Abstract
Free full text

The genome sequence of Tadarida brasiliensis I. Geoffroy Saint-Hilaire, 1824 [Molossidae; Tadarida]
Abstract
We present a genome assembly from an individual male Tadarida brasiliensis (The Brazilian free-tailed bat; Chordata; Mammalia; Chiroptera; Molossidae). The genome sequence is 2.28 Gb in span. The majority of the assembly is scaffolded into 25 chromosomal pseudomolecules, with the X and Y sex chromosomes assembled.
Species taxonomy
Eukaryota; Metazoa; Eumetazoa; Bilateria; Deuterostomia; Chordata; Craniata; Vertebrata; Gnathostomata; Teleostomi; Euteleostomi; Sarcopterygii; Dipnotetrapodomorpha; Tetrapoda; Amniota; Mammalia; Theria; Eutheria; Boreoeutheria; Laurasiatheria; Chiroptera; Yangochiroptera; Molossidae; Tadarida; Tadarida brasiliensis, (I. Geoffroy Saint-Hilaire, 1824) (NCBI:txid9438; subordinal taxonomy updated per Teeling et al, ( Teeling et al., 2005).
Introduction
Tadarida brasiliensis, commonly known as the Mexican free-tailed bat or the Brazilian free-tailed bat, is medium-sized New World insectivorous bat. Belonging to the family Molossidae, Tadarida is one of 21 genera that comprise the 4 th largest family in Chiroptera ( Simmons, 2023). The genus of Tadarida contains 8 species, with T. brasiliensis being the only New World bat of the genus ( Figure 1). While some genera with Molossidae show support for monophyly, Tadarida does not show evidence for forming a monophyletic clade ( Agnarsson et al., 2011). Since T. brasiliensis is the only New World bat of this genus, a subgenus classification of Rhizomops has been previously proposed ( Legendre, 1984), but morphological and genetic evidence does not support this distinction ( Gregorin & Cirranello, 2016; Ammerman et al., 2012). Genetic evidence from four genes produces a clade specifically formed of T. brasiliensis, Tadarida aegyptiaca and Sauromys petrophilus ( Ammerman et al., 2012), but more recent analysis using morphological evidence does not support this clade ( Gregorin & Cirranello, 2016). The closest relative of T. brasiliensis is T. aegyptiaca, with T. brasiliensis showing a higher relation to Old World molossids with the last shared common ancestor with the New World clade being 29 mya ( Ammerman et al., 2012).
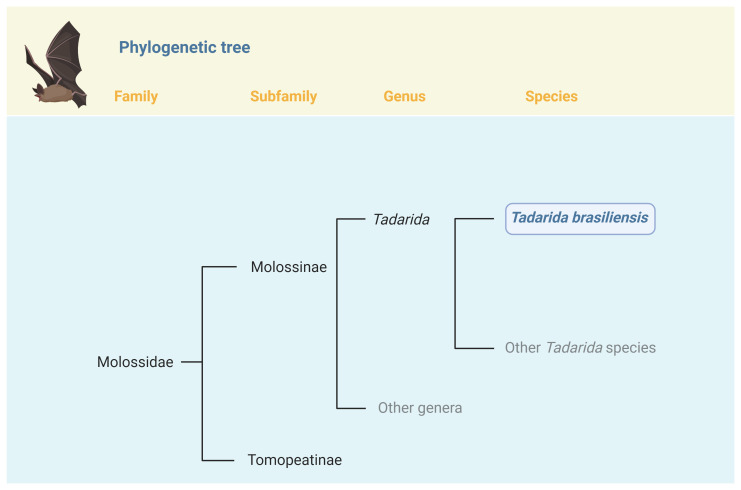
Tadarida brasiliensis is one of 8 species currently recognized in the genus Tadarida (Rafinesque, 1814). Tadarida belongs to the subfamily Molossinae ( Gervais, 1856), which currently includes 20 genera and 132 species. FIgure created with BioRender.com.
T. brasiliensis is one of the most widely distributed mammals in the New World, and one of the most abundant bat species. According to the International Union for Conservation of Nature (IUCN), T. brasiliensis is listed as Least Concern ( Barquez et al., 2015). The geographic range of the species includes most of the United States, Mexico, Central America, and southwestern South America, including Greater and Lesser Antilles ( Wilkins, 1989). Due to the species’ large range, and proposed behavioral and morphological differences, T. brasiliensis was once thought to comprise up to nine subspecies ( Schwartz, 1955). However, the population structure from genetic studies and morphological evidence do not confirm any subspecies classification and support gene flow between mainland and island populations ( Morales et al., 2016; Morales et al., 2018). Instead, phenotypic differences are hypothesized to be correlated to climatic variation across regions and individuals are recommended to be categorized as migratory or non-migratory ( Morales et al., 2016). The large geographic range is most likely related to the species’ large dispersal capabilities.
One of the defining traits of T. brasiliensis is the “free tail”, where the tail extends beyond the uropatagium which is characteristic of the Molossidae family ( Figure 2A). T. brasiliensis is the smallest of the New World molossids with an adult weight range of 11–14 g, average total body length of 95 mm, and the average forearm length of 42 mm ( Schmidly, 2018). The species has a short velvety brown pelage, with long hairs on the feet that extend past the toes ( Wilkins, 1989). The snout is short with wrinkled lips. The ears, when pressed forward, do not extend past the snout and do not meet at the midline. The species is not strongly sexually dimorphic, but reproductively active males have an enlarged gular gland which is a sebaceous gland found on the suprasternal neck region ( Krutzsch et al., 2002). This gland shows seasonal functionality and during the breeding season secretes a thick, oily, odorous substance in reproductive adult males.
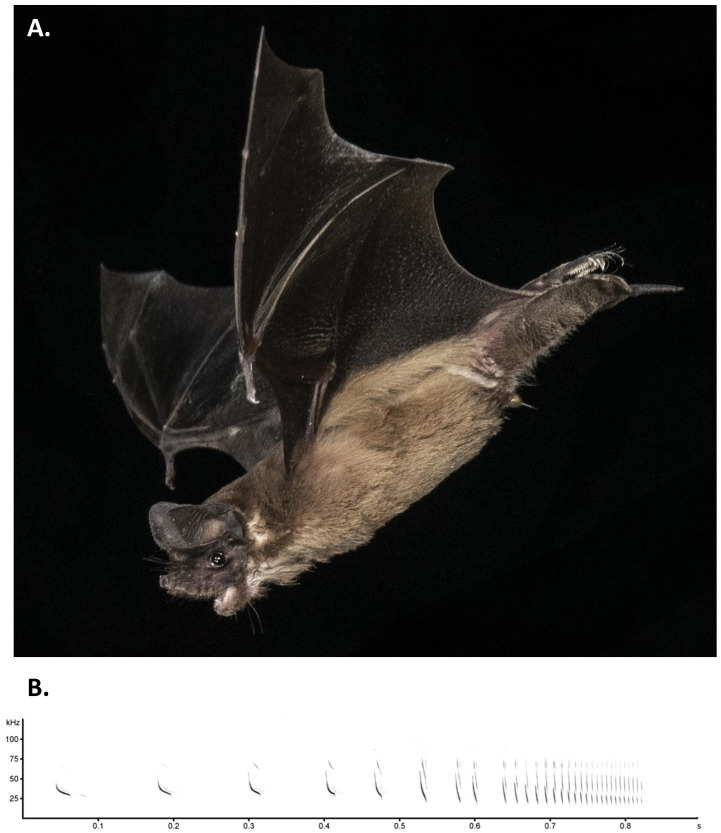
Tadarida brasiliensis A) Adult individual of the Mexican free-tailed bat, Tadarida brasiliensis. Note the tail extending beyond the uropatagium, short wrinkled snout, and hairs extending beyond toes. Photo courtesy of Brock and Sherri Fenton with permission, Windsor Cave, Jamaica. B) An echolocation pulse sequence emitted by Tadarida brasiliensis while foraging over a pond. This sequence begins with a typically shallow frequency-modulated search-phase pulse and ends with a terminal buzz.
T. brasiliensis is known for forming large colonies, with a single colony sometimes containing several million individuals. The densest populations of the species are found in Texas, where in the summer an estimated 95–104 million bats (primarily females forming maternity colonies) occupy a few select caves known as guano caves ( Schmidly, 2018). T. brasiliensis primarily roost in caves or man-made structures such as buildings or under bridges but have also been found in hollow trees in the southeastern US ( Wilkins, 1989).
This species is classified as a migratory bat with some of the longest recorded bat migrations. T. brasiliensis is estimated to have an annual migration as far as 1500 km ( Villa-R & Cockrum, 1962), traveling from central and southwestern United States southward into Mexico ( Cockrum, 1969). While shorter seasonal movements in temperate bats are not uncommon, mostly in response to unfavorable climate ( Popa-Lisseanu & Voigt, 2009), longer migrations as seen in T. brasiliensis are rarer in bats. While temperature changes could be triggering this movement, another potential reason for this annual trip is the seasonal availability and distribution of food resources, such as movements of migratory moths ( Russell et al., 2005). Even during nightly foraging trips, individuals may travel more than 160 km from their roost to a foraging site and back in one evening, maintaining horizontal flight speeds of up to 44 m/s ( Davis et al., 1962; McCracken et al., 2016). This high dispersal capability may be responsible for the lack of distinct population structure across the wide species range. However, there are differences in migratory behavior based on geographic location and sex. Previous banding experiments propose sedentary or non-migratory subpopulations in parts of the United States ( Cockrum, 1969), but no genetic differentiation has been found to account for this difference in migratory phenotype ( Russell et al., 2005). Also, females seem to travel further with more males forming resident populations in the winter or not traveling as far into Mexico ( Russell et al., 2005).
T. brasiliensis is an aerial insectivore that relies upon echolocation to navigate and forage for prey ( Figure 2B). In open spaces this species emits a shallow frequency-modulated echolocation pulse that descends from approximately 25 to 20 kHz over a 15–20 ms duration. When approaching obstacles or prey, pulse durations are progressively shortened to 2 ms while the starting frequency of the fundamental harmonic is concomitantly raised to approximately 50 kHz, with higher (2 nd and 3 rd) non-overlapping harmonics also becoming prominent in the signal ( Schwartz et al., 2007; Simmons et al., 1978). T. brasiliensis is known to forage at high altitudes, with recorded foraging activity on migratory noctuid moths ( Lepidoptera) occurring as high as 3000 m ( Williams et al., 1973). However, T. brasiliensis can also forage near ground and their diverse diet varies seasonally depending on species abundance and availability ( McCracken et al., 2021; Ross, 1961).
Previously, there has only been a short read genome assembly of T. brasiliensis available (GenBank accession: GCA_004025005.1) which was generated as a part of the Zoonomia Project ( Zoonomia, 2020). Notable sequencing projects utilizing this assembly consist of comparative genomics analyses of diet, visual system adaptations based on foraging style, immunity and metabolic adaptations, and longevity across several bat species ( Blumer et al. 2022; Davies et al., 2020; Fushan et al., 2015; Moreno Santillan et al., 2021; Potter et al., 2021). As for future applications with the new reference quality long read genome assembly reported herein, T. brasiliensis has previously been proposed as an ideal mammal model for the genetic and epigenetic basis of migration, mostly due to the widespread species range, abundant population, and variation of the phenotype ( Merlin & Liedvogel, 2019). Functional genomics projects should be fruitful in this species compared to other North American bats due to their high population levels and therefore less conservation pressure, as sample sizes are often a limiting factor. Although there is continued potential for comparative projects across multiple bat species, access to this genome assembly will hopefully encourage more work regarding the genetic basis for T. brasiliensis’ unique traits.
Genome sequence report
The genome was sequenced from a single male Tadarida brasiliensis collected from the Texas A&M campus of College Station, Brazos County, Texas, USA. A total of 39-fold coverage in Pacific Biosciences Hi-Fi long reads (contig N50 86 Mb) was generated after removal of all reads shorter than 10kb. Primary assembly contigs were scaffolded with chromosome confirmation Hi-C data. The final assembly has a total length of 2.28 Gb in 147 sequence scaffolds with a scaffold N50 of 111 Mb ( Table 1). The majority, 98.44%, of the assembly sequence was assigned to 25 chromosomal-level scaffolds, representing 23 autosomes (numbered by sequence length, and the X and Y sex chromosomes). Chromosomal pseudomolecules in the genome assembly of Tadarida brasiliensis are shown in Table 2. The assembly has a BUSCO ( Simao et al., 2015) completeness of 96.3% using the laurasiatheria reference set. While not fully phased, the assembly deposited is of one haplotype.
Table 1.
| Project accession data | |
|---|---|
| Assembly identifier | DD_mTadBra1_pri |
| Species | Tadarida brasiliensis |
| Specimen | mTadbra1 |
| NCBI taxonomy ID | txid9438 |
| BioProject | Bat1K: Accession:
PRJNA489245; ID: 489245 Tadarida: Accession: PRJNA972445ID: 972445 |
| BioSample ID | SAMN35075070 |
| Isolate information | Male - muscle |
| Raw data accessions | |
| Pacific Biosciences
SEQUEL II | SRX20532109 |
| Hi-C Illumina | SRX20532110, SRX20532111 |
| 10X linked-reads | SRX20532112 |
| Assembly accession | GCA_030848825.1 |
| Accession of Alternative haplotype | GCA_030848815.1 |
| Span (Mb) | 2,283,188,801 |
| Number of contigs | 168 |
| Contig N50 length (Mb) | 86,333,355 |
| Number of scaffolds | 147 |
| Scaffold N50 length (Mb) | 111,098,925 |
| Longest scaffold (Mb) | 250,357,659 |
Table 2.
ENA accession Chromosome Size (bp) GC%. The chromosome number of Tadarida brasiliensis is 2n= 50 .
| Genbank accession | Chromosome | Size (bp) | GC% |
|---|---|---|---|
| CM061257.1 | 1 | 250,357,659 | 41.0 |
| CM061258.1 | 2 | 131,366,638 | 41.5 |
| CM061259.1 | 3 | 123,954,994 | 43.2 |
| CM061260.1 | 4 | 112,652,436 | 40.2 |
| CM061261.1 | 5 | 112,580,834 | 42.5 |
| CM061262.1 | 6 | 111,418,324 | 40.6 |
| CM061263.1 | 7 | 111,368,843 | 42.0 |
| CM061264.1 | 8 | 111,098,925 | 44.0 |
| CM061265.1 | 9 | 107,746,758 | 40.7 |
| CM061266.1 | 10 | 105,253,767 | 39.5 |
| CM061267.1 | 11 | 101,937,717 | 40.8 |
| CM061268.1 | 12 | 93,068,937 | 43.3 |
| CM061269.1 | 13 | 90,219,467 | 42.9 |
| CM061270.1 | 14 | 78,395,959 | 42.9 |
| CM061271.1 | 15 | 70,705,328 | 43.6 |
| CM061272.1 | 16 | 70,395,922 | 41.3 |
| CM061273.1 | 17 | 66,982,268 | 46.3 |
| CM061274.1 | 18 | 66,967,517 | 43.7 |
| CM061275.1 | 19 | 53,271,390 | 44.8 |
| CM061276.1 | 20 | 52,736,693 | 46.6 |
| CM061277.1 | 21 | 38,494,270 | 43.7 |
| CM061278.1 | 22 | 27,110,749 | 44.9 |
| CM061279.1 | 23 | 15,501,133 | 47.4 |
| CM061280.1 | X | 134,431,381 | 39.4 |
| CM061281.1 | Y | 9,583,748 | 46.7 |
Methods
The T. brasiliensis specimen was an adult male individual collected on the evening of October 15, 2018. The bat was caught by hand net as it left a roost located in a building on the Texas A&M campus in College Station, Brazos County, Texas, USA. Capture, handling, and sampling were approved by the local institutional Animal Care and Use Committee (Texas A&M University animal use protocol # 2017-0163D) and by Texas Parks and Wildlife scientific collecting permit SPR-1104-610.
Upon capture, a dichotomous key ( Schmidly, 2018) was utilized to confirm species identity. The bats were identified as T. brasiliensis based on specific morphological features such as forearm measures and other external features. Additionally, other molossids found in Texas ( Eumops perotis, Nyctinomops femorosaccus, Nyctinomops macrotis) are much larger compared to T. brasiliensis. At the capture location, there is limited range overlap with other molossids except for N. macrotis which is an uncommon species. However, these two species can be distinguished based on ear morphology as N. macrotis’ ears meet on the midline of the head whereas T. brasiliensis’ ears do not.
After collection from the field, the specimen was brought back to the laboratory and spent 5 months in captivity before tissue extraction and sample preparation. The animal was euthanized with pentobarbital overdose on February 28, 2019. Tissue samples collected were blood, brain (left hemisphere, cerebellum, front and back cortex, dorsal and ventral striatum), liver, heart (ventricle), left and right lung, spleen, left and right kidney, arm muscle, and left and right testicle. In total, 23 tissue samples were collected. All tissue samples were flash frozen in liquid nitrogen and stored in a -80°C freezer until shipment with the cold chain maintained. All data were recorded and reported in accordance with the ARRIVE guidelines ( Kilkenny et al., 2010) – see data availability section and Table 1.
Phenol-chloroform extraction of genomic DNA
Snap-frozen muscle tissue of Tadarida brasiliensis has been grinded into a fine powder in liquid nitrogen. Powdered muscle tissue was lysed overnight at 55°C in high-salt tissue lysis buffer (400 mM NaCl, 20 mM Tris base pH 8.0. 30 mM EDTA pH 8.0, 0,5% SDS, 100 ug/ml Proteinase K). RNA was removed by incubating in 50 mg/ml RNase A for 1 hour at 37°C. Cell debris have been removed by a centrifugation step. High molecular weight genomic DNA (HMW gDNA) was purified with two washes of Phenol-Chloroform-IAA equilibrated to pH 8.0, followed by two washes of Chloroform-IAA, and precipitated in ice-cold 100% Ethanol. HMW gDNA was collected by centrifugation, the gDNA pellet was washed twice in 70% cold Ethanol, dried for 10 min at 37°C, and eluted in 1x TE. Fragment length of the HMW gDNA was between 30 and 300 kb as shown by pulse field gel electrophoresis (PFGE) (Pippin Pulse, SAGE Science, Beverly, MA).
Extraction of megabase-size gDNA
a) Bionano-plug based megabase-size gDNA extraction for Bionano optical mapping. Megabase-size gDNA was extracted from liver tissue according to the Bionano Prep™ Animal tissue DNA isolation soft tissue protocol (Document number 30077, Bionano, San Diego, CA). In brief, liver tissue was homogenized in a tissue grinder directly followed by a mild Ethanol fixation. The homogenized tissue was embedded into agarose plugs and treated with Proteinase K and RNase A. Genomic DNA has been extracted from agarose plugs by agarose treatment and purified by drop dialysis against 1x TE. PFGE revealed megabase-size DNA molecule length of 50 kb up to 600 kb.
b) Bionano-SP based megabase-size gDNA extraction for 10x linked Illumina reads. A second batch of megabase-size gDNA from snap-frozen kidney tissue was extracted with the beta version of the Bionano Prep SP Animal Tissue DNA Isolation Protocol (Document number 30339, Bionano, San Diego, CA). In brief, snap-frozen kidney tissue was homogenized with the Tissue Ruptor (Qiagen) on ice in a chaotropic buffer containing ethanol and tissue lysis took place by adding Proteinase K. Cell debris have been removed by centrifugation. The released gDNA was bound to a Nanobind disk (a novel nano structured silica on the outside of the thermoplastic paramagnetic disk) upon the addition of salting buffer and isopropanol. After several washing steps, the gDNA was eluted from the Nanobind disk. PFGE revealed mega-size DNA molecule length of 50 kb up to 600 kb.
PacBio HiFi library preparation and sequencing
Three HiFi libraries of Phenol-chloroform extraction of genomic DNA (HMW gDNA) of Tadarida brasiliensis have been prepared as recommended by Pacific Biosciences according to the ‘Guidelines for preparing HiFi SMRTbell libraries using the SMRTbell Express Template Prep Kit 2.0 (PN 101-853-100, version 01). In summary, HMW gDNA has been sheared to 20 and 25 kb fragments, respectively, with the MegaRuptor™ device (Diagenode). 10 ug sheared gDNA have been used for library preparation. All PacBio SMRTbell™ libraries were size selected for fragments larger than 9 to 13 kb, 13 kb, and 15 kb with the BluePippin™ device according to the manufacturer’s instructions. The size selected libraries run on six Sequel II SMRT cells with the SEQUEL II sequencing kit 2.0 for 30 hours on the SEQUEL II of the DRESDEN concept Genome Center (DcGC), Germany. Circular consensus sequences were called making use of the default SMRTLink tools.
Bionano optical mapping of megabase-size gDNA
Megabase-size gDNA of Tadarida brasiliensis was labelled as described in the Bionano Prep direct label and stain (DLS) protocol (Document number 30206). These DNAs were tagged with the nicking-free DLE enzyme. One flow cell of the labelled gDNA was run on the Bionano Saphyr instrument at the DcGC and circa 200X genome coverage of molecules longer than 150 kb was achieved.
10x linked reads
Linked Illumina reads were generated by using the 10x Genomics Chromium™ genome application following the Genome Reagent Kit Protocol v2 (Document CG00043, Rev B, 10x Genomics, Pleasonton, CA). In brief, 1 ng of mega-size genomic DNA was partitioned across over 1 Million Gel bead-in-emulsions (GEMS) using the Chromium™ devise. Individual gDNA molecules were amplified in these individual GEMS in an isothermal incubation using primers that contain a specific 16 bp 10x barcode and the Illumima ® R1 sequence. After breaking the emulsions, pooled amplified barcoded fragments were purified, enriched, and went into Illumina sequencing library preparation as described in the protocol. Pooled Illumina libraries were sequenced to a 100X genome coverage on an Illumina NovaSeq instrument at the DKMS Life Science Lab gGmbH, Dresden, Germany.
Hi-C chromatin confirmation capture
Chromatin confirmation capturing was done making use of the ARIMA-Hi-C (Material Nr. A510008) and the Hi-C+ Kit (Material Nr. A410110) and followed the user guide for animal tissues (ARIMA-Hi-C kit, Document A160132 v01 and ARIMA-Hi-C 2.0 kit Document Nr: A160162 v00). In brief, circa 50 mg flash-frozen powdered tissue was crosslinked chemically. The crosslinked genomic DNA was digested with the restriction enzyme cocktail consisting of two and four restriction enzymes, respectively. The 5’-overhangs are filled in and labelled with biotin. Spatially proximal digested DNA ends were ligated and finally the ligated biotin containing fragments were enriched and went for Illumina library preparation, which followed the ARIMA user guide for Library preparation using the Kapa Hyper Prep kit (ARIMA Document Part Number A160139 v00). The barcoded Hi-C libraries run on a NovaSeq6000 with 2x 150 cycles.
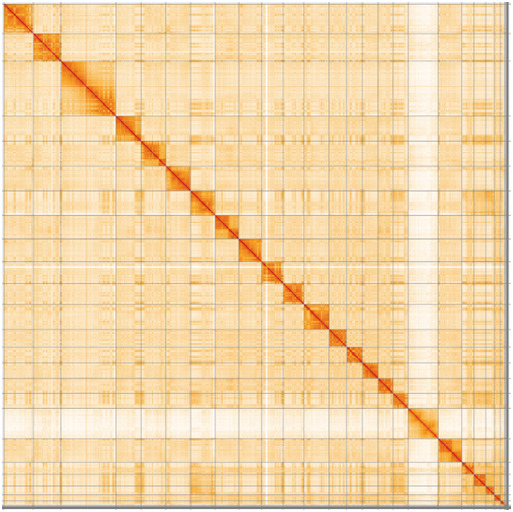
Assembly was carried out following the Vertebrate Genome Project pipeline v2.0 ( Rhie et al., 2020) as follows. HiFi reads were created with ccs (v6.0.0). HiFiasm (v0.16.0) was used to create the initial contig set. Haplotypic duplication was identified and removed with purge dups (v1.2.5) ( Guan et al., 2020). The quality of the assembly was evaluated using Merqury ( Rhie et al., 2020) and BUSCO ( Manni et al., 2021). Scaffolding with 10X data was carried out with Scaff10X (commit bc3a0cb), Bionano data with Bionano Solve (v 3.6.1) and Hi-C data ( Rao et al., 2014) with SALSA2 (commit e6e3c77) ( Ghurye et al., 2019). HiGlass ( Kerpedjiev et al., 2018) was implemented to generate Hi-C contact maps and perform manual curation of scaffolds into chromosomes. Figure 3, Figure 4, Figure 5 & Figure 6 were generated using BlobToolKit ( Challis et al., 2020). Software utilized for T. brasiliensis analysis are depicted in Table 3.
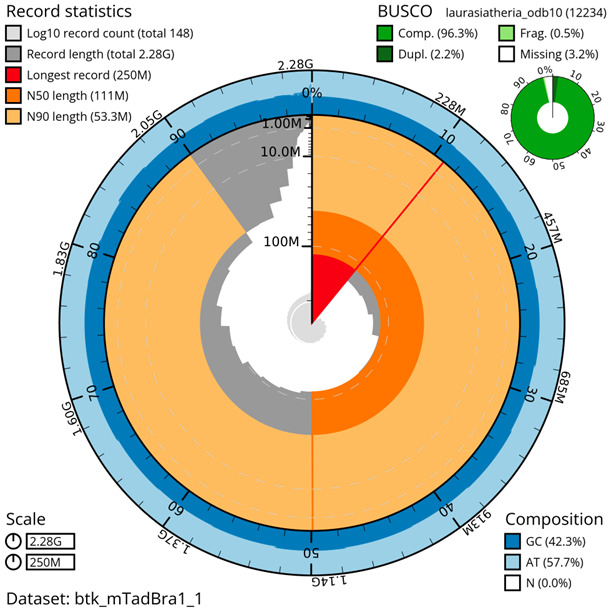
The larger snail plot depicts scaffold statistics including N50 length (bright orange) and base composition (blue). The smaller plot shows BUSCO completeness in green.
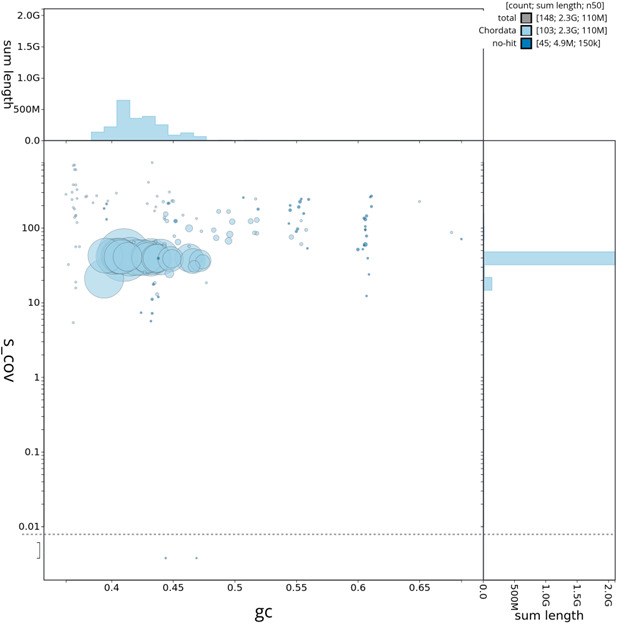
Individual chromosomes and scaffolds are represented by each circle. The circles are sized in proportion to chromosome/scaffold length. Histograms show the sum length of chromosome/scaffold size along each axis. Color of circles indicate taxonomic hits of each Phylum represented in the assembly.
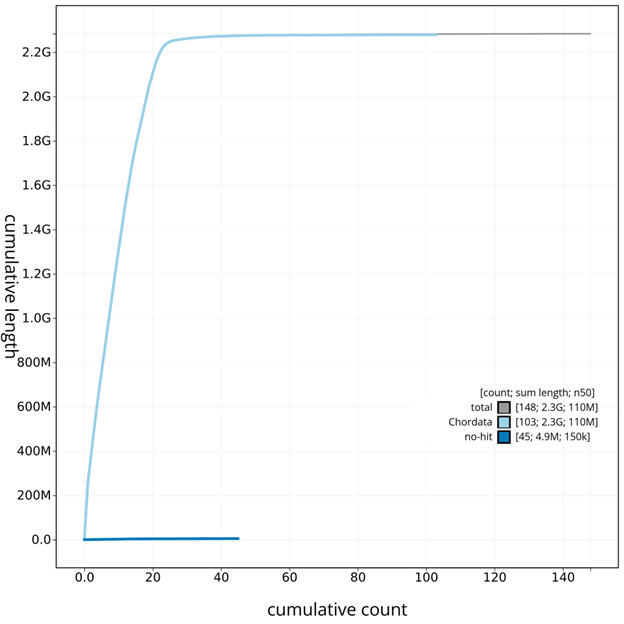
The grey line shows the cumulative length for all chromosomes/scaffolds in the assembly. Colored lines represent Phylum represented in the assembly.
Table 3.
| Software tool | Version | Source |
|---|---|---|
| bamUtil | 1.0.15 | https://genome.sph.umich.edu/wiki/BamUtil:_bam2FastQ |
| FastQC | 0.11.9 | https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ |
| MultiQC | 1.13 | https://github.com/ewels/MultiQC |
| Genomescope | 2.0 | https://github.com/tbenavi1/genomescope2.0 |
| HiFiasm | 0.16.0 | https://github.com/chhylp123/hifiasm |
| purge_dups | 1.2.5 | https://github.com/dfguan/purge_dups |
| BUSCO | 5.3.2 | https://busco.ezlab.org/ |
| Merqury | 1.3 | https://github.com/marbl/merqury |
| Assembly-stats | 17.02 | https://github.com/rjchallis/assembly-stats |
| Scaff10X | 4.2 | https://github.com/wtsi-hpag/Scaff10X |
| Bionano Solve | 3.6.1 | https://bionano.com/software-downloads/ |
| Arima-HiC Mapping Pipeline | - | https://github.com/ArimaGenomics/mapping_pipeline |
| SALSA | 2.2 | https://github.com/marbl/SALSA |
| HiGlass | 1.11.7 | https://github.com/higlass/higlass |
| samtools | 1.9 | https://www.htslib.org/ |
| BlobToolKit | 3.2.7 | https://github.com/blobtoolkit/blobtoolkit |
Acknowledgments
We would like to thank the long-read team of the DRESDEN-concept Genome center. For information on The Bat1K Consortium, please see www.bat1k.com.
Funding Statement
SCV was supported by a UKRI Future Leaders Fellowship, (MR/T021985/1), an ERC Consolidator Grant (101001702; BATSPEAK), and a Max Planck Research Group awarded by the Max Planck Society. ECT is a Wellcome collaborator and the Irish Research Council Laureate Award IRCLA/2017/58 and Science Foundation Ireland Future Frontiers 19/FFP/6790. MS and CW were supported by the US Office of Naval Research (ONRN00014-17-1–2736).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data availability
Underlying data
The T. brasiliensis genome sequencing initiative is part of the Bat1K genome sequencing project. The genome assembly is released openly for reuse.
The genome assembly can be found in the European Nucleotide Archive: T. brasiliensis (Brazilian free-tailed bat). Accession number: PRJNA972445, https://identifiers.org/ena.embl:PRJNA972445 ( Bat1K, 2023a).
NCBI BioProject: T. brasiliensis isolate: mTadBra1 (Brazilian free-tailed bat). Accession number: PRJNA972445, https://www.ncbi.nlm.nih.gov/bioproject/PRJNA972445 under the Bat1K BioProject PRJNA489245 ( Bat1K, 2023b).
This Whole Genome Shotgun project has been deposited in the DDBJ/ENA/GenBank repositories under the accession number GCA_030848815.1. The individual sequences are available here: https://www.ebi.ac.uk/ena/browser/view/GCA_030848815.1 ( Bat1K, 2023c).
Data accession identifiers are reported in Table 1.
References
- Agnarsson I, Zambrana-Torrelio CM, Flores-Saldana NP, et al. : A time-calibrated species-level phylogeny of bats (Chiroptera, Mammalia). PLoS Curr. 2011;3: RRN1212. 10.1371/currents.RRN1212 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Ammerman LK, Lee DN, Marie Tipps T, et al. : First molecular phylogenetic insights into the evolution of free-tailed bats in the subfamily Molossinae (Molossidae, Chiroptera). J Mammal. 2012;93(1):12–28. 10.1644/11-MAMM-A-103.1 [CrossRef] [Google Scholar]
- Barquez R, Diaz M, Gonzalez E, et al. : Tadarida brasiliensis. The IUCN Red List of Threatened Species 2015: e.T21314A22121621.2015; Accessed on 9 June 2023. 10.2305/IUCN.UK.2015-4.RLTS.T21314A22121621.en [CrossRef]
- Bat1K: T. brasiliensis (Brazilian free-tailed bat) genome assembly. European Nucleotide Archive. [Dataset],2023a. https://identifiers.org/ena.embl:PRJNA972445
- Bat1K: T. brasiliensis isolate: mTadBra1 (Brazilian free-tailed bat). NCBI BioProject. [Dataset],2023b. https://www.ncbi.nlm.nih.gov/bioproject/PRJNA972445
- Bat1K: Whole Genome Shotgun project of T. brasiliensis. DDBJ/ENA/GenBank. [Dataset],2023c. https://www.ebi.ac.uk/ena/browser/view/GCA_030848815.1
- Blumer M, Brown T, Freitas MB, et al. : Gene losses in the common vampire bat illuminate molecular adaptations to blood feeding. Sci Adv. 2022;8(12): eabm6494. 10.1126/sciadv.abm6494 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Challis R, Richards E, Rajan J, et al. : BlobToolKit - Interactive Quality Assessment of Genome Assemblies. G3 (Bethesda). 2020;10(4):1361–1374. 10.1534/g3.119.400908 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Cockrum EL: Migration in the guano bat Tadarida brasiliensis. Museum of Natural History, University of Kansas,1969;303–336. [Google Scholar]
- Davies KTJ, Yohe LR, Almonte J, et al. : Foraging shifts and visual preadaptation in ecologically diverse bats. Mol Ecol. 2020;29(10):1839–1859. 10.1111/mec.15445 [Abstract] [CrossRef] [Google Scholar]
- Davis RB, Herreid CF, Short HL: Mexican free-tailed bat in Texas. Ecol Monogr. 1962;32(4):311–346. 10.2307/1942378 [CrossRef] [Google Scholar]
- Fushan AA, Turanov AA, Lee SG, et al. : Gene expression defines natural changes in mammalian lifespan. Aging Cell. 2015;14(3):352–365. 10.1111/acel.12283 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Gervais MP: Documents zoologiques pour servir à la Monographie des Chéiroptères Sud-Américains. Paris, P. Bertrand, Libraire-Éditeur,1856. [Google Scholar]
- Ghurye J, Rhie A, Walenz BP, et al. : Integrating Hi-C links with assembly graphs for chromosome-scale assembly. PLoS Comput Biol. 2019;15(8): e1007273. 10.1371/journal.pcbi.1007273 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Gregorin R, Cirranello A: Phylogeny of Molossidae Gervais (Mammalia: Chiroptera) inferred by morphological data. Cladistics. 2016;32(1):2–35. 10.1111/cla.12117 [Abstract] [CrossRef] [Google Scholar]
- Guan D, McCarthy SA, Wood J, et al. : Identifying and removing haplotypic duplication in primary genome assemblies. Bioinformatics. 2020;36(9):2896–2898. 10.1093/bioinformatics/btaa025 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Kerpedjiev P, Abdennur N, Lekschas F, et al. : HiGlass: web-based visual exploration and analysis of genome interaction maps. Genome Biol. 2018;19(1): 125. 10.1186/s13059-018-1486-1 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Kilkenny C, Browne WJ, Cuthill IC, et al. : Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. J Pharmacol Pharmacother. 2010;1(2):94–99. 10.4103/0976-500X.72351 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Krutzsch PH, Fleming TH, Crichton EG: Reproductive biology of Male Mexican Free-Tailed bats ( Tadarida brasiliensis mexicana). J Mammal. 2002;83(2):489–500. Reference Source [Google Scholar]
- Legendre S: Étude odontologique des représentants actuels du groupe Tadarida (Chiroptera, Molossidae). Implications phylogéniques, systématiques et zoogeographiques. Verh Schweiz Naturforsch Ges. 1984;91:399–442. 10.5962/bhl.part.81886 [CrossRef] [Google Scholar]
- Manni M, Berkeley MR, Seppey M, et al. : BUSCO Update: Novel and Streamlined Workflows along with Broader and Deeper Phylogenetic Coverage for Scoring of Eukaryotic, Prokaryotic, and Viral Genomes. Mol Biol Evol. 2021;38(10):4647–4654. 10.1093/molbev/msab199 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- McCracken GF, Safi K, Kunz TH, et al. : Airplane tracking documents the fastest flight speeds recorded for bats. R Soc Open Sci. 2016;3(11): 160398. 10.1098/rsos.160398 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- McCracken GF, Lee YF, Gillam EH, et al. : Bats flying at high altitudes. 50 Years of Bat Research: Foundations and New Frontiers,2021;189–205. 10.1007/978-3-030-54727-1_12 [CrossRef] [Google Scholar]
- Merlin C, Liedvogel M: The genetics and epigenetics of animal migration and orientation: birds, butterflies and beyond. J Exp Biol. 2019;222(Pt Suppl 1): jeb191890. 10.1242/jeb.191890 [Abstract] [CrossRef] [Google Scholar]
- Morales AE, De-la-Mora M, Pinero D: Spatial and environmental factors predict skull variation and genetic structure in the cosmopolitan bat Tadarida brasiliensis. J Biogeogr. 2018;45(7):1529–1540. 10.1111/jbi.13243 [CrossRef] [Google Scholar]
- Morales AVF, Velazco PM, Simmons NB, et al. : Environmental niche drives genetic and morphometric structure in a widespread bat. J Biogeogr. 2016;43(5):1057–1068. 10.1111/jbi.12666 [CrossRef] [Google Scholar]
- Moreno Santillán DD, Lama TM, Gutierrez Guerrero YT, et al. : Large-scale genome sampling reveals unique immunity and metabolic adaptations in bats. Mol Ecol. 2021;30(23):6449–6467. 10.1111/mec.16027 [Abstract] [CrossRef] [Google Scholar]
- Popa-Lisseanu AG, Voigt CC: Bats on the Move. J Mammal. 2009;90(6):1283–1289. 10.1644/09-MAMM-S-130R2.1 [CrossRef] [Google Scholar]
- Potter JHT, Davies KTJ, Yohe LR, et al. : Dietary Diversification and Specialization in Neotropical Bats Facilitated by Early Molecular Evolution. Mol Biol Evol. 2021;38(9):3864–3883. 10.1093/molbev/msab028 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Rao SS, Huntley MH, Durand NC, et al. : A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell. 2014;159(7):1665–1680. 10.1016/j.cell.2014.11.021 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Rhie A, Walenz BP, Koren S, et al. : Merqury: reference-free quality, completeness, and phasing assessment for genome assemblies. Genome Biol. 2020;21(1): 245. 10.1186/s13059-020-02134-9 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Ross A: Notes on the food habits of bats. J Mammal. 1961;42(1):66–71. 10.2307/1377243 [CrossRef] [Google Scholar]
- Russell AL, Medellín RA, McCracken GF: Genetic variation and migration in the Mexican free-tailed bat ( Tadarida brasiliensis mexicana). Mol Ecol. 2005;14(7):2207–2222. 10.1111/j.1365-294X.2005.02552.x [Abstract] [CrossRef] [Google Scholar]
- Schmidly DJB, R D: Spatial and environmental factors predict skull variation and genetic structure in the cosmopolitan bat Tadarida brasiliensis. J Biogeogr. 2018;45(7):1529–1540. [Google Scholar]
- Schwartz A: The Status of the Species of the Brasiliensis Group of the Genus Tadarida. J Mammal. 1955;36(1):106–109. [Google Scholar]
- Schwartz CT, Tressler J, Keller H, et al. : The tiny difference between foraging and communication buzzes uttered by the Mexican free-tailed bat, Tadarida brasiliensis. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2007;193(8):853–863. 10.1007/s00359-007-0237-7 [Abstract] [CrossRef] [Google Scholar]
- Simão FA, Waterhouse RM, Ioannidis P, et al. : BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics. 2015;31(19):3210–3212. 10.1093/bioinformatics/btv351 [Abstract] [CrossRef] [Google Scholar]
- Simmons JA, Lavender WA, Lavender BA, et al. : Echolocation by free-tailed bats ( Tadarida). J Comp Physiol. 1978;125:291–299. 10.1007/BF00656863 [CrossRef] [Google Scholar]
- Simmons NBC, AL: Bat Species of the World: A Taxonomic and Geographic Database. Version: 1.4.2023. [Google Scholar]
- Teeling EC, Springer MS, Madsen O, et al. : A molecular phylogeny for bats illuminates biogeography and the fossil record. Science. 2005;307(5709):580–584. 10.1126/science.1105113 [Abstract] [CrossRef] [Google Scholar]
- Villa BR, Cockrum EL: Migration in the Guano Bat Tadarida brasiliensis mexicana (Saussure). J Mammal. 1962;43(1):43–64. 10.2307/1376879 [CrossRef] [Google Scholar]
- Wilkins KT: Tadarida brasiliensis. Mammalian Species. 1989;331:1–10. [Google Scholar]
- Williams TC, Ireland LC, Williams JM: High altitude flights of the free-tailed bat, Tadarida brasiliensis, observed with radar. J Mammal. 1973;54:807–821. 10.2307/1379076 [CrossRef] [Google Scholar]
- Zoonomia C: A comparative genomics multitool for scientific discovery and conservation. Nature. 2020;587(7833):240–245. 10.1038/s41586-020-2876-6 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
Reviewer response for version 1
The first long-read genome assembly of Tadarida brasiliensis (Brazilian free-tailed bat) is reported in this work. Genomic studies will be beneficial for this species due to its unique traits, including as a mammalian model for the genetic and epigenetic basis of migration.
The sequencing methods and genome assembly are comprehensively outlined and appropriately designed based on the pipeline developed by the Vertebrate Genome Project of the Genome 10K Consortium. The combination of advanced sequencing technologies (HiFi, Hi-C, and Bionano) fittingly addressed the challenge of generating a high-quality haplotype genome assembly in the absence of a reference sequence. Post-assembly quality control revealed 96% of the genes have complete sequences.
The results are clearly and comprehensively detailed. The sequence data is also made available, allowing further use for downstream analysis such as annotation, comparative genomics, and functional studies.
I only have minor comments:
The short-read genome sequence of T. brasiliensis is already available. Although the authors argued on the importance of genomics research for this species, the need to generate a long-read genome assembly should be clearly communicated.
Does Figure 2 refer to the specific individual caught for this study or are these data obtained from other studies? While the expertise of the authors on bat species identification is unquestionable, actual data may be added to validate the claim on the bat’s identity, especially since it has close morphological features with other bats such as N. macrotis.
The sequencing coverage was 39X for HiFi and 100X for Bionano. How was the sequencing coverage decided? Was this estimated from the genome size of the organism?
The section Genome Sequence Report describes that the assembly obtained from HiFi long reads were scaffolded with Hi-C data. However, the role of Bionano genome mapping in the analysis was not clarified.
In the section Genome Sequence Report, it was claimed that 98.44%, of the assembly sequence was assigned to 25 chromosomal-level scaffolds. However, this statistic was not mentioned in the data section. How was this value obtained? Please clarify.
It would be worthwhile to mention in the main text that the alternative haplotype is also available.
The sequencing was appropriately performed for one individual bat, but it was noted that different tissues were used (muscle, kidney, liver) for each sequencing technology. While there may be a technical explanation for this, this should be communicated.
The data follow the format for similar manuscripts in this journal, however Figures 3-6 may not be understandable to a non-expert. Additional information in the figure caption or main text can help guide readers in the interpretation of data.
Were the authors able to make a comparison of the short-read and long-read genomes for T. brasiliensis?
Are sufficient details of methods and materials provided to allow replication by others?
Yes
Is the rationale for creating the dataset(s) clearly described?
Partly
Are the datasets clearly presented in a useable and accessible format?
Partly
Are the protocols appropriate and is the work technically sound?
Yes
Reviewer Expertise:
genomics, wildlife and disease spillover
I confirm that I have read this submission and believe that I have an appropriate level of expertise to confirm that it is of an acceptable scientific standard, however I have significant reservations, as outlined above.
Reviewer response for version 1
The manuscript represents valuable research on the genome of the Brazilian free-tailed bat.
Approaches and methodologies are based on strong and well-documented protocols, as shown in the excellent implementation of results.
The language of the manuscript is according to the good scientific practice. Such a report is valuable and will help future research on bat genomes.
Minor comments:
Keywords - please replace or delete Tadarida brasiliensis and genome sequence, because they are used in the title and can not be used as keywords. For example, use Brazilian free-tailed bat instead Tadarida brasiliensis.
Species taxonomy - include also Vespertilionoidea, before Molossidae.
Are sufficient details of methods and materials provided to allow replication by others?
Yes
Is the rationale for creating the dataset(s) clearly described?
Yes
Are the datasets clearly presented in a useable and accessible format?
Yes
Are the protocols appropriate and is the work technically sound?
Yes
Reviewer Expertise:
Chiroptera, phylogeny, phylogeography, taxonomy
I confirm that I have read this submission and believe that I have an appropriate level of expertise to confirm that it is of an acceptable scientific standard.
Reviewer response for version 1
The authors report the first long-read assembly of the Mexican free-tailed bat (Tadarida brasiliensis). Bats are important models for a range of scientific study areas such as longevity, immunity, social structure, and migration. The authors provide an excellent introduction to this species as well as the rationale for generating a new assembly. I only have a few comments;
1. The BioNano data does not appear to have been made publicly available and hence the report does not comply fully with Wellcome Open Research Data Policy. This should be uploaded to ENA as an 'Analysis' entry .https://ena-docs.readthedocs.io/en/latest/submit/analyses/bionano-maps.html
2. In the methods section the authors mention they generate 10x Genomics and BioNano data, but there's no mention of using this in the Genome sequence report.
3. In sentence three of the Introduction change, "While some genera with Molossidae .." to "While some genera within Molossidae ..."
Are sufficient details of methods and materials provided to allow replication by others?
Yes
Is the rationale for creating the dataset(s) clearly described?
Yes
Are the datasets clearly presented in a useable and accessible format?
Partly
Are the protocols appropriate and is the work technically sound?
Yes
Reviewer Expertise:
Genome assembly and conservation genomics.
I confirm that I have read this submission and believe that I have an appropriate level of expertise to confirm that it is of an acceptable scientific standard, however I have significant reservations, as outlined above.
Reviewer response for version 1
In this study, Webster et al. report the genome of Tadarida brasiliensis. I find that the rationale is well described and the methods are extensive, including Bionano, PacBio HiFi, 10x linked reads, and HiC. The genome quality looks excellent, and data are already available. I see no need for additional improvements or revisions to the manuscript.
Are sufficient details of methods and materials provided to allow replication by others?
Yes
Is the rationale for creating the dataset(s) clearly described?
Yes
Are the datasets clearly presented in a useable and accessible format?
Yes
Are the protocols appropriate and is the work technically sound?
Yes
Reviewer Expertise:
Evolutionary biology and genetics/genomics
I confirm that I have read this submission and believe that I have an appropriate level of expertise to confirm that it is of an acceptable scientific standard.
Articles from Wellcome Open Research are provided here courtesy of The Wellcome Trust
Citations & impact
This article has not been cited yet.
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/160288277
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
Data Citations
- (1 citation) DOI - 10.2305/IUCN.UK.2015-4.RLTS.T21314A22121621.en
BioProject
- (1 citation) BioProject - PRJNA489245
GCA - NCBI genaome assembly (3)
- (1 citation) GCA - GCA_030848825.1
- (1 citation) GCA - GCA_030848815.1
- (1 citation) GCA - GCA_004025005.1
Nucleotide Sequences (Showing 29 of 29)
- (1 citation) ENA - CM061275
- (1 citation) ENA - CM061274
- (1 citation) ENA - CM061273
- (1 citation) ENA - CM061272
- (1 citation) ENA - CM061271
- (1 citation) ENA - CM061270
- (1 citation) ENA - SRX20532109
- (1 citation) ENA - CM061259
- (1 citation) ENA - CM061258
- (1 citation) ENA - CM061257
- (1 citation) ENA - CM061279
- (1 citation) ENA - CM061278
- (1 citation) ENA - CM061277
- (1 citation) ENA - CM061276
- (1 citation) ENA - CM061264
- (1 citation) ENA - CM061263
- (1 citation) ENA - CM061262
- (1 citation) ENA - CM061261
- (1 citation) ENA - CM061260
- (1 citation) ENA - CM061281
- (1 citation) ENA - CM061280
- (1 citation) ENA - CM061269
- (1 citation) ENA - CM061268
- (1 citation) ENA - CM061267
- (1 citation) ENA - CM061266
- (1 citation) ENA - CM061265
- (1 citation) ENA - SRX20532112
- (1 citation) ENA - SRX20532111
- (1 citation) ENA - SRX20532110
Show less
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The genome sequence of <i>Molossus alvarezi</i> González-Ruiz, Ramírez-Pulido and Arroyo-Cabrales, 2011 (Chiroptera, Molossidae).
Wellcome Open Res, 9:522, 12 Sep 2024
Cited by: 0 articles | PMID: 39411461 | PMCID: PMC11474149
The genome sequence of Molossusnigricans (Chiroptera, Molossidae; Miller, 1902).
Wellcome Open Res, 8:198, 05 May 2023
Cited by: 0 articles | PMID: 37600588 | PMCID: PMC10435916
The genome sequence of Rhynchonycteris naso, Peters, 1867 (Chiroptera, Emballonuridae, Rhynchonycteris).
Wellcome Open Res, 9:361, 10 Jul 2024
Cited by: 0 articles | PMID: 39239167 | PMCID: PMC11375410
The genome sequence of the particolored bat, Vespertilio murinus Linnaeus, 1758.
Wellcome Open Res, 9:403, 26 Jul 2024
Cited by: 0 articles | PMID: 39239168 | PMCID: PMC11375412
Funding
Funders who supported this work.
European Research Council (2)
Grant ID: 101001702
Grant ID: 101001702; BATSPEAK
Irish Research Council Laureate Award (1)
Grant ID: IRCLA/2017/58
Max Planck Society (1)
Grant ID: Max Planck Research Group
Science Foundation Ireland Future Frontiers (1)
Grant ID: 19/FFP/6790
UKRI Future Leaders Fellowship (1)
Grant ID: MR/T021985/1
Grant ID: ONRN00014-17-1–2736

 a,6,9 and The Bat1K Consortium
a,6,9 and The Bat1K Consortium