Abstract
Aims
Atrial fibrillation (AF) and concomitant cardiometabolic disease processes interact and combine to lead to adverse events, such as stroke, heart failure, myocardial infarction, and cardiovascular death. Circulating biomolecules provide quantifiable proxies for cardiometabolic disease processes. The aim of this study was to test whether biomolecule combinations can define phenotypes in patients with AF.Methods and results
This pre-specified analysis of the EAST-AFNET 4 biomolecule study assigned patients to clusters using polytomous variable latent-class analysis based on baseline concentrations of 13 precisely quantified biomolecules potentially reflecting ageing, cardiac fibrosis, metabolic dysfunction, oxidative stress, cardiac load, endothelial dysfunction, and inflammation. In each cluster, rates of cardiovascular death, stroke, or hospitalization for heart failure or acute coronary syndrome, the primary outcome of EAST-AFNET 4, were calculated and compared between clusters over median 5.1 years follow-up. Findings were independently validated in a prospective cohort of 748 patients with AF (BBC-AF; median follow-up 2.9 years).Unsupervised biomolecule analysis assigned 1586 patients (71 years old, 46% women) into four clusters. The highest risk cluster was dominated by elevated bone morphogenetic protein 10, insulin-like growth factor-binding protein 7, N-terminal pro-B-type natriuretic peptide, angiopoietin 2, and growth differentiation factor 15. Patients in the lowest risk cluster showed low concentrations of these biomolecules. Two intermediate-risk clusters differed by high or low concentrations of C-reactive protein, interleukin-6, and D-dimer. Patients in the highest risk cluster had a five-fold higher cardiovascular event rate than patients in the low-risk cluster. Early rhythm control was effective across clusters (Pinteraction = 0.63). Sensitivity analyses and external validation in BBC-AF replicated clusters and risk gradients.Conclusion
Biomolecule concentrations identify cardiometabolic subphenotypes in patients with AF at high and low cardiovascular risk.Free full text

Blood-based cardiometabolic phenotypes in atrial fibrillation and their associated risk: EAST-AFNET 4 biomolecule study
Abstract
Aims
Atrial fibrillation (AF) and concomitant cardiometabolic disease processes interact and combine to lead to adverse events, such as stroke, heart failure, myocardial infarction, and cardiovascular death. Circulating biomolecules provide quantifiable proxies for cardiometabolic disease processes. The aim of this study was to test whether biomolecule combinations can define phenotypes in patients with AF.
Methods and results
This pre-specified analysis of the EAST-AFNET 4 biomolecule study assigned patients to clusters using polytomous variable latent-class analysis based on baseline concentrations of 13 precisely quantified biomolecules potentially reflecting ageing, cardiac fibrosis, metabolic dysfunction, oxidative stress, cardiac load, endothelial dysfunction, and inflammation. In each cluster, rates of cardiovascular death, stroke, or hospitalization for heart failure or acute coronary syndrome, the primary outcome of EAST-AFNET 4, were calculated and compared between clusters over median 5.1 years follow-up. Findings were independently validated in a prospective cohort of 748 patients with AF (BBC-AF; median follow-up 2.9 years).
Unsupervised biomolecule analysis assigned 1586 patients (71 years old, 46% women) into four clusters. The highest risk cluster was dominated by elevated bone morphogenetic protein 10, insulin-like growth factor–binding protein 7, N-terminal pro-B-type natriuretic peptide, angiopoietin 2, and growth differentiation factor 15. Patients in the lowest risk cluster showed low concentrations of these biomolecules. Two intermediate-risk clusters differed by high or low concentrations of C-reactive protein, interleukin-6, and D-dimer. Patients in the highest risk cluster had a five-fold higher cardiovascular event rate than patients in the low-risk cluster. Early rhythm control was effective across clusters (Pinteraction = 0.63). Sensitivity analyses and external validation in BBC-AF replicated clusters and risk gradients.
Conclusion
Biomolecule concentrations identify cardiometabolic subphenotypes in patients with AF at high and low cardiovascular risk.
Graphical Abstract
Graphical Abstract
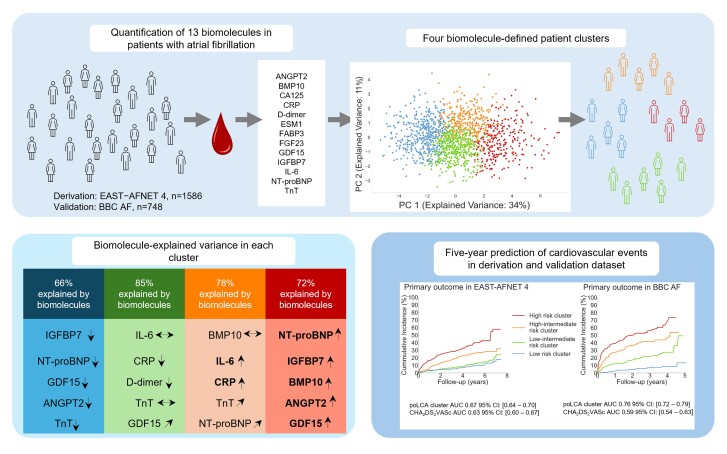
The quantification of 13 biomolecules pre-selected for their ability to provide quantitative proxies for cardiovascular disease processes assigns patients with recently diagnosed atrial fibrillation and comorbidities to four clusters. The main biomolecules contributing to the clustering process are N-terminal pro-B-type natriuretic peptide, insulin-like growth factor–binding protein 7, bone morphogenetic protein 10, angiopoietin 2, growth differentiation factor 15, interleukin-6, and C-reactive protein. These biomolecules explain 66–85% of the assignment to a cluster. The four clusters differ in their risk of cardiovascular events (cardiovascular death, stroke, or unplanned hospitalisation for heart failure or acute coronary syndrome) over a 5-year time horizon. Early rhythm control therapy is effective in all four clusters (Pinteraction = 0.63). The clusters and their associated risk are replicated in an independent cohort.
Time of primary review: 9 days
1. Introduction
Several chronic and interacting disease processes1,2 contribute to the development of atrial fibrillation (AF) and cardiovascular events in patients with AF.3,4 More than 80% of patients with AF suffer from comorbidities, such as hypertension, heart failure, diabetes, or coronary, cerebral, or peripheral artery disease at the time of diagnosing AF.4,5 These include cardiometabolic dysfunction,6 systemic and atrial thromboinflammation,7,8 vascular and endothelial dysfunction,7,8 and cardiomyocyte dysfunction.9 Quantification of underlying disease processes and their interactions may identify drivers of cardiovascular complications in patients with AF.
Circulating biomolecules provide quantitative proxies of cardiovascular disease processes, including at early, subclinical stages.1,2 For example, slight, chronic elevations of circulating troponin concentrations are associated with subclinical myocyte injury and increased cardiovascular risk,10 including in patients with AF.11 Quantification of selected biomolecules in a single blood draw can further refine the prediction of stroke and bleeding risk in patients with AF.11–13 Whether a selection of biomolecules aiming to represent different cardiovascular and inflammatory disease processes can be used to identify cardiometabolic subphenotypes in patients with AF has not been tested.
This pre-specified secondary analysis of the EAST-AFNET 4 biomolecule study embedded into the Early treatment of Atrial fibrillation for STroke prevention (EAST-AFNET 4) trial14 quantified 13 biomolecules reflecting different disease processes in AF that were defined a priori.1 Unsupervised clustering methods capturing interactions between biomolecules were applied to identify patients at risk of cardiovascular events based on biomolecule concentrations. Additionally, independent validation was performed in a prospective registry of patients with AF (BBC-AF15).
2. Methods
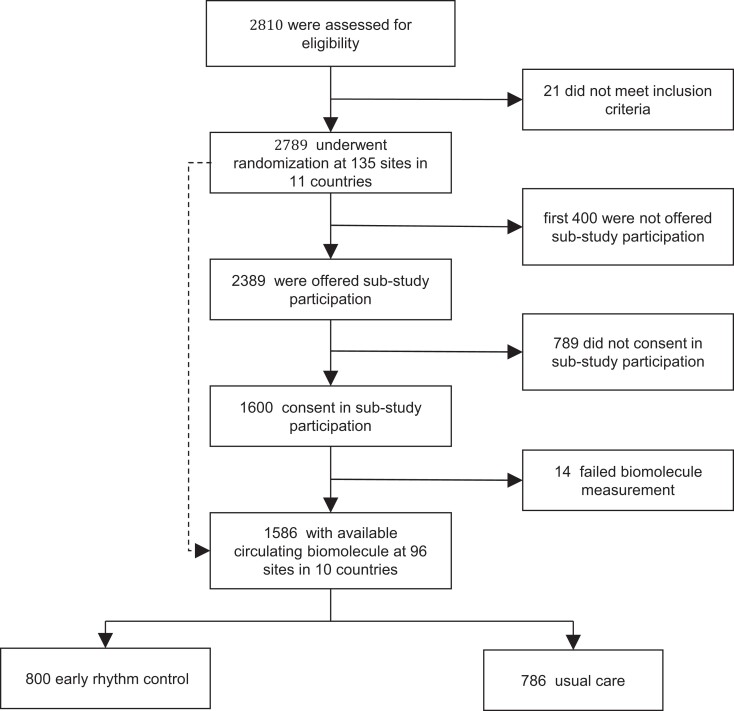
The main analyses described here report a pre-specified analysis of the EAST-AFNET 4 biomolecule study (see Supplementary material online, Protocol Appendix).16Post hoc exploratory analyses were added to better understand the main findings (Figure Figure11).
2.1. Derivation cohort (EAST-AFNET 4)
The early treatment of AF for stroke prevention trial (EAST-AFNET 4) randomized patients with recently diagnosed AF and stroke risk factors to systematic early rhythm control or usual care, including symptom-based rhythm control.14 All patients were followed up for a median of 5.1 years. The primary outcome of the trial was a composite of stroke, cardiovascular death, and unplanned hospitalization for heart failure or acute coronary syndrome.14 Details of the EAST-AFNET 4 biosample study collecting a baseline blood sample from 1586 patients enrolled in the EAST-AFNET 4 trial have been published.16 In brief, all consenting patients provided a blood sample at baseline. Samples were shipped to the core biostorage facility at UKE Hamburg, spun, shock frozen, and stored at −8°C. EAST-AFNET 4, and its biomolecule substudy were approved at all study sites. Written informed consent was obtained from all patients. This study complied with the Declaration of Helsinki.
2.2. Validation cohort (BBC-AF subcohort)
Details of the BBC-AF cohort have been described before.15 In brief, consecutive patients eligible for recruitment had electrocardiogram (ECG)-diagnosed AF or presented with at least two cardiovascular conditions (congestive heart failure, hypertension, diabetes, prior stroke, or vascular disease) to a large teaching hospital (Sandwell and West Birmingham NHS Trust). Patients who did not have a diagnosis of AF underwent 7-day ambulatory ECG monitoring to rule out undiagnosed ECG-documented AF. For this analysis, only patients with ECG-documented AF were included. All patients underwent a detailed interview, physical examination, 12-lead ECG, and transthoracic echocardiography at the time of recruitment. Follow-up data for events were collected by assessing local hospital records corroborated against Hospital Episode Statistics data, general practitioner records, and mortality data from NHS Digital, at 2.5 years after the final patient was recruited.17 The follow-up duration was calculated as the time between the baseline assessment date and an event or the date of record review, where no events were documented. This study complied with the Declaration of Helsinki, was approved by the National Research Ethics Service Committee (IRAS ID 97753), and was sponsored by the University of Birmingham. All patients provided written, informed consent.
2.3. Selection of biomolecules and their quantification
The methodological approach taken for this work tried to combine the multifaceted information of several circulating biomolecules and the reliability of high-precision assays. A group of scientists within the EU-funded CATCH-ME consortium searched literature and scouted meetings for disease mechanisms related to AF and to cardiovascular events in patients with AF. One summary of this initial effort has been published.1 In a next step, biomolecules that could potentially reflect these disease processes (called ‘health modifiers’ in Fabritz et al.1) were identified based on a literature and patent search enriched with knowledge available in the EU Horizon 2020 CATCH-ME consortium. A modified Delphi expert consensus process was conducted to identify biomolecules representing these disease mechanisms available for high-precision quantification. Thirteen biomolecules were identified (Table Table11): angiopoietin 2 (ANGPT2), bone morphogenetic protein 10 (BMP10), cancer antigen 125, C-reactive protein (CRP), D-dimer, endothelial-specific molecule 1, fatty acid–binding protein 3 (FABP3), fibroblast growth factor 23 (FGF23), growth differentiation factor 15 (GDF15), insulin-like growth factor–binding protein 7 (IGFBP7), interleukin-6 (IL-6), N-terminal pro-B-type natriuretic peptide (NT-proBNP), and cardiac troponin T (TnT).
Table 1
Disease mechanisms hypothesized to be related to AF and AF-related complications and corresponding circulating biomolecules selected for quantification in this study
| Disease process (‘health modifier’) | Biomolecule selected as quantifiable proxy of this disease process |
|---|---|
| Ageing | Growth differentiation factor 15 (GDF15) Cancer antigen 125 (CA125) |
| Loss of cardiomyocytes | Cardiac troponin (TnT) |
| Replacement of cardiomyocytes with extracellular matrix | Fibroblast growth factor 23 (FGF23) Insulin-like growth factor–binding protein 7 (IGFBP7) |
| Adaptive changes to an increased work load | N-terminal pro-B-type natriuretic peptide (NT-proBNP) |
| Delayed left atrial activation | See two rows above: replacement of cardiomyocytes with extracellular matrix |
| Spontaneous electrical activity | Possibly bone morphogenetic protein 10 (BMP10) |
| Genetic predisposition to AF | Tested using genomic analysis (see supplementary material and Kany et al.16) |
| Infiltration of fat cells in the atria and activation of atrial fat tissue | Fatty acid–binding protein 3 (FABP3) |
| Elevated atrial oxidative stress | Bone morphogenetic protein 10 (BMP10) |
| Renal dysfunction | Creatinine, also fibroblast growth factor 23 (FGF23) |
| Prothrombotic dysregulation: endothelial | Angiopoietin 2 (ANGPT2) Endothelial-specific molecule 1 (ESM1) |
| Prothrombotic dysregulation: humoral | D-dimer |
| Additional processes: systemic inflammation | Interleukin-6 (IL-6) C-reactive protein (CRP) |
The list of candidates for disease mechanisms (‘health modifiers’) is copied from Box 2 in Fabritz et al.1 with one added mechanism (systemic inflammation, last line). The biomolecules selected as quantifiable proxies of each disease process in this study are shown in the right column. The selection process is detailed in the Methods section of this paper and in Fabritz et al.1 This table aligns biomolecules with selected disease processes. The assignment of biomolecules to one mechanism is an oversimplification as the concentrations of biomolecules will be influenced by several mechanisms (see Figure Figure11 in Fabritz et al.1).
Biomolecules were centrally quantified using pre-commercial and commercial high-throughput, high-precision platforms (Roche, Penzberg, Germany). The biomolecule quantification was provided as an in-kind contribution by Roche to the EU Horizon 2020 CATCH-ME consortium. Absolute protein concentrations were centrally quantified in ethylenediaminetetraacetic acid (EDTA) plasma. Run controls and calibrators were measured twice each run, and lab staff involved were blinded to clinical status and data. Blood samples were shipped to and quantifications were conducted at the Roche biomolecule research facility in Penzberg, Germany. This is the first analysis of the biomolecules in the EAST-AFNET 4 trial substudy.
2.4. Data pre-processing and clustering
Biomolecule concentrations were 1% winsorized and Blom-transformed18 and each patient was assigned into one quintile for each biomolecule. These quintiles were used to cluster patients using polytomous variable latent-class analysis (poLCA). K-means clustering was used as sensitivity analysis. Unsupervised models were developed using LCA, available in the package poLCA19 in R (https://www.r-project.org/). LCA was performed on 13 biomarker variables. Patients with any missing biomolecule concentrations were excluded. The models were created in a bootstrapping fashion by repeating 100 times with data resampling with replacement. Each data resampling was performed with a fixed initialization seed to ensure reproducibility. Models between 2 and 10 clusters were assessed. The Bayesian information criterion (BIC) was used to assess the best number of clusters by penalizing models with too many parameters. The number of clusters with the lower BIC score was counted over all bootstraps. This most frequent number of clusters was used to create the final model using the original data, without resampling. We also fitted k-means clustering models against the same Blom-transformed biomolecules without conversion of those into categorical variables. To assess the optimal number of cluster groups, we followed the exact same approach as for poLCA models. As base model instance, we made use of SciKit-learns algorithm (https://scikit-learn.org/stable/index.html) with Lloyd algorithm,20k-means++21 as initial cluster centroid selection strategy and let the algorithm run for 10 iterations with different centroid seeds to fit the model against our data set.
2.5. Phenotypic description
Clusters were formed agnostic to any clinical data. Patients’ characteristics were summarized for each cluster. Body mass index (BMI) was categorized into obese (BMI ≥ 30) and non-obese. The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation22,23 and categorized into normal kidney function (eGFR ≥ 60 mL/min) and chronic kidney disease. The left ventricular ejection fraction was categorized into groups of ≥50 and <50%. Differences between categorical variables were calculated using generalized logistic mixed models with the study centre as random effect. For continuous variables, linear mixed models with study centre as random effect were used for normally distributed and non-normally distributed variables. P-values resulted from Analysis of Deviance Table (Type II Wald χ2 tests). The R packages lme4 and car were used for this analysis. In the non-multicentric BBC-AF cohort, t-test and χ2 test were applied for quantification of differences between continuous and categorical variables.
2.6. Commonality analysis
Biomarkers are biologically secreted and reabsorbed reflecting different disease processes. They are excreted or shed based on common pathways (e.g. secretion via the kidney), creating collinearity in the data. The presence of multicollinearity, as can be demonstrated using a correlation matrix or calculating the variance inflation factor, complicates the interpretation of regression model outcomes, since both unique and shared variances are contributing to an effect on the outcome. Commonality analysis allows an exploration of relationships between biomarkers by decomposing the R2 of the regression model or respectively the pseudo R2 of the binomial regression model to quantify unique and shared variances of each biomarker in explaining the outcome. The analysis returns 2k − 1 commonality coefficients (k = number of variables entered).
2.7. Dominance analysis
As commonality analysis is one way to assess the relative importance of predictors (p; the 13 biomolecules) on an outcome (the cluster group), dominance analysis (DA) is a different approach that makes two distinct contributions. First, it measures the relative importance of a predictor in a pairwise fashion and secondly, it does this in the context of all
2.8. Change in clustering after removal of biomolecules
To generate a more global understanding of the contribution of each biomolecule to the clustering, the patient clustering was repeated with reduced feature sets by removing one, two, three, four, or five of the biomolecules. For each possible biomolecule combination of the reduced feature sets, we estimated the optimal number of clusters, predicted the clusters, and used those to partition the patients again. We computed the adjusted rand index (ARI) for those new partitions in comparison with the original ones derived from the poLCA model fitted against 13 biomolecules and predicting four clusters. The biomolecule clusters were used as predictors in Cox proportional hazards (PH) model instances, with the first primary composite outcome as an event of interest to obtain hazard ratios and c index.
2.9. Risk of cardiovascular events
Cox PH models were fitted using cluster membership as the predictor to predict a composite outcome of cardiovascular death, heart failure hospitalization, stroke or systemic embolism, and acute coronary syndrome. To infer hazard ratios, we used models with the centre as shared frailty term and the R package survival. For sensitivity analyses, we added age, sex, and randomization group as confounding variables to the models. To compare the unsupervised cluster assignment with existing predictors, separate risk prediction models were built using (i) CHA₂DS₂VASc score (congestive heart failure, hypertension, age, diabetes, stroke, vascular disease, sex category), (ii) ABC stroke12 and bleeding13 scores, (iii) discretized17 Troponin T, and (iv) NT-proBNP quartiles. For the ABC scores, published criteria12,13 were computed. The ABC (age, biomarker, clinical history)-stroke and ABC-bleeding risk scores incorporate clinical variables and cardiovascular biomarkers to estimate risk of stroke or systemic embolic events and bleeding, respectively, in patients with AF.
In the BBC-AF validation cohort, there are 68 missing values for the first primary composite outcome and 59 missing values for the first primary safety outcome for either the event-status information or the time-to-event information. We dropped those participants from the primary analysis, but added a best- and worst-case scenario analysis. For the best-case scenario, all missing event values were set to one (occurrence of an event) and for the best-case scenario all event values were set to zero (no occurrence of an event). We imputed all missing time-to-event data by the median censoring time.
To calculate the area under the receiver operating characteristic curve (AUC), we fitted unadjusted Cox PH models without centre as frailty as this information is missing in the validation data set, and our aim was to measure the discriminatory power of inter-cohort generalizable models. We used the Python lifelines26 and Sklearn packages for this analysis. The ABC scores12,13 and genomic risk scores were used as continuous variables, and all other predictors were discretized as proposed in the literature (details in legend to Supplementary material online, Figure S5).
2.10. Genomic risk scores
Genomic DNA extraction was performed from buffy coat samples derived from EDTA blood samples. DNA samples were sent to the Broad Institute of MIT and Harvard in Cambridge, MA, USA. After quality control of the DNA, array genotyping (Infinium PsychArray-24 v1.2 BeadChip) and imputation with the TOPMed Freeze 5 data set as reference were performed. Previously published polygenic risk scores (PRSs) for the risk of AF (PRS-AF) and ischaemic stroke risk (PRS stroke) were computed using PLINK2.16 The sum scores were obtained, and PRS calculations were based on TOPMed imputed genotype dosages, ensuring an imputation quality measure exceeding 0.3 for each variant on every chromosome. Following quality control and imputation, a total of 6 363 335 single-nucleotide variants (out of 6 730 541) were utilized to calculate PRS-AF, and 516 013 single-nucleotide variants (out of 530 933) were employed for PRS-stroke calculation.
3. Results
3.1. Biomolecule concentrations define four distinct clusters of patients
Clinical features of the EAST-AFNET 4 biomolecule study were similar to the patient population enrolled in the main trial (Table Table22). Unsupervised clustering of patients based on concentrations of the 13 biomolecules without any clinical information identified four distinct patient clusters (Figure Figure22A) with overlapping clinical characteristics (Table Table22).
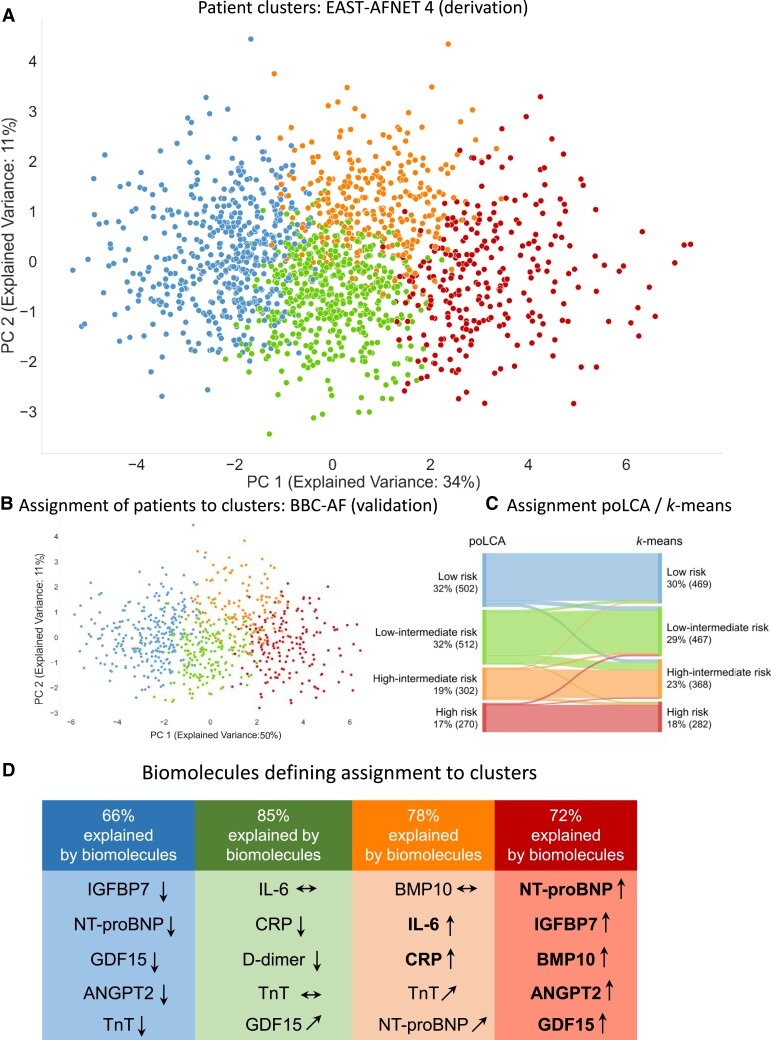
Unsupervised clustering based on concentrations of 13 biomolecules reflecting different cardiovascular disease mechanisms assigns patients to four clusters. (A) Projection of patients into two-dimensional space spanned by first two principal components derived by applying principal component analysis on the 13 biomolecules in EAST-AFNET 4. Clustering was performed without any clinical information, relying on biomolecule concentrations only. (B) Application of the assignment rules derived in the EAST-AFNET 4 trial assigns patients in the validation cohort to four clusters with similar frequencies. The validation cohort consisted of all patients with AF enrolled into the BBC-AF cohort, a prospective cohort study enrolling patients with cardiovascular conditions presenting to a large NHS teaching trust. (C) Sankey plot comparing the partitioning of the EAST participants to cluster groups based on 13 biomolecules resulting from k-means and poLCA clustering. Both methods create comparable clusters. (D) List of the top five biomolecules explaining variance in each cluster. The highest risk cluster was dominated by high concentrations of NT-proBNP, IGFBP7, BMP10, ANGPT2, and GDF15. The lowest risk cluster showed low concentrations of these biomolecules. Elevated concentrations of IL-6, CRP, and low concentrations of D-dimer contribute additional information to the variance in the two intermediate-risk clusters.
Table 2
Distribution of patient characteristics in the four biomolecule-derived patient clusters in the EAST-AFNET 4 biomolecule data set
| Characteristic | Cluster in EAST-AFNET4 by poLCA | P-value | |||
|---|---|---|---|---|---|
| Blue cluster n = 502 (32%) | Green cluster n = 512 (32%) | Orange cluster n = 302 (19%) | Red cluster n = 270 (17%) | ||
| Randomized to early rhythm control | 257 (51.2) | 263 (51.4) | 142 (47.0) | 138 (51.1) | 0.8 |
| CHA2DS2VASc score | 3.0 (2.0–3.0) | 3.0 (2.0–4.0) | 3.0 (3.0–4.0) | 4.0 (3.0–5.0) | <0.001 |
| Age >65 | 332 (66%) | 433(85%) | 253(84%) | 239(89%) | <0.001 |
| Female sex | 233 (46%) | 238 (46%) | 119 (39%) | 123 (46%) | 0.2 |
| BMI | 28.6 (25.6–31.6) | 28.0 (25.2–31.4) | 30.0 (26.7–33.4) | 29.1 (25.7–33.3) | 0.026 |
| Obese, defined as BMI ≥ 30 | 190 (38%) | 177 (35%) | 151 (50%) | 114 (42%) | 0.026 |
| Arterial hypertension | 435 (87%) | 446 (87%) | 270 (89%) | 249 (92%) | <0.001 |
| Diabetes mellitus | 98 (20%) | 112 (22%) | 100 (33%) | 86 (32%) | 0.7 |
| Stable heart failure NYHA Stages II–IV or left ventricular ejection fraction <50% | 108 (22%) | 134 (26%) | 86 (28%) | 147 (54%) | <0.001 |
| Prior stroke or TIA | 53 (11%) | 63 (12%) | 46 (15%) | 33 (12%) | 0.8 |
| History of myocardial infarction or revascularization by stenting or bypass surgery | 58 (12%) | 65 (13%) | 68 (23%) | 61 (23%) | 0.8 |
| Chronic kidney disease | 38 (7.6) | 99 (19.3) | 73 (24.2) | 118 (43.7) | <0.001 |
| Chronic obstructive lung disease | 27 (5.4%) | 37 (7.2%) | 29 (9.6%) | 31 (11%) | 0.3 |
| Peripheral artery disease | 12 (2.4%) | 19 (3.7%) | 18 (6.0%) | 21 (7.8%) | 0.3 |
Continuous and discrete numeric parameters are shown as median (interquartile range), nominal features as number of patients (%). Chronic kidney disease was classified based on estimated creatinine clearance calculated using the CKD-Epi formula.
Almost all patients were clearly assigned to a cluster (Figure Figure22A). In the validation data set BBC-AF, the classification criteria derived in the EAST-AFNET 4 data set sorted patients into similar clusters with similar sizes (Figure Figure22B). The definition of four clusters was robust within poLCA and also when another method, k-means, was applied to the data set (Figure Figure22C).
The cluster later shown to be the high-risk cluster was dominated by elevated concentrations of BMP10, IGFBP7, NT-proBNP, ANGPT2, and GDF15. Patients in the cluster with the lowest risk of cardiovascular events showed low concentrations of these biomolecules. Two intermediate-risk clusters differed by high or low concentrations of thromboinflammatory markers (CRP, IL-6, and D-dimer; Figure Figure22D).
Clinical features differed between clusters, illustrated, e.g. by ages between 68 years (low-risk cluster) and 72–74 years (low-intermediate risk cluster, high-intermediate risk cluster, high-risk cluster; Table Table22) or differences in rates of obesity (the intermediate high-risk cluster had the highest rate of obese patients; Table Table22). The estimated CHA2DS2VASc score was similar between three of the four clusters. Only the lowest risk cluster included younger patients with fewer clinical stroke risk factors (Table Table22). Clinical features were slightly different in the validation data set, BBC-AF (see Supplementary material online, Table S1). The distribution of clinical features across clusters was similar in BBC-AF (see Supplementary material online, Table S2). Biomolecule concentrations are shown for each cluster in Table Table33.
Table 3
Biomolecule concentrations in the clusters
| Blue cluster n = 502 (32%) | Green cluster n = 512 (32%) | Orange cluster n = 302 (19%) | Red cluster n = 270 (17%) | Overall range min.—max. | |
|---|---|---|---|---|---|
| IL-6 (pg/mL) | 1.8 (1.5–2.5) | 2.1 (1.5–2.9) | 4.6 (3.6–7.1) | 4.7 (3.2–7.7) | 1.50–38.83 |
| NT-proBNP (pg/mL) | 154.8 (84.3–303.7) | 560.2 (302.0–1024.5) | 461.3 (223.2–844.5) | 1527.5 (919.8–541.5) | 27.49–5409 |
| TnT (ng/L) | 8.0 (6.3–10.4) | 11.0 (8.6–14.5) | 15.1 (11.1–22.6) | 19.1 (13.9–29.5) | 3.53–79.27 |
| GDF15 (pg/mL) | 937.0 (760.6–226.8) | 1295.5 (1006.0–699.8) | 1716.5 (1329.0–333.5) | 2499.0 (1910.2–409.5) | 507.7–8007 |
| CRP (mg/L) | 1.4 (0.6–3.0) | 1.6 (0.7–2.7) | 4.7 (2.5–8.9) | 4.5 (2.1–11.7) | 0.08–88.63 |
| D-dimer (µg/mL) | 0.1 (0.1–0.2) | 0.1 (0.1–0.2) | 0.3 (0.1–0.5) | 0.3 (0.2–0.7) | 0–3.54 |
| CA125 (U/mL) | 9.9 (7.3–12.9) | 11.3 (8.0–14.8) | 11.8 (8.2–17.1) | 15.8 (11.0–25.5) | 3.46–97.98 |
| ANGPT2 (ng/mL) | 1.8 (1.5–2.3) | 2.8 (2.1–3.9) | 2.6 (2.0–3.4) | 4.9 (3.3–7.5) | 0.95–12.58 |
| BMP10 (ng/mL) | 1.9 (1.7–2.1) | 2.3 (2.0–2.5) | 2.0 (1.7–2.2) | 2.8 (2.4–3.1) | 1.30–3.89 |
| ESM1 (ng/mL) | 1.8 (1.5–2.2) | 2.1 (1.8–2.5) | 2.0 (1.5–2.8) | 2.6 (2.1–4.0) | 0.98–10.58 |
| FABP3 (ng/mL) | 26.4 (23.0–31.1) | 32.2 (27.2–37.3) | 35.2 (30.6–45.4) | 45.4 (34.9–55.4) | 15.52–92.53 |
| FGF23 (pg/mL) | 116.0 (97.3–150.0) | 153.6 (126.5–192.0) | 176.4 (129.8–236.5) | 254.4 (189.4–400.6) | 68.11–1352.70 |
| IGFBP7 (ng/mL) | 87.8 (81.3–95.0) | 106.1 (98.4–114.6) | 103.6 (92.7–114.7) | 135.0 (121.6–157.4) | 68.52–208.46 |
All biomolecule concentrations are given as median and interquartile range. Note that some of the most relevant biomolecules by explained variance show a relatively small range of concentrations, e.g. BMP10 and IGFBP7, compared with other biomolecules with known predictive effects in patients with cardiovascular diseases and a high range of values, e.g. TnT or NT-proBNP.
3.2. Risk of outcome events in each cluster
Each cluster had a distinct risk of primary and safety outcomes (Figures Figures33A and and33C). Patients in the highest risk cluster had a five-fold higher rate of primary outcomes than patients in the lowest risk cluster in the derivation (Figure Figure33A) and validation (Figure Figure33B) data sets. Each component of the composite outcome moved in the same direction as the composite for the primary outcomes (Table Table44, Figures33A and33B) and for the safety outcome (Table Table55, Figures Figures33C and 33D). The clustering using k-means resulted in a similar risk gradient across clusters (see Supplementary material online, Figure S3). Early rhythm control was effective across all biomolecule clusters (Pinteraction = 0.63, Supplementary material online, Table S5). The clustering model outperformed other risk scores for most of the tested outcomes (see Supplementary material online, Figures S5–S8). For the first primary composite outcome, the poLCA cluster model yielded an AUC 0.76 [95% confidence interval (CI): 0.72–0.79], the next best predictive model relied on the ABC bleeding score and yielded an AUC 0.74 (95% CI: 0.70–0.78) in the validation data set. Hazard ratios for the clustering were higher or similar to hazard ratios obtained by applying established risk prediction models using clinical features, combinations of clinical features and biomolecules, or a single biomolecule (see Supplementary material online, Table S5).
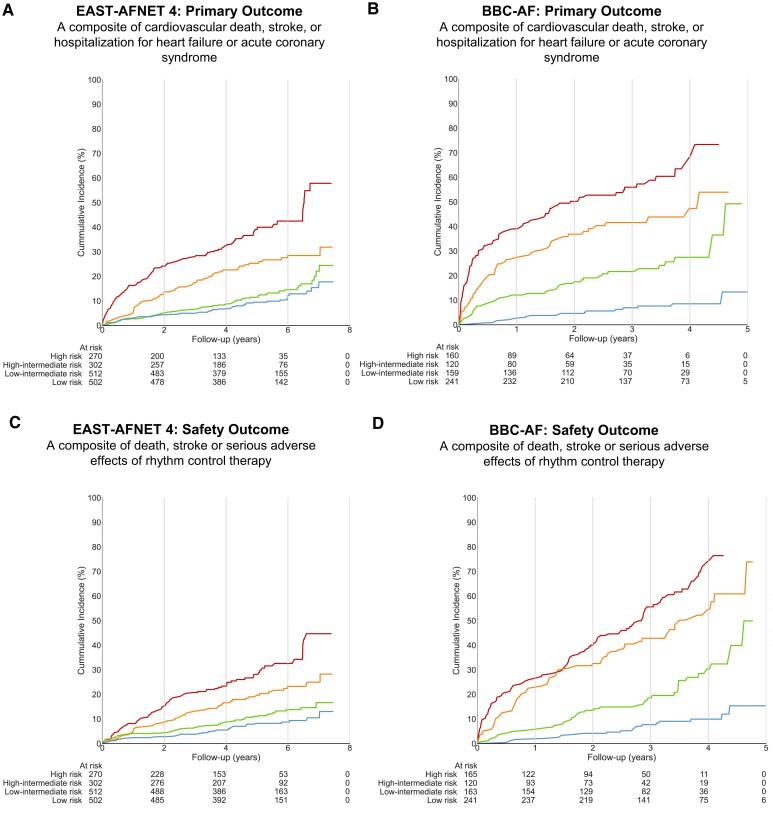
Risk of cardiovascular events for each biomolecule cluster. (A) Aalen–Johansen curves for cluster groups from poLCA clustering model for the first primary outcome in EAST-AFNET 4, a composite of all-cause mortality, stroke, or unplanned hospitalization for heart failure or acute coronary syndrome. (B) Aalen–Johansen curves for cluster groups from poLCA clustering model for the first primary outcome in BBC-AF, a composite of all-cause mortality, stroke, or unplanned hospitalization for heart failure or acute coronary syndrome. Administrative censoring has been applied to events occurring after number of at-risk patients dropped below five. (C) Aalen–Johansen curves for cluster groups from poLCA clustering model for the safety outcome in EAST-AFNET 4. (D) Aalen–Johansen curves for cluster groups from poLCA clustering model for the safety outcome in BBC-AF. Administrative censoring has been applied to events occurring after number of at-risk patients dropped below five.
Table 4
Efficacy outcomes in the EAST-AFNET 4 biomolecule data set for each biomolecule cluster
| EAST-AFNET 4 (derivation) | ||||
|---|---|---|---|---|
| Low-risk (blue) cluster n = 502 (32%) | Low-intermediate risk (green) cluster n = 512 (32%) | High-intermediate risk (orange) cluster n = 302 (19%) | High-risk (red) cluster n = 270 (17%) | |
| Stroke | 10/2587 (0.4) | 16/2634 (0.6) | 11/1450 (0.8) | 14/1160 (1.2) |
| Cardiovascular death | 10/2624 (0.4) | 17/2667 (0.6) | 21/1474 (1.4) | 42/1190 (3.5) |
| Unplanned heart failure hospitalization | 20/2585 (0.8) | 37/2598 (1.4) | 43/1367 (3.1) | 65/1024 (6.3) |
| Unplanned hospitalization for acute coronary syndrome | 17/2581 (0.7) | 14/2627 (0.5) | 18/1429 (1.3) | 14/1151 (1.2) |
| BBC-AF (Validation) | ||||
|---|---|---|---|---|
| Low-risk (blue) cluster n = 268 (36%) | Low-intermediate risk (green) cluster n = 185 (25%) | High-intermediate risk (orange) cluster n = 123 (16%) | High-risk (red) cluster n = 172 (23%) | |
| Stroke | 2/843 (0.2) | 5/516 (1.0) | 5/288 (1.7) | 7/360 (1.9) |
| Cardiovascular death | 5/814 (0.6) | 19/509 (3.7) | 30/292 (10.3) | 72/366 (19.7) |
| Unplanned heart failure hospitalization | 15/826 (1.8) | 32/469 (6.8) | 29/248 (11.7) | 56/269 (20.8) |
| Unplanned hospitalization for acute coronary syndrome | 0/870 (0.0) | 3/546 (0.5) | 0/297 (0.0) | 0/378 (0.0) |
Given are patients with event per observation-years and the annualized event rate in per cent (in parentheses).
Table 5
Safety outcomes in the EAST-AFNET 4 biomolecule data set and in the BBC-AF data set
| EAST-AFNET 4 (derivation) | ||||
|---|---|---|---|---|
| Low-risk (blue) cluster n = 502 (32%) | Low-intermediate risk (green) cluster n = 512 (32%) | High-intermediate risk (orange) cluster n = 302 (19%) | High-risk (red) cluster n = 270 (17%) | |
| Death | 18/2624 (0.7) | 32/2667 (1.2) | 44/1474 (3.0) | 64/1190 (5.4) |
| Stroke | 10/2587 (0.4) | 16/2634 (0.6) | 11/1450 (0.8) | 14/1160 (1.2) |
| Major adverse events related to rhythm control | 41/2583 (1.6) | 61/2597 (2.3) | 62/1436 (4.3) | 81/1150 (7.0) |
| BBC-AF (validation) | ||||
|---|---|---|---|---|
| Low-risk (blue) cluster n = 268 (36%) | Low-intermediate risk (green) cluster n = 185 (25%) | High-intermediate risk (orange) cluster n = 123 (16%) | High-risk (red) cluster n = 172 (23%) | |
| Death | 23/814 (2.8) | 39/509 (7.7) | 55/292 (18.8) | 104/366 (28.4) |
| Stroke | 2/843 (0.2) | 5/516 (1.0) | 5/288 (1.7) | 7/360 (1.9) |
| Major bleeding | 6/1098 (0.5) | 18/744 (2.4) | 17/464 (3.7) | 22/680 (3.2) |
| Low-risk (blue) cluster/reference group | Low-intermediate risk (green) cluster/other risk group | High-intermediate risk (orange) cluster/other risk group | High-risk (red) cluster/other risk group | |
|---|---|---|---|---|
| EAST-AFNET 4 (derivation data set) | ||||
| Biomolecule clusters | 1 (reference) | 1.3 (0.9–1.9) | 2.7 (1.9–3.6) | 5.2 (3.7–7.2) |
| CHA2DS2VASc | 1 (reference) | 1.5 (1.0–2.2) | 2.4 (1.6–3.34) | 3.8 (2.8–5.3) |
| ABC stroke | No events | 1 (reference) | 2.7 (2.2–3.4) | 4.7 (3.1–6.9) |
| ABC bleeding | 1 (reference) | 1.9 (1.1–3.3) | 4.8 (2.7–8.3) | |
| NT-proBNP (quartiles) | 1 (reference) | 1.5 (1.2–2.1) | 2.1 (1.5–2.8) | 4.7 (3.2–6.9) |
| TnT (discretized) | 1 (reference) | 1.2 (0.9–1.7) | 2.3 (1.7–3.1) | 4.4 (3.1–6.2) |
| PRS AF | 1 (reference) | 0.9 (0.7–1.3) | 0.9 (0.7–1.3) | |
| PRS stroke | 1 (reference) | 1.1 (0.8–1.4) | 1.3 (0.9–1.9) | |
| BBC-AF (validation data set) | ||||
| Biomolecule clusters BBC-AF (validation) | 1 (reference) | 4.0 (2.3–7.0) | 8.3 (4.80–14.4) | 14.1 (8.4–23.7) |
| CHA2DS2VASc | 1 (reference) | 1.9 (1.2–3.0) | 1.6 (0.9–2.5) | 2.3 (1.5–3.4) |
| ABC stroke | No events | 1 (reference) | 4.3 (2.6–6.9) | 8.2 (5.0–13.4) |
| ABC bleeding | 1 (reference) | 3.4 (2.4–4.7) | 4.8 (3.4–6.9) | |
| NT-proBNP (quartiles) | 1 (reference) | 2.3 (1.2–4.4) | 6.3 (3.4–11.7) | 10.2 (5.7–18.3) |
| TnT (discretized) | 1 (reference) | 2.7 (1.5–4.8) | 5.5 (3.1–9.6) | 7.2 (4.2–12.5) |
Numbers show patients with events and annualized event rates in per cent. The safety outcome component ‘major adverse events related to rhythm control therapy’ was not exactly defined in BBC-AF. Therefore, the clinically relevant outcome ‘major bleeding’ was used.
3.3. Biomolecule combinations are required to assign patients to cluster groups
To estimate the relevance of each biomolecule for the assignment to a patient cluster, we computed the ARI and the C statistic for each combination of biomolecules as further post hoc analyses. Removal of five or more biomolecules consistently yielded ARIs of 0.55 or less, indicating that almost half, or more, of the patients were no longer assigned to their original cluster (Figure Figure44A). Figure Figure44B plots the ARI and the C statistic for each possible model with fewer biomolecules. While risk prediction remains reasonable with only two to three biomolecules (C statistic estimates 0.67–0.69), assignment to cluster requires more information.
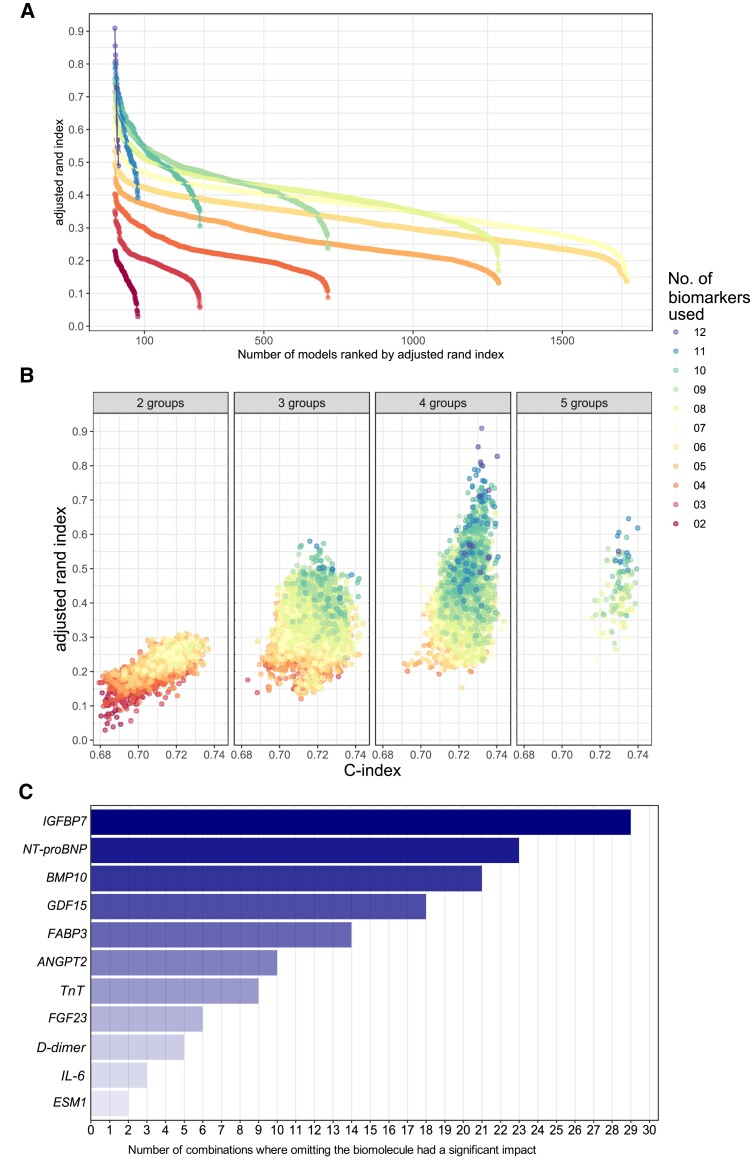
Effects of removing biomolecules on clusters. (A) Plot of ARIs for each simulated clustering process when using less biomolecules. Each colour indicates one number of biomolecules (2–12) used for clustering. Each dot represents one simulated set of clusters. ARIs are shown for each model in ascending order from right to left. (B) Plot of the ARI, a measure of the assignment of a patient to a biomolecule-derived cluster, and of the corresponding c index, for each virtual model using poLCA clustering with a reduced number of biomolecules (2–12). Each dot represents one clustering model. The colour indicates the number of biomolecules used. Models relying on eight or less biomolecules (yellow, orange, and red colours) consistently yield ARIs below 0.55. Only models using 7–12 biomolecules achieve correct assignment of patients to biomolecule clusters. The c index, a summarized measure of the accuracy of risk prediction, changes only marginally (x axis). (C) Importance of each biomolecule included in this study based on the effect of its removal from the clustering process. For each number of biomolecules (2–12), the five clusters with the lowest rand indices were selected. The missing biomolecules were listed and ranked by number of clusters lacking that biomolecule. This list provides an estimate of the importance of each biomolecule in the clustering process.
To estimate the relevance of each biomolecule for the assignment of patients to the risk clusters, another in silico exercise was performed: For each number of biomolecules, the five models with the lowest ARIs were selected. The missing biomolecules were listed and counted. Biomolecules whose removal often lead to a low ARI were considered relevant for the clustering process. Figure Figure44C provides a list of all biomolecules sorted by the number of relevant removals in this exercise.
3.4. Sensitivity analyses
Removing or adding biomolecules using forward and backward selections resulted in similar rankings of biomolecules (see Supplementary material online, Figure S4 and Table S6). For each cluster, the unique and common contribution to the clustering was calculated (see Supplementary material online, Tables S8 and S9). The number of biomolecule-based clusters remained constant at four clusters when one to five biomolecules were randomly removed from the data set (see Supplementary material online, Table S10). These analyses identified several key biomolecules relevant for patient clustering, including IGFBP7, NT-proBNP, BMP10, ANGPT2, and the thromboinflammatory biomolecules CRP, IL-6, and D-dimer (Figure Figure44C).
4. Discussion
4.1. Main findings
Integrating information contained in 13 biomolecules that were selected as potential quantifiable proxies for different disease processes with relevance in AF defines four distinct clusters of patients with AF. Each cluster has a unique biomolecule pattern and risk of cardiovascular events. The findings were robust in sensitivity analyses and in an independent prospective cohort of patients with AF. The biomolecules contributing to the clustering identify shared disease mechanisms in subphenotypes of AF that are associated with cardiovascular events.
Clustering patients based on biomolecules potentially reflecting overlapping disease processes, as done by the unsupervised analyses used here, suggests possible disease mechanisms related to cardiovascular complications in patients with AF. The clustering may enable the development of stratified therapies that may differ in patients with similar clinical features by highlighting treatable underlying disease processes linked to cardiometabolic dysfunction and load (BMP10, NT-proBNP, and IGFBP7), endothelial dysfunction and shear stress (ANGPT2 and BMP10), and increased thromboinflammation (CRP, IL-6, and D-dimer). Six biomolecules related to atrial cardiomyocyte dysfunction and vascular smooth muscle cell dysregulation (BMP10),9,27 endothelial cell dysfunction (IGFBP728,29 and ANGPT230), atrial and ventricular volume load (NT-proBNP), myocardial metabolism (FABP3), and mitochondrial dysfunction (GDF15) contributed most to the biomolecule-based clustering. Patients at high risk showed elevated biomolecule concentrations related to cardiomyocyte dysfunction, disturbed metabolism, and increased endothelial stress. Intermediate risk patients were further differentiated into intermediate-high and intermediate-low risk by elevated concentrations of thromboinflammatory biomolecules (CRP, IL-6, d-dimer). Patients with low concentrations of these biomolecules have a very low event rate on current therapy. The results were similar using different unsupervised clustering techniques (Figure Figure22C), in sensitivity analyses, and in an independent data set (BBC-AF, 748 patients, Figures Figures22 and 33). Pending further validation, the results highlight that patients with AF can be stratified using circulating biomolecules without added clinical information. The distinct signature of biomolecules in each cluster suggests that treatments beyond oral anticoagulation, treatment of concomitant conditions, and early rhythm control may be needed to further reduce their risk.
This work was performed in two data sets of patients with AF and cardiovascular comorbidities (Table Table22). Some have argued that patients with AF are a model population for elderly patients with multiple cardiovascular diseases. While the present results show that the biomolecule clusters identified here define groups of patients with AF with distinct biomolecule patterns and outcome risks, it is conceivable that similar biomolecule patterns and outcome associations can be found in cardiovascular patients without AF. Our findings call for future research into the effects of biomolecules on cardiovascular function. IGFBP7 (also called angiomedullin) is released following activation of transforming growth factor (TGF)-beta in fibroblasts and in cardiomyocytes,28 including in heart failure.29 Its elevation highlights cardiac fibroblasts as a potential target for risk-reducing therapies in AF. Further research into the reasons for ANGPT2 elevations in patients with AF may identify treatable disease mechanisms.30 Its relevance for patient clustering, especially in context with IGFBP7 and BMP10, suggests relevant interactions between endothelial cells and cardiomyocytes. Some of the biomolecules used here are associated with systemic or general cardiovascular disease mechanisms. Future studies are needed to understand the associations of the biomolecule clusters identified here with cardiac rhythm and with outcomes in patients without AF. Such work will determine to what extent the clusters identified here are specific in their application to patients with AF.
A growing array of medical,14,31 interventional,14,32 and surgical33 treatment options in patients with AF illustrates the need to identify treatable, risk-modifying disease processes in these patients. The promising effects of SGLT2 inhibitors on AF and the first results on PPAR1 inhibitors on preventing and reversing AF hold promise for metabolic interventions.34 Our analysis suggests first testing such interventions in patients assigned to the high-risk cluster in this analysis.
This analysis was not designed to select patients for a specific therapy, but the patient clusters can potentially form a basis for selecting therapy responders: BMP10 is almost exclusively secreted from atrial cardiomyocytes9,35 and secreted BMP10 regulates vascular smooth muscle cells,27 rendering atrial-specific therapies such as rhythm control, but also antihypertensive therapy and metabolic interventions useful in patients with elevated BMP10 concentrations.9,36,37 Reducing inflammation using specific interleukin-targeting antibodies such as canakinumab38 or the general anti-inflammatory agent colchicine39–41 may be most effective in patients in the intermediate high-risk cluster defined by inflammatory biomolecules.
4.2. Comparison with other risk estimation scores and limitations of the C statistic
As expected, the biomolecule-based clustering process evaluated here provides better risk estimation than the CHA2DS2VASc score.12 Its C statistic was better than or comparable with other proposed risk scores, including the ABC stroke and bleeding scores12,13 (Figure Figure44B). In view of the summative nature of the C statistic, this may not come as a surprise. The UK Biobank provided the first insights into the added value of multiple biological measurements for risk prediction.42 Previous work on biomarkers tested the predictive value of a biomolecule when added to clinical characteristics,43 or for single biomolecules on their own,10,44 prior to combining biomolecules into scores.12,13 Classical statistical methods, including forward and backward selection, did not identify these biomolecules, probably due to a different handling of shared and common information.
4.3. Comparison with proteomic methods
Proteomic technologies now enable the quantification of thousands of proteins from a small sample of plasma or blood. Earlier iterations of these technologies contributed to the discovery of AF-related biomolecules quantified here, e.g. FGF23,15 while RNA sequencing contributed to the discovery of BMP109 as a biomolecule of interest in patients at risk of AF. Others used proteomic analyses in all-encompassing analyses of circulating proteins related to cardiovascular events in patients with AF.11 Such proteomic analyses are hypothesis free but necessarily highlight proteins with large concentration ranges that can be quantified with high precision using proteomic technologies. These proteomic methods will be extremely helpful in identifying additional proteins related to AF. Such work is likely to confirm, refine, and extend the present findings. The present analysis pre-selected 13 biomolecules hypothesized to reflect different modifiable disease processes that can be quantified at high precision. These biomolecules were used to identify groups of patients who share pathophysiological patterns based on these biomolecules. This method identifies groups of patients with different predominant disease mechanisms. It may be useful alongside continued hypothesis-free research aiming to identify additional disease mechanisms leading to age-related diseases45 and chronic cardiovascular diseases.11
4.4. Strengths and limitations
The biomolecule-based clusters developed here are agnostic to clinical information. They can be used to identify disease processes and estimate risk in anonymized samples without clinical information and in settings where clinical assessment is not available or feasible. Another feature of the present clustering is its ability to identify patients with AF at risk of cardiovascular events beyond stroke. This broadens the potential therapeutic benefits for patients. A novel methodology chosen here is the preselection and simultaneous quantification of 13 distinct biomolecules chosen for their potential relevance in AF.1 Biomolecules were identified in a semi-formalized a priori process and centrally quantified using high-precision assays. While this can be viewed as a strength in view of the selection process, the precision of the measurements, and the disease processes reflected by these biomolecules, it is also a weakness as it limits the analyses to these biomolecules. Another strength of the analysis is the collection of samples in a broad range of care settings in a cohort of adequately treated patients with AF in the context of a clinical trial with centrally adjudicated outcomes, externally monitored data collection, and external validation in a cohort of patients with AF enrolled in a routine care setting.
The study has important limitations. (i) Assessment of smaller number of biomolecules is limited to in silico calculations. (ii) Almost all patients received guideline-recommended anticoagulation, rate and rhythm control, and often effective treatment of concomitant conditions. The clusters presented here will require independent assessment in patients not receiving these therapies, which might be difficult given the ethical need to treat patients according to evidence. (iii) A limitation is the lack of follow-up samples that would enable assessment of treatment effects, the lack of an untreated population of patients with AF, and the lack of validation in data sets of patients without AF. (iv) While BMP10, NT-proBNP, FABP3, and troponin are proteins released by the heart, the other markers are more systemic in nature, and cannot differentiate between cardiac, vascular, and other origins of the measured biomolecules. On the contrary, especially the vascular and inflammatory molecules might reflect ongoing systemic changes associated with cardiovascular outcomes unrelated to cardiac defects. (v) Quantification of plasma biomolecules in a single sample may have missed smaller, but pathophysiologically relevant changes in the heart or atria diluted by systemic production and elimination of circulating biomolecules. (vi) This study is limited to the 13 biomolecules quantified. Sequencing of cardiac tissue,46 quantification of circulating RNAs, and advanced proteomics47,48 enable hypothesis-free quantification of many molecules at once. These methods will discover additional molecules and may help refine the disease processes suggested in this analysis. (vii) This study cannot evaluate whether the biomolecule combinations identified here truly identify patients who are likely to respond to cardiometabolic, anti-inflammatory, or other disease-process-modifying therapies. This will need prospective testing, e.g. by using the biomolecule clusters identified here as inclusion criteria in interventional trials. (viii) The EAST-AFNET 4 cohort is predominantly of Caucasian ethnicity. The validation cohort has a more mixed ethnicity.49 Further validation in other ethnicities is needed. (ix) While some of the biomolecules can be measured in clinical routine as in vitro diagnostic devices with regulatory approval, other assays are not approved for use in clinical routine and available for research use only. (x) Clinical features were not used for clustering. This enables application to samples without precise information on clinical features but limits cause-specific interpretation.
In conclusion, these findings open the possibility that combinations of pre-selected plasma biomolecules, as studied here, and potentially unbiased plasma multiomics can define distinct AF subphenotypes and thereby advance the management of this condition. Future studies are needed to determine whether such subphenotypes can be used to select therapies and to identify therapy responders.
Contributor Information
Larissa Fabritz, Department of Cardiology, University Heart and Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Martinistraße 52, 20246 Hamburg, Germany. University Center of Cardiovascular Science, University Heart and Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany. German Center for Cardiovascular Research (DZHK), partner site Hamburg/Kiel/Lübeck, Germany. AFNET, Münster, Germany. Institute of Cardiovascular Sciences, University of Birmingham, Wolfson Drive, Birmingham, UK.
Winnie Chua, Institute of Cardiovascular Sciences, University of Birmingham, Wolfson Drive, Birmingham, UK.
Victor R Cardoso, Institute of Cardiovascular Sciences, University of Birmingham, Wolfson Drive, Birmingham, UK.
Christoph Al-Taie, Department of Cardiology, University Heart and Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Martinistraße 52, 20246 Hamburg, Germany. University Center of Cardiovascular Science, University Heart and Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany. German Center for Cardiovascular Research (DZHK), partner site Hamburg/Kiel/Lübeck, Germany.
Katrin Borof, Department of Cardiology, University Heart and Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Martinistraße 52, 20246 Hamburg, Germany. University Center of Cardiovascular Science, University Heart and Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany.
Anna Suling, Institute of Medical Biometry and Epidemiology, University Medical Center Hamburg-Eppendorf, Hamburg, Germany.
Linda Krause, Institute of Medical Biometry and Epidemiology, University Medical Center Hamburg-Eppendorf, Hamburg, Germany.
Shinwan Kany, Department of Cardiology, University Heart and Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Martinistraße 52, 20246 Hamburg, Germany. German Center for Cardiovascular Research (DZHK), partner site Hamburg/Kiel/Lübeck, Germany. Cardiovascular Disease Initiative, The Broad Institute of MIT and Harvard, Cambridge, MA, USA. Cardiovascular Research Center, Massachusetts General Hospital, Boston, MA, USA.
Christina Magnussen, Department of Cardiology, University Heart and Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Martinistraße 52, 20246 Hamburg, Germany. German Center for Cardiovascular Research (DZHK), partner site Hamburg/Kiel/Lübeck, Germany. Center for Population Health Innovation (POINT), University Heart and Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany.
Karl Wegscheider, Institute of Medical Biometry and Epidemiology, University Medical Center Hamburg-Eppendorf, Hamburg, Germany.
Guenter Breithardt, University Hospital Münster, Münster, Albert-Schweitzer-Straße 1A, 48149 Münster, Germany.
Harry J G M Crijns, Department of Cardiology, University Hospital Maastricht, Maastricht, The Netherlands.
A John Camm, Clinical Sciences, St George´s University, London, UK.
George Gkoutos, Institute of Cardiovascular Sciences, University of Birmingham, Wolfson Drive, Birmingham, UK.
Patrick T Ellinor, Cardiovascular Disease Initiative, The Broad Institute of MIT and Harvard, Cambridge, MA, USA. Cardiovascular Research Center, Massachusetts General Hospital, Boston, MA, USA.
Andreas Goette, Vincenz-Krankenhaus, Am Busdorf 2, 33098 Paderborn, Germany.
Ulrich Schotten, AFNET, Münster, Germany. Department of Physiology, Maastricht University, Maastricht, The Netherlands.
Ursula-Henrike Wienhues-Thelen, Roche Diagnostics, Nonnenwald 2, 82377 Penzberg, Germany.
Tanja Zeller, Department of Cardiology, University Heart and Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Martinistraße 52, 20246 Hamburg, Germany. University Center of Cardiovascular Science, University Heart and Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany. German Center for Cardiovascular Research (DZHK), partner site Hamburg/Kiel/Lübeck, Germany.
Renate B Schnabel, Department of Cardiology, University Heart and Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Martinistraße 52, 20246 Hamburg, Germany. University Center of Cardiovascular Science, University Heart and Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany. German Center for Cardiovascular Research (DZHK), partner site Hamburg/Kiel/Lübeck, Germany.
Antonia Zapf, Institute of Medical Biometry and Epidemiology, University Medical Center Hamburg-Eppendorf, Hamburg, Germany.
Paulus Kirchhof, Department of Cardiology, University Heart and Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Martinistraße 52, 20246 Hamburg, Germany. University Center of Cardiovascular Science, University Heart and Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany. AFNET, Münster, Germany. Institute of Cardiovascular Sciences, University of Birmingham, Wolfson Drive, Birmingham, UK.
Funding
The EAST-AFNET 4 trial and its biomolecule study were funded by BMBF, DZHK, AFNET, European Society of Cardiology, through EHRA, St Jude Medical–Abbott, Sanofi, and the German Heart Foundation. The biomolecule analysis was funded by EU Horizon 2020 grant agreement number 633196 (CATCH-ME) and EU Horizon 2020 grant agreement number 965286 (MAESTRIA). Further support came from EU IMI 116074 (BigData@Heart), British Heart Foundation (PG/20/22/35093; AA/18/2/34218), German Center for Cardiovascular Research supported by the German Ministry of Education and Research (DZHK, grant numbers FKZ 81X2800182, 81Z0710116, and 81Z0710110), German Research Foundation (Ki 509167694), and Leducq Foundation. L.F. received institutional research grants by EU 633196 (CATCH-ME) and EU 965286 (MAESTRIA). British Heart Foundation (AA/18/2/34218), German Center for Cardiovascular Research supported by the German Ministry of Education and Research (DZHK). P.T.E. is supported by grants from the National Institutes of Health (RO1HL092577, RO1HL157635) and from the American Heart Association (18SFRN34230127, 961045), U.S. by grants of the Dutch Heart Foundation (CVON2014-09, RACE V Reappraisal of Atrial Fibrillation: Interaction between hyperCoagulability, Electrical remodelling, and Vascular Destabilisation in the Progression of AF, and grant number 01-002-2022-0118, EmbRACE: Electro-Molecular Basis and the theRapeutic management of Atrial Cardiomyopathy, fibrillation, and associated outcomEs), S.K. by a Walter Benjamin Fellowship from the Deutsche Forschungsgemeinschaft (521832260). C.M. receives study-specific funding from the German Center for Cardiovascular Research (DZHK; promotion of women scientists programme; FKZ 81X3710112), the Deutsche Stiftung für Herzforschung, the Dr Rolf M. Schwiete Stiftung unrelated to the current work. R.B.S. has received funding from the European Research Council under the European Union’s Horizon 2020 research and innovation programme under the grant agreement no. 648131, from the European Union’s Horizon 2020 research and innovation programme under the grant agreement no. 847770 (AFFECT-EU), from the European Union’s Horizon Europe research and innovation programme under the grant agreement ID: 101095480 and German Center for Cardiovascular Research (DZHK e.V.; 81Z1710103 and 81Z0710114); German Ministry of Research and Education (BMBF 01ZX1408A) and ERACoSysMed3 (031L0239); and Wolfgang Seefried project funding German Heart Foundation.
Data availability
Data will be made available upon request. Please contact ed.nremmilffohrov-ztenznetepmok@ofni.
References
Articles from Cardiovascular Research are provided here courtesy of Oxford University Press
Full text links
Read article at publisher's site: https://doi.org/10.1093/cvr/cvae067
Read article for free, from open access legal sources, via Unpaywall:
https://academic.oup.com/cardiovascres/advance-article-pdf/doi/10.1093/cvr/cvae067/57211270/cvae067.pdf
Citations & impact
This article has not been cited yet.
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/162412644
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Biomarker-based prediction of sinus rhythm in atrial fibrillation patients: the EAST-AFNET 4 biomolecule study.
Eur Heart J, ehae611, 31 Aug 2024
Cited by: 0 articles | PMID: 39215973
Early rhythm-control therapy for atrial fibrillation in patients with a history of stroke: a subgroup analysis of the EAST-AFNET 4 trial.
Lancet Neurol, 22(1):45-54, 01 Jan 2023
Cited by: 11 articles | PMID: 36517170
Disturbed atrial metabolism, shear stress, and cardiac load contribute to atrial fibrillation after ablation: AXAFA biomolecule study.
Europace, 26(2):euae028, 01 Feb 2024
Cited by: 2 articles | PMID: 38266130 | PMCID: PMC10873713
[Cluster Analysis and Ablation Success Rate in Atrial Fibrillation Patients Undergoing Catheter Ablation].
Sichuan Da Xue Xue Bao Yi Xue Ban, 55(3):687-692, 01 May 2024
Cited by: 0 articles | PMID: 38948279
Funding
Funders who supported this work.
AFNET
American Heart Association (2)
Grant ID: 961045
Grant ID: 18SFRN34230127
BMBF
British Heart Foundation (2)
Accelerator Award (round 1)
Paulus Kirchhof, Birmingham, University of
Grant ID: AA/18/2/34218
Defining clusters of patients with atrial fibrillation at risk of heart failure and death
Paulus Kirchhof, University of Birmingham
Grant ID: PG/20/22/35093
CATCH-ME (1)
Grant ID: EU 965286 (
DZHK
Deutsche Stiftung für Herzforschung
Dr Rolf M. Schwiete Stiftung
Dutch Heart Foundation (2)
Grant ID: CVON2014-09
Grant ID: 01-002-2022-0118
EHRA
ERACoSysMed3 (1)
Grant ID: 031L0239
European Research Council
European Union's Horizon 2020 research and innovation programme (2)
Grant ID: 648131
Grant ID: 847770
European Union's Horizon Europe research and innovation programme (1)
Grant ID: 101095480
European Union’s Horizon 2020 research and innovation programme (2)
Grant ID: 847770
Grant ID: 648131
European Union’s Horizon Europe research and innovation programme (1)
Grant ID: 101095480
German Heart Foundation (1)
Grant ID: EU 633196
German Ministry of Education and Research (3)
Grant ID: FKZ 81X2800182
Grant ID: 81Z0710116
Grant ID: 81Z0710110
German Ministry of Research and Education (1)
Grant ID: BMBF 01ZX1408A
German Research Foundation (1)
Grant ID: Ki 509167694
Leducq Foundation (1)
Grant ID: EU 633196
Loewenstein Medical
MAESTRIA
NDD
NHLBI NIH HHS (1)
Grant ID: R01 HL157635
NIH HHS (1)
Grant ID: RO1HL092577
National Institutes of Health (2)
Grant ID: RO1HL092577
Grant ID: RO1HL157635
Sanofi
St Jude Medical-Abbott
St Jude Medical–Abbott
promotion of women scientists programme (1)
Grant ID: FKZ 81X3710112