Abstract
Objective
This study aimed to explore the impact of co-antiseizure medication (co-ASM) optimization on the effectiveness and tolerability of adjunctive cenobamate (CNB) in patients with drug-resistant epilepsy in a real-world setting.Methods
This unicentric, retrospective, observational study included adults with focal-onset seizures who had received ≥2 previous ASMs. The main effectiveness endpoints included responder rates and seizure frequency reduction at 3, 6, and 12-month visits. The number of co-ASMs and defined daily dose (DDD) were analyzed at every visit. Safety endpoints included adverse drug reactions (ADRs).Results
Thirty-four patients with a median epilepsy duration of 22 years and a median of 15.5 seizures/month were analyzed. The median number of prior ASMs was 12, and the mean number of co-ASMs was 2.9 (SD 1). There was a reduction in seizure frequency/month from baseline to the last visit (p < 0.0001). Between baseline and the end of the study, the mean number of co-ASMs in the per-protocol (PP) population was reduced from 2.9 to 1.6 (p < 0.0001), and DDD was reduced from 3.6 to 1.4 (p < 0.0001). Sodium channel blockers (carbamazepine and lacosamide) and GABAergic drugs (clobazam) were the agents with the most significant reductions in DDD after 12 months. The percentage of patients in the PP population with ≥3 co-ASMs was reduced from 61.8% at baseline to 14.3% at 12 months; 1 patient was receiving CNB as monotherapy at the last visit. At the last visit, 85.7% of the PP population were ≥50% responders, and 33.3% were seizure-free. The percentage of patients with ADRs in the PP population was 71.9% at 3 months and 52.3% at 12 months.Significance
Following rational polytherapy, optimization of co-ASM management during CNB treatment allowed high seizure freedom rates despite meaningful reductions in co-medication, while also achieving both good tolerability and patient satisfaction scores in a highly drug-resistant population.Plain language summary
Many patients with epilepsy still have seizures, even after being treated with several different epilepsy drugs. In this study of 34 patients from a Spanish clinic, we show that the epilepsy drug cenobamate can reduce the number of seizures in these patients, even after many other epilepsy drugs have failed. We also show that patients treated with cenobamate can reduce the dose or even stop taking certain other epilepsy drugs. This allows them to simplify their treatment and reduce adverse effects while still keeping control of their epilepsy.Free full text

Treatment simplification to optimize cenobamate effectiveness and tolerability: A real‐world retrospective study in Spain
Associated Data
Abstract
Objective
This study aimed to explore the impact of co‐antiseizure medication (co‐ASM) optimization on the effectiveness and tolerability of adjunctive cenobamate (CNB) in patients with drug‐resistant epilepsy in a real‐world setting.
Methods
This unicentric, retrospective, observational study included adults with focal‐onset seizures who had received ≥2 previous ASMs. The main effectiveness endpoints included responder rates and seizure frequency reduction at 3, 6, and 12‐month visits. The number of co‐ASMs and defined daily dose (DDD) were analyzed at every visit. Safety endpoints included adverse drug reactions (ADRs).
Results
Thirty‐four patients with a median epilepsy duration of 22 years and a median of 15.5 seizures/month were analyzed. The median number of prior ASMs was 12, and the mean number of co‐ASMs was 2.9 (SD 1). There was a reduction in seizure frequency/month from baseline to the last visit (p
years and a median of 15.5 seizures/month were analyzed. The median number of prior ASMs was 12, and the mean number of co‐ASMs was 2.9 (SD 1). There was a reduction in seizure frequency/month from baseline to the last visit (p <
< 0.0001). Between baseline and the end of the study, the mean number of co‐ASMs in the per‐protocol (PP) population was reduced from 2.9 to 1.6 (p
0.0001). Between baseline and the end of the study, the mean number of co‐ASMs in the per‐protocol (PP) population was reduced from 2.9 to 1.6 (p <
< 0.0001), and DDD was reduced from 3.6 to 1.4 (p
0.0001), and DDD was reduced from 3.6 to 1.4 (p <
< 0.0001). Sodium channel blockers (carbamazepine and lacosamide) and GABAergic drugs (clobazam) were the agents with the most significant reductions in DDD after 12
0.0001). Sodium channel blockers (carbamazepine and lacosamide) and GABAergic drugs (clobazam) were the agents with the most significant reductions in DDD after 12 months. The percentage of patients in the PP population with ≥3 co‐ASMs was reduced from 61.8% at baseline to 14.3% at 12
months. The percentage of patients in the PP population with ≥3 co‐ASMs was reduced from 61.8% at baseline to 14.3% at 12 months; 1 patient was receiving CNB as monotherapy at the last visit. At the last visit, 85.7% of the PP population were ≥50% responders, and 33.3% were seizure‐free. The percentage of patients with ADRs in the PP population was 71.9% at 3
months; 1 patient was receiving CNB as monotherapy at the last visit. At the last visit, 85.7% of the PP population were ≥50% responders, and 33.3% were seizure‐free. The percentage of patients with ADRs in the PP population was 71.9% at 3 months and 52.3% at 12
months and 52.3% at 12 months.
months.
Significance
Following rational polytherapy, optimization of co‐ASM management during CNB treatment allowed high seizure freedom rates despite meaningful reductions in co‐medication, while also achieving both good tolerability and patient satisfaction scores in a highly drug‐resistant population.
Plain Language Summary
Many patients with epilepsy still have seizures, even after being treated with several different epilepsy drugs. In this study of 34 patients from a Spanish clinic, we show that the epilepsy drug cenobamate can reduce the number of seizures in these patients, even after many other epilepsy drugs have failed. We also show that patients treated with cenobamate can reduce the dose or even stop taking certain other epilepsy drugs. This allows them to simplify their treatment and reduce adverse effects while still keeping control of their epilepsy.
1. INTRODUCTION
The primary goal of antiseizure medication (ASM) treatment in epilepsy is to achieve seizure freedom without intolerable side effects. 1 Currently, over one‐third of patients with epilepsy have uncontrolled seizures despite treatment with multiple ASMs. 2 The International League Against Epilepsy defines drug‐resistant epilepsy (DRE) as the failure to achieve seizure freedom by using two ASMs at the correct dose, either in sequential monotherapy or combination. 3 Few patients with DRE achieve seizure freedom, and the odds of achieving seizure freedom decrease drastically with the failure of each additional ASM prescribed. 2 This situation has a relevant impact on patients' lives since DRE is often disabling and associated with more comorbidities, including psychological and social dysfunction, and an increased risk of premature death. 4
Furthermore, patients with epilepsy, especially DRE, experience a high burden of polypharmacy, leading to a higher probability of side effects and lower adherence rates. 5 Since the ultimate goal of epilepsy treatment is to achieve seizure freedom with the fewest adverse events (AEs), drug overload reduction seems to be a smart strategy to optimize the efficacy, tolerability, and adherence of a newly initiated ASM. In this scenario, there is an urgent need to develop breakthrough ASMs that increase seizure freedom rates with a balanced tolerability profile, allowing a reduction of co‐ASM overload in a rational polytherapy fashion.
Cenobamate (CNB) is a newly marketed ASM with a unique, dual, and complementary mechanism of action, 6 , 7 , 8 acting as a positive allosteric modulator of GABA‐A receptors (at a non‐benzodiazepine binding site) and as a sodium channel blocker (SCB) selectively acting on the persistent sodium current. 7 , 8 In Europe, it is indicated for the adjunctive treatment of focal‐onset seizures with or without secondary generalization in adults with epilepsy who have not been adequately controlled despite treatment with ≥2 ASMs. 6 CNB was authorized by the Food and Drug Administration (FDA) for the treatment of partial‐onset seizures in adult patients, 9 and by the European Medicines Agency (EMA), 6 based on efficacy and safety results versus placebo in several randomized trials in adults with focal‐onset seizures taking one to three ASMs. 10 Long‐term data on CNB in the open‐label studies support the results from the double‐blind placebo‐controlled trials. 11 , 12 , 13
Given the high seizure freedom rates reported in the clinical trials 10 , 14 , 15 and the potential of CNB to reduce co‐ASM overload, 16 the main objective of our research was to explore the effectiveness and safety of co‐ASM optimization after initiating CNB in patients with DRE in a real‐world setting. This investigation will enhance the evidence in routine clinical practice.
2. MATERIALS AND METHODS
2.1. Study design
This single‐center, retrospective, observational study was conducted at a Spanish center and is a sub‐analysis of a unicenter cohort included in the multicenter study published by Villanueva et al. 17 The study adhered to the Declaration of Helsinki and Good Clinical Practice, and the protocol was approved by the local institutional review board and health authorities of the La Fe Policlinic University Hospital (Valencia, Spain). Data were retrieved from the electronic medical records of patients at the Centro de Neurología Avanzada (Seville, Spain).
2.2. Study population
The retrospective study included adults with a diagnosis of refractory focal onset seizures who initiated CNB through a Named Patient Program (NPP) approved by the Spanish Agency of Medicines and Medical Devices (AEMPS) as part of routine clinical practice between August 2020 and June 2022. Subjects were 18 years of age or older with diagnosed focal onset seizures that had not responded to more than two previous well‐indicated ASMs. All subjects were granted access to CNB through the expanded access program (EAP). Exclusion criteria were severe hepatic impairment, end‐stage renal disease, nonfocal seizures, inaccurate or unreliable clinical records according to participating physicians, initiation of CNB ≤3
years of age or older with diagnosed focal onset seizures that had not responded to more than two previous well‐indicated ASMs. All subjects were granted access to CNB through the expanded access program (EAP). Exclusion criteria were severe hepatic impairment, end‐stage renal disease, nonfocal seizures, inaccurate or unreliable clinical records according to participating physicians, initiation of CNB ≤3 months before the closing of the database, and participation in other CNB clinical trials.
months before the closing of the database, and participation in other CNB clinical trials.
2.3. Study endpoints
When available, study participants' demographic and clinical characteristics were collected at baseline. Clinical characteristics included years of disease evolution, age at initiation of CNB, previous epilepsy surgery, comorbidities, and the number of previous ASMs. The timepoints at which the effectiveness and safety of CNB were analyzed were baseline and 3, 6, and 12 months after CNB initiation, corresponding to standardized visits.
months after CNB initiation, corresponding to standardized visits.
The effectiveness population consisted of patients with ≥1 efficacy measurement obtained during the 12 months after initiating CNB, while the safety population included those who received ≥1 dose of CNB. The median number of seizures per month experienced over the 3
months after initiating CNB, while the safety population included those who received ≥1 dose of CNB. The median number of seizures per month experienced over the 3 months prior to the initiation of CNB was used to establish baseline seizure frequency.
months prior to the initiation of CNB was used to establish baseline seizure frequency.
The main effectiveness endpoints were the evolution of the co‐ASM number, the CNB dose titration, and the total defined daily dose (DDD). The DDD for each co‐ASM was derived using the WHO methodology (https://www.whocc.no/atc_ddd_index/). It is important to note that this DDD value does not include the DDD of cenobamate.
Other effectiveness variables were seizure frequency, seizure freedom (proportion of patients with no seizures since the previous visit), and responder rates (≥50%, ≥75%, ≥90%, and 100%; defined as the percentage of subjects with a reduction in the frequency of their focal seizures compared with the previous time point) at each time point.
Safety and tolerability were assessed using the incidence of adverse drug reactions (ADRs; i.e., AEs considered by the investigator to be related to CNB treatment or to the pharmacokinetic or pharmacodynamic interactions of other ASMs with CNB). The primary safety endpoint was the proportion of patients with ADRs at each time point. Other secondary safety endpoints included the proportion of patients with AEs leading to treatment discontinuation at each time point.
To assess the patients' experiences, the Patient Global Impression‐Improvement (PGI‐I) scale score was collected after 12 months of follow‐up. This is a questionnaire based on a seven‐point Likert scale in which the answers ranged from 1 (“Much better”) to 7 (“Much worse”) compared with the baseline situation. Similarly, the investigators completed the Clinical Global Impression‐Improvement (CGI‐I) scale survey.
months of follow‐up. This is a questionnaire based on a seven‐point Likert scale in which the answers ranged from 1 (“Much better”) to 7 (“Much worse”) compared with the baseline situation. Similarly, the investigators completed the Clinical Global Impression‐Improvement (CGI‐I) scale survey.
Analyses were performed in the per‐protocol (PP) and the intention‐to‐treat (ITT) populations. The PP population included all subjects who had completed 3, 6, or 12 months and had not discontinued treatment. Subjects who were ongoing and yet to reach months 6 and 12 were not included in the analysis at months 6 and 12, respectively. The ITT set included all subjects who had completed 3, 6, or 12
months and had not discontinued treatment. Subjects who were ongoing and yet to reach months 6 and 12 were not included in the analysis at months 6 and 12, respectively. The ITT set included all subjects who had completed 3, 6, or 12 months, even if they had discontinued cenobamate, assuming the worst outcome for missing data.
months, even if they had discontinued cenobamate, assuming the worst outcome for missing data.
2.4. Statistical analysis
Subject disposition, AEs, CGI‐I, and PGI‐scores, the number of co‐ASMs per subject, co‐ASM DDD, CNB dose levels, seizure frequency, and reductions in seizure frequency from baseline were appropriately summarized using descriptive statistics, frequency, and percentage. The number of co‐ASMs per subject at each visit and the DDD were analyzed using a paired t‐test, testing the null hypothesis of no change from baseline for each subject.
3. RESULTS
3.1. Patient disposition and baseline characteristics
The electronic medical records of patients receiving CNB as part of the EAP program were screened; 34 subjects were selected for the study as they met the inclusion/exclusion criteria and had received at least one dose of CNB.
The numbers of subjects in the PP population were 32, 29, and 21 at 3, 6, and 12 months of follow‐up, respectively. Concerning the ITT population, the numbers of subjects were 34, 33, and 28 at 3, 6, and 12
months of follow‐up, respectively. Concerning the ITT population, the numbers of subjects were 34, 33, and 28 at 3, 6, and 12 months of follow‐up, respectively. The retention rate of CNB was 94% (32/34 patients), 88% (29/33 patients), and 77% (21/28 patients) at 3, 6, and 12
months of follow‐up, respectively. The retention rate of CNB was 94% (32/34 patients), 88% (29/33 patients), and 77% (21/28 patients) at 3, 6, and 12 months of follow‐up, respectively (Figure S1). Seven patients discontinued CNB, including two in the first 3
months of follow‐up, respectively (Figure S1). Seven patients discontinued CNB, including two in the first 3 months (due to lack of tolerability or seizure worsening), two between months 3 and 6 (due to pneumonia‐associated encephalopathy or seizure worsening), and three between months 6 and 12 (two due to lack of effectiveness and one due to seizure worsening). Although four patients in the study experienced skin rash with SCB, none had a skin reaction due to CNB.
months (due to lack of tolerability or seizure worsening), two between months 3 and 6 (due to pneumonia‐associated encephalopathy or seizure worsening), and three between months 6 and 12 (two due to lack of effectiveness and one due to seizure worsening). Although four patients in the study experienced skin rash with SCB, none had a skin reaction due to CNB.
In our cohort, most subjects were female (58.8%), the mean ±
± SD age of disease onset was 13.6
SD age of disease onset was 13.6 ±
± 11.4
11.4 years, the median (IQR) duration of epilepsy was 22 (11) years, and more than half of individuals showed some comorbidity (58.8%). Approximately one‐quarter of subjects (23.5%) had received previous epilepsy surgery. The mean age of treatment initiation with CNB was 36.7
years, the median (IQR) duration of epilepsy was 22 (11) years, and more than half of individuals showed some comorbidity (58.8%). Approximately one‐quarter of subjects (23.5%) had received previous epilepsy surgery. The mean age of treatment initiation with CNB was 36.7 ±
± 10.5
10.5 years, and the median (IQR) number of previous ASMs was 12 (7). Finally, the median (IQR) monthly focal seizure frequency at baseline was 15.5 (44) (Table 1).
years, and the median (IQR) number of previous ASMs was 12 (7). Finally, the median (IQR) monthly focal seizure frequency at baseline was 15.5 (44) (Table 1).
TABLE 1
Patient demographics and disease characteristics at baseline.
| Demographics and characteristics | All patients N = = 34 34 |
|---|---|
| Gender | |
| Female, n (%) | 20 (58.8%) |
| Mean age, years (SD) | 36.7 (10.5) |
| Age at epilepsy onset, years | |
| Mean (SD), [range] | 13.6 (11.4), [41] |
| Median (IQR) | 10 (17) |
| Duration of epilepsy, years | |
| Mean (SD), [range] | 23.1 (11.5), [49] |
| Median (IQR) | 22 (11) |
| Monthly seizure frequency | |
| Mean (SD), [range] | 57.8 (156), [898] |
| Median (IQR) | 15.5 (44) |
| Etiology | |
| Structural | 13 |
| Genetic | 1 |
| Infectious | 0 |
| Metabolic | 0 |
| Autoimmune | 1 |
| Unknown | 19 |
| Number of previous ASMs | |
| Mean (range) | 12.1 (13) |
| Median (IQR) | 12 (7) |
| Number of concomitant ASMs | |
| Mean (SD) | 2.9 (1) |
| Median (IQR) | 3 (1) |
| Prior surgery, n (%) | 8 (23.5%) |
| Comorbidities, n (%) | 20 (58.8%) |
| Encephalopathy/Intellectual disability, n (%) | 8 (23.5%) |
Abbreviations: ASMs, antiseizure medications; IQR, interquartile range; SD, standard deviation.
3.2. Safety
In the PP population, 23/32 (71.9%) participants reported at least one ADR at 3 months of follow‐up. The most frequent ADRs were somnolence and dizziness. The other reported ADRs were ataxia and diplopia.
months of follow‐up. The most frequent ADRs were somnolence and dizziness. The other reported ADRs were ataxia and diplopia.
At 12 months of follow‐up, 11/21 (52.3%) participants in the PP population reported at least one ADR; all ADRs occurred less frequently at 12
months of follow‐up, 11/21 (52.3%) participants in the PP population reported at least one ADR; all ADRs occurred less frequently at 12 months than at 3
months than at 3 months. The most frequently reported ADR at 12
months. The most frequently reported ADR at 12 months was somnolence (10/21 participants; 47.6%). The percentage of patients reporting dizziness reduced from 56.3% at 3
months was somnolence (10/21 participants; 47.6%). The percentage of patients reporting dizziness reduced from 56.3% at 3 months of follow‐up to 14.3% at 12
months of follow‐up to 14.3% at 12 months. Similarly, the percentage of patients reporting ataxia improved from 37.5% at 3
months. Similarly, the percentage of patients reporting ataxia improved from 37.5% at 3 months to 4.76% at 12
months to 4.76% at 12 months, and the percentage reporting diplopia reduced from 18.8% to 4.76% during this period. See Table 2 for more detail.
months, and the percentage reporting diplopia reduced from 18.8% to 4.76% during this period. See Table 2 for more detail.
TABLE 2
Frequency and percentage of adverse events for subjects treated with cenobamate (PP population).
| ADR | Month 3 (n = = 32) 32) | Month 6 (n = = 29) 29) | Month 12 (n = = 21) 21) |
|---|---|---|---|
| Somnolence | 22 (68.8) | 17 (58.6) | 10 (47.6) |
| Dizziness | 18 (56.3) | 10 (34.5) | 3 (14.3) |
| Ataxia | 12 (37.5) | 5 (17.2) | 1 (4.76) |
| Diplopia | 6 (18.8) | 2 (6.90) | 1 (4.76) |
| Other ADR | 3 (9.38) | 0 (0) | 0 (0) |
Abbreviation: ADR, adverse drug reaction.
3.3. Co‐ASM management
At the baseline visit, patients treated with CNB received a mean of 2.9 ±
± 1 co‐ASMs, which decreased significantly to 1.6
1 co‐ASMs, which decreased significantly to 1.6 ±
± 0.9 (p
0.9 (p <
< 0.0001) after one year of treatment with CNB (PP population; Figure 1A).
0.0001) after one year of treatment with CNB (PP population; Figure 1A).

Total reduction in co‐ASMs during follow‐up (PP population). (A) Evolution of the number of co‐ASMs per subject. (B) Mean DDD for subjects treated with CNB by study period. (C) Evolution of percentage of subjects receiving different numbers of co‐ASMs; differences in height between corresponding shaded areas (i.e., the same color) within the bars and areas immediately to the right of the bars indicate discontinuations or incomplete follow‐up between timepoints. Vagus nerve stimulation and a ketogenic diet were removed from the analysis. p‐values are based on a paired t‐test versus baseline; **p <
< 0.0001; ***p
0.0001; ***p <
< 0.0001. Co‐ASMs, antiseizure medications co‐administered with cenobamate; DDD, daily defined dose; PP, per protocol.
0.0001. Co‐ASMs, antiseizure medications co‐administered with cenobamate; DDD, daily defined dose; PP, per protocol.
The total DDD per patient significantly reduced over time, from a mean of 3.6 ±
± 1.3 at baseline to 1.4
1.3 at baseline to 1.4 ±
± 1.2 (p
1.2 (p <
< 0.001) at 1
0.001) at 1 year of treatment with CNB. The evolution of total DDD per patient throughout the study for the PP population is shown in Figure 1B.
year of treatment with CNB. The evolution of total DDD per patient throughout the study for the PP population is shown in Figure 1B.
After CNB initiation, the number of individuals receiving multiple co‐ASMs decreased significantly. In the PP population, 21/34 (61.8%) patients received three or more co‐ASMs at baseline, which reduced to 3/21 (14.3%) patients after 12 months. In contrast, the number of patients treated with one or two co‐ASMs increased from 13/34 (38.2%) at baseline to 17/21 (81%) after 12
months. In contrast, the number of patients treated with one or two co‐ASMs increased from 13/34 (38.2%) at baseline to 17/21 (81%) after 12 months. At the end of the study, 1 (4.8%) subject was receiving CNB as the only ASM (Figure 1C).
months. At the end of the study, 1 (4.8%) subject was receiving CNB as the only ASM (Figure 1C).
The most frequently used co‐ASMs at baseline were carbamazepine (CBZ) in 20/34 (58.8%) participants, clobazam (CLB) in 16/34 (47.1%) participants, and brivaracetam (BRV) and lacosamide (LCM) in 9/34 (26.5%) participants each. Reductions in total DDD and in the number of participants taking each different co‐ASM were observed during follow‐up; these occurred for ASMs with different mechanisms of action (Figures 2 and S2; Table S2). The greatest reductions in mean co‐ASM total DDD between baseline and month 12 occurred for SCB (mainly CBZ and LCM) and GABAergics (CLB) (Tables S1 and S2; Figures S2 and S3).
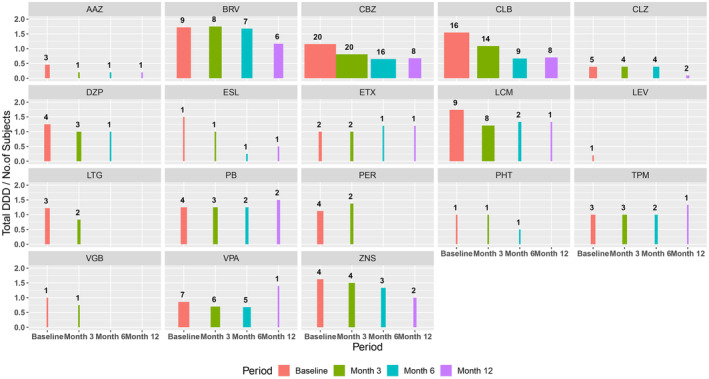
Reduction in co‐ASM DDD during follow‐up for each drug. The height of the bars represents DDD and the width represents the number of subjects on each drug (the numerical value is also displayed over each graph). AAZ, acetazolamide; BRV, brivaracetam; CBZ, carbamazepine; CLB, clobazam; CLZ, clonazepam; co‐ASMs, antiseizure medications co‐administered with cenobamate; DDD, daily defined dose; DZP, diazepam; ESL, eslicarbazepine; ETX, ethosuximide; LCM, lacosamide; LEV, levetiracetam; LTG, lamotrigine; PB, phenobarbital; PER, perampanel; PHT, phenytoin; TPM, topiramate; VGB, vigabatrin; VPA, valproic acid; ZNS, zonisamide.
For all ADRs studied, the mean DDD of SCB and GABAergics was reduced to a significantly greater extent relative to baseline in patients with versus without ADRs at month 3; these differences were maximal at month 6 (Figure 3B). At the 6‐month timepoint, the mean percent DDD for SCB and GABAergics dropped to 26.6 ±
± 22.9% of baseline in patients with any ADR at month 3 versus 58.4
22.9% of baseline in patients with any ADR at month 3 versus 58.4 ±
± 32.9% of baseline in patients without an ADR at month 3; corresponding values at the 12‐month timepoint were 14.2
32.9% of baseline in patients without an ADR at month 3; corresponding values at the 12‐month timepoint were 14.2 ±
± 27.0% versus 43.3%
27.0% versus 43.3% ±
± 34.4% of baseline, respectively. This same comparison showed a significant relative reduction of the mean DDD for SCB and GABAergics when analyzing individual ADRs at month 6 for diplopia (17.3
34.4% of baseline, respectively. This same comparison showed a significant relative reduction of the mean DDD for SCB and GABAergics when analyzing individual ADRs at month 6 for diplopia (17.3 ±
± 4.3% vs. 42.0
4.3% vs. 42.0 ±
± 32.0% of baseline; p
32.0% of baseline; p <
< 0.005), dizziness (20.1
0.005), dizziness (20.1 ±
± 15.9% vs. 50.5
15.9% vs. 50.5 ±
± 37.7% of baseline; p
37.7% of baseline; p <
< 0.005), and somnolence (26.4
0.005), and somnolence (26.4 ±
± 23.6% vs. 56.3
23.6% vs. 56.3 ±
± 32.5% of baseline; p
32.5% of baseline; p <
< 0.01), but not for ataxia (19.1
0.01), but not for ataxia (19.1 ±
± 17.4% vs. 43.6
17.4% vs. 43.6 ±
± 32.1% of baseline; p
32.1% of baseline; p =
= 0.057). Additionally, patients who presented with ADRs at 3
0.057). Additionally, patients who presented with ADRs at 3 months of follow‐up showed a higher mean DDD of SCB at baseline when compared with participants with no ADRs (Figure 3A), although these differences were not statistically significant.
months of follow‐up showed a higher mean DDD of SCB at baseline when compared with participants with no ADRs (Figure 3A), although these differences were not statistically significant.
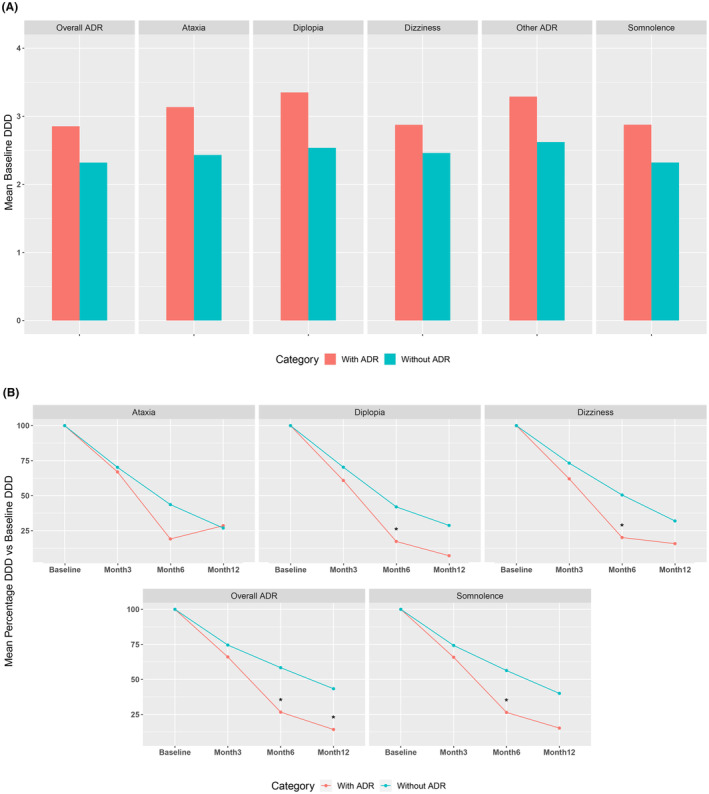
Differential initial dose and management of co‐ASMs between participants with and without ADRs at month 3 of follow‐up. (A) Mean DDD of GABAergics or SCB at baseline for participants with and without ADRs at month 3; (B) Percentage reduction in the mean DDD of GABAergics or SCB from baseline for participants with and without ADRs at month 3. p‐values are based on a paired t‐test comparing “with” versus “without” ADR; *p <
< 0.05. ADR, adverse drug reaction; DDD, daily defined dose; SCB, sodium channel blockers.
0.05. ADR, adverse drug reaction; DDD, daily defined dose; SCB, sodium channel blockers.
3.4. Cenobamate dose
The mean CNB daily dose in the PP population was 207.8 ±
± 25.7
25.7 mg at 3
mg at 3 months, 267.2
months, 267.2 ±
± 53.9
53.9 mg at 6
mg at 6 months, and 326.2
months, and 326.2 ±
± 80
80 mg at 12
mg at 12 months (See Table S3 for more detail).
months (See Table S3 for more detail).
3.5. Effectiveness
Responder rates at the ≥50%, ≥75%, ≥90%, and 100% levels are shown in Figure 4 for the PP population. Responder rates at the ≥50% level were 81.2%, 82.8%, and 85.7% at 3, 6, and 12 months of follow‐up, respectively. Responder rates at the ≥75% level were 62.5%, 69%, and 71.4% at 3, 6, and 12
months of follow‐up, respectively. Responder rates at the ≥75% level were 62.5%, 69%, and 71.4% at 3, 6, and 12 months, respectively. At the ≥90% level, responder rates at 3, 6, and 12
months, respectively. At the ≥90% level, responder rates at 3, 6, and 12 months were 43.8%, 58.6%, and 47.6%, respectively. The percentages of seizure‐free patients (100% responder rate) at 3, 6, and 12
months were 43.8%, 58.6%, and 47.6%, respectively. The percentages of seizure‐free patients (100% responder rate) at 3, 6, and 12 months were 21.9%, 44.8%, and 33.3%, respectively. Efficacy results for the ITT population are shown in Figure S4.
months were 21.9%, 44.8%, and 33.3%, respectively. Efficacy results for the ITT population are shown in Figure S4.

Responder rates during the study, by period. PP population includes all subjects who completed Month 3/6/12 and had not discontinued treatment. PP, per protocol.
At the end of the follow‐up period, 41.2% of participants reported clinically significant satisfaction with the drug in terms of an improvement in seizure frequency (i.e., PGI‐I score 1 [“Very much better”] or 2 [“Much better”]; Table S4).
4. DISCUSSION
Retrospective real‐world studies with newly approved ASMs complement data from clinical trials and inform specialists about the performance of new medications in populations not captured during clinical development. Furthermore, EAPs allow patients with no other options to access drugs and physicians to gain experience before commercialization. 18 Only a few other studies have analyzed the effectiveness and tolerability of CNB in patients with focal‐onset DRE in a real‐world setting as part of the Spanish EAP. 15 , 17 , 19 These studies and the present investigation demonstrate that adjunctive CNB is effective and safe in clinical practice. Specifically, our study revealed that polytherapy optimization while initiating treatment with CNB results in high seizure freedom rates. In real‐world studies, these rates are even higher than those reported by clinical trials. Besides, a high level of satisfaction and a favorable tolerability profile accompanied the improvement in clinical variables.
Analysis of the baseline characteristics reveals that most subjects had not undergone previous epilepsy surgery. Our cohort includes DRE patients with ≥12 previous ASMs. This population, the so‐called absolute drug‐resistant (failure of ≥6 ASMs), had a probability of achieving seizure freedom close to zero. 2 , 20 , 21 However, after treatment with CNB, a quarter of patients in the ITT population achieved seizure freedom at month 12.
Despite an individualized approach to epilepsy therapy and the availability of many ASM combinations, physicians may find themselves in a loop of transitional polytherapy with DRE patients. 16 In this population, polytherapy is associated with a high number of adverse effects, which are stronger predictors of quality of life than seizures. Besides, treatment complexity has been associated with lower adherence and pharmacological interactions, possibly impacting effectiveness and safety. 22 , 23
A “start low, go slow” titration approach has improved tolerability when starting a new ASM. When adding a new ASM, the adjustment of co‐ASMs to achieve seizure control with the lowest drug load is also key to minimizing tolerability issues.
24
In addition, if tolerability issues arise when adding a new ASM, lowering the doses of co‐ASMs that failed to achieve seizure freedom is an option rather than discontinuing the new ASM. In patients with DRE, such a strategy allows continuation of the new ASM titration and reduction of adverse effects without negatively affecting seizure control,
25
allowing seizure freedom to be reached in the optimal scenario. The titration schedule of CNB (every 2 weeks) and its demonstrated early efficacy
26
,
27
allow physicians to increase the CNB dose slowly while reducing co‐ASMs.
weeks) and its demonstrated early efficacy
26
,
27
allow physicians to increase the CNB dose slowly while reducing co‐ASMs.
A panel of epileptologists developed ASM dose‐reduction recommendations for initiating CNB in adults. 28 , 29 These include proactive dose adjustments before the report of side effects for clobazam, SCBs, phenytoin, and phenobarbital. Reactive dose adjustments in response to side effects are recommended for all other ASMs at standard doses. A study conducted by Steinhoff et al. 26 has demonstrated the feasibility of reducing co‐ASM DDD in patients with focal‐onset seizures who initiated CNB. Similarly, in a post hoc analysis by Rosenfeld et al., 16 patients who continued CNB tended to have more significant reductions in co‐ASM doses than those who discontinued the drug.
Our study highlights the therapeutic relevance of managing co‐ASMs while escalating CNB doses to improve the effectiveness‐tolerability balance. At the beginning of the study, 61.8% of patients in the PP population received three or four co‐ASMs. In comparison, after 12 months, this proportion was only 14.3%, significantly increasing the rate of subjects treated with only one or two co‐ASMs after 1
months, this proportion was only 14.3%, significantly increasing the rate of subjects treated with only one or two co‐ASMs after 1 year (more than three‐quarters of the PP population). Improvement in tolerability was followed by a high reduction in seizure frequency. A third of patients in the PP population achieved seizure freedom at month 12, and patients showed high retention rates after reducing or discontinuing ≥1 co‐ASM. It is also worth noting that co‐ASM doses were reduced for many patients very early in the CNB titration.
year (more than three‐quarters of the PP population). Improvement in tolerability was followed by a high reduction in seizure frequency. A third of patients in the PP population achieved seizure freedom at month 12, and patients showed high retention rates after reducing or discontinuing ≥1 co‐ASM. It is also worth noting that co‐ASM doses were reduced for many patients very early in the CNB titration.
Following the principles of rational therapy, the highest reduction in drug load after starting CNB in our cohort occurred with SCB and GABAergics (mainly carbamazepine, lacosamide, and clobazam). This reduction of co‐ASMs was most evident in those patients who presented with ADRs, which might have allowed us to achieve better tolerability results than in previously reported studies.
Furthermore, as both an enzyme inducer and inhibitor of different CYPs, CNB affects the metabolism of other ASMs, such as clobazam and its active metabolite N‐desmethylclobazam (N‐CLB).
30
Potential interactions between CNB and clobazam have been described,
6
,
31
and their combination results in a large and clinically significant increase in the serum concentration of N‐CLB.
32
In our study, some patients who received a single ASM and were seizure‐free experienced seizure recurrence at month 6. However, seizure freedom was achieved again after re‐introducing very low clobazam doses (2.5 mg/day in most cases). This potentially beneficial synergy between CNB and low clobazam doses between months 6 and 12 in five of our patients has been observed in another series published by Osborn et al.
33
As CNB has CYP3A4‐induction effects, decreasing levels of clobazam with higher doses of CNB are to be expected. However, we proactively reduced clobazam to reduce pharmacodynamic interactions that, in our experience, are a major contributor to adverse effects. Consequently, after the introduction of CNB, the management of polypharmacy in this study achieved very good effectiveness results despite very low numbers of co‐ASMs.
mg/day in most cases). This potentially beneficial synergy between CNB and low clobazam doses between months 6 and 12 in five of our patients has been observed in another series published by Osborn et al.
33
As CNB has CYP3A4‐induction effects, decreasing levels of clobazam with higher doses of CNB are to be expected. However, we proactively reduced clobazam to reduce pharmacodynamic interactions that, in our experience, are a major contributor to adverse effects. Consequently, after the introduction of CNB, the management of polypharmacy in this study achieved very good effectiveness results despite very low numbers of co‐ASMs.
Notably, there were no serious ADRs in our study, and ADRs could be adequately addressed or resolved. Although four of the patients in the study had suffered a skin rash with SCB before initiating treatment with CNB, none experienced skin reactions during CNB treatment. At 3 months of follow‐up, the rate of ADRs was comparable to rates described in previous studies.
33
Most importantly, the ADR rate was significantly reduced at 6 and especially at 12
months of follow‐up, the rate of ADRs was comparable to rates described in previous studies.
33
Most importantly, the ADR rate was significantly reduced at 6 and especially at 12 months of follow‐up. The low rate of ADRs occurred in parallel with the simplification of co‐ASMs, especially of GABAergics and SCB. As stated previously, co‐ASMs were significantly reduced in all patients. However, the reduction of co‐administered GABAergics and SCB occurred fastest and to the greatest extent when ADRs were detected. Therefore, reactive co‐ASM reduction might have allowed us to reduce ADRs. It is also important to note that GABAergic and SCB DDD showed a tendency to be highest in patients who presented with ADRs 3
months of follow‐up. The low rate of ADRs occurred in parallel with the simplification of co‐ASMs, especially of GABAergics and SCB. As stated previously, co‐ASMs were significantly reduced in all patients. However, the reduction of co‐administered GABAergics and SCB occurred fastest and to the greatest extent when ADRs were detected. Therefore, reactive co‐ASM reduction might have allowed us to reduce ADRs. It is also important to note that GABAergic and SCB DDD showed a tendency to be highest in patients who presented with ADRs 3 months after initiation of CNB. Thus, the appearance of ADRs should be more closely followed in patients with higher dosages of these ASM groups in order to optimize ADR management.
months after initiation of CNB. Thus, the appearance of ADRs should be more closely followed in patients with higher dosages of these ASM groups in order to optimize ADR management.
CNB may also be distinguished from other ASMs by the high rates of seizure freedom not seen in previous placebo‐controlled trials, which has the potential to improve quality of life significantly.
15
,
34
Other retrospective studies, including in patients receiving CNB during the EAP,
14
,
15
reported lower seizure freedom rates than those observed in our study. We believe these results might have been possible due to the high doses of CNB that were reached in our study, which might have been better tolerated thanks to the previously described reduction of co‐ASMs. Along with the marked seizure freedom rates observed, the reported 35.7% rate of patients in the ITT population with a ≥90% reduction in seizure frequency at 12 months is also remarkable. Also, the retention rate of CNB after 12
months is also remarkable. Also, the retention rate of CNB after 12 months of therapy was high since 77% of the subjects were still on CNB treatment at the end of the study.
months of therapy was high since 77% of the subjects were still on CNB treatment at the end of the study.
The high effectiveness results shown in our study were obtained thanks to several factors. First, CNB has a well‐characterized safety profile and exhibits predictable side effects, which are mainly mild to moderate and, in most cases, dose dependent.
32
Second, there was a low rate of tolerability issues, which might have been due to the management and reduction of co‐ASMs. Third, the long half‐life of CNB allows for a single daily dose. Fourth, the biweekly titration schedule and the low starting dose (12.5 mg) recommended in the Summary of Product Characteristics (SmPC)
6
favor tolerability and allow co‐ASM reduction,
35
alongside the early efficacy of CNB.
mg) recommended in the Summary of Product Characteristics (SmPC)
6
favor tolerability and allow co‐ASM reduction,
35
alongside the early efficacy of CNB.
This research also served to accumulate experience in the management of CNB, so there is increasing knowledge regarding how to titrate and deal with the tolerability issues related to the addition of CNB in highly polymedicated patients with DRE. Finally, it is also noteworthy that the high PGI‐I scores were encouraging, as many participants were satisfied with the results achieved with CNB.
This study has several limitations, including its retrospective, single‐center design. Also, results are mainly focused on the per‐protocol population, although efficacy data for the ITT population are also provided. Additionally, seizure counts in observational studies are less accurate than in clinical trials. The limited sample and the lack of adherence data suggest that the results should be interpreted cautiously. Nonetheless, our results align with those previously reported in clinical trials and other real‐world studies, providing additional evidence of the effectiveness and tolerability of adjunctive CNB in polymedicated patients with highly resistant focal epilepsy. In addition, this study may inform other physicians about the importance of treatment optimization while introducing CNB to avoid drug overload, and the subsequent impact on tolerability, adherence, and treatment satisfaction. More real‐world studies are needed to consolidate our findings.
In conclusion, the results of this study demonstrate that high seizure freedom rates, together with a significant dose reduction in the number and dose of co‐ASMs, can be achieved with the use of adjunctive CNB in patients with absolute drug‐resistant focal‐onset seizures.
AUTHOR CONTRIBUTIONS
JRU led clinical research, data collection, review of the analysis of results, and approved the final version of the manuscript. JMSC and RHR collected data, reviewed and revised manuscript drafts, and approved the final version.
CONFLICT OF INTEREST STATEMENT
Juan Jesús Rodríguez‐Uranga has been a paid consultant for EISAI Farmaceutica, GW Pharma, Jazz Pharmaceutical Iberia, UCB Pharma, Bial, Novartis, Livanova, Angelini Pharma, Arvelle Therapeutics, GlaxoSmithKline, Pfizer, and Esteve. Juan M. Sánchez Caro has served as a consultant for Angelini Pharma and Nutricia. Roshan Hariramani Ramchandani has carried out consulting work for Eisai. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
ACKNOWLEDGMENTS
The authors are grateful for the funding received from Angelini Pharma. They also acknowledge María Romero and Ian Marshall for preparing and writing this manuscript on behalf of Springer Healthcare.
Notes
Rodríguez‐Uranga JJ, Sánchez‐Caro JM, Hariramani Ramchandani R. Treatment simplification to optimize cenobamate effectiveness and tolerability: A real‐world retrospective study in Spain. Epilepsia Open. 2024;9:1345–1356. 10.1002/epi4.12959 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
REFERENCES
Articles from Epilepsia Open are provided here courtesy of Wiley Periodicals Inc. on behalf of International League Against Epilepsy
Full text links
Read article at publisher's site: https://doi.org/10.1002/epi4.12959
Read article for free, from open access legal sources, via Unpaywall:
https://onlinelibrary.wiley.com/doi/pdfdirect/10.1002/epi4.12959
Citations & impact
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/163923983
Article citations
Treatment simplification to optimize cenobamate effectiveness and tolerability: A real-world retrospective study in Spain.
Epilepsia Open, 9(4):1345-1356, 27 May 2024
Cited by: 1 article | PMID: 38800945 | PMCID: PMC11296129
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Spanish consensus on the management of concomitant antiseizure medications when using cenobamate in adults with drug-resistant focal seizures.
Epilepsia Open, 9(3):1051-1058, 04 Apr 2024
Cited by: 4 articles | PMID: 38573131 | PMCID: PMC11145622
Adjunctive cenobamate in people with focal onset seizures: Insights from the Italian Expanded Access Program.
Epilepsia, 65(10):2909-2922, 14 Aug 2024
Cited by: 0 articles | PMID: 39140704
Cenobamate add-on therapy for drug-resistant focal epilepsy.
Cochrane Database Syst Rev, 8:CD014941, 01 Aug 2024
Cited by: 1 article | PMID: 39087564
Review
Cenobamate as an Early Adjunctive Treatment in Drug-Resistant Focal-Onset Seizures: An Observational Cohort Study.
CNS Drugs, 38(9):733-742, 03 Aug 2024
Cited by: 1 article | PMID: 39096467 | PMCID: PMC11316687
Funding
Funders who supported this work.

 1
1



