Abstract
Free full text

Resistance caused by decreased penetration of beta-lactam antibiotics into Enterobacter cloacae.
Abstract
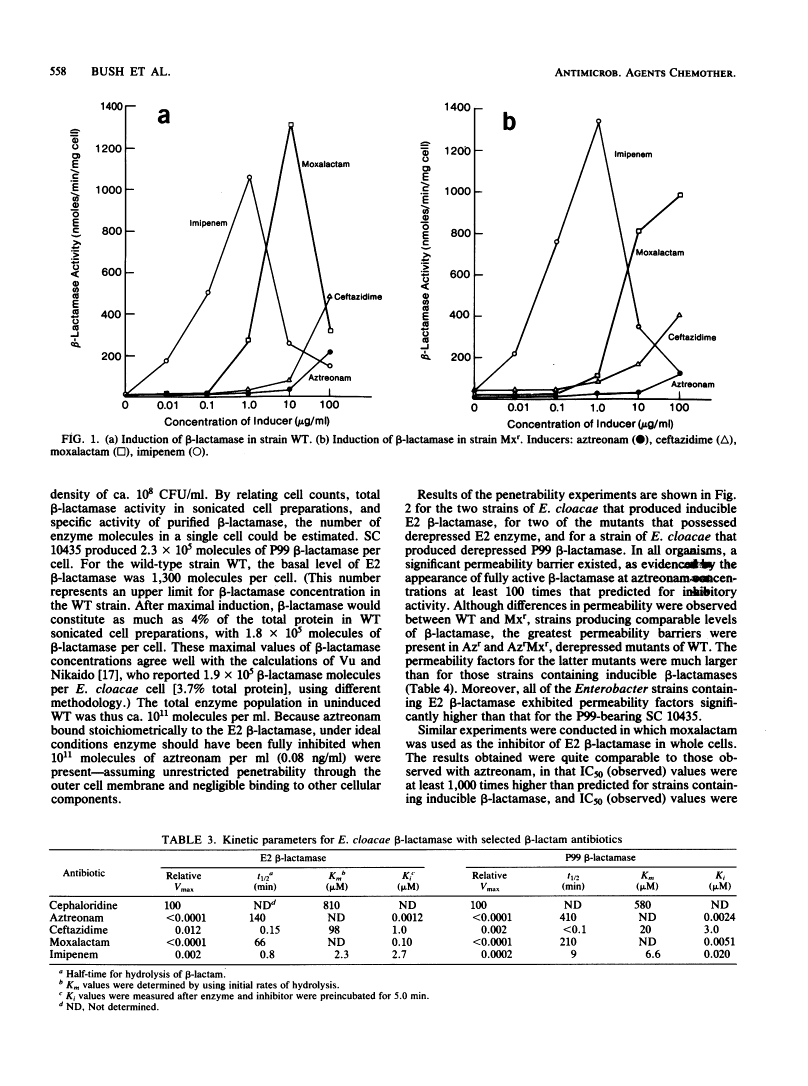
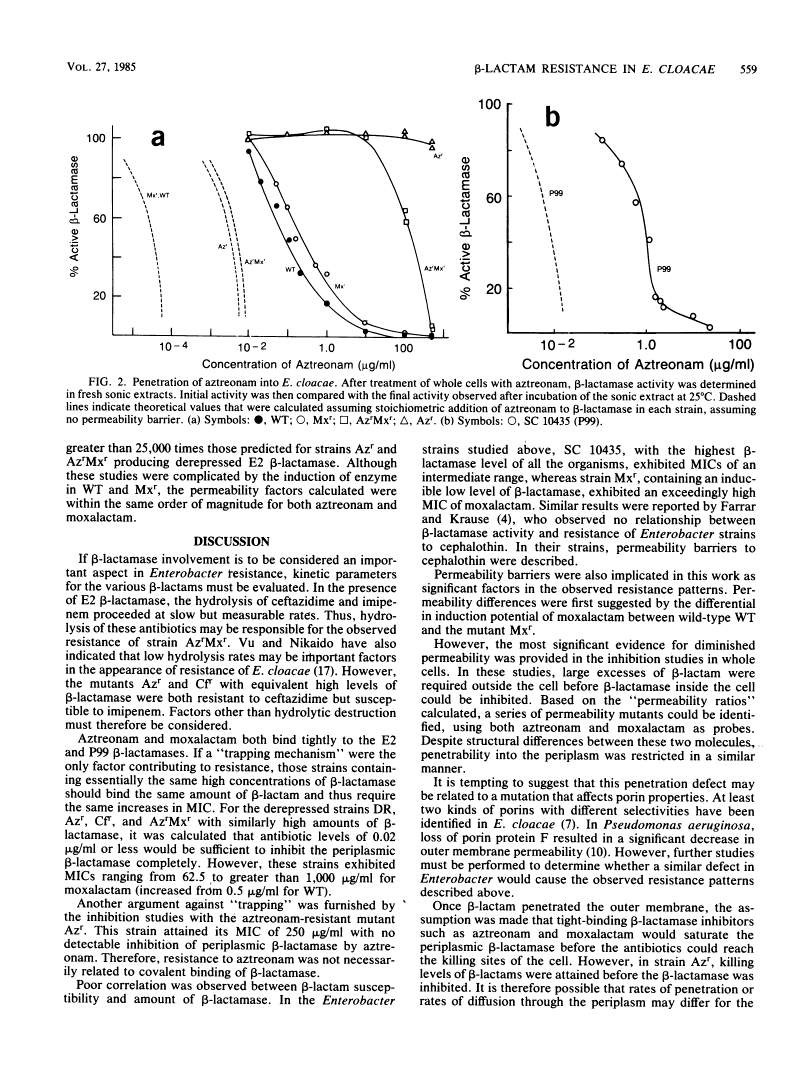
Strains of Enterobacter cloacae were selected on the basis of resistance to aztreonam, ceftazidime, moxalactam, or imipenem. All strains produced the same E2 beta-lactamase, with an isoelectric point greater than 9.5 and with high hydrolytic activity in the presence of cephaloridine. Resistance to beta-lactams could not be correlated with the amount of beta-lactamase present in the various strains. beta-Lactamase activity was induced strongly by moxalactam and imipenem in the wild-type and moxalactam-resistant strains, with beta-lactamase representing as much as 4% of the total cellular protein after induction (2 X 10(5) molecules per cell). Ceftazidime and aztreonam were poor inducers. None of the antibiotics studied was readily hydrolyzed by the E2 beta-lactamase; aztreonam and moxalactam inhibited the enzyme with apparent Ki values of 1.2 and 100 nM, respectively. Aztreonam, which bound covalently to the E2 beta-lactamase with a half-life of 2.3 h at 25 degrees C, was used to measure penetrability of beta-lactam into the periplasmic space of the resistant E. cloacae strains. In all of the E2-producing organisms studied, a significant permeability barrier existed. A maximum concentration of 0.02 microgram of aztreonam per ml should have saturated the periplasmic beta-lactamase in the highest enzyme producers studied. However, fully active beta-lactamase was observed in the periplasm of cells exposed to aztreonam at concentrations at least 1,000-fold higher than that theoretically necessary to inhibit the total enzyme within the cell. Thus, the major cause for resistance to beta-lactam antibiotics in these E. cloacae strains was lack of penetration across the outer membrane.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.1M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruderlein H, Daniel R, Perras D, DiPaola A, Fideliá A, Belleau B. An investigation of the destruction of the beta-lactam ring of penems by the albumin drug-binding site. Can J Biochem. 1981 Oct;59(10):857–866. [Abstract] [Google Scholar]
- Bush K, Freudenberger JS, Sykes RB. Interaction of azthreonam and related monobactams with beta-lactamases from gram-negative bacteria. Antimicrob Agents Chemother. 1982 Sep;22(3):414–420. [Europe PMC free article] [Abstract] [Google Scholar]
- Dixon M. The graphical determination of K m and K i . Biochem J. 1972 Aug;129(1):197–202. [Europe PMC free article] [Abstract] [Google Scholar]
- Farrar WE, Krause JM. Relationship Between beta-Lactamase Activity and Resistance of Enterobacter to Cephalothin. Infect Immun. 1970 Nov;2(5):610–616. [Europe PMC free article] [Abstract] [Google Scholar]
- Gootz TD, Sanders CC, Goering RV. Resistance to cefamandole: derepression of beta-lactamases by cefoxitin and mutation in Enterobacter cloacae. J Infect Dis. 1982 Jul;146(1):34–42. [Abstract] [Google Scholar]
- Kaneko M, Yamaguchi A, Sawai T. Purification and characterization of two kinds of porins from the Enterobacter cloacae outer membrane. J Bacteriol. 1984 Jun;158(3):1179–1181. [Europe PMC free article] [Abstract] [Google Scholar]
- Kojo H, Shigi Y, Nishida M. A novel method for evaluating the outer membrane permeability to beta-lactamase-stable beta-lactam antibiotics. J Antibiot (Tokyo) 1980 Mar;33(3):310–316. [Abstract] [Google Scholar]
- Kojo H, Shigi Y, Nishida M. Enterobacter cloacae outer membrane permeability to ceftizoxime (FK 749) and five other new cephalosporin derivatives. J Antibiot (Tokyo) 1980 Mar;33(3):317–321. [Abstract] [Google Scholar]
- Nicas TI, Hancock RE. Pseudomonas aeruginosa outer membrane permeability: isolation of a porin protein F-deficient mutant. J Bacteriol. 1983 Jan;153(1):281–285. [Europe PMC free article] [Abstract] [Google Scholar]
- Ross GW, Boulton MG. Purification of beta-lactamases on QAE-sephadex. Biochim Biophys Acta. 1973 Jun 6;309(2):430–439. [Abstract] [Google Scholar]
- Sanders CC, Sanders WE, Jr, Goering RV. In vitro antagonism of beta-lactam antibiotics by cefoxitin. Antimicrob Agents Chemother. 1982 Jun;21(6):968–975. [Europe PMC free article] [Abstract] [Google Scholar]
- Seeberg AH, Tolxdorff-Neutzling RM, Wiedemann B. Chromosomal beta-lactamases of Enterobacter cloacae are responsible for resistance to third-generation cephalosporins. Antimicrob Agents Chemother. 1983 Jun;23(6):918–925. [Europe PMC free article] [Abstract] [Google Scholar]
- Sykes RB, Bonner DP, Bush K, Georgopapadakou NH. Azthreonam (SQ 26,776), a synthetic monobactam specifically active against aerobic gram-negative bacteria. Antimicrob Agents Chemother. 1982 Jan;21(1):85–92. [Europe PMC free article] [Abstract] [Google Scholar]
- Then RL, Angehrn P. Trapping of nonhydrolyzable cephalosporins by cephalosporinases in Enterobacter cloacae and Pseudomonas aeruginosa as a possible resistance mechanism. Antimicrob Agents Chemother. 1982 May;21(5):711–717. [Europe PMC free article] [Abstract] [Google Scholar]
- Vu H, Nikaido H. Role of beta-lactam hydrolysis in the mechanism of resistance of a beta-lactamase-constitutive Enterobacter cloacae strain to expanded-spectrum beta-lactams. Antimicrob Agents Chemother. 1985 Mar;27(3):393–398. [Europe PMC free article] [Abstract] [Google Scholar]
- Waterworth PM, Emmerson AM. Dissociated resistance among cephalosporins. Antimicrob Agents Chemother. 1979 Apr;15(4):497–503. [Europe PMC free article] [Abstract] [Google Scholar]
- Zimmermann W, Rosselet A. Function of the outer membrane of Escherichia coli as a permeability barrier to beta-lactam antibiotics. Antimicrob Agents Chemother. 1977 Sep;12(3):368–372. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Antimicrobial Agents and Chemotherapy are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/aac.27.4.555
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc180094?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1128/aac.27.4.555
Article citations
Emerging Strategies to Combat β-Lactamase Producing ESKAPE Pathogens.
Int J Mol Sci, 21(22):E8527, 12 Nov 2020
Cited by: 10 articles | PMID: 33198306 | PMCID: PMC7697847
Review Free full text in Europe PMC
Role of TEM-1 β-Lactamase in the Predominance of Ampicillin-Sulbactam-Nonsusceptible Escherichia coli in Japan.
Antimicrob Agents Chemother, 63(2):e02366-18, 29 Jan 2019
Cited by: 3 articles | PMID: 30455244 | PMCID: PMC6355572
Community-Acquired Urinary Tract Infection by Escherichia coli in the Era of Antibiotic Resistance.
Biomed Res Int, 2018:7656752, 26 Sep 2018
Cited by: 65 articles | PMID: 30356438 | PMCID: PMC6178185
Review Free full text in Europe PMC
Past and Present Perspectives on β-Lactamases.
Antimicrob Agents Chemother, 62(10):e01076-18, 24 Sep 2018
Cited by: 304 articles | PMID: 30061284 | PMCID: PMC6153792
Review Free full text in Europe PMC
Electronic Public Health Registry of Extensively Drug-Resistant Organisms, Illinois, USA.
Emerg Infect Dis, 21(10):1725-1732, 01 Oct 2015
Cited by: 21 articles | PMID: 26402744 | PMCID: PMC4593443
Review Free full text in Europe PMC
Go to all (56) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Contribution of beta-lactamase hydrolysis and outer membrane permeability to ceftriaxone resistance in Enterobacter cloacae.
Antimicrob Agents Chemother, 31(10):1589-1595, 01 Oct 1987
Cited by: 30 articles | PMID: 3501699 | PMCID: PMC174996
Factors that influence the evolution of beta-lactam resistance in beta-lactamase-inducible strains of Enterobacter cloacae and Pseudomonas aeruginosa.
J Infect Dis, 155(5):936-941, 01 May 1987
Cited by: 24 articles | PMID: 3104483
Overproduced beta-lactamase and the outer-membrane barrier as resistance factors in Serratia marcescens highly resistant to beta-lactamase-stable beta-lactam antibiotics.
J Gen Microbiol, 135(5):1275-1290, 01 May 1989
Cited by: 17 articles | PMID: 2695600
The emergence of beta-lactam resistance among strains of Enterobacter cloacae and Pseudomonas aeruginosa.
Pediatr Infect Dis J, 8(9 suppl):S100-3; discussion S128-32, 01 Sep 1989
Cited by: 0 articles | PMID: 2510122
Review