
 Parental illness Parental illness | 1.059 (0.891 to 1.258) | 1.230 (1.054 to 1.435) | 1.885 (1.578 to 2.251) |
 Parental death Parental death | 1.032 (0.876 to 1.215) | 1.013 (0.874 to 1.174) | 0.980 (0.816 to 1.176) |
 Parental quarrel/divorce Parental quarrel/divorce | 1.214 (1.011 to 1.458) | 1.181 (1.000 to 1.394) | 1.262 (1.038 to 1.536) |
 Relative poverty Relative poverty | 1.092 (0.944 to 1.264) | 1.210 (1.061 to 1.379) | 1.333 (1.136 to 1.564) |
 Food insufficiency Food insufficiency | 1.403 (1.197 to 1.645) | 1.372 (1.190 to 1.582) | 1.780 (1.472 to 2.152) |
 Domestic violence Domestic violence | 1.132 (0.982 to 1.305) | 1.340 (1.178 to 1.523) | 1.597 (1.360 to 1.876) |
 Bullying Bullying | 1.051 (0.889 to 1.243) | 1.215 (1.046 to 1.411) | 1.443 (1.208 to 1.724) |
| Covariates | |||
 Female Female | 1.384 (1.127 to 1.698) | 1.573 (1.308 to 1.893) | 2.499 (1.981 to 3.154) |
 Rural Rural | 0.932 (0.797 to 1.089) | 0.921 (0.800 to 1.061) | 0.976 (0.819 to 1.162) |
 Marital status (ref: other status) Marital status (ref: other status) | 1.337 (1.135 to 1.574) | 1.700 (1.464 to 1.974) | 2.309 (1.897 to 2.811) |
 Educational attainment (ref: below high school) Educational attainment (ref: below high school) | 0.925 (0.720 to 1.190) | 1.085 (0.869 to 1.355) | 1.421 (1.089 to 1.855) |
 Receiving Dibao assistance (ref: no) Receiving Dibao assistance (ref: no) | 1.261 (0.982 to 1.619) | 1.422 (1.134 to 1.783) | 2.026 (1.565 to 1.721) |
 Types of medical insurance (ref: none) Types of medical insurance (ref: none) | |||
  Urban employee Urban employee | 1.075 (0.689 to 1.679) | 1.241 (0.826 to 1.865) | 1.516 (0.907 to 2.534) |
  Urban and rural resident Urban and rural resident | 1.019 (0.691 to 1.502) | 1.130 (0.791 to 1.615) | 1.166 (0.739 to 1.839) |
  Others Others | 1.084 (0.602 to 1.951) | 1.154 (0.676 to 1.972) | 1.347 (0.686 to 2.646) |
 Smoking (ref: no) Smoking (ref: no) | 1.059 (0.872 to 1.286) | 1.120 (0.941 to 1.335) | 1.381 (1.108 to 1.721) |
 Drinking (ref: no) Drinking (ref: no) | 0.980 (0.837 to 1.149) | 0.952 (0.825 to 1.098) | 0.840 (0.702 to 1.004) |
 Physical activity (ref: none) Physical activity (ref: none) | |||
  Low intensity Low intensity | 0.966 (0.774 to 1.206) | 1.009 (0.825 to 1.233) | 0.863 (0.676 to 1.101) |
  Moderate intensity Moderate intensity | 1.186 (0.938 to 1.498) | 1.297 (1.049 to 1.603) | 1.047 (0.811 to 1.352) |
  High intensity High intensity | 1.016 (0.805 to 1.283) | 1.082 (0.877 to 1.337) | 0.824 (0.638 to 1.064) |
The reference category was group 1 (‘maintaining-low’).
ELA, early-life adversity; ref, reference; RRR, relative risk ratio.
Model 2 (table 4) presents the results of the multinomial logistic regressions for the relationship between the accumulation of different ELA experiences and multimorbidity trajectories. Individuals who had accumulated one to two types, three to four types and five to seven types of ELA experiences reported a higher relative risk of being in the ‘low onset-rapidly increasing’ group, the ‘middle onset-moderately increasing’ group and the ‘chronically-high’ group than in the ‘maintaining-low’ group.
Table 4
Multinomial logistic regressions for ELA experiences and multimorbidity trajectories (model 2)
| Variables | Group 2 ‘Low onset-rapidly increasing’ | Group 3 ‘Middle onset-moderately increasing’ | Group 4 ‘Chronically-high’ |
RRR (95% CI) CI) | RRR (95% CI) CI) | RRR (95% CI) CI) | |
| ELA (ref: none) | |||
 1–2 1–2 | 1.694 (1.284 to 2.233) | 1.512 (1.189 to 1.922) | 1.975 (1.384 to 2.819) |
 3–4 3–4 | 2.066 (1.557 to 2.741) | 2.148 (1.680 to 2.746) | 3.746 (2.621 to 5.355) |
 5–7 5–7 | 2.625 (1.836 to 3.755) | 3.427 (2.505 to 4.690) | 7.555 (4.993 to 11.431) |
| Covariates | |||
 Female Female | 1.377 (1.129 to 1.679) | 1.540 (1.287 to 1.841) | 2.435 (1.946 to 3.046) |
 Rural Rural | 0.937 (0.805 to 1.090) | 0.922 (0.804 to 1.058) | 0.974 (0.822 to 1.154) |
 Marital status (ref: other status) Marital status (ref: other status) | 1.362 (1.163 to 1.595) | 1.743 (1.508 to 2.014) | 2.362 (1.953 to 2.856) |
 Educational attainment (ref: below high school) Educational attainment (ref: below high school) | 0.943 (0.737 to 1.207) | 1.133 (0.912 to 1.408) | 1.480 (1.142 to 1.918) |
 Receiving Dibao assistance (ref: no) Receiving Dibao assistance (ref: no) | 1.256 (0.990 to 1.595) | 1.370 (1.104 to 1.700) | 1.962 (1.534 to 2.510) |
 Types of medical insurance (ref: none) Types of medical insurance (ref: none) | |||
  Urban employee Urban employee | 1.054 (0.682 to 1.629) | 1.183 (0.794 to 1.762) | 1.495 (0.902 to 2.477) |
  Urban and rural resident Urban and rural resident | 1.002 (0.684 to 1.466) | 1.101 (0.775 to 1.562) | 1.162 (0.714 to 1.822) |
  Others Others | 1.007 (0.565 to 1.793) | 1.071 (0.635 to 1.809) | 1.170 (0.602 to 2.275) |
 Smoking (ref: no) Smoking (ref: no) | 1.065 (0.882 to 1.288) | 1.104 (0.931 to 1.310) | 1.381 (1.116 to 1.710) |
 Drinking (ref: no) Drinking (ref: no) | 0.974 (0.834 to 1.137) | 0.965 (0.839 to 1.109) | 0.869 (0.731 to 1.035) |
 Physical activity (ref: none) Physical activity (ref: none) | |||
  Low intensity Low intensity | 0.990 (0.800 to 1.226) | 0.991 (0.817 to 1.201) | 0.893 (0.706 to 1.130) |
  Moderate intensity Moderate intensity | 1.227 (0.978 to 1.538) | 1.270 (1.035 to 1.557) | 1.058 (0.826 to 1.355) |
  High intensity High intensity | 1.049 (0.837 to 1.315) | 1.074 (0.877 to 1.317) | 0.888 (0.693 to 1.138) |
The reference category was group 1 (‘maintaining-low’).
*P<0.05, **P<0.01, ***P<0.001.
ELA, early-life adversity; ref, reference; RRR, relative risk ratio.
Sensitivity analyses
The results remained generally consistent when restricting the GBTM analyses to only 12 chronic conditions, when using a different set of ELA indicators or different categorisation for the accumulation of ELA, and when stratifying by gender and urban–rural residence (see online supplemental tables 3, 5–9 for details).
Discussion
Using population-based longitudinal data, we identified four distinct long-term trajectories of multimorbidity development among Chinese older adults and linked different types and accumulation of ELA experiences with these long-term multimorbidity trajectories. Exposure to food insufficiency and parental quarrel/divorce had the most prominent associations with health deterioration. Individuals who had accumulated different ELA experiences had a significantly higher relative risk of developing more severe multimorbidity trajectories over time.
The development of multimorbidity in older adults has been widely reported, but only a few studies have examined the heterogeneity of multimorbidity trajectories in older adults.12 41 Our findings confirmed the existence of heterogeneous long-term multimorbidity trajectories in Chinese older adults. The ‘maintaining-low’ (19.1%) group and the ‘low onset-rapidly increasing’ (22%) group were both characterised by a low starting point, whereas the ‘low onset-rapidly increasing’ group reported the onset of diseases around the age of 65 and a faster development of new disease diagnosis from age 80. A similar trajectory of health development in later life has been consistently reported in previous studies among older Chinese populations across a variety of health outcomes, including frailty and functional disability.42 43 This might be because increasing life expectancy and decreasing incidence of infectious diseases have led to an increase in chronic disease in old age.44 Individuals from the largest group, ‘middle onset-moderately increasing’ (40.2%), were characterised by an average of one condition in their 50s and a gradual progression of new condition diagnosis over time. The ‘chronically-high’ group comprised individuals with the highest number of conditions throughout the age range from 50 to 85, accounting for approximately 20% of the study population. Such a similar group has also been found in several previous studies,12 13 with an estimated proportion ranging between 7.5% and 27.8%.
Our findings indicated that exposure to specific types of ELA was associated with more severe long-term multimorbidity trajectories. Experience of childhood food insufficiency and parental quarrel/divorce had the most prominent associations with health deterioration. Specifically, our finding was consistent with previous studies linking childhood food insufficiency with a higher risk of chronic conditions in middle and old age.25 45 46 This might be due to the fact that individuals shape physiological characteristics in early life that make them capable of maintaining homeostasis in metabolism when challenged by a metabolic load.25 47 Food insufficiency in childhood may lead to malnutrition, which may impair the development of metabolic capacity and increase the susceptibility to chronic diseases in the long term.47 Additionally, our finding of the harmful effects of parental quarrel/divorce on long-term multimorbidity trajectories extends the literature on the importance of early-life dysfunctional family relationships in physical and mental health in adulthood.48 49 One possible explanation might be that parental quarrel/divorce would cause psychological stress in childhood,48 which causes behavioural proclivities (such as unhealthy lifestyle choices) and hormonal dysregulation (such as altered endocrine patterns) over the lifespan,50 giving rise to increased risks of chronic diseases.44 By linking specific types of ELA experiences with the longitudinal development of multimorbidity, our study provided new evidence for the critical period model that childhood adverse experiences may exert long-lasting, irreversible health-damaging effects that lead to significant health disparities in old age.22 26
Previous studies have also documented associations between the accumulation of different ELA experiences and an increased risk of multimorbidity.23 51 The current study makes further contributions by linking the accumulation of different ELA experiences with distinctive long-term trajectories of multimorbidity. Our findings supported our hypothesis that individuals who experienced more accumulated ELAs were more likely to develop a more severe multimorbidity trajectory over time. This finding was consistent with the accumulation of risk model,52 suggesting that cumulative disadvantages of ELA may contribute to the persistent widening of health inequalities.31 Initial disadvantages may cause individuals to be exposed to subsequent risk factors, such as interrupted education in adolescence, employment disadvantages and unhealthy lifestyles in adulthood, leading to greater disparities in health status in old age.53 54 On the one hand, ELAs such as poverty and food insufficiency may cause excessive wear and tear in the body. As people age, they are at increasing risks of chronic diseases such as arthritis. On the other hand, since China’s reform and opening up in the late 1970s, with socioeconomic development, individuals who have experienced childhood adversity may have more compensatory energy intake,55 leading to hypertension, diabetes and other cardiometabolic diseases. Notably, the influence of ELA on the multimorbidity trajectories observed above remained significant even after adjusting for demographic features, socioeconomic factors and health behaviour factors. Consistent with the existing literature linking a range of different individual factors with later-life health development,10 33 our results revealed that factors such as gender, marital status, educational attainment, Dibao assistance, smoking or not smoking and physical activity exerted independent effects on multimorbidity trajectories. Specifically, being female, being married, having high school and above educational attainment, receiving Dibao assistance, smoking and engaging in moderate physical activity intensity were significantly associated with a higher risk of developing more severe multimorbidity trajectories. Our study contributes to the literature by providing new evidence that ELA serves as an explanatory factor influencing long-term trajectories of multimorbidity. Future research could build on our study by further examining the impact of ELA on multimorbidity trajectories in interaction with various confounders (eg, gender and marital status).
Our study has some key strengths. First, taking advantage of time-varying data on history of chronic conditions, we captured the entire course of individuals’ development of multimorbidity from the first chronic disease onset to old age, with the longest time spanning 35 years. Second, our study is among the first to empirically establish the longitudinal association between ELA and long-term differential multimorbidity trajectories. Third, our study linked two important aspects of ELA experiences (specific types and accumulation of ELA) with multimorbidity trajectories, offering a more nuanced picture for testing the life-course models concerning the pathways through which ELA influences health development. Nevertheless, this study also has some limitations. First, our measures of ELA and multimorbidity were retrospective and based on self-reports, which may have introduced recall bias into the data. Second, we did not consider the frequency and intensity of ELA, which were limited by data unavailability. Additionally, the issue of omitted variables may have persisted in our study, and future research is encouraged to employ more comprehensive data sets to investigate the relationship between potentially influential confounders (eg, genetic inheritance and resilience) and multimorbidity trajectories. Third, our sample excluded individuals over 85 years old, which may have incurred some sample selection bias. Our research findings should thus be interpreted with caution with regard to the possible underestimation of multimorbidity prevalence and trajectories in the oldest segments of the population. Considering this limitation, future research could focus on developing inclusive sampling strategies to ensure a more comprehensive representation of individuals aged 85 and above. This approach would contribute to a more comprehensive view of multimorbidity across the full age spectrum.
Conclusion
In conclusion, our study indicates that the development of multimorbidity shows considerable heterogeneity within the older Chinese population with respect to the onset and increased rate of conditions, and such heterogeneity can be explained by ELA experiences. The findings highlight the critical role of childhood in an individual’s physical and psychological development, as childhood adversity can even influence the trajectory of multimorbidity in old age. Our findings could have the following implications. First, poverty alleviation programmes or rural revitalisation programmes should pay specific attention to poor children and endeavour to provide them with better living conditions, thereby reducing the burden of multimorbidity in later life. Second, expanding existing child health services to include child support interventions that advance nurturing care is essential for a multisectoral effort to support families and benefit children.56 For example, services that improve the nutritional status of infants and young children as well as social work practice that identifies and intervenes in children’s unfavourable developmental environments (eg, family violence and parental quarrels) may be helpful. Third, clinicians should develop early preventive interventions for susceptible middle-aged and older adults (such as those with ELA experience, female gender, lower education, etc) so as to alleviate the rapid increases in chronic diseases in later adulthood. The integral treatment or management of multimorbidity should be implemented to mitigate the deleterious health consequences of ELA. Moreover, future studies that identify mechanisms linking ELA with multimorbidity are needed to inform health management strategies and social policies that support long-term health in middle-aged and older adults.
Supplementary Material
Acknowledgments
The authors thank the CHARLS research team, the field team and every respondent for their time and efforts devoted to the CHARLS project.
Footnotes
Contributors: HL (guarantor), XZhao: conceptualisation, methodology, writing—original draft, writing—review and editing. MZ, XZhang: writing—original draft, formal analysis. All authors contributed to the planning, conduct and reporting of this study. All authors had full access to all data and can take responsibility for the integrity of the data analysis.
Funding: This work was supported by the National Natural Science Foundation of China (grant number 72274222, 72004236) and the Humanities and Social Science Fund of the Ministry of Education of China (grant number 23YJC840043).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available. Data are available in a public, open access repository. Data were derived from the China Health and Retirement Longitudinal Study (CHARLS). Researchers who want to use these data can visit http://charls.pku.edu.cn/
Ethics statements
Patient consent for publication
Not required.
Ethics approval
CHARLS was approved by the Peking University Ethical Review Committee. The current study is a secondary analysis of the deidentified CHARLS public data. The Ethics Review Committee granted an exempt research determination to the current study.
References
Articles from BMJ Open are provided here courtesy of BMJ Publishing Group
Citations & impact
Impact metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1136/bmjopen-2023-075834
Article citations
Exploring the association between Frailty Index and low back pain in middle-aged and older Chinese adults: a cross-sectional analysis of data from the China Health and Retirement Longitudinal Study (CHARLS).
BMJ Open, 14(5):e085645, 27 May 2024
Cited by: 1 article | PMID: 38802272 | PMCID: PMC11131124
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Patterns of multimorbidity trajectories and their correlates among Korean older adults.
Age Ageing, 50(4):1336-1341, 01 Jun 2021
Cited by: 13 articles | PMID: 33570586
Adverse Childhood Experiences and Subsequent Chronic Diseases Among Middle-aged or Older Adults in China and Associations With Demographic and Socioeconomic Characteristics.
JAMA Netw Open, 4(10):e2130143, 01 Oct 2021
Cited by: 62 articles | PMID: 34694390 | PMCID: PMC8546496
Early-life adversity and edentulism among Chinese older adults.
BMC Oral Health, 22(1):542, 25 Nov 2022
Cited by: 0 articles | PMID: 36434640 | PMCID: PMC9700936
Early life adversity as a risk factor for cognitive impairment and Alzheimer's disease.
Transl Neurodegener, 12(1):25, 12 May 2023
Cited by: 13 articles | PMID: 37173751 | PMCID: PMC10182702
Review Free full text in Europe PMC
Funding
Funders who supported this work.
Humanities and Social Science Fund of Ministry of Education of China (1)
Grant ID: 23YJC840043
the National Natural Science Foundation of China (2)
Grant ID: 72274222
Grant ID: 72004236

 2
2