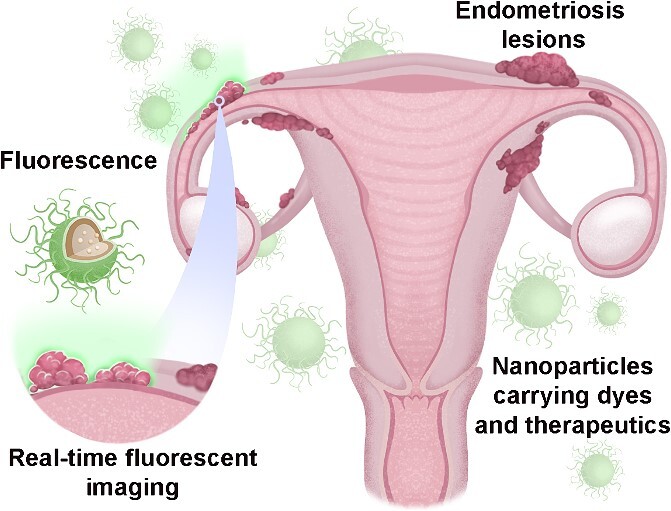
Abstract
Free full text

Targeted nanoparticles for imaging and therapy of endometriosis†
Abstract
In this brief review, we discuss our efforts to validate nanoplatforms for imaging and treatment of endometriosis. We specifically highlight our use of nonhuman primates and primate tissues in this effort. Endometriosis is a painful disorder of women and nonhuman primates where endometrium-like tissue exists outside of the uterus. There are no reliable, specific, and noninvasive diagnostic tests for endometriosis. Laparoscopic imaging remains the gold standard for identifying small endometriotic lesions in both women and monkeys. Visualizing and surgically removing microscopic lesions remains a clinical challenge. To address this challenge, we have created nanoparticle reagents that, when administered intravenously, enter endometriotic lesions both passively and by targeting endometriotic cells. The particles can carry payloads, including near-infrared fluorescent dyes and magnetic nanoparticles. These agents can be used for imaging and thermal ablation of diseased tissues. We evaluated this approach on macaque endometriotic cells, human and macaque endometrium engrafted into immunodeficient mice, in endometrium subcutaneously autografted in macaques, and in rhesus monkeys with spontaneous endometriosis. Employing these models, we report that nanoplatform-based reagents can improve imaging and provide thermal ablation of endometriotic tissues.
Introduction
Nanomedicine is a rapidly emerging field that employs nanoscale agents (particles <200 nm in diameter) as carriers for imaging and therapeutic agents for the visualization and treatment of various diseases [1]. The systemic administration of agents via nanocarriers can improve their solubility and stability, increase systemic half-life, enhance accumulation at diseased tissues, and provide controlled drug release [2]. Our group has evaluated nanoplatforms for detecting and removing various cancers and recently proposed this approach for imaging and treating endometriosis [2–4]. In recent reports, we extensively describe the synthesis of the nanoplatforms, mechanisms for nanoparticle accumulation and retention in lesions after systemic administration, and strategies for targeting the particles to endometriotic cells [2–4]. This review highlights these studies and updates our ongoing work on nanoplatforms for imaging and treating endometriosis in primate models.
Endometriosis is the appearance, growth, and infiltration of endometrium-like tissue at sites outside the uterus [5]. The endometriotic tissues create a gynecological disorder that is associated with infertility and debilitating chronic pelvic pain [6, 7]. Endometriosis affects ~10% of reproductive-aged women, representing ~190 million women worldwide [8]. Menstruating Old World nonhuman primates (NHPs), including macaques and baboons, also develop endometriosis, making them suitable animals for studies of the disorder.
Despite recent progress on biomarkers [9, 10] to identify ectopic endometrium, no clinically approved noninvasive or minimally invasive tests are available to diagnose the disease in either NHPs or women [11, 12]. The reported endometriosis rates vastly underestimate the disease’s impact, and it is estimated that 11% of endometriosis cases remain clinically undiagnosed [13, 14]. The lack of high-resolution, less invasive approaches to characterize early-stage lesions or assess disease progression is a well-recognized roadblock to developing new medical therapies [15]. Diagnosis based on visual laparoscopic examination remains the “gold standard” for identifying and “staging” the disease, and the appearance and presentation of endometriotic lesions in women have been extensively described [16]. However, endometriotic tissues can have multiple forms and color manifestations [17]. Some lesions appear pigmented due to a high level of hemosiderin, giving them a dark blue or brown color, while many are clear or white and more challenging to identify intraoperatively. Moreover, visual characterization of microscopic lesions represents a challenge during surgical treatments and may contribute to the high rate of recurrence (>30%) after surgical intervention.
There is growing evidence that nanoplatforms can be applied in real-time to detect disorders, including endometriosis, to provide imaging for point-of-care tests and remove diseased tissues [18]. Nanoplatforms can carry a wide array of payloads that include photoacoustic [19], fluorescent [20], magnetic [21], and acoustic molecules with the potential for high-resolution imaging [19] and treatment [22]. When administered intravenously, these nanocarriers can deliver the payloads through leaky vessels to the disease tissue bed. Moreover, nanoparticles that accept surface modifications, including specific ligands, can target diseased cells and tissues, including endometriosis [2]. A broad premise of our work has been that nanoplatforms have value in treating endometriotic lesions and thereby reducing the recurrence rate after surgery.
Endometriosis models
Experimental models are indispensable for validating nanoplatform-based delivery because these novel reagents have not been fully vetted in human clinical trials. Unfortunately, no single in vitro or in vivo model fully recapitulates endometriotic disease in women [23]. Mouse models are preferred because of their low cost and ease of availability and maintenance. However, studies in rodents have limitations, the most important being that rodents do not menstruate or develop spontaneous endometriotic disease. Thus, findings derived from pure rodent models do not necessarily translate to new therapies for women [23]. Our studies employ a multi-model approach for assessing nanoplatforms to address this roadblock. These models include in vitro treatment of primary endometrial and endometriotic cells, in vivo treatment of human and macaque endometriotic tissue transplanted into severe combined immunodeficient (SCID) mice, and in vivo studies in macaques with either transplanted or spontaneous endometriosis lesions. For instance, our initial nanoparticle targeting studies were conducted in isolated human and macaque endometrial or endometriotic cells in vitro [2]. Cell and organ culture approaches have the advantage of low cost and the ease of examining the outcome of cell proliferation and viability cell. However, in vitro models fail to display endometriotic disease’s tissue architecture or cyclic characteristics fully. Therefore, we extended our studies to assess the nanoplatform in human and macaque endometrium (or endometriosis) transplanted into SCID mice [24, 25]. In this model (Figure 1a–d), the mice were treated sequentially with estradiol (E2) and progesterone (P4) at levels that recapitulate the normal cycle in women and monkeys. Inducting several artificial 28-day “menstrual” cycles in the mice reduces the impact of surgery on the graft sites and nanoparticle uptake. During these cycles, the grafts bleed and form lesions histologically similar to endometriosis in women and monkeys [25]. To address the impact of immunodeficiency in our xenograft models, we extended our studies to include auto-transplanted endometrium at heterologous sites in macaques [26]. The monkeys are treated with Silastic capsules that release ovarian steroid hormones to recapitulate the primate menstrual cycle. In the autograft model, the grafted tissues express matrix metalloproteinase enzymes, bleed during menstruation, and form blood-filled lesions (Figure 1e–h) that are histologically similar to endometriosis in macaques and women [26, 27]. The “artificial” cycles also permitted the scheduling of nanoparticle administration to hormone-controlled phases of the menstrual cycle. Recently, we extended our studies to spontaneous endometriosis in rhesus macaques to validate the uptake of our nanoplatform in the naturally occurring disease. All animal studies reported herein were reviewed and approved by the ONPRC/OHSU Institutional Animal Care and Use Committee (IACUC).
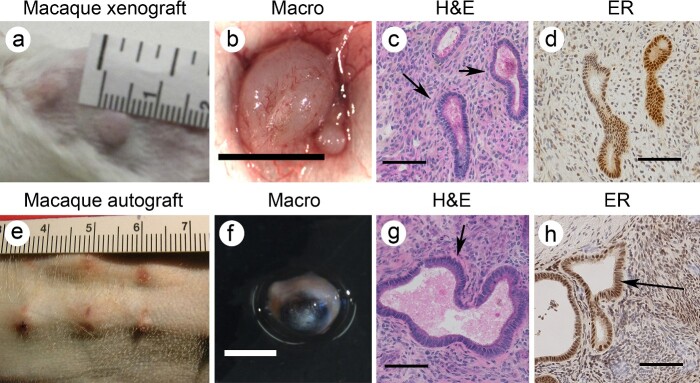
Representative images of macaque endometrial xenografts in SCID mice (a–d) and macaque endometrial autografts (e–h). In both cases, the grafts develop endometriosis-glands (arrow) and stroma that stained positive for estrogen receptor alpha (ER). Scale bar in (b), and (f) =
= 5 mm; scale in (c,d) and (g,h)
5 mm; scale in (c,d) and (g,h) =
= 50 mm.
50 mm.
Passive and active targeting of nanoparticles to endometriosis
Nanoparticles have been investigated for detecting and treating cancer, where the particles appear to pass through immature permeable vessels and accumulate in the extracellular tumor tissue bed [28–31]. This passive targeting of tumors by nanoparticles is termed an enhanced permeability and retention (EPR) effect (Figure 2a). The EPR effect is amplified at sites with hypervascularization and high vascular permeability and can occur in other disorders where vascular permeability is increased [30, 32]. Particle size, composition, and surface charge are critical in the EPR effect [33]. Our work has shown that systemically injected polymeric nanoparticles can extravasate through hyperpermeable, leaky vessels and accumulate in the tissue bed. For instance, poly(ethylene glycol)-poly(ε-caprolactone) (PEG-PCL)-based polymeric nanoparticles with a hydrodynamic size of ~40 nm and slightly negative surface charge (−3 mV) efficiently accumulate in both endometriotic and cancer tissues engrafted into immunodeficient mice [4, 34]. These particles pass from the vasculature and penetrate the endometriotic tissue bed, which can remain for days and weeks [4, 33].
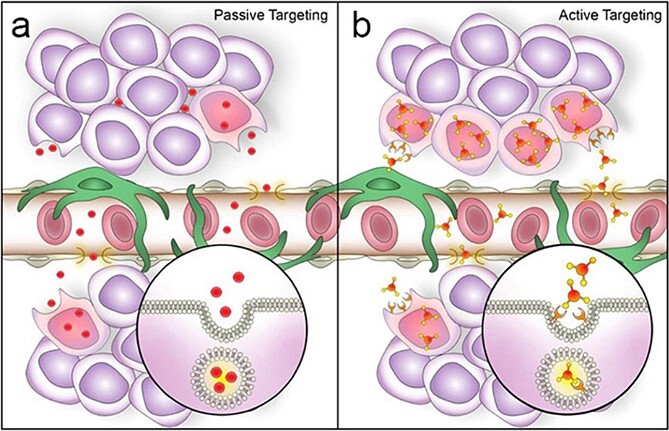
Comparison of passive versus active nanoparticle accumulation in endometriotic lesions. During passive targeting (a) nanoparticles extravasate from systemic circulation into lesion interstitium through permeable blood vessels. During active targeting (b) nanoparticles equipped with tissue-specific ligands first extravasate passively and then bind to endometriotic cells and are internalized. Published with permisson from Small [2].
Another approach actively targets nano reagents to endometriotic cells [35, 36]. To accomplish targeting, specific ligands to the particle surface that can bind specific receptors on disease cells. Upon binding, the particles are internalized into the target cells (Figure 2b). Endometriosis and tumors share many pathophysiologic features [2]. Both exhibit angiogenesis-dependent growth [37–39] mediated by paracrine factors, including vascular endothelial growth factor [VEGF], bradykinin, prostaglandins, and nitric oxide, are highly expressed in many tumors [40–42] and in endometriotic tissues [39, 43]. Employing this approach has been reported for ligands that bind overexpressed receptors in endometriotic cells. While several targeting moieties have been explored [33, 44–47], our work has focused on VEGF receptor 2 (KDR), which is highly expressed in endometriotic lesions [48]. We incorporated a heptapeptide (ATWLPPR) VEGFR/KDR antagonist as a targeting ligand, demonstrating a high affinity for the KDR receptor [49]. To do this, we conjugated the carboxylic acid-terminated PEG-PCL to the peptide by coupling the carboxylic group of PEG to the terminal amine group in the peptide sequence. Our results revealed that the KDR peptide enhances nanoparticle binding to macaque endometriosis stromal cells by 2.5 times but not to endometrium and kidney cells with low expression of the KDR receptor [3].
Imaging methodologies
Non-surgical imaging modalities, including ultrasound (US), magnetic resonance imaging (MRI), computed tomography (CT), and positron emission tomography (PET), are potential noninvasive diagnostic tools for clinical assessment of endometriosis. Ultrasound [50] and MRI imaging [51] have been evaluated to identify larger endometrioma and deep infiltrating endometriosis. While the precision of these evaluated imaging modalities is improving, none currently appear to detect overall pelvic endometriosis with enough accuracy to replace laparoscopic surgery [52]. We recently reported PET-CT imaging of rhesus macaques (Macaca mulatta) using radiotracers that target estrogen receptor (ER) and progesterone receptor (PR) [16α-[18F] fluoroestradiol (FES) and 12-[18F] fluoro-furanyl-nor-progesterone (FFNP)] in individuals with and without endometriosis [53]. While PET/CT with FES and FFNP-supported estrogen and progesterone receptor levels are altered in individuals with endometriosis, the PET/CT failed to provide sufficient resolution to characterize disease burden [53]. Therefore, these techniques hold promise for future clinical applications, but the specificity of high-resolution contrast agents specific to endometriosis has hindered their use as “first-line” diagnostic tools. Moreover, this method exposes the patient to significant radiation doses that may not be suitable for repeated diagnostic assessment.
Fluorescence-guided surgical imaging
Nanoplatforms are well-suited as contrast agents for advanced imaging because they can be injected intravenously (IV) and act as vehicles for delivering contrast agents to endometriotic sites [4]. We have focused on two imaging strategies: near-infrared (NIR) fluorescence [4] and magnetic particle MRI [3, 4, 54]. An added advantage is that both strategies can provide thermal therapy, including NIR photothermal therapy (PTT) and magnetic thermal therapy (MTT), to ablate lesions.
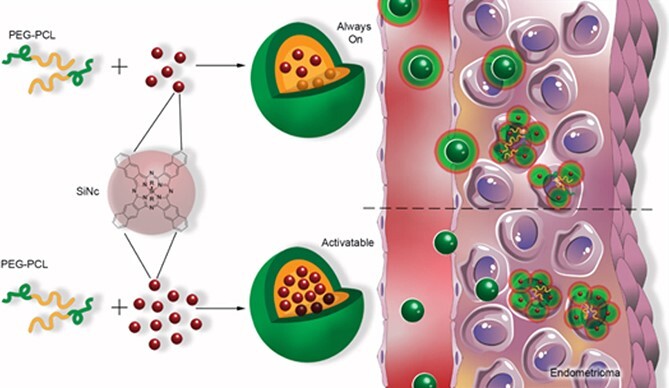
As indicated above, laparoscopic surgery remains the gold standard for identifying endometriotic tissues. However, during laparoscopic surgery, the success of the procedure is dependent on the surgeon’s ability to distinguish between endometriotic and non-disease tissues. The development of real-time fluorescent surgical imaging techniques can significantly improve the detection of small lesions. Fluorescence with visible light during surgery cannot penetrate tissues deeply because energy absorbance and scatter limit imaging to surface features. We have employed NIR light, which has relatively high tissue penetration and lower scattering [51, 55], to address this hurdle. NIR fluorescence-guided surgery is a promising route to ensure real-time demarcation of endometriotic lesions, thereby decreasing the possibility of recurrence or healthy tissue damage during ablation. We selected silicon naphthalocyanine (SiNc), a NIR fluorescent dye, to demonstrate this approach to detect endometriotic tissues [4]. SiNc is a promising contrast agent in fluorescence-guided surgery, but its application is limited by water solubility (<1 ng/mL) [34]. The SiNc was loaded into PEG-PCL nanoparticles (SiNc-NP) that efficiently accumulated in endometriotic lesions following systemic administration to overcome this hurdle. The SiNc molecules are tightly packed inside the hydrophobic core of PEG–PCL polymer, resulting in self-quenching of fluorescence. Upon internalization into target cells, the PEG–PCL nanoparticles disintegrate, “unpacking” the SiNc molecules to produce fluorescence (Figure 3). This feature provides high-contrast imaging of endometriotic tissues because the particles are “activatable” after internalization by endometriotic cells. Our studies confirmed that 24 h post systemic injection, the “activatable” SiNc-NPs accumulated in the subcutaneous endometriotic grafts, resulting in clear delineation of endometriotic lesions through NIR fluorescence. These observations were made using an FDA-approved intraoperative imaging system, Fluobeam 800.
MRI with iron-oxide particles
In theory, MRI can be employed to detect endometriosis for lesions that are not visible under US [56]. Unfortunately, US and MRI suffer from low sensitivity for small and deep infiltration endometriotic lesions [55]. Hemorrhagic components associated with endometriosis lead to hyperintense T1 and T2-weighted MR images (T1WI/T2WI) of endometriotic lesions [51]. While the sensitivity of T1/T2 imaging is superior to that of ultrasonography and PET/CT for deep infiltrating endometriosis, anatomical and histologic variation can produce a wide range of false-positive results [52, 57, 58]. These include fibrous connective tissues, benign and malignant tumors, feces, surgical materials, and post-treatment scars. Therefore, contrasting T1 and T2-weighted images combined with T1 contrast agents such as paramagnetic gadolinium complexes can improve lesion resolution [57].
We synthesized magnetic hexagonal cobalt-doped iron oxide nanoparticles that provide a high-contrast endometriotic lesion to the background signal under MRI. As described above, we loaded these nanoparticles into PEG-PCL vehicles and modified them with KDR-targeting ligands (Figure 4 [3]).
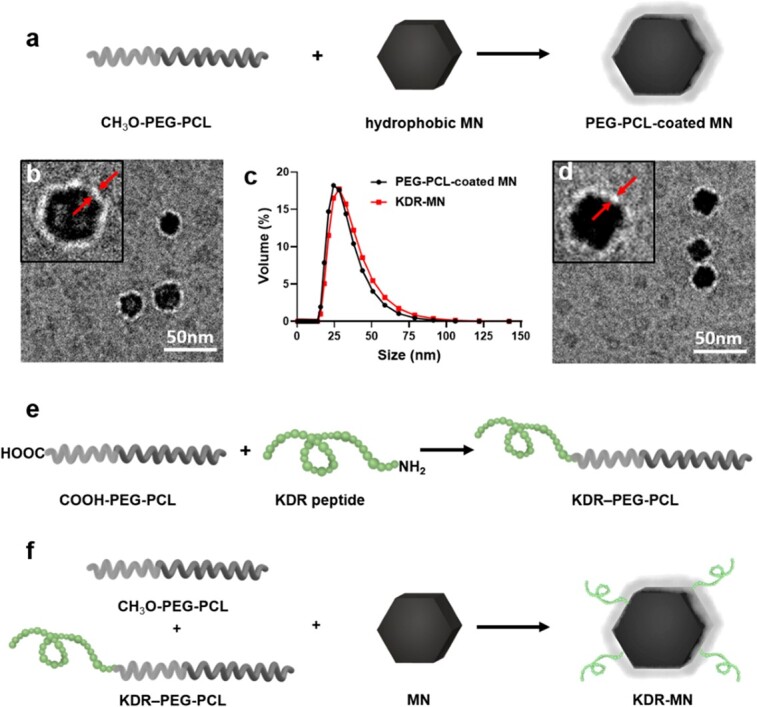
Schematic (a) depicts the synthesis of hydrophobic hexagonal MN into PEG-PCL-based polymeric carriers. Representative cryo-TEM images and dynamic light scattering profiles of non-targeted MN (b, c) and KDR-targeted MN (c, d). Arrowsindicate the PEG-PCL layer. (e–f) Two-step preparation of KDR-MN. Published with permission from Small [3].
We initially evaluated the particles on cultured endometriotic and endometrial cells in vitro. We demonstrated that the KDR targeting magnetic particles (KDR-MN) accumulated in endometriotic cells at approximately a two-fold greater level than in endometrial and kidney control cell cultures [3]. Examination in silico of the KDR-MN particles by T2-weighted MRI revealed that the particles displayed a significant concentration-dependent signal loss. Therefore, these KDR-MN particles provide a reliable negative contrast agent for MRI of endometriosis in vivo.
To demonstrate that our targeted KDR-MN nanoparticles efficiently accumulate in endometriotic lesions following systemic administration, mice bearing multiple endometriotic grafts (Figure 5a) were IV injected with KDR-MN (3 mg Fe/kg). To further confirm that PEG-PCL-based polymeric vehicles also delivered the prepared iron oxide-based MN, endometriotic grafts were dissected and stained with Prussian blue used to detect iron. As shown in Figure 5b, Prussian blue staining is distributed throughout the endometriosis tissue from the edge to the center. These results demonstrated that systemically injected KDR-MN can efficiently reach the periphery of endometriotic grafts via passive targeting and penetrating the endometriotic lesions. Microscopic localization of Prussian blue-stained sections of various organs, including endometriosis grafts, collected at 24 h and 5 days post administrations revealed that the spleen and liver sequester a significant amount of KDR-MN after intravenous injection and eliminate them from the body. Iron staining was negligible in the other organs, including the liver, indicating that nanoparticles had been cleared from these tissues [3].
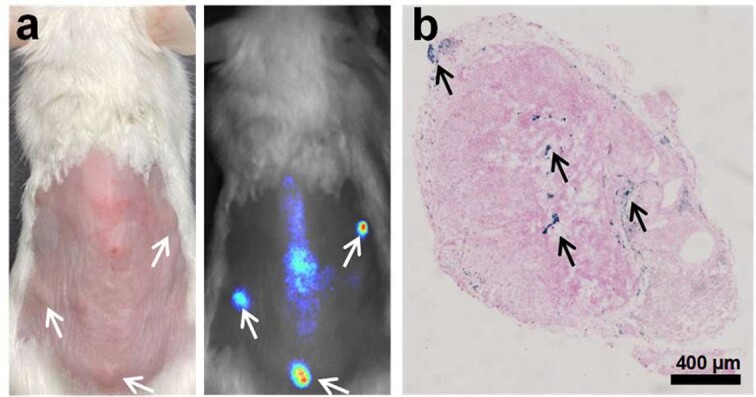
(a) Photograph (left) and NIR fluorescence image (right) of a mouse with macaque endometriotic grafts (arrows) at 5 days after IV injection of KDR targeted MN loaded also loaded with a NIR fluorescence dye (SiNc). (b) Prussian blue iron staining (arrows) section of an endometriotic graft collected from a mouse at 5 days post-injection with KDR-MN. Published with permission from Small [3].
As indicated above, the KDR-MN particles produce a robust negative contrast during T2-weighted MRI. To demonstrate this imaging, mice bearing subcutaneous macaque (or human) endometriotic grafts were subject to T1 and T2 MRI (Figure 6). The acquired images demonstrated significant T2 signal loss associated with KDR-MN uptake in endometriotic grafts relative to the untreated control at the concentration (3 mg Fe/kg).
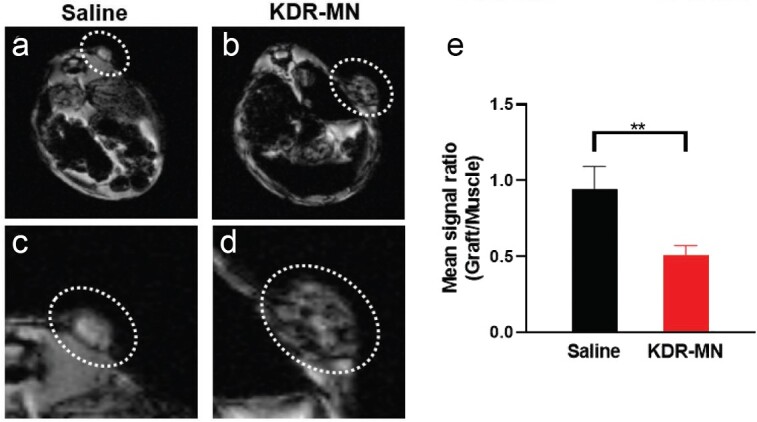
T2-weighted magnetic resonance images (MRI at TE 4.5 ms) were obtained after 24 h in mice injected intravenously with (a,c) saline and KDR-MN (3 mg Fe/kg). (b,d). Image (c,d) are magnified images of (a–b). Note the relatively darker signal in the endometriosis graft with the KDR-MN injected mouse, showing localization of the nanoparticles by MRI and its property as a negative contrast imaging agent. (e) The mean signal ratio (+/− SD) between grafts, normalized to skeletal muscle in mice injected with saline (n =
= 3/group), unpaired t-test was used for statistical comparisons (**p
3/group), unpaired t-test was used for statistical comparisons (**p <
< 0.01). Reproduced with permission from Small [3].
0.01). Reproduced with permission from Small [3].
One ongoing concern when adapting xenograft models for imaging of endometriosis is the potential confounding effects of surgery and the immunodeficient state of the mice when grafted with macaque or human tissues. To address this concern, in a preliminary study, we identified rhesus macaques with extensive naturally occurring endometriosis that was not associated with prior surgery. Our primate center has a standardized protocol for identifying animals with endometriosis by disease symptoms, including chronic pelvic pain at menses [59]. The endometriotic mass was then confirmed by US and abdominal palpation, and the animals underwent repeated T1 and T2 weighted MRI to map potential endometriotic sites. The animals were systemically treated (IV) with KDR-targeted magnetic particles (3 mg Fe/kg; n =
= 5), and a repeat T2-weighted MRI was performed 24 h after injection. In this study, treatment with the KDR-MN resulted in dense regions of negative contrast associated with the lesions. The animals underwent necropsy, and the lesions identified by MRI were collected along with non-target organs (e.g. bladder, kidney, liver endometrium, myometrium). Endometriosis was confirmed in the KDR-MN labeled sites by histology, revealing endometriotic glands and stroma. Prussian blue staining revealed the uptake of iron in endometriosis (data not shown). Weaker but significant staining was also observed in the liver and minimal or no staining was observed in other non-target organs. These results validate that the KDR-targeted nanoparticles specifically target endometriotic tissues and not sites of recent surgery and that off-target background in non-target organs should be minimal.
5), and a repeat T2-weighted MRI was performed 24 h after injection. In this study, treatment with the KDR-MN resulted in dense regions of negative contrast associated with the lesions. The animals underwent necropsy, and the lesions identified by MRI were collected along with non-target organs (e.g. bladder, kidney, liver endometrium, myometrium). Endometriosis was confirmed in the KDR-MN labeled sites by histology, revealing endometriotic glands and stroma. Prussian blue staining revealed the uptake of iron in endometriosis (data not shown). Weaker but significant staining was also observed in the liver and minimal or no staining was observed in other non-target organs. These results validate that the KDR-targeted nanoparticles specifically target endometriotic tissues and not sites of recent surgery and that off-target background in non-target organs should be minimal.
Nanoplatform-mediated thermal therapy
Our PEG–PCL polymer nanoparticles are excellent carriers for therapeutic agents. In addition to single-agent therapies, [44, 60–62] nanoparticle-based approaches allow concurrent delivery of combinations of therapeutic agents that target multiple therapeutic pathways in endometriosis, resulting in novel combinatorial therapies [63, 64]. For example, it has recently been demonstrated in a mouse model that it is feasible to target angiogenesis, oxidative stress, and MMP activity through the delivery of epigallocatechin gallate (EGCG) and the antibiotic doxycycline (Dox) in a single polymer-based nano delivery system [64]. Loading drugs, including anti-estrogenic compounds, aromatase inhibitors, and anti-metabolic drugs, such as methotrexate, into nanoplatforms that target endometriosis would significantly reduce the unwanted side effects of these drugs.
Phototherapy is a therapeutic modality in which light energy is either focused and used to treat diseases directly [65] or used to activate a photosensitive agent (photosensitizer) to elicit a therapeutic response [66]. In one type of phototherapy, PTT, light-absorbing dye converts light energy into heat energy, generating localized hyperthermia in the diseased tissues. The generated heat leads to cell death [67]. Many of these light-absorbing dyes are hydrophobic. Nanoparticles have proven to be a promising route to improve the efficiency of PTT mainly due to their capability to efficiently deliver photosensitizing agents to diseased tissue, while simultaneously improving water solubility and stability in systemic circulation [66]. Active targeting through surface modification of nanoparticles increases their selectivity and accumulation in disease sites [68].
To our knowledge, two approaches have been reported for nanoparticle-based PTT to eradicate endometriotic lesions [4]. One approach was conducted by Guo et al. who employed non-targeted hollow gold nanospheres (HAuNS) and TNYL tagged HAuNS (TNYL-HAuNS) in the photothermal treatment of endometriotic lesions [33]. TNYL conjugation increased the selective accumulation of nanoparticles in endometrial cells by binding to the overexpressed EphB4 receptor. A temperature increase was observed upon exposure to NIR light of passively and actively targeted gold photosensitizer nanoparticles. Biodistribution profiles revealed significantly higher targeted nanoparticle accumulation in lesions following systemic administration. Exposure of endometriotic grafts to higher intensity NIR light (808 nm, 2 W/cm2) for 10 min on days 2 and 4 after injection of targeted and non-targeted nanoparticles significantly inhibited the growth of lesions.
Our group developed an alternate approach. This approach employed SiNc based nanoparticles and NIR light for photothermal treatment of endometriosis [4, 33]. SiNc was the photosensitizer of choice due to its high NIR absorption rates in the spectral range of 750 nm to 800 nm, corresponding to enhanced heating efficiency [69]. However, one of the challenges hindering SiNc’s PTT application is its poor water solubility [4, 34, 69]. To overcome this hurdle, biocompatible block copolymer PEG-PCL was used as a carrier to encapsulate SiNc in its hydrophobic core and improve its solubility and delivery [4, 34]. In vitro photothermal efficiency of the developed SiNc nanoparticles (SiNc-NP) was investigated by incubating monkey endometriotic stromal cells (30 μg/mL) over 2 days. Exposure of incubated cells to NIR light (780 nm, 0.9 W/cm2) for 15 min increased the intra-cellular temperature up to 53°C, leading to the death of 95% of the cells. SiNc-NP was then administered intravenously (3 mg/kg) to endometriotic lesion-bearing mice via tail vein injection, and whole-body fluorescence imaging showed the nanoparticles efficiently accumulated in endometriotic lesions. Exposure of lesions to NIR light for 15 min, 24 h post-injection, raised the intralesional temperature to 47°C, leading to their eradication within 4 days [4]. Histological analysis confirmed the destruction of glands and stroma in endometriotic grafts, accompanied by a loss of ER and PR, rendering them unresponsive to hormonal stimulation and eliminating their potential for growth under estrogen. What remained in the lesion sites was connective tissues indicative of scaring. In addition, NIR light or SiNc-NP alone had no impact on the viability of endometriotic cells, confirming the photothermal phenomenon is mutually inclusive of both components. This approach was initially developed by us to successfully eradicate tumors in murine cancer models with no sign of acute toxicity [34].
Magnetic thermal therapy
One application of our KDR-MN particles is the potential for thermal eradication of tissues via magnetic hyperthermia. Thermal ablation of cancerous tissues has been proposed following direct administration of the magnetic particles to the cancerous tissue. For systemic delivery, the heterogeneous nanoparticle distribution within cancer tissues following intratumoral administration is a critical issue that could lead to underheating regions within tumors [70, 71].
As described above, our fluorescence imaging studies revealed that KDR-targeted iron oxide nanoparticles (KDR-MN) were also labeled with a fluorescence dye accumulated efficiently in all endometriotic grafts in SCID mice. Prussian blue staining demonstrated that the iron particles are homogeneously distributed throughout the endometriosis tissue (Figure 5b). We evaluated the ability of the systemically injected KDR-MN to generate heat in endometriotic grafts under an alternating magnetic field (AMF) and found that 5 days after IV injection, the KDR-MN elevated the temperature inside of grafts up to 51.6 ±
± 1.2°C. In contrast, non-targeted MN raised only to 49.3
1.2°C. In contrast, non-targeted MN raised only to 49.3 ±
± 1.8°C; the ability of non-targeted and KDR targeted nanoparticles to increase the temperature in grafts above 46°C suggests that both nanoparticles accumulate efficiently in endometriotic tissue via passive targeting. The observed difference in heating rates during AMF exposure is associated with increased retention of passively accumulated KDR-MN in endometriosis grafts compared with non-targeted MN within 5 days after administration. This treatment resulted in elimination of the endometriotic tissues in mice, intravenously injected with KDR-MN. We also confirmed that our nanoparticles efficiently accumulate in lesions after systemic administration in macaques with spontaneous endometriosis and elevate the temperature in lesions up to 45°C upon exposure to AMF.
1.8°C; the ability of non-targeted and KDR targeted nanoparticles to increase the temperature in grafts above 46°C suggests that both nanoparticles accumulate efficiently in endometriotic tissue via passive targeting. The observed difference in heating rates during AMF exposure is associated with increased retention of passively accumulated KDR-MN in endometriosis grafts compared with non-targeted MN within 5 days after administration. This treatment resulted in elimination of the endometriotic tissues in mice, intravenously injected with KDR-MN. We also confirmed that our nanoparticles efficiently accumulate in lesions after systemic administration in macaques with spontaneous endometriosis and elevate the temperature in lesions up to 45°C upon exposure to AMF.
Conclusion and future perspective
Clinically approved imaging modalities for endometriosis are currently limited by the methodological resolution of the lesions during surgery. Noninvasive methods including PET, MRI, and US lack molecular specificity for endometriosis and depend on clinical experience to evaluate the lesions. The studies described above validate that nanoparticle-based imaging has the potential to improve the identification of lesions during surgery and can be applied to noninvasive modalities. Moreover, thermal therapy can be a valuable tool for removing microscopic endometriotic tissues that cannot be treated surgically. Both magnetic and NIR thermal ablation approaches are limited primarily by the existing technology to view the tissues and detect the lesions via MRI or another imaging approach. Technological limitations on imaging and control of thermal ablation aside, these nanocarriers have great potential to act as combined therapeutics to provide both imaging modalities and anti-endometriotic drugs to bear on the lesions within a single treatment. Currently, pharmacologic treatment of endometriosis is limited by the off-target action of the therapies. Our nanoparticle approach provides the option of directly targeting the endometriotic lesions, visualizing that the therapeutic agent has reached its desired target, and evidence that the agents are released within the target. Importantly, we have confirmed in macaques with spontaneous endometriosis that our targeted polymeric nanocarriers are safe, preferentially accumulate in lesions after systemic administration, and deliver magnetic nanoparticles as T2-weighted MRI contrast to enhance the detection of endometriosis lesions. Future studies assessing the impact of thermal ablation of endometriotic tissues remain to be conducted. Imaging of our model systems indicates that connective tissues replace endometriotic cells. It is unknown if this will result in scaring the endometriotic sites and promoting tissue adhesions. Evaluating these nanoparticle-based modalities in clinical trials is an essential next step to demonstrate their potential for imaging and treating endometriosis.
Footnotes
† Grant Support: This work was supported by NIH (grants R21HD088654, R01HD101450 and P51OD011092).
Contributor Information
Ov Slayden, Division of Reproductive and Developmental Sciences, Oregon National Primate Research Center, Oregon Health & Science University, Beaverton, OR, USA.
Fangzhou Luo, Division of Reproductive and Developmental Sciences, Oregon National Primate Research Center, Oregon Health & Science University, Beaverton, OR, USA.
Youngrong Park, Department of Pharmaceutical Sciences, College of Pharmacy, Oregon State University, Portland, OR, USA.
Abraham S Moses, Department of Pharmaceutical Sciences, College of Pharmacy, Oregon State University, Portland, OR, USA.
Ananiya A Demessie, Department of Pharmaceutical Sciences, College of Pharmacy, Oregon State University, Portland, OR, USA.
Prem Singh, Department of Pharmaceutical Sciences, College of Pharmacy, Oregon State University, Portland, OR, USA.
Tetiana Korzun, Department of Pharmaceutical Sciences, College of Pharmacy, Oregon State University, Portland, OR, USA. School of Medicine, Oregon Health & Science University, Portland, OR, USA.
Olena Taratula, Department of Pharmaceutical Sciences, College of Pharmacy, Oregon State University, Portland, OR, USA.
Oleh Taratula, Department of Pharmaceutical Sciences, College of Pharmacy, Oregon State University, Portland, OR, USA.
References
 for treatment planning. Int J Hyperthermia 2020;
for treatment planning. Int J Hyperthermia 2020; 37:76–99. [Abstract] [Google Scholar]
37:76–99. [Abstract] [Google Scholar]Articles from Biology of Reproduction are provided here courtesy of Oxford University Press
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Nanoparticle-Based Platform for Activatable Fluorescence Imaging and Photothermal Ablation of Endometriosis.
Small, 16(18):e1906936, 06 Apr 2020
Cited by: 26 articles | PMID: 32250034 | PMCID: PMC7210057
A protocol for creating endometriosis in rhesus macaques (Macaca mulatta).
J Med Primatol, 52(6):405-413, 17 Oct 2023
Cited by: 0 articles | PMID: 37849073
Decidualization of Endometriosis in Macaques.
Vet Pathol, 53(6):1252-1258, 08 Jun 2016
Cited by: 11 articles | PMID: 27281017 | PMCID: PMC5368640
Cancer-associated mutations in endometriosis: shedding light on the pathogenesis and pathophysiology.
Hum Reprod Update, 26(3):423-449, 01 Apr 2020
Cited by: 31 articles | PMID: 32154564
Review
Funding
Funders who supported this work.
NICHD NIH HHS (1)
Grant ID: R01 HD101450
NIH (3)
Grant ID: P51OD011092
Grant ID: R01HD101450
Grant ID: R21HD088654
NIH HHS (2)
Grant ID: P51 OD011092
Grant ID: R21HD088654