Abstract
Free full text

Targeting CD38 with isatuximab and a novel CD38/CD3×CD28 trispecific T-cell engager in older patients with acute myeloid leukemia
TO THE EDITOR:
Acute myeloid leukemia (AML) is the most common acute leukemia in adults and its prevalence significantly increases in older patients.1 Furthermore, while the 5-year rate of overall survival (OS) for adults younger than 60 is around 40%, it decreases to 10% in patients above this age.2 The treatment landscape of AML is evolving from a curative/intensive vs palliative/low-intensity binary approach into new strategies that incorporate mutation-specific targeted therapies, apoptosis-inducing small molecules, and immunotherapy.3 Nonetheless, older patients who are unable to receive intensive chemotherapy with acceptable side effects4 and patients with relapsed/refractory disease5 continue to have dismal survival rates. Thus, the identification of new targets and treatment modalities is an unmet need.
The use of anti-CD38 monoclonal antibodies has significantly prolonged the OS of patients with multiple myeloma (MM). Accordingly, daratumumab6, 7, 8, 9, 10 and isatuximab11,12 are approved for the treatment of newly diagnosed and relapsed/refractory disease. In AML, there are limited data about the potential role of CD38 as a therapeutic target,13, 14, 15 with virtually no information about CD38 levels in older patients.16 Thus, here we analyzed the frequency and pattern of CD38 expression in patients with AML ineligible to receive intensive chemotherapy and performed preclinical studies to investigate the antitumor efficacy of 2 CD38-targeting antibodies, isatuximab and a novel CD38/CD3 × CD28 trispecific T-cell engager (CD38-TCE).
A complete description of methods and techniques used in this study is available in supplemental Data. Briefly, CD38 expression was evaluated using EuroFlow standardized operating procedures17,18 in bone marrow (BM) aspirates from 241 newly diagnosed patients with AML with a median age of 75, who were enrolled in the PETHEMA-FLUGAZA phase 3 clinical trial.19 Preclinical studies were performed in BM aspirates from 25 patients with AML (median age, 70; range, 34-84). The KG-1, MOLM-13 and OCI-AML3 cell lines were used to investigate the mode of action and antitumor activity of 2 anti-CD38 antibodies, isatuximab and an experimental CD38-TCE (SAR442257, Sanofi R&D, Cambridge, MA).20 Isotypes of each antibody lacking the CD38-binding domain were used as control. BM aspirates and peripheral blood mononucleated cells (PBMC) from age-matched healthy adults (n = 16) were used as control in preclinical studies. All samples were collected after informed consent was given by each participant, according to the local ethics committee and the Declaration of Helsinki.
Expression of CD38 was observed in 239 of the 241 (99.2%) older patients with AML (Figure 1A), of which homogeneous CD38 expression was observed in 134/241 (55.6%) and heterogeneous CD38 expression was observed in 105/241 (43.6%) of cases. Homogeneous CD38 expression was more frequent in patients with normal karyotype compared with altered karyotype (66/97 [68%] vs 53/117 [45%]; P = .001) and in those with favorable/intermediate cytogenetic risk compared with adverse cytogenetic risk according to the 2010 Medical Research Council criteria (98/156 [63%] vs 31/77 [40%]; P = .001). Nonetheless, the percentage of CD38+ tumor cells at diagnosis had no impact in OS (Figure 1B). In patients with paired samples, CD38 density in tumor cells did not decrease over time from diagnosis to the assessment of measurable residual disease (n = 10) or till relapse (n = 3), (Figure 1C-D; supplemental Figure 1). Altogether, these results demonstrate that CD38 is present in older patients with AML, although with lower density when compared with that observed in plasma cells from healthy adults (Figure 1E) and from patients with MM21 (data not shown).
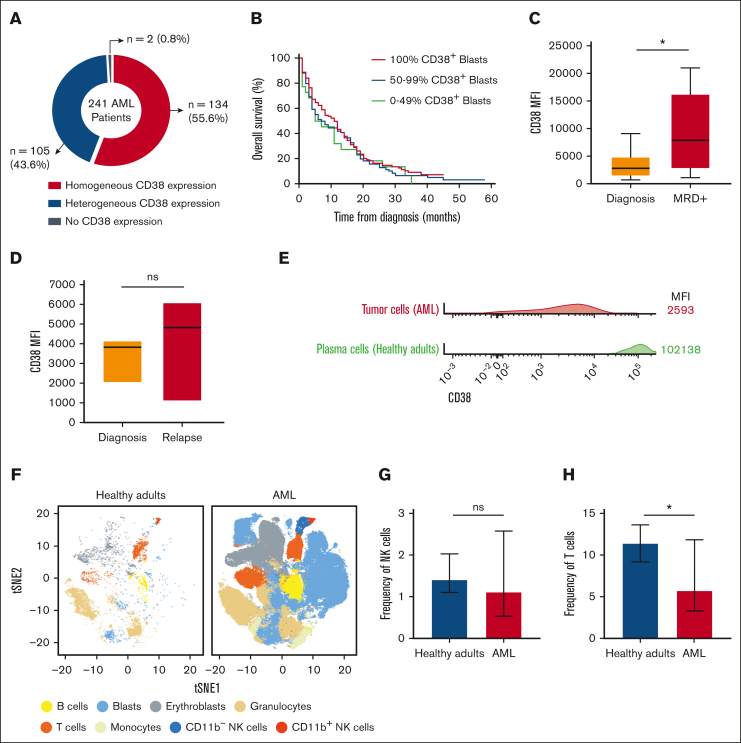
CD38 expression and tumor immune composition in older patients with AML. (A) Distribution of 241 older patients with newly diagnosed AML according to the pattern of CD38 expression: negative, heterogeneous, and homogeneous positive. (B) OS of the 241 older patients with AML treated in the PETHEMA/FLUGAZA phase 3 clinical trial, stratified according to the percentage of CD38+ tumor cells at diagnosis: <50% (green), 50% to 99% (blue) and 100% (red). (C-D) CD38 expression in patient-matched tumor cells at diagnosis, complete response after 3 induction cycles in patients with measurable residual disease (MRD, n = 10), and at relapse (n = 3). (E) Mean fluorescence intensity (MFI) of CD38 in tumor cells from the 241 patients with AML and in BM plasma cells from 10 healthy adults. (F) t-Distributed Stochastic Neighbor Embedding (t-SNE) projection nucleated cells in the BM of older patients with AML and healthy adults (n = 10). (G-H) Frequency of BM NK and T lymphocytes among total alive nucleated cells in BM from patients with AML vs healthy adults. All panels,  P < .05; ns, non-significant.
P < .05; ns, non-significant.
Because the 2 anti-CD38 antibodies being investigated in this study induce tumor lysis through the respective binding and activation of natural killer (NK) and T lymphocytes,20,21 the abundance of both cell types in the BM of older patients with AML was analyzed (n = 240). Based on semiautomated multidimensional flow cytometry immunophenotyping (Figure 1F), the presence of BM NK and T lymphocytes was noted in most patients at diagnosis (Figure 1G-H). Therefore, older patients with AML display immune effector cells in the primary site of tumor expansion that, together with the presence of CD38 in tumor cells described above, support the preclinical investigation of isatuximab and CD38-TCE.
Two AML cell lines with CD38 density comparable with that of patients with AML (MOLM-13 and OCI-AML3) and another with significantly lower CD38 levels (KG-1) were selected for preclinical studies. Treatment of AML cell lines and NK lymphocytes or monocyte-derived macrophages isolated from healthy adults (n = 3) with isatuximab (70 nM), enhanced antibody dependent cellular cytotoxicity and phagocytosis, respectively, particularly in the 2 cell lines displaying the highest CD38 density (Figure 2A-B). Furthermore, there was significant tumor lysis in BM aspirates from patients with AML (n = 17; P = .0002), specifically in those with homogeneous (median of 19.5%; P = .004) vs heterogeneous (median of 4%; P = .125) CD38 expression (Figure 2C). These results suggest that, similar to MM,21 the mode of action of isatuximab in AML might be influenced by CD38 levels (Figure 2D) and that patients with heterogeneous CD38 expression are less likely to respond to an anti-CD38 monoclonal antibody.
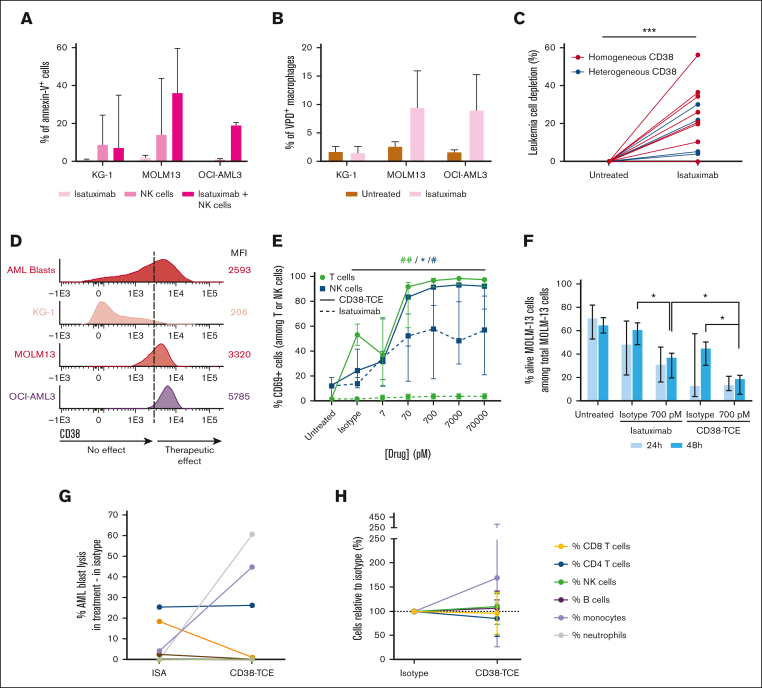
Targeting CD38 with isatuximab and a CD38/CD3 × CD28 trispecific T-cell engager (CD38-TCE). The KG-1 as well as the MOLM-13 and OCI-AML3 AML cell lines were selected because of their respective low and bright density of CD38. (A) Triplicates of antibody-dependent cellular cytotoxicity (ADCC) were performed after 24 hours of treatment with isatuximab (70 nM) in coculture with NK lymphocytes from healthy adults (1:1 effector to target cell [E:T] ratio). ADCC was measured as the percentage of annexin-V+ tumor cells. (B) Triplicates of antibody-dependent cellular phagocytosis (ADCP) in coculture with monocyte-derived macrophages obtained from healthy adults (1:2 Φ:AML cell ratio) were performed after labeling with violet proliferation dye (VPD) and pretreatment with isatuximab (70 nM) for 30 minutes. ADCP was measured as the percentage of CD14/CD300e/VPD triple-positive macrophages after 2 hours of coculture. (C) Tumor depletion in BM aspirates from older patients with AML (n = 17) after treatment with isatuximab (70 nM) during 24 hours. Results are represented as the relative increment observed in treated vs untreated samples. (D) Using the Infinicyt software (Cytognos, Salamanca, Spain), we merged the fetal calf serum files of blasts from older patients with AML (n = 241) with those from KG-1, MOLM-13 and OCI-AML3 cell lines. The mean fluorescence intensity (MFI) of CD38 (measured with the clone HB7 conjugated with APCH7; BD Biosciences, San Jose, CA) is represented by population-band histograms, and ordered from the lowest into the highest expression detected among AML cell lines. The MFI of CD38 required to trigger ADCC and ADCP induced by isatuximab is represented by a hypothetical vertical dash line for a schematic representation of the results described in panels A-B. (E) MOLM-13 cells were labeled with VPD, pretreated with isatuximab or the CD38-TCE and their respective isotypes for 30 minutes, and cocultured with PBMC from healthy adults (n = 6) in a 10:1 E:T ratio. After 48 hours, the activation of effector cells was measured according to the percentage of CD69+ T and NK lymphocytes. For statistical significance, the highest dose was compared with the isotype-treated condition: green and blue symbols represent T and NK cells, respectively;  and # indicated treatment with isatuximab and the CD38-TCE, respectively. (F) Tumor cell death of MOLM-13 induced by pretreatment with isatuximab and the CD38-TCE was determined using annexin V staining after 24 and 48 hours of coculture in the presence of PBMC from healthy adults. (G) Tumor cell death in primary samples from patients with AML (n = 8) after treatment with isatuximab (700 pM) and the CD38-TCE (700 pM) for 48 hours. The percentage of tumor cell death was calculated as follows: [(% leukemic cells in untreated – % of leukemic cells in treated sample)/% of leukemic cells in untreated] × 100. Each colored line is a patient sample, and the 2 cases having tumor cells resistant to isatuximab while being sensitive to the CD38-TCE are represented by the pink and gray lines. (H) After 48 hours of treatment with the CD38-TCE (700 pM), the percentages of neutrophils, monocytes, NK, B, as well as CD4 and CD8 T lymphocytes among CD45+ nucleated cells in BM were normalized to those observed upon treatment with the isotype, which was set up to 100%. In all panels, 1 symbol (
and # indicated treatment with isatuximab and the CD38-TCE, respectively. (F) Tumor cell death of MOLM-13 induced by pretreatment with isatuximab and the CD38-TCE was determined using annexin V staining after 24 and 48 hours of coculture in the presence of PBMC from healthy adults. (G) Tumor cell death in primary samples from patients with AML (n = 8) after treatment with isatuximab (700 pM) and the CD38-TCE (700 pM) for 48 hours. The percentage of tumor cell death was calculated as follows: [(% leukemic cells in untreated – % of leukemic cells in treated sample)/% of leukemic cells in untreated] × 100. Each colored line is a patient sample, and the 2 cases having tumor cells resistant to isatuximab while being sensitive to the CD38-TCE are represented by the pink and gray lines. (H) After 48 hours of treatment with the CD38-TCE (700 pM), the percentages of neutrophils, monocytes, NK, B, as well as CD4 and CD8 T lymphocytes among CD45+ nucleated cells in BM were normalized to those observed upon treatment with the isotype, which was set up to 100%. In all panels, 1 symbol ( or #), P < .05; 2 symbols, P < .01; 3 symbols, P < .001.
or #), P < .05; 2 symbols, P < .01; 3 symbols, P < .001.
Leukemic cells with reduced CD38 expression could potentially be lysed by other immunotherapeutic modalities which are less dependent on the density of the target antigen, such as anti-CD38 chimeric antigen receptor T cells and T-cell engagers.22, 23, 24 Thus, additional preclinical studies were performed with the CD38-TCE that includes an anti-CD28 costimulatory signal to enhance T-cell activation and increase tumor lysis at lower drug concentrations.20 Indeed, treatment of MOLM-13 and PBMC from healthy adults (n = 6) with increasing concentrations of the CD38-TCE resulted in a significant expansion of CD69+ T lymphocytes, in contrast to the null effect of isatuximab (Figure 2E). Interestingly, treatment with the CD38-TCE also activated NK lymphocytes at a higher degree than isatuximab. Additional experiments with purified T or NK lymphocytes demonstrated that activation of NK lymphocytes induced by the CD38-TCE was likely a T-cell–dependent bystander effect (supplemental Figure 2).
The simultaneous activation of 2 effector cell types by the CD38-TCE could potentially result in greater tumor depletion when compared with isatuximab, which only activated NK lymphocytes. Accordingly, treatment of PBMC (n = 6) and MOLM-13 cells (effector-to-target cell ratio of 10:1) with isatuximab or the CD38-TCE (both 700 pM) for 48 hours, showed that tumor depletion was significantly higher with the latter (Figure 2F). Tumor depletion in some BM aspirates from patients with AML (n = 8) was observed with CD38-TCE, including leukemic cells from 2 cases that were resistant to isatuximab (Figure 2G). Conversely, tumor cells from 1 patient were sensitive to isatuximab and not to the CD38-TCE. Of note, the efficacy of the CD38-TCE was not significantly associated with CD38 expression in tumor cells and the antigen was not shed from the surface after binding (supplemental Figure 3).
There were no clinical (eg, age, disease stage, tumor burden) or immune (eg, effector-to-target cell ratio or frequency of BM neutrophils, monocytes, basophils, eosinophils, and NK, CD4+, CD8+, and B lymphocytes) features significantly associated with responsiveness to isatuximab or CD38-TCE (supplemental Figure 4). In addition, the percentage of CD28+ T lymphocytes in primary AML samples was not associated with the magnitude of tumor depletion induced by the CD38-TCE (supplemental Figure 5).
The percentages of immune cell types in BM aspirates from patients with AML remained unaltered after treatment with the CD38-TCE (Figure 2H). Thus, preclinical experimentation suggests that there was no apparent on-target off-tumor toxicity, which might be related with the median affinity (Kd = −20 nM) of anti-CD3ε and the monovalent CD28 binding.20,25 As expected, normal CD34+ progenitors were undetectable in BM aspirates from patients with AML and on-target off-tumor toxicity could not be analyzed. In healthy adults, CD34+ progenitors display higher CD38 density than the immune cells in which no apparent on-target off-tumor toxicity was observed (supplemental Figure 6).
In summary, our results suggest a potential role for CD38 as a therapeutic target in AML. However, because not all patients express CD38 homogeneously and the density of the antigen is lower than that observed in other hematological malignancies such as MM, the immunotherapeutic modality would have to be tailored according to the pattern and levels of CD38 expression in tumor cells. Furthermore, the high CD38 density in CD34+ progenitors could suggest that treatment duration may require adaptation to mitigate the possibility of hematological adverse events, particularly with the CD38-TCE. Further in vivo studies are warranted to investigate this hypothesis.
Conflict-of-interest disclosure: B.P. reports honoraria for lectures from and membership on advisory boards with Amgen, Bristol Myers Squibb, Celgene, Janssen, Merck, Novartis, Roche, and Sanofi; unrestricted grants from Celgene, EngMab, Sanofi, and Takeda; and consultancy for Celgene, Janssen, Sanofi, and Takeda. The remaining authors declare no competing financial interests.
Acknowledgments
Acknowledgments: The authors acknowledge all PETHEMA investigators involved in the FLUGAZA clinical trial: Manuel Mateo Pérez Encinas, Guillermo Debén Ariznavarreta, Lorenzo Algarra Algarra, Pascual Fernández Abellán, Susana Vives Polo, Olga Salamero, Amaia Balerdi Malcorra, Juan Miguel Bergua Burgués, Adriana Gascón Buj, Jorge Labrador, Josefina Serrano, Fernando Ramos Ortega, Esperanza Lavilla Rubira, María Ángeles Foncillas García, Pilar Herrera Puente, María del Pilar Martínez Sánchez, Alfons Serrano, Raúl Córdoba Mascuñano, Adriana Simiele Narvarte, Belén Vidriales, Mercedes Colorado Araujo, José Francisco Falantes González, Pau Montesinos Fernández, María del Mar Tormo Díaz, María José Sayas Lloris, Magdalena Sierra, Maite Olave Rubio, José Luis López Lorenzo, María Ángeles Fernández Fernández, Aránzazu Alonso Alonso, and María Lourdes Amador Barciela. The buffy coats used as a source of healthy adults’ PBMC were obtained from Navarra Blood and Tissue Bank/Navarrabiomed Biobank (Navarra Health Department).
This study was supported by the University of Navarra (Plan de Investigación Universidad de Navarra), the Centro de Investigación Biomédica en Red – Área de Oncología - del Instituto de Salud Carlos III (CB16/12/00369; and CB16/12/00489), the Instituto de Salud Carlos III/Subdirección General de Investigación Sanitaria (FIS No. PI16/01661), and the Scientific Foundation of the Spanish Association Against Cancer (FCAECC, INVES211176MART). This study was supported internationally by the Cancer Research UK, FCAECC and American International Recruitment Council under the Accelerator Award Program and by an independent grant from Sanofi.
Contribution: E.M.-S., T.J., B.P., and A.Z. conceived the idea and designed the study protocol; E.M.-S., T.J., C.S., B.P., and A.Z. analyzed flow cytometry data; E.M.-S., L.B., T.J., and A.Z. performed in vitro and ex vivo experiments; P.S.K., K.B., H.W., H.V.d.V., F.P., J.F.S.M., A.A., J.B., R.R.-V., S.V., D.M.-C., and P.M. provided study material and/or patients’ samples; E.M.-S. and A.Z. performed statistical analysis; E.M.-S., T.J., B.P., and A.Z. wrote the manuscript; and all authors reviewed and approved the manuscript.
Footnotes
 E.M.-S., P.M., B.P., and A.Z. are joint last authors and contributed equally to this study.
E.M.-S., P.M., B.P., and A.Z. are joint last authors and contributed equally to this study.
Data are available on request from the corresponding author, Bruno Paiva (se.vanu@aviapb).
The full-text version of this article contains a data supplement.
Supplementary Material
References
Articles from Blood Advances are provided here courtesy of The American Society of Hematology
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Pre-Clinical Assessment of SAR442257, a CD38/CD3xCD28 Trispecific T Cell Engager in Treatment of Relapsed/Refractory Multiple Myeloma.
Cells, 13(10):879, 20 May 2024
Cited by: 0 articles | PMID: 38786100 | PMCID: PMC11120574
A CD38-directed, single-chain T-cell engager targets leukemia stem cells through IFN-γ-induced CD38 expression.
Blood, 143(16):1599-1615, 01 Apr 2024
Cited by: 0 articles | PMID: 38394668
Application of CD38 monoclonal antibody in kidney disease.
Front Immunol, 15:1382977, 10 May 2024
Cited by: 0 articles | PMID: 38799465 | PMCID: PMC11116655
Review Free full text in Europe PMC
Targeting Multiple Myeloma with AMG 424, a Novel Anti-CD38/CD3 Bispecific T-cell-recruiting Antibody Optimized for Cytotoxicity and Cytokine Release.
Clin Cancer Res, 25(13):3921-3933, 27 Mar 2019
Cited by: 75 articles | PMID: 30918018



