Abstract
Introduction
Late-onset Pompe disease (LOPD) is characterized by a progressive myopathy resulting from a deficiency of acid α-glucosidase enzyme activity. Enzyme replacement therapy has been shown to be effective, but long-term treatment results vary. Avalglucosidase alfa demonstrated non-inferiority to alglucosidase alfa in a phase 3 study, allowing in France compassionate access for advanced LOPD patients unresponsive to alglucosidase alfa.Methods
Data from the French Pompe registry were analyzed for patients who benefited from a switch to avalglucosidase alfa with at least 1 year of follow-up. Respiratory (forced vital capacity [FVC]) and motor functions (Six-Minute Walk Test [6MWT]) were assessed before and 1 year after switching. Individual changes in FVC and 6MWT were expressed as slopes and statistical analyses were performed to compare values.Results
Twenty-nine patients were included (mean age 56 years, 11 years of prior treatment). The FVC and 6MWT values remained stable. The individual analyses showed a stabilization of motor worsening: -1 m/year on the 6MWT after the switch versus -63 m/year the year before the switch (i.e., a worsening of 33%/year before vs. an improvement of 3%/year later). Respiratory data were not statistically different.Discussion
At the group level, gait parameters improved slightly with a stabilization of previous worsening, but respiratory parameters showed limited changes. At the individual level, results were discordant, with some patients with a good motor or respiratory response and some with further worsening.Conclusion
Switching to avalglucosidase alfa demonstrated varied responses in advanced LOPD patients with failing alglucosidase alfa therapy, with a general improvement in motor stabilization.Free full text

Real‐life effectiveness 1 year after switching to avalglucosidase alfa in late‐onset Pompe disease patients worsening on alglucosidase alfa therapy: A French cohort study
Abstract
Introduction
Late‐onset Pompe disease (LOPD) is characterized by a progressive myopathy resulting from a deficiency of acid α‐glucosidase enzyme activity. Enzyme replacement therapy has been shown to be effective, but long‐term treatment results vary. Avalglucosidase alfa demonstrated non‐inferiority to alglucosidase alfa in a phase 3 study, allowing in France compassionate access for advanced LOPD patients unresponsive to alglucosidase alfa.
Methods
Data from the French Pompe registry were analyzed for patients who benefited from a switch to avalglucosidase alfa with at least 1 year of follow‐up. Respiratory (forced vital capacity [FVC]) and motor functions (Six‐Minute Walk Test [6MWT]) were assessed before and 1
year of follow‐up. Respiratory (forced vital capacity [FVC]) and motor functions (Six‐Minute Walk Test [6MWT]) were assessed before and 1 year after switching. Individual changes in FVC and 6MWT were expressed as slopes and statistical analyses were performed to compare values.
year after switching. Individual changes in FVC and 6MWT were expressed as slopes and statistical analyses were performed to compare values.
Results
Twenty‐nine patients were included (mean age 56 years, 11
years, 11 years of prior treatment). The FVC and 6MWT values remained stable. The individual analyses showed a stabilization of motor worsening: –1
years of prior treatment). The FVC and 6MWT values remained stable. The individual analyses showed a stabilization of motor worsening: –1 m/year on the 6MWT after the switch versus –63
m/year on the 6MWT after the switch versus –63 m/year the year before the switch (i.e., a worsening of 33%/year before vs. an improvement of 3%/year later). Respiratory data were not statistically different.
m/year the year before the switch (i.e., a worsening of 33%/year before vs. an improvement of 3%/year later). Respiratory data were not statistically different.
Discussion
At the group level, gait parameters improved slightly with a stabilization of previous worsening, but respiratory parameters showed limited changes. At the individual level, results were discordant, with some patients with a good motor or respiratory response and some with further worsening.
Conclusion
Switching to avalglucosidase alfa demonstrated varied responses in advanced LOPD patients with failing alglucosidase alfa therapy, with a general improvement in motor stabilization.
INTRODUCTION
Late‐onset Pompe disease (LOPD) is a recessively inherited disease muscle glycogenosis (GSDII) due to the deficiency of the lysosomal enzyme acid alpha‐glucosidase (GAA). This enzyme deficiency leads to glycogen storage and disturbance of autophagy in skeletal muscle, with progressive proximal and axial muscle weakness, associated with diaphragmatic weakness resulting in respiratory insufficiency [1]. Enzyme replacement therapy (ERT) with recombinant GAA (rhGAA) alglucosidase alfa, which was marketed in 2006, improved or stabilized the disease in patients with LOPD, but a large number of patients declined after 3–5 years of treatment [1, 2, 3, 4]. Avalglucosidase alfa is a next‐generation recombinant human GAA ERT, designed for enhanced targeting of mannose‐6‐phosphate (M6P) receptor‐mediated uptake [5, 6, 7]. A phase 3 study (“COMET”) comparing both ERTs showed a non‐inferiority of avalglucosidase alfa compared with rare alglucosidase alfa in naïve patients; after 49
years of treatment [1, 2, 3, 4]. Avalglucosidase alfa is a next‐generation recombinant human GAA ERT, designed for enhanced targeting of mannose‐6‐phosphate (M6P) receptor‐mediated uptake [5, 6, 7]. A phase 3 study (“COMET”) comparing both ERTs showed a non‐inferiority of avalglucosidase alfa compared with rare alglucosidase alfa in naïve patients; after 49 weeks, patients treated with avalglucosidase alfa exhibited greater improvements in forced vital capacity (FVC) (percent predicted) and Six‐Minute Walk Test (6MWT) distance than patients treated with alglucosidase alfa [8]. As a consequence of this clinical trial, avalglucosidase alfa received marketing authorization in several countries for treatment of patients with LOPD.
weeks, patients treated with avalglucosidase alfa exhibited greater improvements in forced vital capacity (FVC) (percent predicted) and Six‐Minute Walk Test (6MWT) distance than patients treated with alglucosidase alfa [8]. As a consequence of this clinical trial, avalglucosidase alfa received marketing authorization in several countries for treatment of patients with LOPD.
Based on these results, it has been possible in France to prescribe avalglucosidase alpha for patients initially treated with alglucosidase alfa with the criterion of non‐responsiveness to this treatment, since 11 June 2020 with a temporary authorization for use (compassionate access). This was followed with an early access program for the patients since 22 September 2022 (“Haute Autorité de Santé”, Decision 2022.0330/DC/SEM). The criteria for treatment failure were defined during collegial discussions in the monthly meetings of the French neuromuscular network (FILNEMUS): significant worsening of respiratory and/or motor parameters after at least 1 year of alglucosidase alfa treatment, without intercurrent disease that could account for this clinical deterioration such as severe COVID, hip fracture, vertebral fracture, or significant weight variations. To direct the switch and try to define “a poor alglucosidase alfa responder” among our patients, we also considered the initial response during this discussion when considering the data from the French registry: for instance, to be considered as a good initial response, the attended response should be an improvement of at least 1.4% per year for the first 2
year of alglucosidase alfa treatment, without intercurrent disease that could account for this clinical deterioration such as severe COVID, hip fracture, vertebral fracture, or significant weight variations. To direct the switch and try to define “a poor alglucosidase alfa responder” among our patients, we also considered the initial response during this discussion when considering the data from the French registry: for instance, to be considered as a good initial response, the attended response should be an improvement of at least 1.4% per year for the first 2 years of alglucosidase alfa treatment for the 6MWT [1, 9].
years of alglucosidase alfa treatment for the 6MWT [1, 9].
The aim of this study was to provide real‐life data on the effectiveness of a treatment switch for a selected adult population with failure of alglucosidase alfa, based on the cohort of LOPD patients prospectively followed in the French Pompe registry. It seems important to report these results because all the patients who were included in the phase 3 study “COMET” were non‐treated “naïve” patients, and therefore there currently is no information available on the benefit of avalglucosidase alfa in patients previously treated with alglucosidase alfa.
METHODS
The French Pompe Registry
Data were extracted from the French Pompe Registry (about 150 active patients in 2019–2020). This registry falls within the scope of Chapter IX of the French Data Protection Act and follows the Recommendations of Ethics and Good Practices in Epidemiology (version France ‐ 2007). The protocol, patient information note/consent, and all other relevant documents have been submitted to the competent authorities for approval in accordance with national legislation. In particular, the authorizations of the National Commission for Information Technology and Freedoms (CNIL No. 909139) and the Consultative Committee for the Treatment of Information in the field of Health (CCTIRS No. 08.461) were obtained.
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Endpoints
Respiratory function was assessed by measuring the FVC (in a sitting position, expressed as volume), whereas motor function was assessed with the 6MWT (in meters) [10]. In order to better capture the individual variations, we also expressed respective variations of the FVC and the 6MWT for each patient for 1 year (deltaFVC and delta6MWT respectively, in liters and meters), the year before the switch (pre‐), and the year after the switch (post‐), also expressed as slopes in percent per patient. For instance, the post‐slope6MWT for one patient was “6MWT at the switch moment–6MWT 1 year after)/6MWT at the switch moment (in meters)”. A negative slope indicated an improvement, both for FVC and 6MWT, whereas a positive slope showed worsening. Finally, to compare the good and bad responders’ characteristics, we considered as significant a delta of slope of 10% after the switch [11].
We chose to define a statistical analysis window of 1 year before the switch (to mirror 1
year before the switch (to mirror 1 year after the switch) but we also present the variations in the three previous years in Data S1 and S2.
year after the switch) but we also present the variations in the three previous years in Data S1 and S2.
Statistical analysis
The results are presented as the mean and standard deviation for continuous variables with a normal distribution (according to the Shapiro–Wilk test) and as the median and range for non‐normally distributed continuous variables. FVC and 6MWT as the slopesFVC and slopes6MWT were compared before the switch and 1 year later with a paired sample t‐test (Student's or Wilcoxon according to normality). Categorical variables are expressed as the number and percentage. The significance threshold was set to p
year later with a paired sample t‐test (Student's or Wilcoxon according to normality). Categorical variables are expressed as the number and percentage. The significance threshold was set to p =
= 0.05 for all analyses.
0.05 for all analyses.
Statistical analyses were performed using JASP version 0.18.1 (JASP Team, 2020).
RESULTS
A total of 29 patients were included in this study (Data S1 and S2). At the time of switch, the mean age was 56.2 (±14.9) years with 10.9 (±3.8) years of treatment duration with alglucosidase alfa. Patients were aged 43.0 (±14.1) years at the time of diagnosis, and 45.3
(±14.1) years at the time of diagnosis, and 45.3 (±13.7) years at the commencement of ERT.
(±13.7) years at the commencement of ERT.
The FVC at the time of the switch was 1993 (±1009) mL and was stable (p =
= 0.56) 1 year after the switch at 1937 (±912) mL. The median (and quartiles) represented the following percent predicted values: 49.5% [41.5; 70.5]. The 6MWT also was not different (p
0.56) 1 year after the switch at 1937 (±912) mL. The median (and quartiles) represented the following percent predicted values: 49.5% [41.5; 70.5]. The 6MWT also was not different (p =
= 0.72): 213 (±147) m at the time of the switch versus 188 (±130)
0.72): 213 (±147) m at the time of the switch versus 188 (±130) m 1
m 1 year after the switch. Nevertheless, one patient was not able to perform the 6MWT before the switch, but recorded 60
year after the switch. Nevertheless, one patient was not able to perform the 6MWT before the switch, but recorded 60 m after the switch, although two further patients who had lost their walking ability before the switch did not regain this function 1
m after the switch, although two further patients who had lost their walking ability before the switch did not regain this function 1 year later.
year later.
The individual slopesFVC showed a decrease of 162 (±320) mL in pre‐ versus an increase of 19 (±303)
mL in pre‐ versus an increase of 19 (±303) mL in post‐ (p
mL in post‐ (p =
= 0.79), representing pre‐slopesFVC of 7.68% (±15.90%) and post‐slopesFVC of −3.21% (±18.25%) (p
0.79), representing pre‐slopesFVC of 7.68% (±15.90%) and post‐slopesFVC of −3.21% (±18.25%) (p =
= 0.07; Figure 1a).
0.07; Figure 1a).
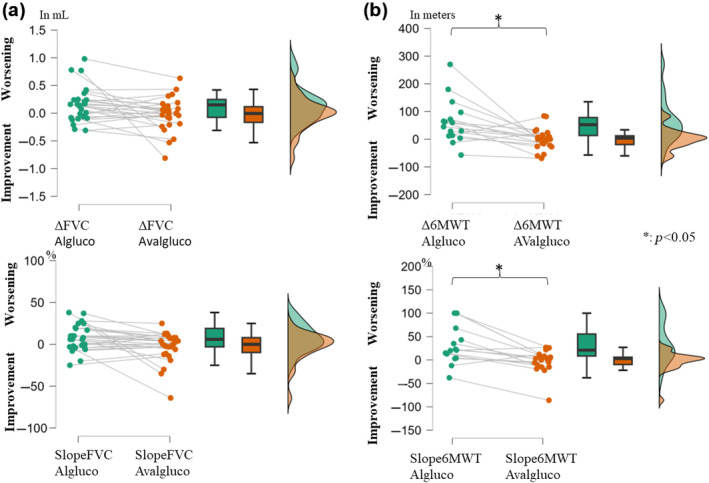
Changes in the forced vital capacity (FVC) (a) and Six‐Minute Walk Test (6MWT) (b) in milliliters/meter (mL/m) (upper) or in percent (%) of the personal value (lower), the year before the switch (left, in green), and the year after the switch (right, in orange). A positive value indicates a worsening whereas a negative value indicates an improvement. The graphs show (from left to right) a point‐per‐point representation, box plots, and violin plots (distributions of numeric data for one or more groups using density curves) for each variable. Note the significant change in 6MWT after the switch with a stabilization of the gait function. Both delta and slopes for 6MWT were significantly different before and after the switch (no significant difference for the FVC delta and slopes). Algluco, alglucosidase alfa; AValgluco, avalglucosidase alfa.
The individual delta6MWT showed a decrease of 63 (±79) m in pre‐ versus a decrease of 1 (±37) m in post‐ (p
m in post‐ (p =
= 0.008), representing pre‐slopes6MWT of 33.00% (±42.04%) and post‐slopes6MWT of −2.46% (±22.86%) (p
0.008), representing pre‐slopes6MWT of 33.00% (±42.04%) and post‐slopes6MWT of −2.46% (±22.86%) (p =
= 0.02; Figure 1b).
0.02; Figure 1b).
Concerning the differences between good and bad responders on FVC, the good responders (n =
= 7) were more severe with respect to their respiratory data at the time of the switch (Table 1) with a FVC of 43.6% (±16.7%) of percent predicted versus 79.8% (±25.3%) of percent predicted for patients with a further worsening (n
7) were more severe with respect to their respiratory data at the time of the switch (Table 1) with a FVC of 43.6% (±16.7%) of percent predicted versus 79.8% (±25.3%) of percent predicted for patients with a further worsening (n =
= 5). Concerning the good responders on the 6MWT (n
5). Concerning the good responders on the 6MWT (n =
= 6) compared to the bad responders (=4), no data were significant. In accordance with the definition, the delta and slope of FVC differed between good and bad respiratory responders, but only during the year following the switch, similar to the delta and slopes of the 6MWT for good and bad motor responders. However, there was no link between the motor variations and respiratory response and vice versa. Only two patients exhibited both motor and respiratory improvement.
6) compared to the bad responders (=4), no data were significant. In accordance with the definition, the delta and slope of FVC differed between good and bad respiratory responders, but only during the year following the switch, similar to the delta and slopes of the 6MWT for good and bad motor responders. However, there was no link between the motor variations and respiratory response and vice versa. Only two patients exhibited both motor and respiratory improvement.
TABLE 1
Differences between good and bad responders.
| Parameter | Delta FVC ≥ ≥ 10% 10% | N | Mean | SD | P‐value | Delta 6MWT ≥ ≥ 10% 10% | N | Mean | SD | P‐value |
|---|---|---|---|---|---|---|---|---|---|---|
| Demographics | ||||||||||
| Diagnosis age (years) | Improvement | 7 | 33.57 | 10.57 | 0.367 | Improvement | 6 | 38.83 | 17.62 | 0.336 |
| Worsening | 5 | 41.80 | 11.74 | Worsening | 4 | 48.50 | 17.79 | |||
| Weight at switch (kg) | Improvement | 7 | 76.85 | 17.19 | 0.410 | Improvement | 6 | 75.60 | 10.25 | 0.437 |
| Worsening | 5 | 72.80 | 24.18 | Worsening | 4 | 67.67 | 19.66 | |||
| ERT beginning age (years) | Improvement | 7 | 40.43 | 8.87 | 0.684 | Improvement | 6 | 43.00 | 15.95 | 0.331 |
| Worsening | 5 | 44.00 | 10.42 | Worsening | 4 | 52.50 | 19.84 | |||
| ERT switch age (years) | Improvement | 7 | 51.00 | 10.83 | 0.745 | Improvement | 6 | 54.33 | 14.86 | 0.257 |
| Worsening | 5 | 52.80 | 13.88 | Worsening | 4 | 64.25 | 23.99 | |||
| ERT duration before switch (years) | Improvement | 7 | 10.57 | 3.60 | 0.463 | Improvement | 6 | 11.33 | 4.89 | 1.000 |
| Worsening | 5 | 8.80 | 3.83 | Worsening | 4 | 11.75 | 4.65 | |||
| Respiratory parameters | ||||||||||
| FVC at switch (L) | Improvement | 7 | 1.821 | 1.166 | 0.167 | Improvement | 6 | 1.883 | 1.179 | 0.548 |
| Worsening | 5 | 2.652 | 0.970 | Worsening | 3 | 2.027 | 0.256 | |||
| FVC at switch (% of the theorical values) | Improvement | 7 | 43.6 | 16.7 | 0.010 | Improvement | 6 | 49.4 | 16.3 | 0.795 |
| Worsening | 5 | 79.8 | 25.3 | Worsening | 3 | 53.7 | 16.3 | |||
| FVC in supine position (L) | Improvement | 2 | 1.195 | 0.629 | 0.333 | Improvement | 1 | 1.040 | ||
| Worsening | 2 | 2.640 | 0.622 | Worsening | 2 | 1.950 | 0.438 | |||
| FVC in supine position (% predicted) | Improvement | 2 | 37.50 | 14.85 | 0.333 | Improvement | 1 | 29.00 | ||
| Worsening | 2 | 78.00 | 32.52 | Worsening | 2 | 59.00 | 15.56 | |||
| Maximal inspiratory pressure (cmH20) | Improvement | 5 | 35.22 | 17.47 | 1.000 | Improvement | 5 | 48.05 | 35.71 | |
| Worsening | 3 | 30.66 | 13.81 | Worsening | 1 | 33.00 | ||||
| Maximal inspiratory pressure (% of theorical values) | Improvement | 5 | 40.80 | 17.20 | 0.905 | Improvement | 5 | 55.53 | 35.69 | |
| Worsening | 4 | 42.18 | 17.26 | Worsening | 1 | 37.00 | ||||
| Maximal expiratory pressure (cmH20) | Improvement | 5 | 48.05 | 24.95 | 0.857 | Improvement | 5 | 47.34 | 8.07 | |
| Worsening | 2 | 37.46 | 16.33 | Worsening | 1 | 32.00 | ||||
| Maximal expiratory pressure (% of theorical values) | Improvement | 5 | 44.40 | 26.89 | 1.000 | Improvement | 5 | 48.72 | 9.26 | |
| Worsening | 3 | 44.34 | 10.01 | Worsening | 1 | 20.00 | ||||
| Motor parameters | ||||||||||
| 6MWT at switch (m) | Improvement | 4 | 100.25 | 91.46 | 0.533 | Improvement | 5 | 161.20 | 111.20 | 0.571 |
| Worsening | 2 | 245.50 | 181.73 | Worsening | 2 | 217.50 | 119.50 | |||
| Motor functional measure dimension 1 | Improvement | 6 | 25.77 | 30.13 | 0.517 | Improvement | 5 | 30.26 | 32.40 | |
| Worsening | 3 | 47.86 | 30.91 | Worsening | 1 | 66.67 | ||||
| Motor functional measure dimension 2 | Improvement | 6 | 76.78 | 11.53 | 0.154 | Improvement | 5 | 76.67 | 24.42 | |
| Worsening | 3 | 89.81 | 6.99 | Worsening | 1 | 100.00 | ||||
| Motor functional measure dimension 3 | Improvement | 6 | 95.19 | 3.01 | 0.596 | Improvement | 5 | 95.44 | 5.43 | |
| Worsening | 3 | 93.65 | 5.50 | Worsening | 1 | 100.00 | ||||
| Total motor functional measure | Improvement | 6 | 61.64 | 16.08 | 0.381 | Improvement | 5 | 61.88 | 22.60 | |
| Worsening | 3 | 73.61 | 12.43 | Worsening | 1 | 86.46 | ||||
| Evolution parameters | ||||||||||
| deltaFVC (Y2–Y1) (L) | Improvement | 6 | 0.018 | 0.248 | 0.126 | Improvement | 6 | 0.113 | 0.227 | 0.697 |
| Worsening | 5 | 1.37 | 1.782 | Worsening | 3 | 0.377 | 0.49 | |||
| slopeFVC (Y2–Y1) (%) | Improvement | 6 | −0.6 | 14.20 | 0.120 | Improvement | 6 | 8.40 | 10.10 | 0.905 |
| Worsening | 5 | 43.8 | 51.70 | Worsening | 3 | 36.00 | 55.50 | |||
| delta6MWT (Y2–Y1) (m) | Improvement | 3 | 47 | 11.79 | 0.400 | Improvement | 5 | 18.40 | 48.73 | 0.857 |
| Worsening | 2 | 10 | 41.01 | Worsening | 2 | 67.50 | 55.86 | |||
| slope6MWT (Y2–Y1) (%) | Improvement | 2 | 40.70 | 0.30 | 0.667 | Improvement | 5 | 10.40 | 48.30 | 1.000 |
| Worsening | 2 | 8.40 | 0.19 | Worsening | 2 | 26.50 | 25.50 | |||
| deltaFVC (Y1–Switch) (L) | Improvement | 7 | 0.179 | 0.220 | 0.383 | Improvement | 6 | 0.122 | 0.175 | 0.381 |
| Worsening | 3 | 0.397 | 0.337 | Worsening | 3 | 0.027 | 0.103 | |||
| slopeFVC (Y1–Switch) (%) | Improvement | 7 | 7.80 | 10.80 | 0.383 | Improvement | 6 | 4.40 | 12.60 | 0.548 |
| Worsening | 3 | 16.60 | 14.30 | Worsening | 3 | 1.80 | 5.50 | |||
| delta6mwt (Y1–Switch) (m) | Improvement | 3 | 126.41 | 90.41 | 0.800 | Improvement | 5 | 159.55 | 110.52 | 0.571 |
| Worsening | 2 | 271.62 | 186.53 | Worsening | 2 | 215.98 | 118.75 | |||
| slope6mwt (Y1–Switch) (%) | Improvement | 3 | 98.10 | 2.00 | 0.800 | Improvement | 5 | 98.40 | 2.00 | 0.857 |
| Worsening | 2 | 99.10 | 0.04 | Worsening | 2 | 99.39 | 4.00 | |||
| deltaFVC (Switch–Y+1) (L) | Improvement | 7 | −0.393 | 0.226 | 0.003 | Improvement | 5 | −0.23 | 0.352 | 0.556 |
| Worsening | 5 | 0.376 | 0.170 | Worsening | 4 | −0.055 | 0.101 | |||
| slopeFVC (Switch–Y+1) (%) | Improvement | 7 | −26.43 | 18.95 | 0.006 | Improvement | 5 | −13.20 | 18.46 | 0.461 |
| Worsening | 5 | 14.60 | 5.94 | Worsening | 4 | −2.75 | 6.80 | |||
| delta6MWT (Switch–Y+1) (m) | Improvement | 7 | 7.43 | 35.83 | 0.222 | Improvement | 6 | −31.33 | 25.00 | 0.010 |
| Worsening | 2 | 9.50 | 0.71 | Worsening | 4 | 56.00 | 31.45 | |||
| slope6MWT (Switch–Y+1) (%) | Improvement | 7 | −1.29 | 13.756 | 0.500 | Improvement | 6 | −28.00 | 28.66 | 0.014 |
| Worsening | 2 | 5.50 | 3.536 | Worsening | 4 | 21.75 | 6.85 | |||
Note Values in bold type represent significant differences. Positive values indicate worsening with time. N varies with missing data.
Abbreviations: 6MWT, Six‐Minute Walk Test; ERT, enzyme replacement therapy; FVC, forced vital capacity; SD, standard deviation; Y1, 1 year before the switch; Y+1, 1
year before the switch; Y+1, 1 year after the switch; Y2, 2
year after the switch; Y2, 2 years before the switch.
years before the switch.
DISCUSSION
This study on the effect of avalgucosidase alfa in LOPD patients previously treated with alglucosidase alfa was based on an analysis of clinical data prospectively collected in the French Pompe registry, thanks to the collaboration of the neuromuscular network FILNEMUS. These data provide important information on the impact of this new ERT in real life for patients suffering from advanced LOPD and declining despite alglucosidase alfa therapy. Although the limited number of patients due to the rarity of this disease and the 1‐year duration of treatment prevent robust statistical analyses, we thought it important to report these data in order to aid decision‐making for future therapies. However, we proposed here to study the evolution of individual variations. It comes from the shared decision‐making process, as switching the ERT was proposed in non‐ or no‐more responding patients. With this approach we demonstrated that the gait parameters were slightly but significantly improved, but with a stabilization or an inversion of the previous worsening (n =
= 8 and 6, respectively). Here too we observed that our analyses sometimes failed to capture the situation, for example, one patient regained the ability to perform the 6MWT, representing an infinite improvement in percentage, but was excluded for the purpose of this calculation. At the group level, no significant differences were observed in raw 6WMT; however, we observed a significant modification of 6MWT evolution in 1
8 and 6, respectively). Here too we observed that our analyses sometimes failed to capture the situation, for example, one patient regained the ability to perform the 6MWT, representing an infinite improvement in percentage, but was excluded for the purpose of this calculation. At the group level, no significant differences were observed in raw 6WMT; however, we observed a significant modification of 6MWT evolution in 1 year, from a decrease of 63
year, from a decrease of 63 m versus 1
m versus 1 m, representing a worsening of 33% of the previous 6MWT in the year before the switch versus an improvement of 2% the year after. These values are generally considered to be clinically meaningful (>30
m, representing a worsening of 33% of the previous 6MWT in the year before the switch versus an improvement of 2% the year after. These values are generally considered to be clinically meaningful (>30 m and >10%). On the contrary, either at the group level or considering the evolution in 1
m and >10%). On the contrary, either at the group level or considering the evolution in 1 year, nothing was significantly different for the FVC, and mean variations were <200 mL and <10% with no clinical difference.
year, nothing was significantly different for the FVC, and mean variations were <200 mL and <10% with no clinical difference.
As regards the respiratory parameters, we failed to demonstrate a modification of the usual values (and more sensitive values as supine FVC or maximal inspiratory pressures were lacking), with individual variations of about ±5% of the percent predicted. However, we observed some good respiratory responders corresponding to more severe patients at the time of the switch. Probably, if the FVC is relatively preserved, it is more difficult to demonstrate an eventual effect. This result is also partially explained by the nonlinear curve of the FVC evolution along the disease course, with more important change, even in percent, when the value is central (about 50% of percent predicted) than where the value is high (about 75%–100% of the percent predicted, considered to be in the normal range if >80%). It seems very important to keep in mind for the interpretation of clinical trial results where the respiratory inclusion criteria vary (30%–85% percent predicted for avalglucosidase alfa [8], and no higher cut‐off for the cipaglucosidase trial [4]).
Regarding the improvement of gait function with no improvement of respiratory function, we also can address a greater placebo effect for the 6MWT with a motivational aspect. Motivation is also important for FVC assessment, but variations are observed more easily as usually three trials are conducted in the same patient, with the best value being recorded (not done for the 6MWT, which is generally not repeated). Or, more plausibly, the effect on the enzymotherapy is different for the diaphragm and other striated muscles.
The novelty of this study is that it also sheds light on personalized data analysis for respiratory and motor parameters, both for variations and slopes (in percent terms). Indeed, it does not seem logical to express variations only as raw values because losing 20 m of the 6MWT does not have the same meaning if the patient achieves 30
m of the 6MWT does not have the same meaning if the patient achieves 30 m (33% loss over 1
m (33% loss over 1 year) as if they achieve 400
year) as if they achieve 400 m (0.5% loss over 1
m (0.5% loss over 1 year); the expressions in percent predicted values can also be modified by variations in age and weight in a given patient. These comments are also valid for the FCV. That is why, as in studies of respiratory diseases where these variables are used to calculate the risk of mortality or the effectiveness of a treatment, it seemed interesting to us to propose criteria for individual variability (see Harari et al. [12] for a review). Further studies are necessary to define response or non‐response criteria for a given ERT, and to compare them with patient‐related outcomes (for activities or quality of life) [13].
year); the expressions in percent predicted values can also be modified by variations in age and weight in a given patient. These comments are also valid for the FCV. That is why, as in studies of respiratory diseases where these variables are used to calculate the risk of mortality or the effectiveness of a treatment, it seemed interesting to us to propose criteria for individual variability (see Harari et al. [12] for a review). Further studies are necessary to define response or non‐response criteria for a given ERT, and to compare them with patient‐related outcomes (for activities or quality of life) [13].
It is also necessary to mention a possible lack of power of this study, linked to the small sample size and missing data due to the study's retrospective nature. Furthermore, a 1‐year follow‐up is a relatively short timeframe in which to observe significant changes, and consequently we will continue to follow the patients to also have 3‐year follow‐up data.
The development of new therapies for LOPD presents a great opportunity. Several mechanisms of muscle impairment are described, starting with glycogen buildup and autophagy processes [14, 15]. Adding mannose‐6‐phosphate was initially designed to improve muscle penetration, for the diaphragm and skeletal muscles, due to the observation that classical ERT was less effective after 3 years of treatment [1, 2, 3, 4]. In this “real‐life” experience, current data are in favor of a benefit of switching ERT from alglucosidase alfa to avalglucosidase alfa in some “declining” patients. However, other patients continued to worsen as regards respiratory and motor functions, highlighting the need for improved investigations to understand the underlying pathophysiology and further adapt therapeutic approaches, such as including substrate reduction therapies. Further studies on the effect of the switching of ERT, on a wider range of patients and over a period of at least 3
years of treatment [1, 2, 3, 4]. In this “real‐life” experience, current data are in favor of a benefit of switching ERT from alglucosidase alfa to avalglucosidase alfa in some “declining” patients. However, other patients continued to worsen as regards respiratory and motor functions, highlighting the need for improved investigations to understand the underlying pathophysiology and further adapt therapeutic approaches, such as including substrate reduction therapies. Further studies on the effect of the switching of ERT, on a wider range of patients and over a period of at least 3 years, will be needed in order to better assess the range of efficiency of avalglucosidase alfa in patients previously treated with alglucosidase alfa.
years, will be needed in order to better assess the range of efficiency of avalglucosidase alfa in patients previously treated with alglucosidase alfa.
In conclusion, we have demonstrated in this real‐life study that patients suffering from LOPD on prior enzymotherapy with alglucosidase alfa, having been switched to avalglucosidase alfa because of respiratory and/or motor worsening, present a significant stabilization of the slope of motor decline. On the respiratory level, no significant change was observed after 1 year of switch. Longer follow‐up assessments over several years, ideally including data from other countries, will be needed in order to draw conclusions in larger cohorts.
year of switch. Longer follow‐up assessments over several years, ideally including data from other countries, will be needed in order to draw conclusions in larger cohorts.
AUTHOR CONTRIBUTIONS
Céline Tard: Conceptualization; investigation; writing – original draft; methodology; validation; visualization; writing – review and editing; software; formal analysis; resources; data curation. Françoise Bouhour: Conceptualization; investigation; supervision; software; formal analysis; methodology; validation; writing – review and editing. Maud Michaud: Conceptualization; investigation; methodology; validation; writing – review and editing; formal analysis; supervision; software. Stephane Beltran: Investigation; validation; writing – review and editing. Maxime Fournier: Investigation; validation; writing – review and editing. Florence Demurger: Investigation; validation; writing – review and editing. Emmeline Lagrange: Investigation; validation; writing – review and editing. Sylvain Nollet: Investigation; validation; writing – review and editing. Sabrina Sacconi: Investigation; validation; writing – review and editing. Jean‐Baptiste Noury: Investigation; validation; writing – review and editing; supervision; methodology. Armelle Magot: Investigation; methodology; validation; writing – review and editing; supervision. Pascal Cintas: Investigation; validation; writing – review and editing. Dimitri Renard: Investigation; validation; writing – review and editing. Joëlle Deibener‐Kaminsky: Investigation; validation; writing – review and editing. Claire Lefeuvre: Investigation; conceptualization; data curation; supervision; validation; methodology; writing – review and editing; formal analysis; funding acquisition. Jean‐Baptiste Davion: Investigation; validation; writing – review and editing. Emmanuelle Salort‐Campana: Investigation; validation; writing – review and editing. Azzeddine Arrassi: Data curation; writing – review and editing; resources; project administration; supervision; investigation. Nadjib Taouagh: Writing – review and editing; data curation; resources; supervision; investigation. Marco Spinazzi: Conceptualization; investigation; writing – review and editing; validation; visualization. Shahram Attarian: Investigation; validation; writing – review and editing; resources; data curation; funding acquisition. Pascal Laforêt: Conceptualization; investigation; writing – review and editing; methodology; validation; visualization; software; data curation; supervision; resources.
CONFLICT OF INTEREST STATEMENT
Related to Pompe disease management, Céline Tard reports personal fees and non‐financial support from Sanofi, and personal fees and non‐financial support from Amicus; Marco Spinazzi reports personal fees and non‐financial support from Sanofi; Jean‐Baptiste Noury reports personal fees and non‐financial support from Sanofi, and personal fees and non‐financial support from Amicus; Sylvain Nollet reports no disclosures; Jean‐Baptiste Davion personal fees and non‐financial support from Sanofi, and non‐financial support from Amicus; Pascal Cintas reports personal fees and non‐financial support from Sanofi, and personal fees and non‐financial support from Amicus; Emmanuelle Salort‐Campana reports personal fees and non‐financial support from Sanofi, personal fees and non‐financial support from Amicus, and non‐financial support from Sparks; Armelle Magot reports personal fees and non‐financial support from Sanofi; Pascal Laforêt reports personal fees and non‐financial support from Sanofi, personal fees and non‐financial support from Amicus, and non‐financial support from Sparks; Claire Lefeuvre reports personal fees and non‐financial support from Sanofi; Stephane Beltran reports no disclosures; Sabrina Sacconi reports personal fees and non‐financial support from Sanofi, personal fees and non‐financial support from Amicus; and Shahram Attarian reports personal fees and non‐financial support from Sanofi, personal fees and non‐financial support from Amicus, and non‐financial support from Sparks.
Supporting information
Data S1.
Data S2.
ACKNOWLEDGMENTS
We thank The Myology Institute for administrative and legal support; and Sanofi and Amicus for financial sponsorship of the French Pompe Registry.
APPENDIX A.
Laforêt P (Garches), Orlikowski D (Garches), Masingue M (Paris), Stojkovic T (Paris), Lefeuvre C (Garches), Béhin A (Paris), Garcia PY (Compiègne), Lallement F (St Brieuc), Bassez G (Paris), Germain D (Garches), Noury JB (Brest), Tard C (Lille), Davion JB (Lille), Salort‐Campana E (Marseille), Kouton L (Marseille), Attarian S (Marseille), Verschueren A (Marseille), Sacconi S (Nice), Furby A (St‐Etienne), Kaminsky AL (St‐Etienne), Lacour A (St‐Etienne), Praline J (Tours), Beltran S (Tours), Sole G (Bordeaux), Duval F (Bordeaux), Renard D (Nîmes), Bouibede F (Orléans), Fournier M (Caen), Chapon F (Caen), Minot MC (Rennes), Spinazzi M (Angers), Hubert J (Vannes), Demurger F (Vannes), Magot A (Nantes), Pereon Y (Nantes), Cintas P (Toulouse), Acket B (Toulouse), Nadaj‐Pakleza A (Strasbourg), Bouhour F (Lyon), Morales RJ (Montpellier), Esselin F (Montpellier), Petiot P (Lyon), Jaussaud R (Nancy), Mohamed S (Nancy), Deibener‐Kaminsky J (Nancy), Echaniz‐Laguna YA (Krémlin‐Bicêtre), Magy L (Limoges), Michaud M (Nancy), Lagrange E (Grenoble), Krim E (Pau), Taithe F (Clermont‐Ferrand), Bedat‐Millet AL (Rouen), Nollet S (Besançon), Toquet S (Reims) Diab E (Amiens), Maillet‐Vioud M (Monluçon).
Notes
Tard C, Bouhour F, Michaud M, et al. Real‐life effectiveness 1 year after switching to avalglucosidase alfa in late‐onset Pompe disease patients worsening on alglucosidase alfa therapy: A French cohort study. Eur J Neurol. 2024;31:e16292. 10.1111/ene.16292 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
 years of enzyme therapy in adults with Pompe disease. Neurology. 2019;93:e1756‐e1767. 10.1212/WNL.0000000000008441
[Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
years of enzyme therapy in adults with Pompe disease. Neurology. 2019;93:e1756‐e1767. 10.1212/WNL.0000000000008441
[Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]Articles from European Journal of Neurology are provided here courtesy of John Wiley & Sons Ltd on behalf of European Academy of Neurology (EAN)
Full text links
Read article at publisher's site: https://doi.org/10.1111/ene.16292
Read article for free, from open access legal sources, via Unpaywall:
https://onlinelibrary.wiley.com/doi/pdfdirect/10.1111/ene.16292
Citations & impact
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/165517861
Article citations
Real-life effectiveness 1 year after switching to avalglucosidase alfa in late-onset Pompe disease patients worsening on alglucosidase alfa therapy: A French cohort study.
Eur J Neurol, 31(7):e16292, 08 Apr 2024
Cited by: 1 article | PMID: 38587143 | PMCID: PMC11235864
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Enzyme replacement therapy for late-onset Pompe disease.
Cochrane Database Syst Rev, 12:CD012993, 12 Dec 2023
Cited by: 3 articles | PMID: 38084761
Review
Safety and efficacy of avalglucosidase alfa versus alglucosidase alfa in patients with late-onset Pompe disease (COMET): a phase 3, randomised, multicentre trial.
Lancet Neurol, 20(12):1012-1026, 01 Dec 2021
Cited by: 56 articles | PMID: 34800399
Long-term Safety and Efficacy of Avalglucosidase Alfa in Patients With Late-Onset Pompe Disease.
Neurology, 99(5):e536-e548, 01 Aug 2022
Cited by: 17 articles | PMID: 35618441 | PMCID: PMC9421599
Safety, tolerability, pharmacokinetics, pharmacodynamics, and exploratory efficacy of the novel enzyme replacement therapy avalglucosidase alfa (neoGAA) in treatment-naïve and alglucosidase alfa-treated patients with late-onset Pompe disease: A phase 1, open-label, multicenter, multinational, ascending dose study.
Neuromuscul Disord, 29(3):167-186, 17 Dec 2018
Cited by: 38 articles | PMID: 30770310
Funding
Funders who supported this work.

 1
,
2
1
,
2



