Abstract
Aims
This study aimed to elucidate age-stratified clinical profiles and outcomes in patients with heart failure (HF) with preserved left ventricular ejection fraction (LVEF) (HFpEF).Methods and results
The Chronic Heart Failure Registry and Analysis in the Tohoku District-2 (CHART-2) Study included 2824 consecutive HFpEF patients with LVEF ≥ 50% (mean age 69.0 ± 12.3 years; 67.7% male) with a median follow-up of 9.8 years. We stratified them into five age groups: ≤54 (N = 349, 12.4%), 55-64 (N = 529, 18.7%), 65-74 (N = 891, 31.6%), 75-84 (N = 853, 30.2%), and ≥85 years (N = 202, 7.2%), and we categorized these age groups into younger (≤64 years) and older (≥65 years) groups. We compared the clinical profiles and outcomes of HFpEF patients across age groups. Younger HFpEF groups exhibited a male predominance, elevated body mass index (BMI), and poorly controlled diabetes (haemoglobin A1c > 7.0%). Older HFpEF groups were more likely to be female with multiple comorbidities, including coronary artery disease, hypertension, renal impairment, and atrial fibrillation. The positive association between elevated BMI and HFpEF was more pronounced with lower classes of age from ≥85 to ≤54 years, especially in males. With higher classes of age from ≤54 to ≥85 years, mortality rates increased, and HF death became proportionally more prevalent (Ptrend < 0.001), whereas sudden cardiac death (SCD) exhibited the opposite trend (Ptrend = 0.002). Poorly controlled diabetes emerged as the only predictor of SCD in the younger groups (adjusted hazard ratio 4.26; 95% confidence interval 1.45-12.5; P = 0.008). Multiple comorbidities were significantly associated with an increased risk of HF-related mortality in the older groups.Conclusions
Younger HFpEF patients (≤64 years) exhibit a male predominance, elevated BMI, and poorly controlled diabetes, highlighting the importance of glycaemic control in reducing SCD risk. Older HFpEF patients (≥65 years) are more likely to be female, with multiple comorbidities linked to an increased risk of HF-related mortality. These findings underscore the need for physicians to recognize age-related, distinct HFpEF phenotypes for personalized patient management.Free full text

Age‐stratified profiles and outcomes of patients with heart failure with preserved ejection fraction
Abstract
Aims
This study aimed to elucidate age‐stratified clinical profiles and outcomes in patients with heart failure (HF) with preserved left ventricular ejection fraction (LVEF) (HFpEF).
Methods and results
The Chronic Heart Failure Registry and Analysis in the Tohoku District‐2 (CHART‐2) Study included 2824 consecutive HFpEF patients with LVEF ≥ 50% (mean age 69.0 ± 12.3 years; 67.7% male) with a median follow‐up of 9.8 years. We stratified them into five age groups: ≤54 (N = 349, 12.4%), 55–64 (N = 529, 18.7%), 65–74 (N = 891, 31.6%), 75–84 (N = 853, 30.2%), and ≥85 years (N = 202, 7.2%), and we categorized these age groups into younger (≤64 years) and older (≥65 years) groups. We compared the clinical profiles and outcomes of HFpEF patients across age groups. Younger HFpEF groups exhibited a male predominance, elevated body mass index (BMI), and poorly controlled diabetes (haemoglobin A1c > 7.0%). Older HFpEF groups were more likely to be female with multiple comorbidities, including coronary artery disease, hypertension, renal impairment, and atrial fibrillation. The positive association between elevated BMI and HFpEF was more pronounced with lower classes of age from ≥85 to ≤54 years, especially in males. With higher classes of age from ≤54 to ≥85 years, mortality rates increased, and HF death became proportionally more prevalent (P trend < 0.001), whereas sudden cardiac death (SCD) exhibited the opposite trend (P trend = 0.002). Poorly controlled diabetes emerged as the only predictor of SCD in the younger groups (adjusted hazard ratio 4.26; 95% confidence interval 1.45–12.5; P = 0.008). Multiple comorbidities were significantly associated with an increased risk of HF‐related mortality in the older groups.
Conclusions
Younger HFpEF patients (≤64 years) exhibit a male predominance, elevated BMI, and poorly controlled diabetes, highlighting the importance of glycaemic control in reducing SCD risk. Older HFpEF patients (≥65 years) are more likely to be female, with multiple comorbidities linked to an increased risk of HF‐related mortality. These findings underscore the need for physicians to recognize age‐related, distinct HFpEF phenotypes for personalized patient management.
Introduction
Heart failure (HF) with preserved left ventricular (LV) ejection fraction (LVEF) (HFpEF) is a heterogeneous disorder, and various phenotyping approaches have been employed to enhance patient management. 1 However, as HFpEF is generally considered a disease of the elderly, 2 , 3 there is a lack of information regarding younger HFpEF patients.
We previously demonstrated that in our CHART (Chronic Heart Failure Registry and Analysis in the Tohoku District) Studies, the prevalence of HFpEF increased not only in adults aged 65 years or older but also in those under 65 years, when comparing the CHART‐1 Study (registration period: 2000–04) and the CHART‐2 Study (registration period: 2006–10). 4 , 5 , 6 Indeed, recent studies have highlighted a greater number of HFpEF patients under 65 years than anticipated, characterized by distinct clinical backgrounds and prognosis compared with the older ones. 7 , 8 , 9 Recently, Tromp et al. conducted a pooled analysis of three major HFpEF trials, CHARM Preserved, I‐PRESERVE, and TOPCAT, which revealed divergent traits between older and younger HFpEF patients. 9 Older patients tended to be female with multiple comorbidities, whereas younger patients exhibited a male predominance, obesity, diabetes, and a more favourable prognosis, albeit with an increased risk of cardiovascular (CV) mortality, especially sudden cardiac death (SCD). 9 While age‐related differences in patient profiles and outcomes observed in HFpEF trials are apparent, their generalized applicability remains uncertain, warranting further studies. Additionally, there is a significant knowledge gap regarding whether the impact of clinical profiles on outcomes varies by age. For these reasons, phenotyping HFpEF patients from the viewpoint of age is important for patient management.
Thus, we aimed to comprehensively examine the age‐stratified clinical profiles and outcomes of HFpEF patients in the CHART‐2 Study. 5 , 10
Methods
Study design
The CHART‐2 Study is a multicentre, prospective, observational cohort study, and details of the study design have been described previously (NCT00418041). 5 Briefly, in the CHART‐2 Study, according to the American Heart Association/American College of Cardiology/Heart Failure Society of America (AHA/ACC/HFSA) guidelines, 11 a total of 10 219 consecutive stable patients aged ≥20 years with coronary artery disease (CAD), asymptomatic structural heart disease (stage B), and a current or past history of symptomatic HF (stage C/D) were enrolled at the Tohoku University Hospital and 23 participating hospitals between October 2006 and March 2010. 5 CAD was defined as either organic stenosis requiring revascularization or vasospastic angina documented on an electrocardiogram (ECG) or angiography. 5 The definition of stage B is summarized in Supporting Information, Appendix S1 . HF was diagnosed by attending experienced cardiologists based on the Framingham criteria. 12 The study protocol was approved by the local ethics committees at each participating hospital. Baseline and follow‐up data, including medical history, laboratory and echocardiography data, and clinical outcomes, were collected at the time of enrolment and have been recorded annually thereafter by clinical research co‐ordinators. The cause of death was finally adjudicated by the principal members of the executive office of the CHART‐2 Study based on the death certificate and medical record of each patient. SCD was defined as the unexpected death of a stable patient occurring within 1 h after the onset of symptoms or during sleep. 13 However, when a cause of death (e.g. pulmonary embolism, aortic dissection, or cerebral haemorrhage) was identified by autopsy imaging carried out as necessary, the death was not counted as SCD. External death was defined as a death due to accidents and violence, including environmental events, circumstances, and conditions.
In the present study, we focused on stage C/D chronic HF patients (N = 4876) and carefully selected a cohort of 2824 HFpEF patients with LVEF ≥ 50%, while excluding severe valvular heart disease. We systematically stratified them into five distinct age groups: ≤54 (N = 349, 12.4%), 55–64 (N = 529, 18.7%), 65–74 (N = 891, 31.6%), 75–84 (N = 853, 30.2%), and ≥85 years (N = 202, 7.2%). As prior literature has generally employed an age cut‐off of 65 years to distinguish younger and older HFpEF patients, 7 , 8 , 9 and furthermore, various international organizations, including the World Health Organization (WHO), Centers for Disease Control and Prevention (CDC), European Union (EU), and United Nations (UN), commonly use this age cut‐off to demarcate the older population in their studies and reports, 14 , 15 , 16 , 17 we categorized the patients into those under 65 years (N = 878, 31.1%) and those aged 65 years or older (N = 1946, 68.9%), defining them as the younger and older HFpEF groups, respectively.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation or median with an interquartile range. Comparisons of these variables were performed by one‐way analysis of variance (ANOVA) or the Kruskal–Wallis rank sum test, as appropriate. Categorical variables were expressed as numerals with percentages and were compared by Fisher's exact test.
To examine age‐related differences in clinical profiles in HFpEF patients, we performed binomial and multinomial logistic regression analyses, including the following baseline variables by reference to the previous literature 8 , 9 : sex, body mass index (BMI), CAD, hypertension, diabetes, chronic kidney disease (CKD), atrial fibrillation (AF), New York Heart Association (NYHA) class, LVEF, and B‐type natriuretic peptide (BNP). CKD was diagnosed when the estimated glomerular filtration rate was <60 mL/min/1.73 m2. Diabetes was defined by a history of antidiabetic therapy and/or haemoglobin A1c (HbA1c) ≥ 6.5%. 10 For diabetic patients, as achieving HbA1c ≤ 7.0% is generally recommended to prevent microvascular complications, 18 we thus reclassified diabetes into well‐controlled diabetes (HbA1c ≤ 7.0%) and poorly controlled diabetes (HbA1c > 7.0%).
We performed Cox proportional hazard analyses to compare the risks of all‐cause death, CV death, including SCD and HF death, and non‐CV death across age groups. When evaluating mode of death, we further applied the Fine and Gray competing risk regression model, considering all‐cause death as a competing risk. 19 For multivariable adjustment, the variables listed above were included. Furthermore, to compare predictors of SCD and HF death between the younger and older groups, we performed Cox proportional hazard analyses with forward–backward stepwise variable selection applying the competing risk regression model. 19 To examine how the clinical profiles distinguishing younger and older HFpEF patients impact their outcomes, we employed age and the baseline variables listed above as shared potential confounders, which included most of the clinically relevant predictors of SCD and HF death in HFpEF patients identified from the post hoc analyses of the I‐PRESERVE trial. 20 , 21
In the present study, a P‐value < 0.05 was considered to be statistically significant. All the statistical analyses were performed by R Version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline clinical characteristics across age groups
Table 1 provides a comprehensive overview of baseline clinical characteristics. The male proportion showed an increment with lower classes of age, ranging from 49.5% in ≥85 years to 75.6% in ≤54 years. Concurrently, BMI values and the prevalence of obesity (defined as BMI ≥ 30 kg/m2) escalated with lower classes of age from ≥85 to ≤54 years. However, the obesity rate was remarkably low, even in the youngest group (only 17.7%). Poorly controlled diabetes exhibited a higher prevalence among the younger groups. As expected, multiple comorbidities were more prevalent, and NYHA class was higher among the older groups. Importantly, only 38 patients (1.3%) received implantable cardioverter defibrillator implantation (22 in the younger groups and 16 in the older groups). Serum BNP levels increased with higher classes of age, from ≤54 to ≥85 years.
Table 1
Baseline clinical characteristics across age groups
| ≤54 years (N = 349) | 55–64 years (N = 529) | 65–74 years (N = 891) | 75–84 years (N = 853) | ≥85 years (N = 202) | P‐value | |
|---|---|---|---|---|---|---|
| Age (years) | 45.6 ± 7.8 | 60.0 ± 2.8 | 70.0 ± 2.9 | 78.7 ± 2.7 | 87.5 ± 2.8 | <0.001 |
| Male sex, no. (%) | 264 (75.6) | 391 (73.9) | 615 (69.0) | 543 (63.7) | 100 (49.5) | <0.001 |
| BMI (kg/m2) | 25.5 ± 4.9 | 24.4 ± 3.4 | 24.3 ± 3.6 | 23.5 ± 3.6 | 22.8 ± 3.6 | <0.001 |
| Obesity (BMI ≥ 30 kg/m2), no. (%) | 61 (17.7) | 33 (6.3) | 51 (5.8) | 37 (4.4) | 8 (4.2) | <0.001 |
| Clinical history, no. (%) | ||||||
| CAD | 122 (35.0) | 273 (51.6) | 504 (56.6) | 551 (64.6) | 116 (57.4) | <0.001 |
| Hypertension | 284 (81.4) | 480 (90.7) | 826 (92.7) | 816 (95.7) | 194 (96.0) | <0.001 |
| Diabetes | <0.001 | |||||
| Well‐controlled diabetes | 77 (22.3) | 131 (25.2) | 241 (27.6) | 241 (29.0) | 45 (23.0) | |
| Poorly controlled diabetes | 35 (10.1) | 99 (19.1) | 130 (14.9) | 103 (12.4) | 13 (6.6) | |
| CKD | 68 (19.6) | 155 (29.5) | 379 (42.7) | 506 (59.8) | 145 (71.8) | <0.001 |
| AF | 84 (24.1) | 182 (34.4) | 398 (44.7) | 377 (44.2) | 96 (47.5) | <0.001 |
| HF admission | 180 (51.6) | 209 (39.5) | 356 (40.0) | 408 (47.8) | 116 (57.4) | <0.001 |
| Prior stroke | 34 (9.7) | 85 (16.1) | 184 (20.7) | 226 (26.5) | 53 (26.2) | <0.001 |
| Cancer | 9 (2.6) | 34 (6.4) | 131 (14.7) | 179 (21.0) | 40 (19.8) | <0.001 |
| ICD implantation | 7 (2.0) | 15 (2.8) | 11 (1.2) | 5 (0.6) | 0 (0.0) | 0.003 |
| NYHA class III/IV, no. (%) | 16 (4.6) | 28 (5.3) | 46 (5.2) | 108 (12.7) | 43 (21.5) | <0.001 |
| Haemodynamics | ||||||
| Systolic BP (mmHg) | 125.2 ± 19.2 | 126.0 ± 16.8 | 128.7 ± 17.5 | 130.4 ± 19.3 | 132.6 ± 21.2 | <0.001 |
| Diastolic BP (mmHg) | 76.7 ± 13.1 | 74.6 ± 11.0 | 73.2 ± 11.2 | 71.2 ± 11.6 | 71.4 ± 13.4 | <0.001 |
| Heart rate (b.p.m.) | 72.4 ± 13.9 | 70.5 ± 14.3 | 71.5 ± 14.3 | 71.8 ± 15.3 | 71.9 ± 13.6 | 0.372 |
| Laboratory data | ||||||
| Haemoglobin (g/dL) | 14.4 ± 1.9 | 13.9 ± 1.7 | 13.4 ± 1.8 | 12.5 ± 1.8 | 11.9 ± 1.7 | <0.001 |
| Creatinine (mg/dL) | 0.8 (0.7, 1.0) | 0.8 (0.7, 1.0) | 0.9 (0.7, 1.0) | 0.9 (0.8, 1.2) | 0.9 (0.8, 1.3) | <0.001 |
| eGFR (mL/min/1.73 m2) | 74.8 ± 20.5 | 69.6 ± 19.8 | 62.3 ± 19.2 | 54.3 ± 18.8 | 50.8 ± 21.9 | <0.001 |
| HbA1c (%) | 6.11 ± 0.94 | 6.49 ± 1.19 | 6.32 ± 0.95 | 6.28 ± 0.90 | 6.10 ± 0.72 | <0.001 |
| BNP (pg/mL) | 27.4 (10.6, 75.5) | 54.8 (19.9, 133.5) | 72.8 (32.8, 153.0) | 118.5 (56.1, 249.9) | 168.0 (87.9, 293.5) | <0.001 |
| Echocardiography | ||||||
| LVEF (%) | 63.0 ± 8.6 | 64.7 ± 8.7 | 64.9 ± 9.0 | 65.5 ± 9.0 | 66.7 ± 8.9 | <0.001 |
| LVDd (mm) | 50.2 ± 7.7 | 49.8 ± 7.1 | 48.9 ± 6.9 | 48.5 ± 7.1 | 46.9 ± 8.0 | <0.001 |
| IVSTD (mm) | 11.1 ± 2.8 | 11.3 ± 2.8 | 11.3 ± 2.8 | 11.3 ± 2.8 | 11.3 ± 2.5 | 0.835 |
| PWD (mm) | 10.8 ± 2.5 | 11.0 ± 2.3 | 11.0 ± 2.4 | 10.9 ± 2.4 | 11.0 ± 2.4 | 0.705 |
| RWT | 0.44 ± 0.14 | 0.45 ± 0.13 | 0.46 ± 0.14 | 0.46 ± 0.14 | 0.49 ± 0.15 | 0.009 |
| LVMI (g/m2) | 119.5 ± 45.0 | 127.0 ± 41.3 | 126.5 ± 40.3 | 131.6 ± 43.9 | 135.9 ± 44.2 | <0.001 |
| LAD (mm) | 39.1 ± 7.5 | 41.2 ± 8.6 | 42.5 ± 8.9 | 42.5 ± 9.2 | 42.7 ± 9.5 | <0.001 |
| Mitral E wave (m/s)a | 0.76 ± 0.32 | 0.75 ± 0.33 | 0.80 ± 0.39 | 0.76 ± 0.32 | 0.82 ± 0.32 | 0.055 |
| Mitral A wave (m/s)b | 0.68 ± 0.24 | 0.74 ± 0.21 | 0.80 ± 0.24 | 0.86 ± 0.25 | 0.89 ± 0.27 | <0.001 |
| E/Ac | 1.14 ± 0.48 | 0.97 ± 0.42 | 0.94 ± 0.63 | 0.85 ± 0.55 | 0.93 ± 0.92 | <0.001 |
| Deceleration time (ms)d | 208.2 ± 57.1 | 220.8 ± 70.8 | 224.5 ± 72.6 | 224.5 ± 74.1 | 213.7 ± 68.4 | 0.017 |
| Medications, no. (%) | ||||||
| Beta‐blocker | 208 (59.6) | 272 (51.4) | 386 (43.3) | 351 (41.1) | 49 (24.3) | <0.001 |
| RAS inhibitor | 263 (75.4) | 384 (72.6) | 626 (70.3) | 610 (71.5) | 138 (68.3) | 0.334 |
| MRA | 68 (19.5) | 86 (16.3) | 161 (18.1) | 148 (17.4) | 38 (18.8) | 0.753 |
| Loop diuretic | 126 (36.1) | 188 (35.5) | 358 (40.2) | 408 (47.8) | 109 (54.0) | <0.001 |
| Thiazide diuretic | 12 (3.4) | 18 (3.4) | 21 (2.4) | 42 (4.9) | 9 (4.5) | 0.064 |
| Statin | 132 (37.8) | 226 (42.7) | 365 (41.0) | 343 (40.2) | 47 (23.3) | <0.001 |
| Oral antidiabetic agent | 35 (10.0) | 85 (16.1) | 134 (15.0) | 115 (13.5) | 18 (8.9) | 0.013 |
| Insulin | 7 (2.0) | 39 (7.4) | 45 (5.1) | 31 (3.6) | 0 (0.0) | <0.001 |
AF, atrial fibrillation; BMI, body mass index; BNP, B‐type natriuretic peptide; BP, blood pressure; CAD, coronary artery disease; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; HbA1c, haemoglobin A1c; HF, heart failure; ICD, implantable cardioverter defibrillator; IVSTD, interventricular septal thickness at end‐diastole; LAD, left atrial diameter; LVDd, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; PWD, posterior wall thickness at end‐diastole; RAS, renin‐angiotensin system; RWT, relative wall thickness.
Well‐controlled diabetes status was defined as HbA1c ≤ 7.0%, and poorly controlled diabetes status was defined as HbA1c > 7.0%. Of the overall cohort, 2231 (79.0%)a, 1691 (59.9%)b, 1688 (59.8%)c, and 1805 (63.9%)d were available due to missing data.
LVEF, relative wall thickness, LV mass index, and left atrial diameter exhibited higher values in the older groups. Conversely, LV end‐diastolic diameter was greater in the younger groups. Utilization of beta‐blockers and oral antidiabetic agents was more prevalent in the younger groups. Although loop and thiazide diuretics were more frequently administered to the older groups, the prescription rates of these diuretics were relatively low across age groups.
After correcting for confounders, male sex, elevated BMI, and poorly controlled diabetes were positively correlated with the younger groups (Figure 1 A ). In contrast, the older groups exhibited positive associations with female sex, CAD, hypertension, CKD, AF, NYHA class III/IV, higher LVEF, and elevated serum BNP levels (Figure 1 A ). Additionally, the positive association between elevated BMI and HFpEF became increasingly prominent with lower classes of age from ≥85 to ≤54 years, particularly among males (Figure 1 B ). Importantly, sex differences in age‐related trends in the influence of diabetes control status were not evident (Supporting Information, Figure S1 ).
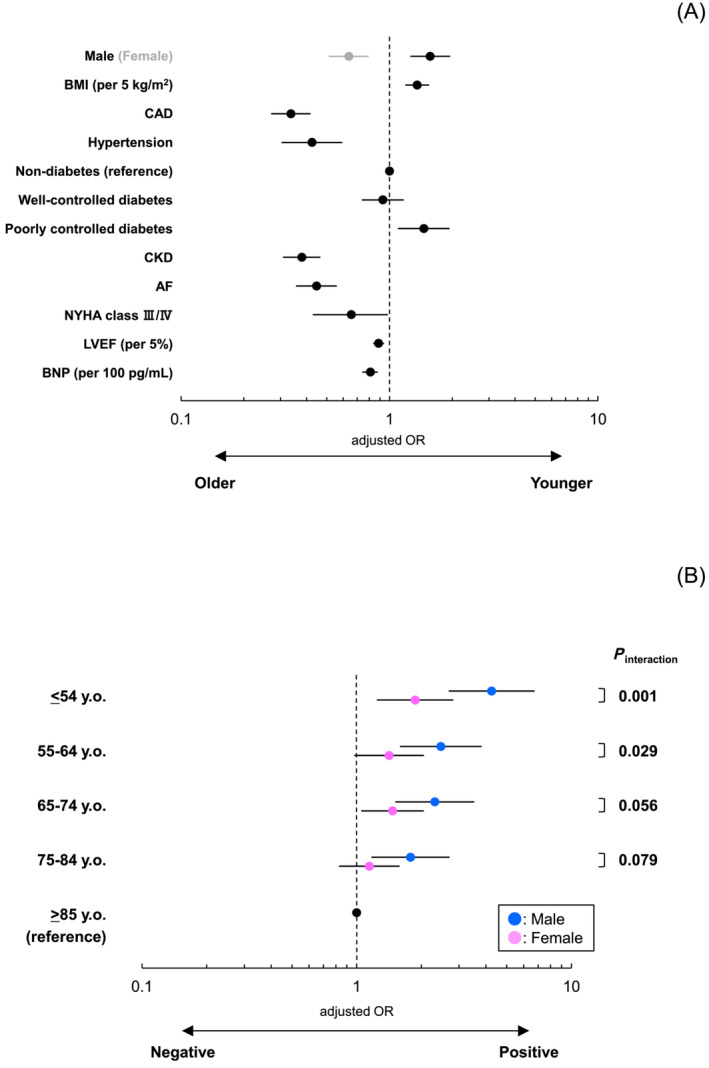
(A) Baseline clinical characteristics associated with younger and older HFpEF patients. In binomial logistic regression analysis, age‐related differences in clinical characteristics among HFpEF patients were examined. The following variables were included for multivariable adjustment: sex, BMI, CAD, hypertension, diabetes, CKD, AF, NYHA class, LVEF, and BNP. Well‐controlled diabetes status was defined as HbA1c ≤ 7.0%, and poorly controlled diabetes status was defined as HbA1c > 7.0%. (B) Sex differences in age‐related trends of the relevance of BMI to HFpEF. In multinomial logistic regression analysis, sex differences in age‐related trends in the relevance of BMI to HFpEF were examined. The following variables were included for multivariable adjustment: sex, BMI, CAD, hypertension, diabetes, CKD, AF, NYHA class, LVEF, and BNP. OR, odds ratio. Other abbreviations are in Table 1 .
Clinical outcomes and causes of death across age groups
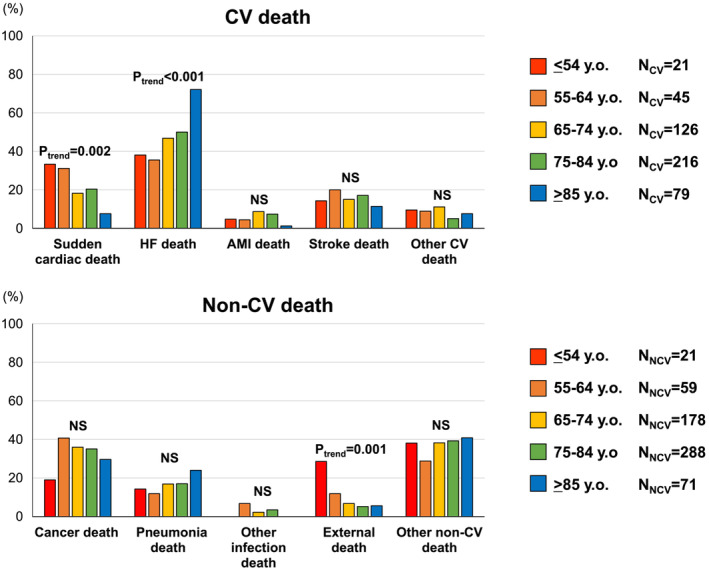
During a median follow‐up period of 9.8 years, 1264 patients experienced mortality. Incidence rates for all‐cause death, CV death, including SCD and HF death, and non‐CV death increased with higher classes of age from ≤54 to ≥85 years (Table 2 ). However, the increasing risk of SCD with age disappeared, unlike other causes of death, after multivariable adjustment applying the competing risk regression model (Table 2 ). The overall population observed a proportion of 38.5% for CV deaths and 48.8% for non‐CV deaths. Notably, statistically significant age‐associated trends in causes of death were evident; SCD and external death became more prevalent with lower classes of age from ≥85 to ≤54 years (P trend = 0.002 and P trend = 0.001, respectively), whereas HF death showed an incremental trend with higher classes of age from ≤54 to ≥85 years (P trend < 0.001) (Figure 2 ).
Table 2
Clinical outcomes across age groups
| Outcomes | Cases/N | Median survival time (years) | Events/1000 person‐years (95% CI) | Crude HR (95% CI) | P‐value | Adjusted HR (95% CI) | P‐value |
|---|---|---|---|---|---|---|---|
| All‐cause death | |||||||
| ≤54 years | 44/349 | 6.03 | 12.2 (8.83–16.3) | 1.00 (reference) | — | 1.00 (reference) | — |
| 55–64 years | 119/529 | 7.01 | 21.9 (18.1–26.2) | 1.80 (1.28–2.55) | 0.001 | 1.59 (1.09–2.31) | 0.015 |
| 65–74 years | 345/891 | 6.19 | 41.7 (37.4–46.3) | 3.51 (2.57–4.80) | <0.001 | 2.94 (2.08–4.16) | <0.001 |
| 75–84 years | 582/853 | 4.94 | 103.4 (95.2–112.2) | 9.45 (6.95–12.8) | <0.001 | 7.06 (4.97–10.0) | <0.001 |
| ≥85 years | 174/202 | 3.71 | 193.0 (165.4–223.9) | 19.8 (14.3–27.6) | <0.001 | 12.9 (8.68–19.1) | <0.001 |
| CV death | |||||||
| ≤54 years | 21/349 | 5.21 | 5.80 (3.59–8.86) | 1.00 (reference) | — | 1.00 (reference) | — |
| 55–64 years | 45/529 | 7.01 | 8.27 (6.03–11.1) | 1.37 (0.82–2.30) | 0.230 | 1.12 (0.65–1.93) | 0.670 |
| 65–74 years | 126/891 | 5.83 | 15.2 (12.7–18.1) | 2.36 (1.49–3.75) | <0.001 | 1.74 (1.06–2.86) | 0.028 |
| 75–84 years | 216/853 | 4.68 | 38.4 (33.4–43.9) | 4.79 (3.60–7.48) | <0.001 | 2.90 (1.76–4.77) | <0.001 |
| ≥85 years | 79/202 | 3.89 | 87.6 (69.4–109.2) | 8.63 (5.32–14.0) | <0.001 | 4.88 (2.73–8.73) | <0.001 |
| Sudden cardiac death | |||||||
| ≤54 years | 7/349 | 4.10 | 1.97 (0.78–3.98) | 1.00 (reference) | — | 1.00 (reference) | — |
| 55–64 years | 14/529 | 6.13 | 2.57 (1.41–4.32) | 1.28 (0.52–3.15) | 0.600 | 1.03 (0.39–2.67) | 0.960 |
| 65–74 years | 23/891 | 4.91 | 2.78 (1.76–4.17) | 1.25 (0.54–2.91) | 0.610 | 0.98 (0.40–2.38) | 0.960 |
| 75–84 years | 44/853 | 4.65 | 7.82 (5.68–10.5) | 2.61 (1.18–5.80) | 0.018 | 1.82 (0.73–4.55) | 0.200 |
| ≥85 years | 6/202 | 3.32 | 6.65 (2.44–14.5) | 1.52 (0.51–4.53) | 0.450 | 1.32 (0.39–4.47) | 0.660 |
| HF death | |||||||
| ≤54 years | 8/349 | 5.41 | 2.21 (0.95–4.35) | 1.00 (reference) | — | 1.00 (reference) | — |
| 55–64 years | 16/529 | 7.25 | 2.94 (1.68–4.77) | 1.28 (0.55–2.98) | 0.570 | 1.08 (0.43–2.72) | 0.860 |
| 65–74 years | 59/891 | 7.31 | 7.13 (5.42–9.19) | 2.83 (1.36–5.92) | 0.006 | 2.25 (1.00–5.05) | 0.050 |
| 75–84 years | 108/853 | 5.16 | 19.2 (15.8–23.2) | 5.91 (2.89–12.1) | <0.001 | 3.73 (1.65–8.43) | 0.002 |
| ≥85 years | 57/202 | 4.07 | 63.2 (47.9–81.9) | 15.2 (7.27–31.9) | <0.001 | 8.78 (3.64–21.2) | <0.001 |
| Non‐CV death | |||||||
| ≤54 years | 21/349 | 7.36 | 5.80 (3.59–8.86) | 1.00 (reference) | — | 1.00 (reference) | — |
| 55–64 years | 59/529 | 7.98 | 10.8 (8.25–14.0) | 1.82 (1.11–2.99) | 0.017 | 1.73 (1.02–2.95) | 0.042 |
| 65–74 years | 178/891 | 6.20 | 21.5 (18.5–24.9) | 3.45 (2.20–5.40) | <0.001 | 3.27 (2.00–5.35) | <0.001 |
| 75–84 years | 288/853 | 4.76 | 51.2 (45.4–57.5) | 6.89 (4.44–10.7) | <0.001 | 5.95 (3.62–9.79) | <0.001 |
| ≥85 years | 71/202 | 3.22 | 78.7 (61.5–99.3) | 7.82 (4.79–12.8) | <0.001 | 6.14 (3.44–11.0) | <0.001 |
CI, confidence interval; CV, cardiovascular; HF, heart failure; HR, hazard ratio.
Other abbreviations are in Table 1 . In Cox proportional hazard analyses applying the competing risk regression model, the following variables were included for multivariable adjustment: sex, BMI, CAD, hypertension, diabetes, CKD, AF, NYHA class, LVEF, and BNP.
Comparison of predictors of sudden cardiac death and heart failure death between the younger and older groups
In the cohort of patients categorized by age, SCD and HF death were characterized by distinct predictive factors. In the younger groups, poorly controlled diabetes showed a significant association with an increased risk of SCD, while in the older groups, elevated serum BNP levels modestly predicted a higher likelihood of SCD (Table 3A ). Parallel analyses were performed to examine predictors of HF death across both age groups. In the younger groups, female sex, CAD, NYHA class III/IV, and elevated serum BNP levels positively correlated with an increased risk of HF death (Table 3B ). Notably, in the older groups, advanced age itself, higher LVEF, and similarly to the younger groups, elevated serum BNP levels were significantly predictive of HF‐related mortality. Additionally, it is also worth noting that comorbidities, such as diabetes, CKD, and AF, were associated with the occurrence of HF‐related mortality, whereas these associations were not observed in the younger groups (Table 3B ).
Table 3A
Comparison of predictors of sudden cardiac death between younger and older heart failure with preserved ejection fraction patients in the competing risk regression model
| Younger HFpEF | Older HFpEF | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariable | Multivariable | Univariable | Multivariable | |||||
| HR (95% CI) | P‐value | HR (95% CI) | P‐value | HR (95% CI) | P‐value | HR (95% CI) | P‐value | |
| Age (per 10 years) | 0.96 (0.59–1.54) | 0.858 | 1.34 (1.01–1.80) | 0.045 | 1.33 (0.93–1.90) | 0.116 | ||
| Male | 2.07 (0.61–7.06) | 0.246 | 1.17 (0.72–1.92) | 0.523 | 1.59 (0.91–2.77) | 0.101 | ||
| BMI (per 5 kg/m2) | 1.01 (0.53–1.91) | 0.984 | 0.83 (0.60–1.16) | 0.279 | ||||
| CAD | 1.07 (0.46–2.52) | 0.873 | 1.35 (0.83–2.20) | 0.228 | ||||
| Hypertension | 3.03 (0.40–22.8) | 0.281 | 1.04 (0.38–2.86) | 0.946 | ||||
| Non‐diabetes | 1.00 (reference) | — | 1.00 (reference) | — | 1.00 (reference) | — | ||
| Well‐controlled diabetes | 1.87 (0.65–5.40) | 0.245 | 2.44 (0.77–7.75) | 0.131 | 0.88 (0.50–1.54) | 0.651 | ||
| Poorly controlled diabetes | 3.34 (1.21–9.20) | 0.020 | 4.26 (1.45–12.5) | 0.008 | 1.46 (0.78–2.73) | 0.239 | ||
| CKD | 0.67 (0.23–2.00) | 0.475 | 0.39 (0.11–1.42) | 0.153 | 1.72 (1.06–2.78) | 0.028 | 1.55 (0.90–2.65) | 0.112 |
| AF | 1.38 (0.57–3.35) | 0.472 | 1.07 (0.68–1.70) | 0.758 | ||||
| NYHA class III/IV | 2.27 (0.53–9.80) | 0.270 | 3.49 (0.87–14.1) | 0.078 | 1.19 (0.57–2.47) | 0.651 | ||
| LVEF (per 5%) | 0.83 (0.58–1.19) | 0.304 | 0.98 (0.87–1.10) | 0.757 | ||||
| BNP (per 100 pg/mL) | 1.11 (0.93–1.32) | 0.239 | 1.11 (1.03–1.21) | 0.009 | 1.10 (0.99–1.21) | 0.064 | ||
CI, confidence interval; HR, hazard ratio; SCD, sudden cardiac death.
Other abbreviations are in Table 1 . In Cox proportional hazard analyses with stepwise variable selection accounting for non‐SCD as a competing risk, potential confounders were defined as follows: age, sex, BMI, CAD, hypertension, diabetes, CKD, AF, NYHA class, LVEF, and BNP.
Table 3B
Comparison of predictors of heart failure death between younger and older heart failure with preserved ejection fraction patients in the competing risk regression model
| Younger HFpEF | Older HFpEF | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariable | Multivariable | Univariable | Multivariable | |||||
| HR (95% CI) | P‐value | HR (95% CI) | P‐value | HR (95% CI) | P‐value | HR (95% CI) | P‐value | |
| Age (per 10 years) | 1.37 (0.80–2.33) | 0.252 | 2.27 (1.90–2.73) | <0.001 | 2.07 (1.63–2.64) | <0.001 | ||
| Male | 0.40 (0.18–0.89) | 0.025 | 0.34 (0.14–0.83) | 0.019 | 0.75 (0.57–0.97) | 0.031 | ||
| BMI (per 5 kg/m2) | 0.84 (0.48–1.47) | 0.549 | 0.82 (0.68–1.00) | 0.047 | ||||
| CAD | 1.99 (0.87–4.55) | 0.103 | 3.63 (1.31–10.1) | 0.013 | 0.92 (0.71–1.20) | 0.547 | ||
| Hypertension | 0.75 (0.26–2.19) | 0.597 | 0.99 (0.57–1.72) | 0.967 | ||||
| Non‐diabetes | 1.00 (reference) | — | 1.00 (reference) | — | ||||
| Well‐controlled diabetes | 1.43 (0.60–3.40) | 0.421 | 1.24 (0.92–1.68) | 0.157 | 1.42 (1.02–1.99) | 0.039 | ||
| Poorly controlled diabetes | 0.53 (0.12–2.32) | 0.401 | 1.37 (0.94–2.00) | 0.098 | 1.87 (1.24–2.83) | 0.003 | ||
| CKD | 1.74 (0.76–3.98) | 0.193 | 2.26 (1.69–3.01) | <0.001 | 1.61 (1.16–2.25) | 0.005 | ||
| AF | 1.12 (0.48–2.60) | 0.800 | 1.94 (1.51–2.50) | <0.001 | 1.56 (1.15–2.11) | 0.004 | ||
| NYHA class III/IV | 7.97 (3.14–20.23) | <0.001 | 4.62 (1.73–12.3) | 0.002 | 2.23 (1.56–3.18) | <0.001 | 1.36 (0.88–2.12) | 0.168 |
| LVEF (per 5%) | 0.94 (0.75–1.18) | 0.605 | 0.89 (0.82–0.96) | 0.003 | 0.88 (0.80–0.96) | 0.005 | ||
| BNP (per 100 pg/mL) | 1.31 (1.15–1.49) | <0.001 | 1.37 (1.18–1.60) | <0.001 | 1.15 (1.10–1.21) | <0.001 | 1.08 (1.01–1.15) | 0.020 |
CI, confidence interval; HF, heart failure; HR, hazard ratio.
Other abbreviations are in Table 1 . In Cox proportional hazard analyses with stepwise variable selection accounting for non‐HF death as a competing risk, potential confounders were defined as follows: age, sex, BMI, CAD, hypertension, diabetes, CKD, AF, NYHA class, LVEF, and BNP.
Discussion
In the present study, we conducted a thorough investigation into the clinical profiles and outcomes of patients with HFpEF, considering diverse age strata. The major findings are as follows: (i) younger HFpEF patients demonstrated distinctive traits, including a male predominance, elevated BMI, and poorly controlled diabetes, whereas older HFpEF patients are more likely to be female with multiple comorbidities such as CAD, hypertension, renal impairment, and AF; (ii) the importance of elevated BMI in relation to HFpEF pathophysiology increased with decreasing age, especially in males; (iii) all‐cause mortality and the proportion of HF death showed an incremental trend with advancing age, whereas the proportion of SCD showed the opposite trend; and (iv) poorly controlled diabetes emerged as an independent predictor of SCD risk in younger HFpEF patients, whereas multiple comorbidities were substantially linked to an elevated risk of HF‐related mortality in older HFpEF patients. These findings suggest that HFpEF exhibits significant variations in its clinical profiles and outcomes depending on age, underscoring the crucial role for physicians to recognize age‐related HFpEF phenotypes for providing personalized patient care.
Age‐stratified distinct clinical profiles and outcomes of heart failure with preserved ejection fraction patients
Recent investigations have underscored notable contrasts between HFpEF patient profiles and outcomes across age segments. 7 , 8 , 9 In concordance with these studies, the present CHART‐2 Study corroborated that male sex and elevated BMI are distinguishing features of younger HFpEF patients. Although our study population demonstrated a significantly lower obesity rate as compared with the prior studies, 7 , 8 , 9 we clarified that higher BMI as a continuous measure was substantially associated with younger HFpEF, highlighting that even in the absence of clinical obesity, elevated BMI itself remains a potential driver of early‐onset HFpEF. Furthermore, we demonstrated for the first time that the positive association between elevated BMI and HFpEF became more pronounced in younger males. This divergence may be attributed to differing patterns of adiposity distribution across sexes. Males typically exhibit visceral adiposity, whereas females tend to exhibit peripheral adiposity, with the distinction being more pronounced before menopause. 22 Treatment with semaglutide, a potent glucagon‐like peptide 1 receptor agonist, resulted in more substantial reductions in symptoms and physical limitations, greater enhancements in exercise function, and increased weight loss compared with placebo for HFpEF patients with obesity. 23 Obesity should not be viewed solely as a comorbidity, but rather, it should be considered a fundamental contributor and a focal point of therapeutic intervention, at least for younger HFpEF patients.
Furthermore, we emphasize the pivotal role of diabetes, particularly when poorly controlled (defined as HbA1c > 7.0%), in characterizing younger HFpEF patients. The accrual of visceral adiposity tends to contribute to insulin resistance and type 2 diabetes, thus establishing a plausible link between elevated BMI, visceral adiposity, and diabetes among younger HFpEF patients. Moreover, given the potential of diabetes to induce LV dysfunction through mechanisms involving hyperglycaemia, hyperinsulinaemia, systemic inflammation, and oxidative stress, 24 , 25 its impact on CV mortality becomes more prominent in the younger population. 26 Indeed, we reported for the first time that poorly controlled diabetes emerged as an independent risk factor for SCD, which was a distinctive cause of death, especially in younger HFpEF patients. When applying the competing risk regression model, the lack of an increasing risk of SCD with age, unlike other causes of death, may underscore the clinical importance of SCD in younger HFpEF patients, albeit partially due to the scarcity of SCD events. To date, several studies have reported that diabetes, especially insulin‐treated diabetes, is a risk factor for SCD in HFpEF patients. 21 , 27 , 28 Cardiac autonomic neuropathy observed in diabetes mellitus, characterized by parasympathetic denervation and enhanced sympathetic tone accompanied by elevated circulating catecholamines, has been demonstrated. 29 Given the increasing prevalence of HFpEF in younger individuals, 3 identifying those at higher risk of SCD is clinically important. In contrast to patients with HF with reduced LVEF (HFrEF), therapeutic strategies to avert SCD in HFpEF patients lack pharmacological or device‐based options. 11 , 30 Our present findings indicate that appropriate glycaemic control may be beneficial to prevent SCD, at least for younger HFpEF patients.
Importantly, in contrast to the report by Tromp et al., 9 HF death remained a significant cause of death for younger HFpEF patients, although the proportion of HF death declined as age decreased. This disparity may be attributed to different study designs, selection criteria, and/or ethnicities among study populations. Moreover, although age‐related trends in causes of death were generally preserved regardless of sex (Supporting Information, Figure S2 ), the rate of HF death was notably higher among younger females aged 55–64 years, which may be attributed to the limited number of CV‐related deaths in younger females. Additionally, in contrast to younger males, CAD was more prevalent, but not statistically significant, in younger females who experienced HF death than in those who experienced other CV deaths excluding HF death (Supporting Information, Table S1 ), possibly explaining the positive association of female sex and CAD with the occurrence of HF‐related mortality in the younger groups. Nevertheless, these observations remain inconclusive due to the insufficient statistical power.
Conversely, multiple non‐cardiac comorbidities, including hypertension, renal impairment, anaemia, AF, diabetes, and chronic obstructive lung disease, have emerged as the determinants and prognostic indicators of CV‐related adverse outcomes in HFpEF patients, who are often typified by older females. 2 , 3 The demographics of this patient cohort closely mirror those of the older HFpEF population in our present study. We demonstrated that, with advancing age, mortality risk, excluding SCD, increased notably, and the proportion of HF deaths gradually rose. Moreover, we clarified for the first time that, in addition to advanced age, these non‐cardiac comorbidities substantially predicted HF death in older HFpEF patients, while indicating that these comorbidities are not causally associated with an increased risk of HF‐related mortality but rather represent the clinical markers of a higher global health risk. Intriguingly, higher LVEF was significantly protective of HF‐related mortality. Prior studies, mostly consisting of acute HF patients, have reported a U‐shaped relationship between LVEF and all‐cause mortality, with the lowest risk being observed at LVEF of 60–65%, 31 , 32 indicating that HF with supranormal LVEF > 65% (HFsnEF) is a high‐risk population. The present study observed a similar U‐shape trend in all‐cause mortality but was not statistically significant (Supporting Information, Figure S3A ). In particular, regarding HF‐related mortality, higher LVEF linearly reduced the risk, at least for older HFpEF patients (Supporting Information, Figure S3B ). Although no prior studies have reported the impact of supranormal LVEF, especially on HF‐related mortality, a recent study suggested that, in contrast to non‐CV‐related mortality risk, CV‐related mortality risk showed a declining trend from HFrEF to HFsnEF. 33
Our observations highlight disparate pathophysiological and prognostic underpinnings across age groups. A larger scale HFpEF population database is warranted to validate and elucidate these clinically pertinent age‐related disparities.
Study limitations
Several limitations should be acknowledged in our study. First, the CHART‐2 Study enrolled only Japanese patients, and caution should be taken when generalizing the present findings to other populations, which calls for external validation studies. Second, as this is a post hoc analysis of an observational study, the impact of missing data (e.g. diastolic functional parameters) and the presence of unmeasured confounders (e.g. ECG data, including the left bundle branch block recognized as a risk factor for SCD 21 ) on the results should be acknowledged. Third, given our selection of HFpEF patients according to LVEF‐based classification in line with AHA/ACC/HFSA guidelines, 11 our HFpEF population comprised a spectrum of diseases, including hypertrophic cardiomyopathy and infiltrative cardiomyopathies (e.g. cardiac amyloidosis and sarcoidosis). However, the sensitivity analysis after excluding these aetiologies showed no substantial change in the results of the present study. Fourth, the relatively small number of patients who experienced SCD in our study may have impacted the statistical power and led to potential data overfitting. Nevertheless, it is worth noting that the proportion of SCD in our study (7.4% of total deaths and 19.3% of CV deaths) closely aligns with figures reported in the Minnesota Heart Survey (10.7% of total deaths and 27.7% of CV deaths) 34 and the Japanese Cardiac Registry of Heart Failure in Cardiology (JCARE‐CARD) (10.7% of total deaths and 18.4% of CV deaths). 35 These studies, which surveyed cause‐specific mortality in HFpEF patients, share similar proportions of SCD and underscore the prevalence of non‐CV death, a characteristic often noted in observational studies of HFpEF patients compared with clinical trials involving individuals with fewer comorbidities. 2 Thus, the relatively small proportion of SCD in our study may be attributed to a significant representation of non‐CV deaths, which accounted for approximately half of all deaths.
Conclusions
Younger HFpEF patients (≤64 years) exhibit a male predominance, elevated BMI, and poorly controlled diabetes, highlighting the importance of glycaemic control in reducing SCD risk. Older HFpEF patients (≥65 years) are more likely to be female, with multiple comorbidities linked to an increased risk of HF‐related mortality. These findings underscore the need for physicians to recognize age‐related, distinct HFpEF phenotypes for personalized patient management.
Funding
This study was supported in part by grants‐in‐aid from the Japanese Ministry of Health, Labour, and Welfare and the Japanese Ministry of Education, Culture, Sports, Science, and Technology.
Supporting information
Appendix S1. Definition of Stage B
Table S1. Comparison of clinical characteristics between younger HFpEF patients under 65 years who experienced HF death and those who experienced other CV death excluding HF death, stratified by sex
Figure S1. Sex differences in age‐related trends of the influence of diabetes control status to HFpEF. In multinomial logistic regression analysis, sex differences in age‐related trends in the relevance of diabetes control status to HFpEF were examined. The following variables were included for multivariable adjustment: sex, BMI, CAD, hypertension, diabetes control status, CKD, AF, NYHA class, LVEF, and BNP. Abbreviations: OR, odds ratio, other abbreviations as in Table 1.
Figure S2. Causes of death in HFpEF patients, stratified by age and sex. Abbreviations: AMI, acute myocardial infarction; CV, cardiovascular; HF, heart failure; NCV, non‐cardiovascular.
Figure S3A. Additive Cox proportional hazard regression model for all‐cause death in HFpEF patients. The shadow area shows the 95% confidence interval. Abbreviations: LVEF, left ventricular ejection fraction.
Figure S3B. Additive Cox proportional hazard regression models for HF death in younger and older HFpEF patients. The shadow area shows the 95% confidence interval. Abbreviations: HF, heart failure; LVEF, left ventricular ejection fraction.
Acknowledgements
We thank all the members of the Tohoku Heart Failure Association and the staff of the Departments of Cardiovascular Medicine and Evidence‐based Cardiovascular Medicine at the Tohoku University Graduate School of Medicine for their contributions.
Notes
Yamanaka, S. , Nochioka, K. , Hayashi, H. , Shiroto, T. , Takahashi, J. , Miyata, S. , Yasuda, S. , Shimokawa, H. , and the CHART‐2 Investigators (2024) Age‐stratified profiles and outcomes of patients with heart failure with preserved ejection fraction. ESC Heart Failure, 11: 2223–2233. 10.1002/ehf2.14798. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
References
Articles from ESC Heart Failure are provided here courtesy of Wiley
Full text links
Read article at publisher's site: https://doi.org/10.1002/ehf2.14798
Read article for free, from open access legal sources, via Unpaywall:
https://onlinelibrary.wiley.com/doi/pdfdirect/10.1002/ehf2.14798
Citations & impact
Impact metrics
Article citations
Age-stratified profiles and outcomes of patients with heart failure with preserved ejection fraction.
ESC Heart Fail, 11(4):2223-2233, 16 Apr 2024
Cited by: 1 article | PMID: 38627993 | PMCID: PMC11287289
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Age-Related Characteristics and Outcomes of Patients With Heart Failure With Preserved Ejection Fraction.
J Am Coll Cardiol, 74(5):601-612, 01 Aug 2019
Cited by: 66 articles | PMID: 31370950
Impact of heart rate changes during hospitalization on outcome in heart failure with preserved ejection fraction.
ESC Heart Fail, 11(5):2901-2912, 21 Mar 2024
Cited by: 1 article | PMID: 38514992 | PMCID: PMC11424277
Epidemiology and one-year outcomes in patients with chronic heart failure and preserved, mid-range and reduced ejection fraction: an analysis of the ESC Heart Failure Long-Term Registry.
Eur J Heart Fail, 19(12):1574-1585, 06 Apr 2017
Cited by: 347 articles | PMID: 28386917
Sudden death in heart failure with preserved ejection fraction and beyond: an elusive target.
Heart Fail Rev, 24(6):847-866, 01 Nov 2019
Cited by: 16 articles | PMID: 31147814
Review
Funding
Funders who supported this work.
Japan Agency for Medical Research and Development (1)
Grant ID: 23ek0109543h0003
Japanese Ministry of Education, Culture, Sports, Science, and Technology
Japanese Ministry of Health, Labour, and Welfare (2)
Grant ID: 15ek0210043h0001
Grant ID: 16ek0210056h0001
Ministry of Health, Labour and Welfare (2)
Grant ID: 15ek0210043h0001
Grant ID: 16ek0210056h0001

 1
1