Abstract
Free full text

Treatment of spinal cord injury with biomaterials and stem cell therapy in non-human primates and humans
Abstract
Spinal cord injury results in the loss of sensory, motor, and autonomic functions, which almost always produces permanent physical disability. Thus, in the search for more effective treatments than those already applied for years, which are not entirely efficient, researches have been able to demonstrate the potential of biological strategies using biomaterials to tissue manufacturing through bioengineering and stem cell therapy as a neuroregenerative approach, seeking to promote neuronal recovery after spinal cord injury. Each of these strategies has been developed and meticulously evaluated in several animal models with the aim of analyzing the potential of interventions for neuronal repair and, consequently, boosting functional recovery. Although the majority of experimental research has been conducted in rodents, there is increasing recognition of the importance, and need, of evaluating the safety and efficacy of these interventions in non-human primates before moving to clinical trials involving therapies potentially promising in humans. This article is a literature review from databases (PubMed, Science Direct, Elsevier, Scielo, Redalyc, Cochrane, and NCBI) from 10 years ago to date, using keywords (spinal cord injury, cell therapy, non-human primates, humans, and bioengineering in spinal cord injury). From 110 retrieved articles, after two selection rounds based on inclusion and exclusion criteria, 21 articles were analyzed. Thus, this review arises from the need to recognize the experimental therapeutic advances applied in non-human primates and even humans, aimed at deepening these strategies and identifying the advantages and influence of the results on extrapolation for clinical applicability in humans.
Introduction
Spinal cord injury (SCI) is considered a devastating neurological condition, with incidence dynamics ranging from 11 to 16 cases per 100,000 people worldwide. In clinical terms, SCI causes the loss of sensory, motor, and autonomic functions, leading to a physical disability that is almost always permanent, with psychological problems (James et al., 2019; Eli et al., 2021). The pathophysiology of SCI consists of a primary event that causes cell death (neuronal, glial, and endothelial cells) and the disruption of axonal connections. Subsequently, axon stumps undergo a process of Wallerian degeneration, which consists of an alteration of the cellular structure of the axon, characterized by axonal degeneration of the distal stump, followed by demyelination and atrophy (Figure 1) (Mietto et al., 2015). The secondary injury cascade begins within a few hours after SCI and is characterized by many cellular, molecular, and biochemical responses that continue to self-destruct to spinal cord tissue and impede neurological recovery. The cascade of physiological secondary events is characterized by obstruction of blood flow, followed by hemorrhage, edema, inflammation, secretion of extracellular matrix molecules, pro- and anti-regenerative biomolecules, lipid peroxidation, and activation of programmed cell death (Figure 1; Mietto et al., 2015; Alizadeh et al., 2019).
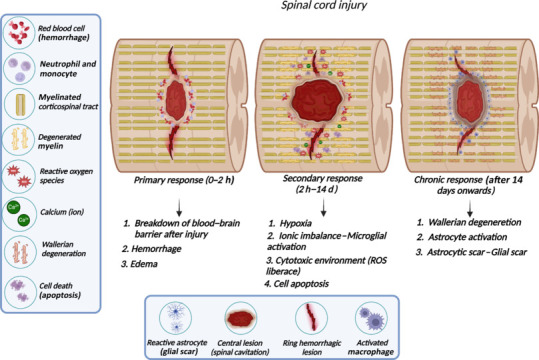
Schematic representation of the pathophysiology of SCI.
The primary response to SCI promotes the breakdown to the ascending and descending tracts and disconnection to the cortex from circuitry below the injury. The trauma also causes the breakdown of the blood–brain barrier and the developing cavities filled by blood near the impact site (area of the epicenter to the lesion). SCI acute phase starts immediately after injury, and finishes when some reflex response can be elicited. The subacute and intermediate phases are characterized by the secondary response beginning a few hours after the injury. These secondary events involve axonal degeneration, infiltration of inflammatory cells (neutrophils and macrophages), apoptotic oligodendrocytes followed by demyelination, synapse alteration, and microenvironment modulation among other responses. The chronic phase in rodents and humans begins 4 and 6 weeks after injury, respectively, and the glial scar delimiting the tissue is quite evident in this phase. Created with BioRender.com. ROS: Reactive oxygen species; SCI: spinal cord injury.
The injury response can be temporally divided into acute, subacute, intermediate, and chronic phases. During the subacute phase, several vasoactive factors are released in physiological response to edema. This mechanism triggers hypoperfusion, hypoxia, and hypoglycemia, which continues with the aforementioned ischemia and cell death (Wang et al., 2021). Ischemia is followed by reperfusion, which triggers an increase in reactive oxygen-free molecules, which form part of the degenerative environment that tends to cause loss of gray matter. The chronic phase is the pathophysiology evolution characterized by glial scar formation. Nowadays, the spinal cord scar is considered a multifaceted interplay that performs dual opposing roles to protect tissue and inhibit repair. The protective glial scar role is related to separating the healthy tissue from pathology after injury to minimize the inflammatory response. The inhibitory role of the glial scar is classically associated with failed axonal re-connectivity due to action as a physical and molecular obstacle to axonal regeneration at the epicenter of the lesion by the scar and scar-associated molecules with signaling changes and the extracellular environment (Chu et al., 2002; Jung et al., 2016; Yu et al., 2018; Bradbury and Burnside, 2019).
Regarding the current treatment of traumatic SCI, the literature shows that this has been limited to supportive care, with mainly physical therapeutic interventions. However, surgical intervention is also estimated within 24 hours after the injury, as long as the type of trauma allows or requires it. Despite this, there is currently no safe and effective treatment available to humans that can regenerate damaged neuronal tracts and restore the loss of neurological functions in patients after the traumatic event. According to a recent research, in search of more effective treatments than those already applied for years, great potential has been demonstrated in biological strategies with stem cell transplantation as a neuroregenerative approach (Wang et al., 2021). Thus, various preclinical studies have shown that scaffold implantation and stem cell transplantation treatments have the capacity to promote functional recovery in experimental animals, until now mostly in rodents. Furthermore, in some clinical trials, the authors have used scaffolds combined with stem cells, showing promising results in the future for the treatment of patients with acute and chronic traumatic SCI, partial or complete. Along these lines, other researchers have pointed to the manufacture of scaffolds and their enrichment with bioactive factors, which offer the possibility of modulating the microenvironment, further enhancing neuronal recovery (Abbas et al., 2020; Llorens-Bobadilla et al., 2020; Soares et al., 2020; Zipser et al., 2022; Gong et al., 2023; Szymoniuk et al., 2023).
The variable neuroanatomical in animal models can impact the response of motor neurons to the execution of some motor actions (Rouiller et al., 1996). In neuroanatomical terms, the difference between rodents and non-human primates (NHP) is quite evident in motor tracts, since the pattern of corticospinal tract (CST) projection is much more complex throughout the evolution of humanity and NHPs when compared to rodents. Rodents have a CST, a descending tract from the motor cortex, projecting mainly to the interneurons of the dorsal horn and the premotor spinal circuits without maintaining direct communication with motor neurons (Figure 2; Lemon et al., 2004; Jacobson et al., 2021; Sun et al., 2021). Conversely, NHPs have a location and function similar to humans (Ko et al., 2019), and like a human exhibit a massive increase in the proportion and size of CST neurons in the neocortex, both in motor and supplementary areas that widely projected from the cortex to the brainstem and into the spinal cord, with CST fibers directed towards the ventral horn with some axons synapses directly to motor neurons (Figure 2; Whishaw et al., 1998; Rosenzweig et al., 2018).
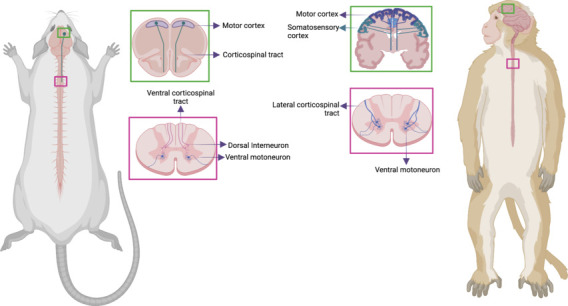
Representative diagram of the main neuroanatomical differences between macaques and rodents.
Special attention is given to the trajectory and communication patterns of the corticospinal tract at both the spinal cord and cerebral cortex levels. In the sections enclosed in the cortex, the graphic highlights the contrasting cortical activation and the differential influence of various areas, predominantly observed in macaques during basic motor responses, like walking or more complex ones, such as fine grips. This stands in contrast to rodents, characterized by a diminished cortical response. The transverse sections of the thoracic spinal cord show variations in neuronal communication, showcasing the interneuronal connectivity prevalent in rodents as opposed to macaques. The latter, akin to humans, exhibit direct communication of the corticospinal tract with motor neurons at the spinal level. Created with BioRender.com.
The difference between rodents and NHP is more evident in the forelimbs muscles, since NHP has developing fine motor skills much more human-like (such reaching and motor grasping, tools using and has precision between the thumb and forefinger in some primates like Rhesus) (Courtine et al., 2007; Han et al., 2019). Although the control of fine motor skills of the forelimb has been tested in rodents and is affected by lesions in the CST, the delicacy in the hand control, specifically in digital dexterity, is much less developed in rodents than in primates and humans, which is also justified by marked differences in the musculoskeletal structure of the forelimb musculature, hand, and fingers between primates and rodents (Whishaw et al., 1998). From the perspective of clinical trials, the evaluation of motor performance in NHP is seen as an advantage due to the neuroanatomical similarity of the motor pathway, and the functional capacities between them, in contrast to rodents. In fact, specific behaviors such as precision grasping, manual precasting, and other hand-grasping tasks performed by macaques and other monkeys bear a strong resemblance to such actions in humans.
Lesions that alter CST neurons in the cortex of rodents give rise to a few sequelae in the execution of gait in rodents, indicating that the motor cortex is not essential to creating the muscle synergies for simple locomotion in rats and mice. However, damage to the CST in the cerebral cortex in rhesus primates causes permanent deficits in motor gait performance, as in humans, where damage to the cortex leads to motor impairment severe enough to compromise independent gait (Nathan, 1994; Courtine et al., 2007) (Figure 2). Thus, the neuroanatomical divergences identified point to a greater complexity and specialization in the organization of the motor pathway in macaques compared to rodents, despite certain general similarities.
In this context, although rodents have been accepted as animal models for preclinical experimental studies of SCI and most experimental research is carried out in rodents, there is increasing awareness of the importance and, above all, the need to determine the safety and effectiveness of these interventions in NHP, before advancing to clinical trials with potentially promising therapies (Slotkin et al., 2017). The literature converges in that NHP models more closely emulate the size, scale, and work protocol for clinical surgical implantation or other types of intervention in SCI. Additionally, NHPs and human beings share many anatomical similarities in the organization of their nervous system structure and even in both normal and pathophysiological processes, which provides greater security to predict the viability and effectiveness of repair in humans (Courtine et al., 2007).
This article focuses precisely on the review of the scientific evidence that supports the application of biological and cellular strategies, specifically in non-human primates, with the purpose of reviewing the advances in intervention in SCI on these species, which form an important part of a transition phase in the experimental process to advance to clinical practice, both for protocol and ethical reasons, they are essential and indispensable in studies carried out in SCI, since they are animal models that would anatomically simulate the most accurate process, both pathophysiological and recovery, of human motor abilities, which until now has not been fully achieved with the development of experimental trials in rodents.
Search Strategy and Selection Criteria
This article was developed through a review of scientific literature published in the areas of neurology, physiotherapy, biology, tissue engineering, and biomedicine in journals indexed from 10 years ago to the present. For its structuring, the guidelines of the PRISMA declaration of bibliographic reviews were applied. Before proceeding to select the articles, the inclusion and exclusion criteria were defined.
Inclusion criteria: Scientific articles that include detailed methodology and results (should be experimental studies, cases, and controls, and review articles); no older than ten years derived from indexed journals; that exclusively include the determined keywords (spinal cord injury, cell therapy, NHP, humans, and advances in tissue bioengineering in spinal cord injury); and be written in English, Spanish or Portuguese.
Exclusion criteria: Articles applied to species other than primates and humans.
The search was in scientific databases such as PubMed, Science Direct, Elsevier, Scielo, Redalyc, Cochrane, and NCBI using the keywords previously cited to find around 110 articles in different languages into the profile to types of studies included (systematic and bibliographic revisions, cases and controls, and case studies). The title and abstract were analyzed, followed by reading the full text from eligible articles, and the results were analyzed and described in figures and table to the text.
The literature search process is presented in Figure 3. The search identified 110 studies of which 21 remained for analysis. Figure 3 shows the flowchart of the articles screening by PRISMA methodology. A total of 110 articles were obtained from the databases, of which 23 were discarded due to replication, and subsequently, 48 more were excluded due to non-compliance with the inclusion criteria. The number of articles and reasons for exclusion are detailed in the side box. Thus, 39 articles were included to be fully read, of which 18 were discarded because full text cannot be obtained, finally including 21 articles in total to this review article.
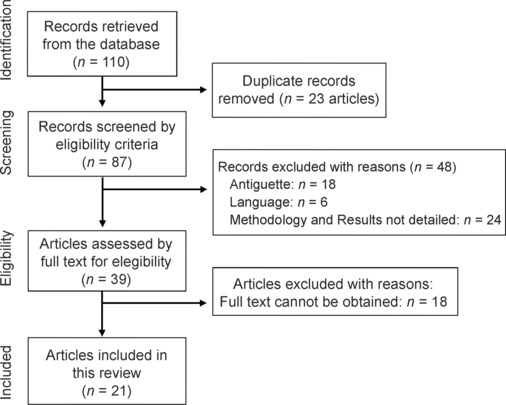
Flow chart of the screening phases of the literature review.
This diagram represents each of the phases described in the methodology applied for the selection of the articles included in the literature review.
Additional Table 1 succinctly summarizes the data from the 21 articles analyzed, presenting the update of the different types of treatments that have been applied in NHP after SCI to describe the utilized biomaterials, experimental population characteristics, and the achieved outcomes, seeking by advances of intervention and the possible advantages that this entails in experimental research with NHP models towards application in humans. Furthermore, it elucidates the evolution of functional recovery resulting from the effects of the employed strategies that demonstrate similarities with human outcomes regarding temporal aspects, motor capacity, and sensory function.
Therapeutic Advances in Spinal Cord Injury in Non-Human Primates
Cell therapy
A few years are coming and a large number of experimental studies have been carried out to develop possible therapeutic options for patients with SCI, which mainly seek to reduce motor sequelae and, therefore, levels of disability. Many studies have shown partial morphological changes, accompanied by improvements in motor behavior in various animal models, rodents, pigs, rabbits, and NHP (Fouad et al., 2005; Blesch and Tuszynski, 2009; Kadoya et al., 2009; Danilov and Steward, 2015; Zhao et al., 2017).
Among this large number of studies, stem cell-based transplantation has been considered a promising therapeutic strategy for successful outcomes, primarily due to the direct replacement of damaged neural tissue, neuroprotective properties to preserve neuronal connections, and the ability to provide a permissive and supportive cell growth substrate for axonal regrowth and/or neuronal plasticity (Ohta et al., 2004; Ferón et al., 2005; Granger et al., 2012; Kanno et al., 2015).
Human neural stem/progenitor cell and human iPSC-neural stem/progenitor cell transplantation in NHP SCI
Yamane et al. (2010) used galectin-1-expressing neural stem cells in SCI at a C3–C4 and T9–T10 level of an adult common marmoset, and they showed that adult marmosets transplanted 9 days after injury showed better motor recovery and the transplanted stem cells were found in the epicenter of the lesion and were capable of differentiating in glial cells. These findings suggest that Gal-neural stem/progenitor cell (NS/PC) and NS/PC could be feasible treatments for SCI (Yamane et al., 2010).
Kobayashi et al. (2012) reported positive therapeutic effects of the use of NS/PC derived from human embryos to treat NHP after SCI, precisely they observed positive results in the motor function recovery, much more visible in manual dexterity. Despite authors suggesting the NS/PCs lineage is very efficient to guide the differentiation into neural cells as neurons, astrocytes, and oligodendrocytes into the injured spinal cord (Yamane et al., 2010; Kawai et al., 2023). However, there are ethical issues to obtain human NS/PC to treat human cases by aborted fetal tissue, and to change this difficult situation, the hiPSCs lineage has been established and developed as a promising cell source for NS/PC transplantation therapy. hiPSC-NS/PC transplantation into subacute SCI has demonstrated ameliorate locomotor function (Kawai et al., 2023).
Tsuji et al. (2019) treated macaque with these stem cells and described a principle of recovery based on a reduction of post-injury demyelination with improvements in the gait pattern, demonstrating a picture very similar to that of humans, with a progressive evolution in the number of steps in walking, along with an evolutionary increase in manual dexterity. For this reason, the projection of the first phase of a protocol in humans with induced pluripotent stem cells, specifically neural/progenitor stem cells (iPSCs-NS/PCs), was proposed. The first-in-human clinical trial of iPSCs and NS/PC with patients with subacute spinal cord injury is underway, using clinical-grade iPSCs and NS/PCs generated at Keio University School of Medicine and Osaka National Hospital. The “iPSC membrane” is manufactured by the iPS Cell Research and Application Center (CiRA) at Kyoto University. Clinical-grade iPSCs and NS/PCs are kept frozen until a few days before surgery, then transplanted into the injured spinal cord of patients in the subacute phase (14–28 days after injury), and then followed for safety reasons for up to 2 years.
Mesenchymal stem cell and their exosome transplantation
Many preclinical studies have been described in the literature in which mesenchymal stem cell (MSC) is used in animal models of SCI, or even studies in macaques or clinical trials in humans, and all researchers described therapeutic potential and safety to this MSC use. Several technical aspects of neuronal MSC transplantation to different sources in various conditions have been tested. MSC transplanted in thoracic macaque SCI and showed benefits in motor recovery for gait readaptation processes, identifying transformations not only at the spinal cord level, but also at the cortical level, which enhances neuroplastic and motor rehabilitation processes (Nemati et al., 2014; Joo et al., 2022).
Exosomes are membrane-bounded extracellular vesicles, secreted by all cells and filled by many biomolecules from donor cells that can influence a large variety of biological activities, including neuroregeneration, neuroprotection, neurorepair, microenvironment modulation, and anti-inflammation effects. Exosome therapy is biocompatible, non-immunogenic, and the macrophages and lysosomes-escaping, and it has become a representative cell-free intervention in regenerative medicine. Also, it is now known that exosomes have the ability to cross the blood–brain barrier and travel long distances toward lesions in the nervous system (Fayazi et al., 2021; Xu et al., 2021).
A variation in cell therapy is the application of stem cell exosomes, which have been applied in large quantities in rodent species; however, in macaques so far the evidence remains low. Go et al. (2020) made MSC exosome transplantation in macaques and 12 weeks later, the monkeys showed improvements in motor function tests and functional grip capacity that were associated with the results found. The exosomes were isolated from human MSC (bone marrow stem cell source) and administered intravenously, first at 24 hours post-injury and again at 14 days post-injury. Also, immunohistochemical analysis shows a greater microglial activation response after the supply of extracellular vesicles (exosomes), which are related to a good anti-inflammatory response, contributing to their time to the generation of cellular changes in the gray matter, specifically in the area of the injury.
Human neural progenitor cell GABA transplantation in non-human primates
The concept of cell therapy related to the use of spinal GABA neurons in SCI in NHP was introduced by Zheng et al. (2023), who collected neural progenitor cells (NPCs) from human spinal GABA neurons, which were resuspended in a culture medium, and then by means of a microinjection pump, they were slowly injected for 3 minutes at the site of injury (T10). With this intervention, the formation of specific human synapses in human neurons was obtained, which was verified by presynaptic and postsynaptic markers (human synaptic synapt and Homer1, respectively). In addition, these synapses were inhibitory as confirmed by the application of GAD65, vesicular transporter of GABA, and gephyrin. In addition, they showed that there was approximately 20.47% differentiation of astrocytes in human cells, which was verified by the stimulation and release of SOX9 and human glial fibrillary acid protein (GFAP). These human astrocytes also expressed vimentin, S100β, connexin 43, and glutamate transporter 1, and facilitated neuronal regeneration procedures, as they were observed to tightly surround the cell body of neurons, support axons, and wrap capillaries, for their survival. Thus, it was concluded, through the evaluation of quadrupedal locomotion, that monkeys that obtained 36 points before SCI, demonstrated severe locomotor disorders, with total paralysis on the left side for 1 week after injury, weight-bearing paralysis on the plantar landing on the right side, and no trunk stability (Gong et al., 2021).
Thanks to studies such as those proposed above, under the principles of cell therapy and the application in species that have a greater similarity with the neuroanatomical and body structure of the human being, it is possible to advance and extrapolate experimental but safe results and procedures from NHP to humans. For these reasons, in another study, Curtis et al. (2018) sought to test the feasibility of transplanting neural stem cells derived from the human spinal cord (NSI-566) for the treatment of chronic SCI in humans.
In this clinical trial, four subjects with SCI T2–T12 received treatment consisting of spinal instrumentation removal, with laminectomy and duratomy, followed by six bilateral stereotactic injections into the NSI-566 midline cell. All subjects tolerated the procedure well and there have been no serious adverse events to date (18 to 27 months post-grafting). In two subjects, one or two levels of neurological improvement were detected using the ISNCSCI motor and sensory scores. These results support the safety of transplantation of NSI-566 at the SCI site, and early signs of potential efficacy in three of the subjects warrant further exploration of NSI-566 cells in dose-escalation studies (Curtis et al., 2018).
Membranes and/or scaffolds combined with cell therapy
Collagen scaffolds with human NPCs, brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor
The use of scaffolds made of different biopolymers is so far a widely used technique in the line of study of nerve regeneration. Xu et al. (2023) developed a study at NHP that once again demonstrated its effectiveness, a little more precise in what would happen in human application. Nineteen adult female rhesus monkeys (Macaca mulatta), 3–4 years old and weighing 4.1–5.8 kg, were used. The authors isolated and cultured 7-week-old human embryonic cells (NPCs), which were inserted into a collagen scaffold with dorsal and ventral neurons (DV-SC), and placed at the site of spinal cord injury at the T8–T9 level. These results demonstrated that the DV-SC scaffold grafted with human NPCs could survive and differentiate into human spinal cord NPCs in mature neurons that still possessed features of dorsal and ventral spinal cord interneurons in SCI lesions of Rhesus monkeys sacrificed 6 months after injury.
DV-SC could also form synapses with host sensory and motor axons in the injured spinal cord of rhesus monkeys (Xu et al., 2023). Therefore, it was shown that the hind leg movement skills in rhesus monkeys implanted with DV-SC continuously improved during the experimental period, the muscle strength of the hind leg was partially restored, and they were able to alternately lift both knees on different occasions. In contrast, Rhesus monkeys in the control group and the scaffolding + NPC group were unable to move their hind leg joints during the entire observation period. These findings revealed that DV-SC implantation is successful and could promote the restoration of electrophysiological motor capacity in the hind legs in rhesus monkeys after spinal cord injury, promoting gait recovery and progressive independence (Xu et al., 2023), making it a potential treatment to be tested in humans.
Chitosan scaffold with neurotrophic factor neurotrophin-3
Chitosan has been one of the most evidence-based biopolymers in scaffold engineering, as it has been repeatedly used in cell therapy studies for neuronal regeneration. Rao et al. (2022) conducted a study applied to macaques, as this biopolymer has been tested primarily on rodents. The authors performed a T7–T9 hemisection of the spinal cord in nine adult female rhesus monkeys (mulatta macaque, 4–6 years old, 5 ± 1 kg). A 10 mm chitosan tube, to which a load of neurotrophin-3 (NT3) (neurotrophic factor) was added, was transplanted into the BF area. Injured animals and those treated with NT3 chitosan showed similar motor adjustments, which demonstrated improvement in motor performance. Consecutive steps on a treadmill before and after spinal cord injury were evaluated and a recovery in gait performance over time was observed.
Additionally, it was possible to demonstrate that, in the injured group, intrahemispheric changes were present. Functional magnetic resonance imaging, combined with Granger causality analysis, showed a functional reorganization of the brain in the early postoperative stage, evidencing interhemispheric interactions in animals treated with chitosan NT3. This allows us to corroborate neuroplastic changes not only at the spinal level, but also at the cortical level, which is quite similar to the human being and favors the restoration of the motor patterns of gait in the NHP with this biotechnology (Rao et al., 2018).
Previously, it was shown that primates (monkeys and humans) can achieve substantially better spontaneous motor recovery than rodents after spinal cord injury, recovering and creating alternative circuits to structural reorganization, such as synaptic remodeling, dendritic spine growth, axonal budding, and reconstruction of circuits in the supra- and sublesional cord can restore the functions after an injury (Ko et al., 2019; Rao et al., 2022).
Human thrombin in fibrinogen loaded with human NPCs and neurotrophic factors
Rosenzweig et al. (2018) subjected nine adult male rhesus monkeys to a C7-level spinal cord injury model and then received the implantation of neural progenitor cells, derived from the human spinal cord. They structured a two-part fibrin matrix membrane that was loaded with a cocktail of neurotrophic factors (brain-derived neurotrophic factor, NT3, glial cell line-derived neurotrophic factor, epidermal growth factor, hepatocyte growth factor, platelet derived growth factor, and vascular endothelial growth factor), and placed it in the BF area, and subsequently followed up to 9 months.
In their view of the results, it was evident that NPCs survived in the lesion cavities of hemisection C7 for 2–9 months. The grafts occupied most of the lesion cavity in all subjects and integrated well with the host’s spinal cord. The more mature neural marker NeuN was also detected two months after engraftment and continued to be expressed over time. The density of cells in the graft was higher two months after implantation, which is related to the evolution in the motor performance of manual gripping.
Graft-derived axons were present 2 mm caudal from the lesion, 2–9 months after grafting, and axons reached distances of up to 50 mm from the graft. Axons emerged rostrally in similar numbers and at similar distances, extended into white matter tracts resting directly against the grafts, and appeared to maintain growth within these same fascicles of white matter to distant points of the graft; this observation suggests that axons may continue to extend through the medullary tracts they first encounter when emerging from the grafts of the injurious focus (Rosenzweig et al., 2018). In monkeys with surviving grafts, the initial period of functional loss or partial spontaneous improvement was 4–8 weeks after injury, followed by a second period of subsequent improvement after 10 weeks. Object manipulation was recovered with a > 25% success rate in 4 out of 5 grafted monkeys, generating high experimental expectations for its application in humans with high BF and/or severe motor hand involvement (Rosenzweig et al., 2018).
Three-dimensional gelatin sponge scaffold as a vehicle for human umbilical cord mesenchymal stem cells
The use of 3D printing technology has currently been used as a bioengineering strategy that can bring great benefits to cell therapeutics. Zeng et al. (2023) conducted a study using fascicularis monkeys aged between 5 and 8 years, who underwent a model of spinal cord injury at the T10 level, and then grafted a D-shaped three-dimensional gelatin sponge scaffold (3D-GS) scaffold membrane to adapt to the gaps created by spinal cord hemisection. This scaffold was structured with an outer layer of poly (lactic-co-glycolic acid) poly resorbable scaffold (PLGA) which was generated by wrapping a thin film of internal PLGA forming a D shape, which was then loaded with human mesenchymal stem cells extracted from the human umbilical cord and combined with nerve growth factors.
The result exhibited that, over the 8-week observation period, the monkeys in both groups gradually regained motor function in the hind leg, although strength, body weight support, gait, coordination, and finger movement remained deficient relative to the monkeys (Zeng et al., 2023). In addition, implantation of the 3D-GS scaffold protected the contralateral residual tissue of the spinal cord against myofibroblast-mediated tissue contraction. In addition, a greater amount of residual tissue, such as medullary white matter nerve tracts, was preserved just after implantation of the 3D-GS scaffold. This scaffold led to a reduction in the accumulation of compressive fibroblasts and improved the site microenvironment or survival of Schwann cells, stromal cells, and even neural stem cells and neurons, ultimately contributing to the structural repair of the injured spinal cord, which was demonstrated with the functional recovery of the monkeys in the functional assessment of their independent gait (Zeng et al., 2023).
NeuroRegen-type scaffolds loaded with autologous bone marrow mononuclear cells
With the trend of structuring membranes with natural and synthetic biopolymers, innovation is evidenced in the literature with the creation of membranes from living tissues derived from animals, which could have compactness with human tissues and bring great benefits in cell therapeutics.
Chen et al. (2020) in view of the positive response to the application of scaffolds with synthetic and natural biopolymers in non-human primates, developed a clinical study in humans with a 3-year follow-up, where they used a male and female population between 18 and 65 years of age with compressive BF between T1 and T12 levels, with a maximum of 4 weeks after the harmful event. Bone marrow mononuclear cells were obtained from patients 2 hours prior to surgery. Mononuclear cells were extracted from the hip bone marrow, more specifically from the iliac crest, using a nucleated cell processing kit, centrifuged under sterile conditions at 2000 r/min for 10 minutes, and then the supernatant plasma was extracted.
The scaffold was constructed from fresh bovine muscles with white aponeurosis, from the local slaughterhouse, which were repeatedly cleaned with cold distilled water for sterilization, and then loaded with bone marrow mononuclear cells and neuronal growth factors, and then implanted in the area of the previous injury (Chen et al., 2020). In the review of the results, different degrees of improvement were observed in terms of sensory level (two cases), sensation of defecation (four cases), physiological erection (five cases), increased sweating (three cases), recovery of superficial sensation (one case), and recovery of deep sensation (one case). In particular, one patient showed an evident recovery of deep sensory localization and numbness to pain sensation with hot water stimulation, but without a clear location. In addition, compared to preoperative scores, the patient’s functional independence measure score and activities of daily living score at 6 and 36 months post-surgery increased significantly (all P < 0.05), while the visual analog scale score was significantly reduced (P < 0.05), indicating that the patient’s ability to live independently and pain improved significantly after surgery (Chen et al., 2020).
Zhao et al. (2017) applied this combined scaffold with human umbilical cord MSCS in humans with SCI with chronic cervical or thoracic injury, and showed improved trunk stability and balance in the sitting position in four patients; in addition, recovery of autonomic neurological function, such as increased skin sweating below the level of injury, was detected in some patients after treatment.
Biodegradable hydrogel scaffold encapsulating human neuroepithelial stem cells and MSCs
In the field of cellular therapeutic strategies, Li et al. (2023) made a great scientific contribution using a total of 9 female rhesus monkeys between 13 and 20 years of age, weighing 4 ± 2 kg, who suffered a BF at the T10 level. For the construction of membranes, methacrylate gelatin (GelMA) and methacrylate hyaluronic acid (HAMA) were prepared, which meet biodegradable requirements and are widely used as tissue engineering scaffolds. Thus, the photosensitive methacrylate (MA) is structured on gelatin and hyaluronic acid (HA) to make MA and HAMA Gel, and then the GH hydrogel is produced by mixing GelMA (G) and HAMA (H), followed by light cross-linking to improve the mechanical properties, and then fabricating the microspheres that encapsulate the cells with the GH hydrogel. They were then loaded with human neuroepithelial stem cells (NESC) and MSCs.
The hydrogel was applied immediately after spinal cord injury and a decrease in the number of GFAP-positive cells was observed. In addition, subsequent staining exhibited that the stem cell grafts had lower activation of microglial cells around the injury focus. This suggests that NESCs or MSCs provide a favorable microenvironment, inhibiting glial scar formation and thus the microglial response.
In all three stem cell groups, genes in the upregulated groups were associated with axon regeneration, CNS development, neurogenesis, myelination, axon envelope, and neurofilament cytoskeleton organization, while downregulated genes were associated with immune response, microglial cell activation, and apoptosis (Li et al., 2023). Taken together, these results suggest that the beneficial effects of NESC and MSCs involve the promotion of neurogenesis and myelination, and the reduction of inflammatory responses, while genes in the negatively regulated groups were associated with CNS development and neurogenesis (Li et al., 2023). Regarding the functional assessment, it should be noted that the paralysis of the left hind limbs did not improve in the 3 control monkeys, even up to 180 days after the injury. Monkeys treated with NESC showed gradual improvements in motor functions over time, regaining the ability to contract the lower extremities, including the toes, knee, and hip of the left hind leg at 90 days, showed significant improvement in their steps, were able to touch the ground, they walked weightily on injured hind legs and were able to move freely and climb into the cage at 180 days, with similar capabilities to healthy monkeys (Li et al., 2023).
Linear order collagen scaffold and linear order collagen scaffold combined with collagen-binding neurotrophin-3
This is another great evidence of the trend to structure membranes from living animal tissues that can be adapted for the construction of human-compatible scaffolds, for which they should initially be tested in primates. Han et al. (2019) structured a closed-order collagen scaffold linear order collagen scaffold (LOCS) membrane that was prepared from bovine aponeurosis. LOCS possessed longitudinally arranged collagen fibers, with recombinant collagen similar to collagen-binding neurotrophin-3 (CBD-NT3) consisting of a native collagen (CBD) and an NT3 binding domain. A total of 15 rhesus primates between 3 and 4 years of age were used, to which a lesion was performed at the T9 level, and the collagen membrane was grafted at the site of the lesion, achieving evidence of a large number of neurons within the area of the lesion in the LOCS + CBD-NT3 group and functional mature neurons such as GABA+ and TH+ were also found in the combined group (Han et al., 2019).
Some axons of neurons at the epicenter of the lesion were shown to be demyelinated and formed synaptic connections in the LOCS + CBD-NT3 group. Therefore, it was speculated that the newly generated neurons could form neural networks to reconnect the residual limbs of the injury, leading to partial functional recovery in monkeys treated with LOCS + CBD-NT3. In the LOCS + CBD-NT3 group, hind leg muscle strength was gradually restored and some monkeys were able to transiently lift their buttocks off the ground, which is considered the most significant event in the entire recovery of neuronal function (Han et al., 2019). In addition, a monkey could occasionally stand without supporting sustainable weight in the combinatorial group. These results are significant in the context of the similar biomechanics that can keep the bipedalism of macaques with humans; therefore, the evolution of their recovery can be extrapolated to what it would be in a human with the same conditions. This allows us to broadly conclude that this model of the complete lesion in NHP and the application of these types of membranes will contribute to effectively evaluating potential interventions and accelerating human clinical transformation in the future.
Autologous neural tissue transplantation on scaffold
Along these lines, two pieces of scientific evidence were found: the first of them provided by Ko et al. (2019), who applied a different therapeutic technique for the treatment of SCI in NHP. A peripheral nerve graft and an acidic fibroblast growth factor were used in six adult rhesus macaques that received spinal cord hemisection at the T8 level.
In the monkeys in the experimental group with the graft, the sural nerve on the right side was removed. The nerve graft was cut into three segments, of these, two were implanted between the two stumps in the white-to-gray direction to attempt to reconstruct the descending spinal tract, and the third segment was implanted in the gray-to-white direction for the ascending spinal tract. The statistical results of the motor assessment indicated a significant difference in hip movement, specifically the right hip, between the two groups, at 8 and 24 weeks postoperatively. At 36 weeks, electromyographic evaluation exhibited how waveform was recovered in two of the three monkeys in the repair group and one of the three monkeys in the control group. On the other hand, it was also evident that the inflammation was mainly in the center of the lesion and decreased gradually and distally.
By way of comparison, the inflammation in the control group was obviously more severe and spread to the undamaged tissue. Hematoxylin-eosin staining revealed hypercellularity after injury and the distribution of hypercellularity was consistent with MRI findings at each corresponding position, indicating a decreased inflammatory response after the repair strategy.
The second study found was published by Sun et al. (2021) using four male Rhesus monkeys (Macaca mulatta) weighing 5–7 kg, which underwent a spinal cord injury with a hemisection at the T8 level, which then underwent a transplant of sural nerve segments at the injured site and a long-term infusion of acidic fibroblast growth factor. To obtain the tissue to be grafted, a sural nerve sample was used, which was extracted, collected, and cut into several segments with approximate lengths of 6 mm; these segments were placed in Hank’s balanced saline solution prior to grafting. Subsequently, the peripheral nerve graft was stabilized using a fibrin-based mixture to fill at the injury site. In the analysis of results, it was shown that all the monkeys that underwent the transplant showed substantially fewer neurological deficiencies than the monkeys in the control group, concerning spasticity, assessed with the Ashworth scale. In addition, it was evidenced that they showed some ability to take measures at the same time, but the improvement in their ability to take action seemed to stop and even began to decrease from the 11-week follow-up. It was concluded that the nerve tissue autograft strategy works in experimental studies and therefore promises evolution in human studies (Sun et al., 2021).
Conclusion
We reviewed the progress of therapeutic strategies for the regeneration of traumatic SCI applied in species closer to humans, such as NHP, and some pilot studies developed in institutionalized patients.
In this review, 39 articles were initially addressed, of which 21 were included due to their relevance according to inclusion criteria, which allows us to conclude one of the first findings, the lack of current evidence from studies carried out in NHP species and humans at an experimental level in both cervical and thoracic spinal cord injury. According to the evidence cited above, and the findings of the review, it is noteworthy that, although much preclinical research has been carried out in recent decades, it is mainly based on the development of therapeutic strategies such as cell transplantation, functional biomaterials and their combined therapy, and even pharmacotherapy, to intervene in these pathophysiological processes at exact stages, are really few studies that have managed to advance to the clinical trial phase in humans. About this situation, there are still many problems that precede the use of these strategies in clinical practice and limit the benefits of human patients from materializing.
About the advantage suggested by carrying out experimental research with non-human primates as animal models of spinal cord injury, several points were found, since, from a neuroanatomical, biomechanical, and somatic perspective in general, the macaque has a greater similarity to humans, so that each of the pathophysiological responses could be compared in a more comparable way than with rodents. It is important to recognize the influence of the cortical response on motor recovery processes in humans, since neuroplasticity is not only evident at the spinal cord level, on the contrary, the cortex, which is directly involved, has a significant influence on motor readaptation, which translates into neuroadaptation if it is translated into neurophysiological terms. Thus, having a more functionally organized cortex like the macaque has, similar to the human being, with a greater presence of pyramidal neurons and motor, pre-motor and somatosensory areas, would allow us to understand the effects of different therapeutic strategies when extrapolated to clinical application in human beings.
That said, regarding the prospects for the future development of biological therapeutic strategies, it is expected that these can focus much more on the application in NHP, since they provide unique advantages over rodents or other animal species, to test and understand the safety and efficacy of reparative interventions and thus, promote functional recovery. In addition, this type of research allows us to simultaneously and comprehensively examine the effects of an intervention on regeneration on multiple variables that cannot be fully explored with rodents, such as the control of fine motor skills in the arms, hands, as well as posture and locomotion (bipeds and quadrupeds) and autonomic function (such as the bladder and bowels).
Thus, the need to structure a research path applicable to humans will lead to the development of new, much more effective interventions in SCI models, in which NHP experiments should probably be included as a first filter, as such studies can point to specific potential benefits, and identify mechanisms of recovery of function and increase safety. The level of efficacy and success of a treatment, before considering running human clinical trials, as this would be the way science would consider homologous and reliable results.
Additional file
Additional Table 1: Abstract table of the latest biological therapeutic approaches for spinal cord injury implemented in macaques.
Additional Table 1
Abstract table of the latest biological therapeutic approaches for spinal cord injury implemented in macaques
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
Data availability statement: All relevant data are within the manuscript and its Additional files.
C-Editors: Zhao M, Liu WJ, Qiu Y; T-Editor: Jia Y
References
- Abbas WA, Ibrahim ME, El-Naggar M, Abass WA, Abdullah IH, Awad BI, Allam NK. Recent advances in the regenerative approaches for traumatic spinal cord injury: materials perspective. ACS Biomater Sci Eng. 2020;6:6490–6509. [Abstract] [Google Scholar]
- Alizadeh A, Dyck SM. and Karimi-Abdolrezaee S. Traumatic spinal cord injury: an overview of pathophysiology, models and acute injury mechanisms. Front Neurol. 2019;10:282. [Europe PMC free article] [Abstract] [Google Scholar]
- Blesch A, Tuszynski MH. Spinal cord injury: plasticity, regeneration and the challenge of translational drug development. Trends Neurosci. 2009;32:41–47. [Abstract] [Google Scholar]
- Bradbury EJ, Burnside ER. Moving beyond the glial scar for spinal cord repair. Nat Commun. 2019;10:3879. [Europe PMC free article] [Abstract] [Google Scholar]
- Chen W, Zhang Y, Yang S, Sun J, Qiu H, Hu X, Niu X, Xiao Z, Zhao Y, Zhou Y, Dai J, Chu T. NeuroRegen scaffolds combined with autologous bone marrow mononuclear cells for the repair of acute complete spinal cord injury: a 3-year clinical study. Cell Transplant. 2020;29:963689720950637. [Europe PMC free article] [Abstract] [Google Scholar]
- Cofano F, Boido M, Monticelli M, Zenga F, Ducati A, Vercelli A, Garbossa D. Mesenchymal stem cells for spinal cord injury: current options, limitations, and future of cell therapy. Int J Mol Sci. 2019;20:2698. [Europe PMC free article] [Abstract] [Google Scholar]
- Courtine G, Bunge MB, Fawcett JW, Grossman RG, Kaas JH, Lemon R, Maier I, Martin J, Nudo RJ, Ramon-Cueto A, Rouiller EM, Schnell L, Wannier T, Schwab ME, Edgerton VR. Can experiments in nonhuman primates expedite the translation of treatments for spinal cord injury in humans? Nat Med. 2007;13:561–566. [Europe PMC free article] [Abstract] [Google Scholar]
- Curtis E, Martin JR, Gabel B, Sidhu N, Rzesiewicz TK, Mandeville R, Van Gorp S, Leerink M, Tadokoro T, Marsala S, Jamieson C, Marsala M, Ciacci JD. A first-in-human, phase I study of neural stem cell transplantation for chronic spinal cord injury. Cell Stem Cell. 2018;22:941–950. [Abstract] [Google Scholar]
- Chu D, Qiu J, Grafe M, Fabian R, Kent TA, Rassin D, Nesic O, Werrbach-Perez K, Perez-Polo R. Delayed cell death signaling in traumatized central nervous system: hypoxia. Neurochem Res. 2002;27:97–106. [Abstract] [Google Scholar]
- Danilov CA, Steward O. Conditional genetic deletion of PTEN after a spinal cord injury enhances regenerative growth of CST axons and motor function recovery in mice. Exp Neurol. 2015;266:147–160. [Europe PMC free article] [Abstract] [Google Scholar]
- Dasari VR, Veeravalli KK, Dinh DH. Mesenchymal stem cells in the treatment of spinal cord injuries: a review. World J Stem Cells. 2014;6:120–133. [Europe PMC free article] [Abstract] [Google Scholar]
- De Almeida FM, Marques SA, Dos Santos ACR, Prins CA, Dos Santos Cardoso FS, Dos Santos Heringer L, Mendonça HR, Martinez AMB. Molecular approaches for spinal cord injury treatment. Neural Regen Res. 2023;18:23–30. [Europe PMC free article] [Abstract] [Google Scholar]
- Diop M, Diop M, Epstein D, Gaggero A, Cherif I, Cherif I, Khiari H, Mallekh R, Hsairi M. Quality of life, health and social costs of patients with spinal cord injury: a systematic review. Eur J Public Health. 2021 10.1093/eurpub/ckab165.177. [Google Scholar]
- Eli I, Lerner DP, Ghogawala Z. Acute traumatic spinal cord injury. Neurol Clin. 2021;39:471–488. [Abstract] [Google Scholar]
- Fayazi N, Sheykhhasan M, Soleimani Asl S, Najafi R. Stem cell-derived exosomes: a new strategy of neurodegenerative disease treatment. Mol Neurobiol. 2021;58:3494–3514. [Europe PMC free article] [Abstract] [Google Scholar]
- Ferón F, Perry C, Cochrane J, Licina P, Nowitzke A, Urquhart S, Geraghty T, Mackay-Sim A. Autologous olfactory ensheathing cell transplantation in human spinal cord injury. Brain. 2005;128:2951–2960. [Abstract] [Google Scholar]
- Fouad K, Schnell L, Bunge MB, Schwab ME, Liebscher T, Pearse DD. Combining Schwann cell bridges and olfactory- ensheathing glia grafts with chondroitinase promotes locomotor recovery after complete transection of the spinal cord. J Neurosci. 2005;25:1169–1178. [Europe PMC free article] [Abstract] [Google Scholar]
- Go V, Bowley BGE, Pessina MA, Zhang ZG, Chopp M, Finklestein SP, Rosene DL, Medalla M, Buller B, Moore TL. Extracellular vesicles from mesenchymal stem cells reduce microglial-mediated neuroinflammation after cortical injury in aged Rhesus monkeys. Geroscience. 2020;42:1–17. [Europe PMC free article] [Abstract] [Google Scholar]
- Golestani A, Shobeiri P, Sadeghi-Naini M, Jazayeri SB, Maroufi SF, Ghodsi Z, Dabbagh Ohadi MA, Mohammadi E, Rahimi-Movaghar V, Ghodsi SM. Epidemiology of traumatic spinal cord injury in developing countries from 2009 to 2020: a systematic review and meta-analysis. Neuroepidemiology. 2022;56:219–239. [Abstract] [Google Scholar]
- Gong CZ, Zheng X, Guo FL, Wang YN, Zhang S, Chen J, Sun XJ, Shah SZA, Zheng YF, Li X, Yin Y, Li Q, Huang XL, Guo T, Han X, Zhang SC, Wang W, Chen H. Human spinal GABA neurons alleviate spasticity and improve locomotion in rats with spinal cord injury. Cell Rep. 2021;34:108889. [Abstract] [Google Scholar]
- Gong W, Zhang T, Che M, Wang Y, He C, Liu L, Zhang S. Recent advances in nanomaterials for the treatment of spinal cord injury. Mater Today Bio. 2023;18:100524. [Europe PMC free article] [Abstract] [Google Scholar]
- Granger N, Blamires H, Franklin RJM, Jeffery ND. Autologous olfactory mucosal cell transplants in clinical spinal cord injury: a randomized double-blinded trial in a canine translational model. Brain. 2012;135:3227–3237. [Europe PMC free article] [Abstract] [Google Scholar]
- Haggerty AE, Oudega M. Biomaterials for spinal cord repair. Neurosci Bull. 2013;29:445–459. [Europe PMC free article] [Abstract] [Google Scholar]
- Han S, et al. Pre-clinical evaluation of CBD-NT3 modified collagen scaffolds in completely spinal cord transected non-human primates. J Neurotrauma. 2019;36:2316–2324. [Abstract] [Google Scholar]
- Jaberi R, Jabbari R, Hajinasrollah M, Mirsadeghi S, Rahmani S, Zarei-Khairabadi M, Rezaei O, Nabavi SM, Kiani S. Transplanted autologous neural stem cells show promise in restoring motor function in monkey spinal cord injury. bioRxiv. 2023 10.1101/2023.05.06.539673. [Google Scholar]
- Jacobson PB, et al. Elezanumab, a human anti-RGMa monoclonal antibody, promotes neuroprotection, neuroplasticity, and neurorecovery following a thoracic hemicompression spinal cord injury in non-human primates. Neurobiol Dis. 2021;155:105385. [Abstract] [Google Scholar]
- James SL, et al. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:56–87. [Europe PMC free article] [Abstract] [Google Scholar]
- Jones LL, Oudega M, Bunge MB, Tuszynski MH. Neurotrophic factors, cellular bridges and gene therapy for spinal cord injury. J Physiol. 2001;533:83–89. [Abstract] [Google Scholar]
- Joo KM, Lee SH, Lee S, Nam H. Review article corresponding author co-corresponding author advances in neural stem cell therapy for spinal cord injury: safety, efficacy, and future perspectives. Neurospine. 2022;19:946–956. [Europe PMC free article] [Abstract] [Google Scholar]
- Jung SY, Seo TB, Kim DY. Treadmill exercise facilitates recovery of locomotor function through axonal regeneration following spinal cord injury in rats. J Exerc Rehabil. 2016;12:284–292. [Europe PMC free article] [Abstract] [Google Scholar]
- Jung HY, Kwon HJ, Kim W, Hahn KR, Moon SM, Yoon YS, Kim DW, Hwang IK. Phosphoglycerate mutase 1 prevents neuronal death from ischemic damage by reducing neuroinflammation in the rabbit spinal cord. Int J Mol Sci. 2020;21:7425. [Europe PMC free article] [Abstract] [Google Scholar]
- Kadoya K, et al. Combined intrinsic and extrinsic neuronal mechanisms facilitate bridging axonal regeneration one year after spinal cord injury. Neuron. 2009;64:165–172. [Europe PMC free article] [Abstract] [Google Scholar]
- Kamada T, Koda M, Dezawa M, Yoshinaga K, Hashimoto M, Koshizuka S, Nishio Y, Moriya H, Yamazaki M. Transplantation of bone marrow stromal cell-derived schwann cells promotes axonal regeneration and functional recovery after complete transection of adult rat spinal cord. J Neuropathol Exp Neurol. 2005;64:37–45. [Abstract] [Google Scholar]
- Kanno H, Pearse DD, Ozawa H, Itoi E, Bunge MB. Schwann cell transplantation for spinal cord injury repair: its significant therapeutic potential and prospectus. Rev Neurosci. 2015;26:121–128. [Abstract] [Google Scholar]
- Kawai M, Nagoshi N, Okano H, Nakamura M. A review of regenerative therapy for spinal cord injury using human iPS cells. N Am Spine Soc J. 2023;13:100184. [Europe PMC free article] [Abstract] [Google Scholar]
- Keirstead HS, Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K, Steward O. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci. 2005;25:4694–4705. [Europe PMC free article] [Abstract] [Google Scholar]
- Ko CC, Tu TH, Chen YT, Wu JC, Huang WC, Cheng H. Monkey recovery from spinal cord hemisection: nerve repair strategies for rhesus macaques. World Neurosurg. 2019;129:e343–351. [Abstract] [Google Scholar]
- Kobayashi Y, Okada Y, Itakura G, Iwai H, Nishimura S, Yasuda A, Nori S, Hikishima K, Konomi T, Fujiyoshi K, Tsuji O, Toyama Y, Yamanaka S, Nakamura M, Okano H. Pre-evaluated safe human iPSC-derived neural stem cells promote functional recovery after spinal cord injury in common marmoset without tumorigenicity. PLoS One. 2012;7:e52787. [Europe PMC free article] [Abstract] [Google Scholar]
- Kojima A, Tator CH. Intrathecal administration of epidermal growth factor and fibroblast growth factor 2 promotes ependymal proliferation and functional recovery after spinal cord injury in adult rats. J Neurotrauma. 2004;19:223–238. [Abstract] [Google Scholar]
- Kucher K, Johns D, Maier D, Abel R, Badke A, Baron H, Thietje R, Casha S, Meindl R, Gomez-Mancilla B, Pfister C, Rupp R, Weidner N, Mir A, Schwab ME, Curt A. First-in-man intrathecal application of neurite growth-promoting anti-nogo- a antibodies in acute spinal cord injury. Neurorehabil Neural Repair. 2018;32:578–589. [Abstract] [Google Scholar]
- Lemon RN, Kirkwood PA, Maier MA, Nakajima K, Nathan P. Direct and indirect pathways for corticospinal control of upper limb motoneurons in the primate. Prog Brain Res. 2004;43:263–279. [Abstract] [Google Scholar]
- Li T, Li P, Yuan H, Chen Y, Zhu X, Xiong L, Zhao S, Li J, Chen T, Ai Z, Cai H, Feng C, Li Y, Wang J, Niu Y, Liu J, Ji W, Zhang L, Wang T. Hydrogel encapsulated stem cells facilitate successful repair after spinal cord injury in rats and monkeys. Res Sq. 2023 10.21203/rs.3.rs-2740238/v1. [Google Scholar]
- Liu S, Schackel T, Weidner N, Puttagunta R. Biomaterial-supported cell transplantation treatments for spinal cord injury: challenges and perspectives. Front Cell Neurosci. 2018;11:430. [Europe PMC free article] [Abstract] [Google Scholar]
- Liu S, Xie YY, Wang B. Role and prospects of regenerative biomaterials in the repair of spinal cord injury. Neural Regen Res. 2019;14:1352–1363. [Europe PMC free article] [Abstract] [Google Scholar]
- Liu T, Zhu W, Zhang X, He C, Liu X, Xin Q, Chen K, Wang H. Recent advances in cell and functional biomaterial treatment for spinal cord injury. Biomed Res Int. 2022;2022:5079153. [Europe PMC free article] [Abstract] [Google Scholar] Retracted
- Llorens-Bobadilla E, Chell JM, Le Merre P, Wu Y, Zamboni M, Bergenstråhle J, Stenudd M, Sopova E, Lundeberg J, Shupliakov O, Carlén M, Frisén J. A latent lineage potential in resident neural stem cells enables spinal cord repair. Science. 2020;370:eabb8795. [Abstract] [Google Scholar]
- Lu P, Woodruff G, Wang Y, Graham L, Hunt M, Wu D, Boehle E, Ahmad R, Poplawski G, Brock J, Goldstein LSB, Tuszynski MH. Long-distance axonal growth from human induced pluripotent stem cells after spinal cord injury. Neuron. 2014;83:789–796. [Europe PMC free article] [Abstract] [Google Scholar]
- Marques SA, Almeida FM, Fernandes AM, Dos Santos Souza C, Cadilhe DV, Rehen SK, Martinez AMB. Predifferentiated embryonic stem cells promote functional recovery after spinal cord compressive injury. Brain Res. 20101349:115–128. [Abstract] [Google Scholar]
- Meshkini A, Sarpoolaki MK, Vafaei A, Mirzaei F, Badripour A, Rafiei E, Khalilzadeh M, Fattahi MR, Iranmehr A. The efficacy of intrathecal methyl-prednisolone for acute spinal cord injury: a pilot study. Heliyon. 2023;9:e15548. [Abstract] [Google Scholar]
- Mietto BS, Mostacada K, Martinez AM. Neurotrauma and Inflammation: CNS and PNS responses. Mediators Inflamm. 2015;2015:251204. [Europe PMC free article] [Abstract] [Google Scholar]
- Mikos AG, Lyman MD, Freed LE, Langer R. Wetting of poly(l-lactic acid) and poly(dl-lactic-co-glycolic acid) foams for tissue culture. Biomaterials. 1994;15:55–58. [Abstract] [Google Scholar]
- Nathan PW. Effects on movement of surgical incisions into the human spinal cord. Brain. 1994;117:337–346. [Abstract] [Google Scholar]
- Nemati S, Jabbari R, Hajinasrollah M, Mehrjerdi NZ, Azizi H, Hemmesi K, Moghiminasr R, Azhdari Z, Talebi A, Mohitmafi S, Dizaj AVT, Sharifi G, Baharvand H, Rezaee O, Kiani S. Transplantation of adult monkey neural stem cells into a contusion spinal cord injury model in Rhesus Macaque monkeys. Cell J. 2014;16:117–130. [Europe PMC free article] [Abstract] [Google Scholar]
- Ohta M, Suzuki Y, Noda T, Ejiri Y, Dezawa M, Kataoka K, Chou H, Ishikawa N, Matsumoto N, Iwashita Y, Mizuta E, Kuno S, Ide C. Bone marrow stromal cells infused into the cerebrospinal fluid promote functional recovery of the injured rat spinal cord with reduced cavity formation. Exp Neurol. 2004;187:266–278. [Abstract] [Google Scholar]
- Okamura RM, Lebkowski J, Au M, Priest CA, Denham J, Majumdar AS. Immunological properties of human embryonic stem cell-derived oligodendrocyte progenitor cells. J Neuroimmunol. 2007;192:134–144. [Abstract] [Google Scholar]
- Rao JS, Zhao C, Zhang A, Duan H, Hao P, Wei RH, Shang J, Zhao W, Liu Z, Yu J, Fan KS, Tian Z, He Q, Song W, Yang Z, Sun YE, Li X. NT3-chitosan enables de novo regeneration and functional recovery in monkeys after spinal cord injury. Proc Natl Acad Sci U S A. 2018;115:E5595–E5604. [Europe PMC free article] [Abstract] [Google Scholar]
- Rao JS, Zhao C, Wei RH, Feng T, Bao SS, Zhao W, Tian Z, Liu Z, Yang ZY, Li XG. Neural regeneration therapy after spinal cord injury induces unique brain functional reorganizations in rhesus monkeys. Ann Med. 2022;54:1867–1883. [Europe PMC free article] [Abstract] [Google Scholar]
- Rosenzweig ES, Brock JH, Lu P, Kumamaru H, Salegio EA, Kadoya K, Weber JL, Liang JJ, Moseanko R, Hawbecker S, Huie JR, Havton LA, Nout-Lomas YS, Ferguson AR, Beattie MS, Bresnahan JC, Tuszynski MH. Restorative effects of human neural stem cell grafts to the primate spinal cord. Nat Med. 2018;24:484. [Europe PMC free article] [Abstract] [Google Scholar]
- Rouiller EM, Moret V, Tanné J, Boussaoud D. Evidence for direct connections between the hand region of the supplementary motor area and cervical motoneurons in the macaque monkey. Eur J Neurosci. 1996;8:1055–1059. [Abstract] [Google Scholar]
- Saremi J, Mahmoodi N, Rasouli M, Ranjbar FE, Mazaheri EL, Akbari M, Hasanzadeh E, Azami M. Advanced approaches to regenerate spinal cord injury: the development of cell and tissue engineering therapy and combinational treatments. Biomed Pharmacother. 2022;146:112529. [Abstract] [Google Scholar]
- Sha
 mardanova GF, Mukhamedshina IO, Rizvanov AA, Salafutdinov II, Chelyshev IA. Effects of transplantation of human umbilical cord blood mononuclear cells, expressing VEGF and FGF2 genes, into the area of spinal cord traumatic lesion. Morfologiia. 2012;142:31–36. [Abstract] [Google Scholar]
mardanova GF, Mukhamedshina IO, Rizvanov AA, Salafutdinov II, Chelyshev IA. Effects of transplantation of human umbilical cord blood mononuclear cells, expressing VEGF and FGF2 genes, into the area of spinal cord traumatic lesion. Morfologiia. 2012;142:31–36. [Abstract] [Google Scholar] - Slotkin JR, Pritchard CD, Luque B, Ye J, Layer RT, Lawrence MS, O’Shea TM, Roy RR, Zhong H, Vollenweider I, Edgerton VR, Courtine G, Woodard EJ, Langer R. Biodegradable scaffolds promote tissue remodeling and functional improvement in non-human primates with acute spinal cord injury. Biomaterials. 2017;123:63–76. [Abstract] [Google Scholar]
- Soares S, von Boxberg Y, Nothias F. Repair strategies for traumatic spinal cord injury, with special emphasis on novel biomaterial-based approaches. Rev Neurol. 2020;176:252–260. [Abstract] [Google Scholar]
- Sun WM, Ma CL, Xu J, He JP. Reduction in post-spinal cord injury spasticity by combination of peripheral nerve grafting and acidic fibroblast growth factor infusion in monkeys. J Int Med Res. 2021;49:3000605211022294. [Europe PMC free article] [Abstract] [Google Scholar]
- Szymoniuk M, Mazurek M, Dryla A, Kamieniak P. The application of 3D-bioprinted scaffolds for neuronal regeneration after traumatic spinal cord injury – A systematic review of preclinical in vivo studies. Exp Neurol. 2023;363:114366. [Abstract] [Google Scholar]
- Tsuji O, Sugai K, Yamaguchi R, Tashiro S, Nagoshi N, Kohyama J, Iida T, Ohkubo T, Itakura G, Isoda M, Shinozaki M, Fujiyoshi K, Kanemura Y, Yamanaka S, Nakamura M, Okano H. Concise review: laying the groundwork for a first‐in‐human study of an induced pluripotent stem cell‐based intervention for spinal cord injury. Stem Cells. 2019;37:6–13. [Europe PMC free article] [Abstract] [Google Scholar]
- Wang TY, Park C, Zhang H, Rahimpour S, Murphy KR, Goodwin CR, Karikari IO, Than KD, Shaffrey CI, Foster N, Abd-El-Barr MM. Management of acute traumatic spinal cord injury: a review of the literature. Front Surg. 2021;8:698736. [Europe PMC free article] [Abstract] [Google Scholar]
- Whishaw IQ, Gorny B, Sarna J. Paw and limb use in skilled and spontaneous reaching after pyramidal tract, red nucleus and combined lesions in the rat: behavioral and anatomical dissociations. Behav Brain Res. 1998;93:167–183. [Abstract] [Google Scholar]
- Xu B, et al. Engineered human spinal cord-like tissues with dorsal and ventral neuronal progenitors for spinal cord injury repair in rats and monkeys. Bioact Mater. 2023;27:125–137. [Europe PMC free article] [Abstract] [Google Scholar]
- Xu L, Mahairaki V, Koliatsos VE. Host induction by transplanted neural stem cells in the spinal cord: further evidence for an adult spinal cord neurogenic niche. Brain. 2012;7:785–797. [Europe PMC free article] [Abstract] [Google Scholar]
- Xu M, Feng T, Liu B, Qiu F, Xu Y, Zhao Y, Zhen GY. Engineered exosomes: desirable target-tracking characteristics for cerebrovascular and neurodegenerative disease therapies. Theranostics. 2021;11:8926–8944. [Europe PMC free article] [Abstract] [Google Scholar]
- Yamane J, Nakamura M, Iwanami A, Sakaguchi M, Katoh H, Yamada M, Momoshima S, Miyao S, Ishii K, Tamaoki N, Nomura T, Okano HJ, Kanemura Y, Toyama Y, Okano H. Transplantation of galectin-1-expressing human neural stem cells into the injured spinal cord of adult common marmosets. J Neurosci Res. 2010;88:1394–1405. [Abstract] [Google Scholar]
- Yao R, Murtaza M, Tello Velasquez J, Todorovic M, Rayfield A, Ekberg J, Barton M, St John J. Olfactory ensheathing cells for spinal cord injury: sniffing out the issues. Cell Transplant. 2018;27:879–889. [Europe PMC free article] [Abstract] [Google Scholar]
- Yu A, Mao L, Zhao F, Sun B. Olfactory ensheathing cells transplantation attenuates chronic cerebral hypoperfusion induced cognitive dysfunction and brain damages by activating Nrf2/HO-1 signaling pathway. Am J Transl Res. 2018;10:3111. [Europe PMC free article] [Abstract] [Google Scholar]
- Zeng X, Wei QS, Ye JC, Rao JH, Zheng MG, Ma YH, Peng LZ, Ding Y, Lai BQ, Li G, Cheng SX, Ling AE, Han I, Zeng YS. A biocompatible gelatin sponge scaffold confers robust tissue remodeling after spinal cord injury in a non-human primate model. Biomaterials. 2023;299:122161. [Abstract] [Google Scholar]
- Zhang J, Chen H, Duan Z, Chen K, Liu Z, Zhang L, Yao D, Li B. The effects of co-transplantation of olfactory ensheathing cells and Schwann cells on local inflammation environment in the contused spinal cord of rats. Mol Neurobiol. 2017;54:943–953. [Abstract] [Google Scholar]
- Zhang Q, Shi B, Ding J, Yan L, Thawani JP, Fu C, Chen X. Polymer scaffolds facilitate spinal cord injury repair. Acta Biomater. 2019;88:57–77. [Abstract] [Google Scholar]
- Zhao Y, Tang F, Xiao Z, Han G, Wang N, Yin N, Chen B, Jiang X, Yun C, Han W, Zhao C, Cheng S, Zhang S, Dai J. Clinical study of neuroregen scaffold combined with human mesenchymal stem cells for the repair of chronic complete spinal cord injury. Cell Transplant. 2017;26:891–900. [Europe PMC free article] [Abstract] [Google Scholar]
- Zheng X, Zhu B, Xu J, Liu D, Huang Y, Chen D, Liu Z, Guo F, Dong Y, Zhu W, Pan D, Zhang SC, Chen H, Wang W. Human spinal GABA neurons survive and mature in the injured nonhuman primate spinal cord. Stem Cell Reports. 2023;18:439–448. [Europe PMC free article] [Abstract] [Google Scholar]
- Zipser CM, Cragg JJ, Guest JD, Fehlings MG, Jutzeler CR, Anderson AJ, Curt A. Cell-based and stem-cell-based treatments for spinal cord injury: evidence from clinical trials. Lancet Neurol. 2022;21:659–670. [Abstract] [Google Scholar]
Articles from Neural Regeneration Research are provided here courtesy of Wolters Kluwer -- Medknow Publications
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.
Cochrane Database Syst Rev, 2(2022), 01 Feb 2022
Cited by: 12 articles | PMID: 36321557 | PMCID: PMC8805585
Review Free full text in Europe PMC
Nanotechnology for the Treatment of Spinal Cord Injury.
Tissue Eng Part B Rev, 27(4):353-365, 22 Jan 2021
Cited by: 13 articles | PMID: 33135599
Review
Animal Models for Treating Spinal Cord Injury Using Biomaterials-Based Tissue Engineering Strategies.
Tissue Eng Part B Rev, 28(1):79-100, 05 Mar 2021
Cited by: 7 articles | PMID: 33267667
Review
Regenerative Therapies for Spinal Cord Injury.
Tissue Eng Part B Rev, 25(6):471-491, 23 Oct 2019
Cited by: 56 articles | PMID: 31452463 | PMCID: PMC6919264
Review Free full text in Europe PMC

 4,*
4,*

