Abstract
Free full text

Association of blood group O with a recurrent risk for acute lower gastrointestinal bleeding from a multicenter cohort study
Abstract
The relationship between blood group and rebleeding in acute lower gastrointestinal bleeding (ALGIB) remains unclear. This study aimed to investigate the association between blood group O and clinical outcomes in patients with ALGIB. The study included 2336 patients with ALGIB whose bleeding source was identified during initial endoscopy (from the CODE BLUE-J Study). The assessed outcomes encompassed rebleeding and other clinical parameters. The rebleeding rates within 30 days in patients with blood group O and those without blood group O were 17.9% and 14.9%, respectively. Similarly, the rates within 1 year were 21.9% for patients with blood group O and 18.2% for those without blood group O. In a multivariate analysis using age, sex, vital signs at presentation, blood test findings, comorbidities, antithrombotic medication, active bleeding, and type of endoscopic treatment as covariates, patients with blood group O exhibited significantly higher risks for rebleeding within 30 days (odds ratio [OR] 1.31; 95% confidence interval [CI] 1.04–1.65; P =
= 0.024) and 1 year (OR 1.29; 95% CI 1.04–1.61; P
0.024) and 1 year (OR 1.29; 95% CI 1.04–1.61; P =
= 0.020) compared to those without blood group O. However, the thrombosis and mortality rates did not differ significantly between blood group O and non-O patients. In patients with ALGIB, blood group O has been identified as an independent risk factor for both short- and long-term rebleeding.
0.020) compared to those without blood group O. However, the thrombosis and mortality rates did not differ significantly between blood group O and non-O patients. In patients with ALGIB, blood group O has been identified as an independent risk factor for both short- and long-term rebleeding.
Introduction
Rebleeding is a serious problem associated with acute lower gastrointestinal bleeding (ALGIB). Previous reports have indicated that the incidence of rebleeding in patients with ALGIB within 30 days ranges from 5.2 to 26%1–4. Managing the risk of rebleeding is crucial in clinical practice, especially considering the current lack of preventive therapies for ALGIB, similar to the use of proton pump inhibitors for upper gastrointestinal bleeding (UGIB)5.
Blood group O has been associated with a high risk for various bleeding disorders6, including traumatic hemorrhage, UGIB, cerebral hemorrhage, postpartum hemorrhage, and epistaxis7–11. Additionally, individuals with blood group O are believed to have a low risk of thrombosis12. Recent findings also suggest that patients with blood group O may have an increased risk of bleeding following endoscopic procedures13. Although previous studies identified age, antithrombotic drug use, and blood transfusion as risk factors for ALGIB rebleeding4,14, the association between blood group and ALGIB rebleeding remains unknown.
To address these knowledge gaps, this multicenter study aimed to determine whether blood group O serves as an independent risk factor influencing the clinical outcomes of patients hospitalized for ALGIB. Furthermore, we explored the association between blood group and colonic diverticular bleeding (CDB), the most common cause of ALGIB15.
Results
Patient characteristics
A total of 10,342 patients admitted with ALGIB were included in the database. After excluding 8,006 patients based on the criteria outlined in Fig. 1, 2336 patients were eligible for analysis. These patients were categorized into two groups: 844 patients with blood group O and 1,492 patients with non-O blood groups (A, B, and AB). Among the ALGIB patients, 1,576 (67.5%) were male, and the mean age was 70.8 years ±
± 13.3 (mean
13.3 (mean ±
± standard deviation). The rebleeding rate in the ALGIB cohort was 22.5% (526/2,336 patients).
standard deviation). The rebleeding rate in the ALGIB cohort was 22.5% (526/2,336 patients).
Table Table11 displays the characteristics and clinical profiles of the enrolled patients stratified according to their ABO blood group status. When comparing the four groups (O, A, B, and AB), a significantly low proportion of patients with group O exhibited a modified Charlson Comorbidity Index (CCI) ≥
≥ 2, platelet count
2, platelet count <
< 150,000/μL, and blood urea nitrogen (BUN)
150,000/μL, and blood urea nitrogen (BUN) >
> 25 mg/dL (P
25 mg/dL (P =
= 0.020, P
0.020, P =
= 0.005, P
0.005, P =
= 0.002, respectively). In comparison between the O and non-O (A, B, and AB) groups, a significantly low proportion of patients with group O were aged
0.002, respectively). In comparison between the O and non-O (A, B, and AB) groups, a significantly low proportion of patients with group O were aged ≥
≥ 65 years, had liver cirrhosis, heart failure, chronic kidney disease, and were using antithrombotic drugs. No significant differences were observed in other variables, including sex, body mass index (BMI), alcohol consumption, smoking, symptoms, and vital signs at admission, other laboratory data, or endoscopic factors.
65 years, had liver cirrhosis, heart failure, chronic kidney disease, and were using antithrombotic drugs. No significant differences were observed in other variables, including sex, body mass index (BMI), alcohol consumption, smoking, symptoms, and vital signs at admission, other laboratory data, or endoscopic factors.
Table 1
Baseline characteristics of acute lower gastrointestinal bleeding patients (n =
= 2,336).
2,336).
| Characteristics | Four-group comparison | P value | Two-group comparison | |||||
|---|---|---|---|---|---|---|---|---|
O (n = = 844) 844) | A (n = = 876) 876) | B(n = = 419) 419) | AB (n = = 197) 197) | O(n = = 844) 844) | non-O (n = = 1,492) 1,492) | P value | ||
Age ≥ ≥ 65 years old 65 years old | 592 (70.1) | 653 (74.5) | 307 (73.2) | 143 (72.6) | 0.229 | 592 (70.1) | 1103 (73.9) | 0.049 |
| Sex, male | 564 (66.8) | 598 (68.3) | 278 (66.4) | 136 (69.0) | 0.832 | 564 (66.8) | 1012 (67.8) | 0.619 |
BMI ≥ ≥ 25 25 | 207 (26.3) | 217 (26.3) | 107 (26.9) | 46 (25.0) | 0.972 | 579 (73.7) | 1038 (73.7) | 0.977 |
| Current drinker | 364 (43.1) | 374 (42.7) | 167 (39.9) | 81 (41.1) | 0.265 | 364 (43.1) | 622 (41.7) | 0.324 |
| Current smoker | 144 (17.1) | 138 (15.8) | 61 (14.6) | 29 (14.7) | 0.511 | 144 (17.1) | 228 (15.3) | 0.200 |
Performance status ≥ ≥ 2 2 | 96 (11.5) | 116 (13.4) | 58 (14.0) | 24 (12.4) | 0.548 | 96 (11.5) | 198 (13.4) | 0.182 |
| Comorbidities | ||||||||
CCI ≥ ≥ 2 2 | 298 (35.3) | 362 (41.3) | 181 (43.2) | 79 (40.1) | 0.020 | 298 (35.3) | 622 (41.7) | 0.002 |
| History of colorectal surgery | 43 (5.1) | 59 (6.7) | 27 (6.4) | 12 (6.1) | 0.533 | 43(5.1) | 98 (5.6) | 0.151 |
| History of colonic diverticular bleeding | 211 (25) | 212 (24.2) | 93 (22.2) | 57 (29.1) | 0.312 | 211 (25.0) | 362 (24.3) | 0.704 |
| Hypertension | 480 (56.9) | 495(56.5) | 246 (58.7) | 101 (51.3) | 0.381 | 480 (56.9) | 842 (56.4) | 0.838 |
| Diabetes mellitus | 156 (18.5) | 181 (20.7) | 90 (21.5) | 42 (21.3) | 0.527 | 156 (18.5) | 313 (21.0) | 0.148 |
| Dyslipidemia | 208 (24.6) | 219 (25.0) | 109 (26.0) | 53 (26.9) | 0.890 | 208 (24.6) | 381 (25.5) | 0.627 |
| Cirrhosis | 6 (0.7) | 17 (1.9) | 11 (2.6) | 4 (2.0) | 0.051 | 6 (0.7) | 32 (2.1) | 0.009 |
| Heart failure | 65 (7.7) | 93 (10.6) | 47 (11.2) | 17 (8.6) | 0.108 | 65 (7.7) | 157 (10.5) | 0.025 |
| Chronic kidney disease | 107 (12.7) | 135 (15.4) | 72 (17.2) | 30 (15.2) | 0.158 | 107 (12.7) | 237 (15.9) | 0.036 |
| Symptoms | ||||||||
| Loss of consciousness | 63 (7.5) | 63 (7.2) | 30 (7.2) | 14 (7.1) | 0.995 | 63 (7.5) | 107 (7.2) | 0.794 |
| Abdominal pain | 50 (5.9) | 54 (6.2) | 31 (7.4) | 18 (9.1) | 0.340 | 50 (5.9) | 103 (6.9) | 0.355 |
Fever > > 38 38 | 23 (2.7) | 41 (4.7) | 14 (3.3) | 9 (4.6) | 0.159 | 23 (2.7) | 64 (4.3) | 0.055 |
| Medications | ||||||||
| NSAIDs | 73 (8.7) | 86 (9.8) | 34 (8.1) | 16 (8.1) | 0.699 | 73 (8.7) | 136 (9.1) | 0.705 |
| COX-2 selective inhibitors | 17 (2.0) | 16 (1.8) | 15 (3.6) | 5 (2.5) | 0.228 | 17 (2.0) | 36 (2.4) | 0.534 |
| Corticosteroids | ||||||||
| Antithrombotic drugs | 335 (39.7) | 385 (44.0) | 190 (45.3) | 86 (43.7) | 0.175 | 335 (39.7) | 661 (44.3) | 0.030 |
| Antiplatelet drugs | 240 (28.4) | 284 (32.4) | 136 (32.5) | 64 (32.5) | 0.257 | 240 (28.4) | 484 (32.4) | 0.044 |
| Anticoagulation drugs | 136 (16.1) | 154 (17.6) | 79 (18.9) | 31 (15.7) | 0.596 | 136 (16.1) | 264 (17.7) | 0.330 |
| Hemodynamics | ||||||||
Systolic blood pressure < < 100 mmHg 100 mmHg | 116 (13.7) | 113 (12.9) | 60 (14.3) | 24 (12.2) | 0.897 | 116 (13.7) | 197 (13.2) | 0.750 |
Heart rate > > 100 bpm 100 bpm | 177 (21.0) | 163 (18.6) | 87 (20.8) | 32 (16.2) | 0.420 | 177 (21.0) | 282 (18.9) | 0.244 |
Shock Index > > 1 1 | 51 (6.0) | 60 (6.8) | 28 (6.7) | 9 (4.6) | 0.671 | 51 (6.0) | 97 (6.5) | 0.643 |
| Laboratory data | ||||||||
White blood cells > > 10,000/μL 10,000/μL | 110 (13.1) | 131 (15.0) | 67 (16.0) | 32 (16.2) | 0.424 | 110 (13.1) | 230 (15.4) | 0.119 |
Hemoglobin < < 12 g/dL 12 g/dL | 473 (56.0) | 525 (59.9) | 241 (57.5) | 111 (56.3) | 0.423 | 473 (56.0) | 877 (58.8) | 0.209 |
Platelet count < < 15 15 × × 104/μL 104/μL | 118 (14.0) | 178 (20.3) | 79 (18.9) | 32 (16.2) | 0.005 | 118 (14.0) | 289 (19.4) | 0.001 |
Blood urea nitrogen > > 25 mg/dL 25 mg/dL | 164 (19.8) | 227 (26.2) | 118 (28.2) | 47 (24.2) | 0.002 | 164 (19.8) | 392 (26.5) | 0.0003 |
PT-INR > > 1.5 1.5 | 72 (9.9) | 96 (12.7) | 40 (10.7) | 17 (10.1) | 0.370 | 72 (9.9) | 153 (11.8) | 0.205 |
| Endoscopic treatment | ||||||||
| Clipping | 455 (53.9) | 470 (53.7) | 236 (56.3) | 100 (50.8) | 0.618 | 455 (53.9) | 806 (54.0) | 0.959 |
| Ligation | 238 (28.2) | 225 (25.7) | 99 (23.6) | 54 (27.4) | 0.334 | 238 (28.2) | 378 (25.3) | 0.143 |
Data are presented as n (%). A two-tailed P-value <
< 0.05 was considered to indicate statistical significance. Bold values indicate P
0.05 was considered to indicate statistical significance. Bold values indicate P <
< 0.05. Analyzed using Fisher’s exact test.
0.05. Analyzed using Fisher’s exact test.
Abbreviations: BMI, body mass index; CCI, Charlson comorbidity index; NSAIDs, Non-Steroidal Anti-Inflammatory Drugs; PT-INR, international normalized ratio of prothrombin time.
Incidence of rebleeding according to ABO blood group
When comparing the four blood groups (O, A, B, and AB), the incidence rates of rebleeding during the observation period were 24.2%, 22.4%, 21.5%, and 18.3%, respectively (P =
= 0.305, χ2 test). The incidence rates of rebleeding within 30 days were 17.9% [151/844], 15.8% [138/876], 13.6% [57/419], and 14.2% [28/197], respectively (P
0.305, χ2 test). The incidence rates of rebleeding within 30 days were 17.9% [151/844], 15.8% [138/876], 13.6% [57/419], and 14.2% [28/197], respectively (P =
= 0.209, χ2 test). Similarly, the incidence rates of rebleeding within 1 year were 21.9% [185/844], 19.3% [169/876], 17.0% [71/419], and 15.7% [31/197], respectively (P
0.209, χ2 test). Similarly, the incidence rates of rebleeding within 1 year were 21.9% [185/844], 19.3% [169/876], 17.0% [71/419], and 15.7% [31/197], respectively (P =
= 0.083, χ2 test). Although no statistically significant difference in the incidence rate of rebleeding was observed among the four groups, a tendency towards a high rebleeding rate was observed in individuals with blood group O.
0.083, χ2 test). Although no statistically significant difference in the incidence rate of rebleeding was observed among the four groups, a tendency towards a high rebleeding rate was observed in individuals with blood group O.
The rebleeding within 30 days and 1 year after the initial colonoscopy for ALGIB patients
Table Table22 presents the rebleeding rates of patients with ALGIB within 30 days and 1 year after initial colonoscopy. In the univariate analysis, the risk of rebleeding within 30 days was higher in blood group O than in the non-O group (odds ratio [OR] 1.24 [95% confidence interval [CI] 0.99–1.55], P =
= 0.063), although not statistically significant. Blood group O was associated with an increased risk of rebleeding within 1 year (OR 1.26 [95% CI; 1.03–1.56], P
0.063), although not statistically significant. Blood group O was associated with an increased risk of rebleeding within 1 year (OR 1.26 [95% CI; 1.03–1.56], P =
= 0.028). In the multivariate analysis using a logistic regression model, after adjusting for other confounding factors, blood group O was confirmed as an independent risk factor for rebleeding within 30 days (adjusted OR [aOR] 1.31 [95% CI 1.04–1.65], P
0.028). In the multivariate analysis using a logistic regression model, after adjusting for other confounding factors, blood group O was confirmed as an independent risk factor for rebleeding within 30 days (adjusted OR [aOR] 1.31 [95% CI 1.04–1.65], P =
= 0.024) and within 1 year (aOR 1.29 [95% CI 1.04–1.61], P
0.024) and within 1 year (aOR 1.29 [95% CI 1.04–1.61], P =
= 0.020). No significant associations were observed between the ABO blood group and rates of transfusion, active bleeding on colonoscopy, extravasation on computed tomography (CT), hospitalization for
0.020). No significant associations were observed between the ABO blood group and rates of transfusion, active bleeding on colonoscopy, extravasation on computed tomography (CT), hospitalization for >
> 8 days, rate of interventional radiology (IVR), rate of surgery, incidence of thrombosis, or mortality within 30 days of colonoscopy. Kaplan–Meier analysis revealed a significantly higher cumulative probability of rebleeding within 30 days (P
8 days, rate of interventional radiology (IVR), rate of surgery, incidence of thrombosis, or mortality within 30 days of colonoscopy. Kaplan–Meier analysis revealed a significantly higher cumulative probability of rebleeding within 30 days (P =
= 0.038, Generalized Wilcoxon test) and 1 year (P
0.038, Generalized Wilcoxon test) and 1 year (P =
= 0.023, Generalized Wilcoxon test) in group O than in the non-O group among patients with ALGIB (Fig. 2). The median follow-up period for rebleeding was 413 days (interquartile range [IQR], 40–1109).
0.023, Generalized Wilcoxon test) in group O than in the non-O group among patients with ALGIB (Fig. 2). The median follow-up period for rebleeding was 413 days (interquartile range [IQR], 40–1109).
Table 2
Clinical outcomes of acute lower gastrointestinal bleeding patients (n =
= 2,336).
2,336).
| Clinical outcome | Group O (n = = 844) 844) | Group non-O (n = = 1,492) 1,492) | Crude OR (95% CI) | P value | Adjusted OR† (95% CI) | P value |
|---|---|---|---|---|---|---|
| Rebleeding within 30-day | 151 (17.9) | 223 (14.9) | 1.24 (0.99–1.55) | 0.063 | 1.31 (1.04–1.65) | 0.024 |
| Rebleeding within 1-year | 185 (21.9) | 271 (18.2) | 1.26 (1.03–1.56) | 0.028 | 1.29 (1.04–1.61) | 0.020 |
| Blood transfusion | 277 (32.8) | 493 (33.0) | 0.99 (0.83–1.18) | 0.912 | 1.14 (0.94–1.39) | 0.168 |
| Active bleeding | 447 (53.0) | 768 (51.5) | 1.06 (0.90–1.26) | 0.489 | 1.10 (0.92–1.31) | 0.284 |
| Extravasation on CT | 186 (22.0) | 337 (22.6) | 0.97 (079–1.19) | 0.760 | 0.92 (0.75–1.14) | 0.455 |
Length of stay ≥ ≥ 8 days 8 days | 428 (50.7) | 755 (50.6) | 1.00 (0.85–1.19) | 0.960 | 1.09 (0.91–1.31) | 0.333 |
| Interventional radiology | 11 (1.3) | 26 (1.7) | 0.75 (0.37–1.51) | 0.416 | 0.77 (0.37–1.59) | 0.479 |
| Surgery | 3 (0.4) | 13 (0.9) | 0.41 (0.12–1.43) | 0.160 | 0.29 (0.06–1.30) | 0.104 |
| Thrombosis | 6 (0.7) | 9 (0.6) | 1.18 (0.42–3.33) | 0.755 | 1.49 (0.51–4.39) | 0.470 |
| Death within 30-day after colonoscopy | 2 (0.2) | 16 (1.1) | 0.22 (0.05–0.96) | 0.043 | 0.32 (0.07–1.42) | 0.136 |
Values are the number and (%). A two-tailed P-value <
< 0.05 was considered to indicate statistical significance. Bold values indicate P
0.05 was considered to indicate statistical significance. Bold values indicate P <
< 0.05. Analyzed using Fisher’s exact test.
0.05. Analyzed using Fisher’s exact test.
Each of the ORs is obtained by multivariate logistic regression analysis.
†ORs were adjusted for the contributing factors of clinically important variables: age ≥
≥ 65, sex, Shock index
65, sex, Shock index >
> 1, active bleeding, ligation and the following 7 factors found to have significance (P
1, active bleeding, ligation and the following 7 factors found to have significance (P <
< 0.1) on univariate analysis: fever
0.1) on univariate analysis: fever >
> 38, heart failure, chronic kidney disease, cirrhosis, antithrombotic drugs, platelets
38, heart failure, chronic kidney disease, cirrhosis, antithrombotic drugs, platelets <
< 15
15 ×
× 104/μL and blood urea nitrogen
104/μL and blood urea nitrogen >
> 25 mg/dL.
25 mg/dL.
CT, computed tomography; OR, odds ratio.
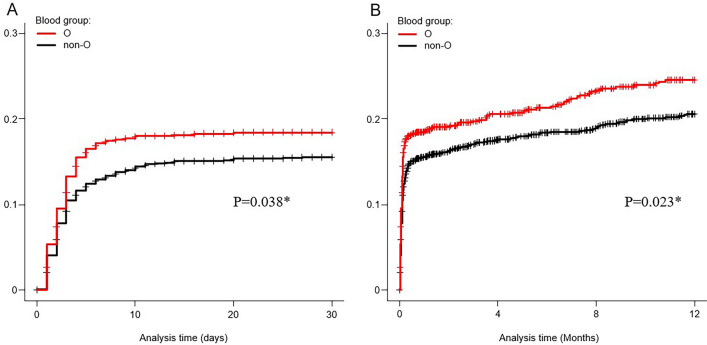
The cumulative probability of rebleeding using Kaplan–Meier method in acute lower gastrointestinal bleeding patients. (A) The cumulative probability of rebleeding within 30-day according to blood group (n =
= 2,336). (B) The cumulative probability of rebleeding within 1-year according to blood group (n
2,336). (B) The cumulative probability of rebleeding within 1-year according to blood group (n =
= 2,336). * P-values were calculated using the Generalized Wilcoxon test.
2,336). * P-values were calculated using the Generalized Wilcoxon test.
The rebleeding within 30 days and 1 year after the initial colonoscopy for CDB patients
Table Table33 presents the rebleeding rates of patients with CDB within 30 days and 1 year after initial colonoscopy. In the cohort, approximately, 1,650 cases were diagnosed with CDB, which was the most frequent final diagnosis among patients with ALGIB (Table (Table4).4). In the univariate analysis, blood group O was identified as a risk factor for rebleeding within 30 days (OR 1.33 [95% CI 1.03–1.71], P =
= 0.029). Moreover, blood group o was also associated with an increased risk of rebleeding within 1 year (OR 1.36 [95% CI 1.08–1.72], P
0.029). Moreover, blood group o was also associated with an increased risk of rebleeding within 1 year (OR 1.36 [95% CI 1.08–1.72], P =
= 0.010). In the multivariate analysis using a logistic regression model, after adjusting for other confounding factors, blood group O was confirmed as an independent risk factor for rebleeding within 30 days (aOR 1.38 [95% CI 1.06–1.80], P
0.010). In the multivariate analysis using a logistic regression model, after adjusting for other confounding factors, blood group O was confirmed as an independent risk factor for rebleeding within 30 days (aOR 1.38 [95% CI 1.06–1.80], P =
= 0.016) and within 1 year (aOR 1.37 [95% CI 1.07–1.74], P
0.016) and within 1 year (aOR 1.37 [95% CI 1.07–1.74], P =
= 0.012). Additionally, no significant association was observed between the ABO blood group and the rate of transfusion, active bleeding on colonoscopy, extravasation on CT, hospitalization for
0.012). Additionally, no significant association was observed between the ABO blood group and the rate of transfusion, active bleeding on colonoscopy, extravasation on CT, hospitalization for >
> 8 days, rate of IVR, rate of surgery, incidence of thrombosis, or mortality within 30 days of colonoscopy. Kaplan–Meier analysis revealed a significantly higher cumulative probability of rebleeding within 30 days (P
8 days, rate of IVR, rate of surgery, incidence of thrombosis, or mortality within 30 days of colonoscopy. Kaplan–Meier analysis revealed a significantly higher cumulative probability of rebleeding within 30 days (P =
= 0.018, Generalized Wilcoxon test) and 1 year (P
0.018, Generalized Wilcoxon test) and 1 year (P =
= 0.007, Generalized Wilcoxon test) in the group with blood group O compared to the non-O group among patients with CDB (Fig. 3).
0.007, Generalized Wilcoxon test) in the group with blood group O compared to the non-O group among patients with CDB (Fig. 3).
Table 3
Clinical outcomes of colonic diverticular bleeding patients (n =
= 1,650).
1,650).
| Clinical outcome | Group O (n = = 614) 614) | Group non-O (n = = 1,036) 1,036) | Crude OR (95% CI) | P value | Adjusted OR† (95% CI) | P value |
|---|---|---|---|---|---|---|
| Rebleeding within 30-day | 129 (21.0) | 173 (16.7) | 1.33 (1.03–1.71) | 0.029 | 1.38 (1.06–1.80) | 0.016 |
| Rebleeding within 1-year | 160 (26.1) | 213 (20.6) | 1.36 (1.08–1.72) | 0.010 | 1.37 (1.07–1.74) | 0.012 |
| Blood transfusion | 216 (35.2) | 348 (33.6) | 1.07 (0.87–1.32) | 0.511 | 1.22 (0.97–1.53) | 0.084 |
| Active bleeding | 335 (54.6) | 548 (52.9) | 1.07 (0.88–1.31) | 0.512 | 1.11 (0.91–1.37) | 0.307 |
| Extravasation on CT | 168 (27.4) | 292 (28.2) | 0.96 (0.77–1.20) | 0.718 | 0.92 (0.73–1.16) | 0.475 |
Length of stay ≥ ≥ 8 days 8 days | 340 (55.4) | 567 (55.7) | 1.03 (0.84–1.25) | 0.799 | 1.10 (0.89–1.37) | 0.366 |
| Interventional radiology | 11 (1.8) | 25 (2.4) | 0.74 (0.36–1.51) | 0.405 | 0.80 (0.38–1.66) | 0.548 |
| Surgery | 1 (0.2) | 4 (0.4) | 0.42 (0.05–3.77) | 0.439 | 0.36 (0.04–3.31) | 0.364 |
| Thrombosis | 5 (0.8) | 7 (0.7) | 1.21 (0.38–3.82) | 0.749 | 1.59 (0.47–5.42) | 0.456 |
| Death within 30-day after colonoscopy | 2 (0.3) | 3 (0.3) | 1.13 (0.19–6.75) | 0.897 | 1.33 (0.21–8.41) | 0.760 |
Values are the number and (%). A two-tailed P-value <
< 0.05 was considered to indicate statistical significance. Bold values indicate P
0.05 was considered to indicate statistical significance. Bold values indicate P <
< 0.05. Analyzed using Fisher’s exact test.
0.05. Analyzed using Fisher’s exact test.
Each of the ORs is obtained by multivariate logistic regression analysis.
†ORs were adjusted for the contributing factors of clinically important variables: age ≥
≥ 65, sex, Shock index
65, sex, Shock index >
> 1, active bleeding, ligation, and the following 7 factors found to have significance (P
1, active bleeding, ligation, and the following 7 factors found to have significance (P <
< 0.1) on univariate analysis: fever
0.1) on univariate analysis: fever >
> 38, heart failure, chronic kidney disease, cirrhosis, antithrombotic drugs, platelets
38, heart failure, chronic kidney disease, cirrhosis, antithrombotic drugs, platelets <
< 15
15 ×
× 104/μL and blood urea nitrogen
104/μL and blood urea nitrogen >
> 25 mg/dL.
25 mg/dL.
CT, computed tomography; OR, odds ratio.
Table 4
Final diagnosis of acute lower gastrointestinal bleeding with stigmata of recent hemorrhage (n =
= 2,336).
2,336).
| Diagnosis | Total (n = = 2,336) 2,336) | Four-group comparison | P value | Two-group comparison | P value | ||||
|---|---|---|---|---|---|---|---|---|---|
O (n = = 844) 844) | A (n = = 876) 876) | B (n = = 419) 419) | AB (n = = 197) 197) | O (n = = 844) 844) | non-O (n = = 1,492) 1,492) | ||||
| Colonic diverticular bleeding | 1,650 (71) | 614 (72.8) | 612 (69.9) | 288 (68.7) | 136 (69.0) | 0.385 | 614 (72.8) | 1,036 (69.4) | 0.091 |
| Postprocedure bleeding | 279 (12) | 105 (12.4) | 106 (12.1) | 42 (10.0) | 26 (13.2) | 0.576 | 105 (12.4) | 174 (11.7) | 0.577 |
| Rectal ulcer | 123 (5) | 34 (4.0) | 52 (5.9) | 25 (6.0)) | 12 (6.1) | 0.255 | 34 (4.0) | 89 (6.0) | 0.044 |
| Colorectal angioectasia | 52 (2) | 14 (1.7) | 19 (2.2) | 17 (4.1) | 2 (1.0) | 0.029 | 14 (1.7) | 38 (2.6) | 0.162 |
| Radiation colitis | 28 (1) | 13 (1.5) | 8 (0.9) | 5 (1.2) | 2 (1.0) | 0.685 | 13 (1.5) | 15 (1.0) | 0.254 |
| Others | 204 (8.7) | 64 (7.6) | 79 (9.0) | 42 (10.0) | 19 (9.6) | 0.463 | 64 (7.6) | 140 (9.4) | 0.138 |
Data are presented as n (%). A two-tailed P-value <
< 0.05 was considered to indicate statistical significance.Bold values indicate P
0.05 was considered to indicate statistical significance.Bold values indicate P <
< 0.05. Analyzed using Fisher’s exact test.
0.05. Analyzed using Fisher’s exact test.
Others includes the following diseases; small intestinal bleeding including presumptive cases, ischemic colitis, hemorrhoid bleeding, infectious colitis, inflammatory bowel disease, Meckel's diverticulum bleeding, drug-induced colonic ulcer.

The cumulative probability of rebleeding using Kaplan–Meier method in colonic diverticular bleeding patients. (A) The cumulative probability of rebleeding within 30-day according to blood group (n =
= 1,650). (B) The cumulative probability of rebleeding within 1-year according to blood group (n
1,650). (B) The cumulative probability of rebleeding within 1-year according to blood group (n =
= 1,650). * P-values were calculated using the Generalized Wilcoxon test.
1,650). * P-values were calculated using the Generalized Wilcoxon test.
Discussion
The findings from this nationwide cohort study provide novel evidence that the risk of rebleeding in patients with ALGIB and CDB varies according to the blood group (Tables (Tables2,2, ,3).3). Notably, supplementary analysis revealed higher short- and long-term cumulative rebleeding rates in patients with ALGIB and CDB in O group than in those in the non-O group (Figs. (Figs.1,1, ,2).2). Conversely, for clinical outcomes other than rebleeding, such as the incidence of thrombosis and short-term mortality, the association with blood group O was not statistically significant (Tables (Tables2,2, ,3).3). In contrast, analysis of similar clinical outcomes in patients with non-diverticular bleeding showed no significant differences based on blood group (Supplementary Table 1). Overall, the results of this retrospective multicenter study indicate that blood grouping is pertinent not only for transfusion compatibility but also for assessing the risk of rebleeding in ALGIB, particularly in CDB.
In this study, individuals with blood group O experienced a higher incidence of rebleeding than those with the non-O group despite exhibiting few risk factors for rebleeding, such as age, platelet count, and use of antithrombotic drugs. This suggests that individuals with blood group O may have additional risk factors for rebleeding. Previous studies have elucidated the underlying mechanism of the association between blood group O and bleeding, indicating a von Willebrand factor (VWF) deficiency. Serum levels of VWF in individuals with blood group O are 25–35% lower than those in individuals with non-O blood group16–20. VWF plays a crucial role in primary hemostasis by bridging injured vascular endothelium and platelets and in secondary hemostasis by activating fibrin clot formation as a factor VIII transport protein. In individuals with a non-O blood group, VWF glycosylation protects them from proteolysis and/or clearance by ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13) and LRP1 (low-density lipoprotein receptor-related protein 1), respectively, resulting in elevated VWF levels compared to those in individuals with blood group O21,22. Furthermore, coagulation factor VIII activity is reported to be low in blood group O23,24. Additionally, platelet function, as analyzed using PFA-10025, is also reduced in individuals with blood group O. These findings provide a theoretical explanation for the diminished hemostatic capacity observed in individuals with blood group O.
Supporting this hypothesis, previous clinical studies have demonstrated that blood group O is a notable risk factor linked to gastrointestinal bleeding7,8,10,26,27. Furthermore, in a meta-analysis, blood group O was identified as an independent risk factor (OR, 1.33) for various bleeding disorders including skin bleeding, cerebral hemorrhage, postpartum hemorrhage, and epistaxis9. Conversely, documented evidence exists that thrombotic events (e.g. deep venous thromboembolism and coronary heart disease) are less prevalent in individuals with blood group O28–31. This reveals discernible differences in clinical outcomes between individuals with blood group O and those without. Genome-wide association analyses have reported a correlation between ABO genes and colonic diverticular disease32–34, suggesting a potential association between blood groups and CDB.
In this study, the discrepancy in cumulative rebleeding rates between patients with blood group O and those with non-O blood groups was most pronounced during the initial week post-admission, after which it maintained a relatively stable trajectory. This observed pattern aligns with the findings of a study that examined the relationship between blood group O and delayed bleeding following endoscopic procedures13, implying the potential influence of analogous mechanisms. The intrinsic primary hemostatic function in individuals with blood group O may be comparatively delicate, potentially explaining the observed surge in early rebleeding events. Once effective thrombogenesis is established, its effect on the fibrinolytic system appears minimal and does not contribute to an increase in long-term rebleeding rates. This phenomenon likely underlies the observed stabilization of the cumulative rebleeding rates over time. The management of rebleeding is crucial in the early stages of ALGIB.
This study has inherent limitations that should be considered. First, this was a retrospective multicenter cohort study utilizing a large dataset compiled from medical records spanning the past 10 years across 49 hospitals, including large academic facilities throughout Japan. However, a detailed investigation of the bleeding site was not performed. Second, while the use of antithrombotic drugs may pose a risk of rebleeding in patients with ALGIB, this study was only able to collect information on the use of antithrombotic drugs at the time of admission. To address this issue, we conducted a sensitivity analysis on the rebleeding risk of blood group O, focusing on cases in which antithrombotic drug status and management (no use/withdrawal/resumption/continuation) were confirmed (n =
= 1829), excluding patients missing information on antithrombotic drug management (n
1829), excluding patients missing information on antithrombotic drug management (n =
= 507). The main results were unchanged after adjusting for antithrombotic drug management, confirming their robustness (Supplementary Table 2). Third, although our findings confirmed that blood group O is a risk factor for rebleeding in ALGIB, whether the particular blood group is also a risk factor for initial bleeding events remains unclear.
507). The main results were unchanged after adjusting for antithrombotic drug management, confirming their robustness (Supplementary Table 2). Third, although our findings confirmed that blood group O is a risk factor for rebleeding in ALGIB, whether the particular blood group is also a risk factor for initial bleeding events remains unclear.
In conclusion, this study demonstrated that blood group O is an independent risk factor for both short- and long-term rebleeding in patients with ALGIB. Notably, the findings from this retrospective multicenter analysis suggest that blood grouping is significant not only for transfusion compatibility but also for its implications in assessing the risk of rebleeding in ALGIB. Therefore, rebleeding should be managed with heightened caution in patients with blood group O who present with ALGIB.
Methods
Patients and study design
This study included 10,342 patients admitted to 49 hospitals for acute hematochezia between January 2010 and December 2019. From the original cohort of patients with acute hematochezia (n =
= 10,342), we excluded those with second and subsequent admissions (n
10,342), we excluded those with second and subsequent admissions (n =
= 2075), those lacking blood group data (n
2075), those lacking blood group data (n =
= 481), those without a definitive diagnosis on colonoscopy based on stigmata of recent hemorrhage (SRH) (n
481), those without a definitive diagnosis on colonoscopy based on stigmata of recent hemorrhage (SRH) (n =
= 5,141), those with UGIB (n
5,141), those with UGIB (n =
= 148), and those with colon cancer (n
148), and those with colon cancer (n =
= 161). The remaining cohort was stratified into two groups: blood group O and non-O. The present study was conducted in accordance with the Declaration of Helsinki of 1964 and its subsequent amendments, or equivalent ethical standards. As this study is a retrospective investigation, the requirement for obtaining informed consent from patients was waived by the central institution, Tokyo Medical University. The central institution has a license committee/institutional review board (IRB) to approve research involving human subjects. The research protocol obtained approval from the Tokyo Medical University IRB (T20190244). The central institution's IRB review was applied to this study, and approval was obtained from the ethics committees and IRB of all participating hospitals (Supplementary Table 3).
161). The remaining cohort was stratified into two groups: blood group O and non-O. The present study was conducted in accordance with the Declaration of Helsinki of 1964 and its subsequent amendments, or equivalent ethical standards. As this study is a retrospective investigation, the requirement for obtaining informed consent from patients was waived by the central institution, Tokyo Medical University. The central institution has a license committee/institutional review board (IRB) to approve research involving human subjects. The research protocol obtained approval from the Tokyo Medical University IRB (T20190244). The central institution's IRB review was applied to this study, and approval was obtained from the ethics committees and IRB of all participating hospitals (Supplementary Table 3).
Variables
All variables were extracted from medical records and electronic endoscopy databases of each participating institution by gastroenterologists or researchers. A total of 42 items, including baseline characteristics such as blood group, age, sex, BMI, drinking habits, smoking, performance status, comorbidities, clinical symptoms, medications, vital signs on admission, initial laboratory findings, endoscopy, and CT results, were collected as previously documented15,35. Comorbidities were evaluated using the modified CCI36, which includes 19 conventional CCI items and additional items for hypertension and hyperlipidemia. The CCI serves as an index for categorizing comorbidities with prognostic relevance and has been extensively validated for gastrointestinal bleeding37. The shock index was defined as heart rate divided by systolic blood pressure38. Contrast-enhanced CT was used to assess extravasation. Detailed information on the endoscopic factors, including the type of SRH and endoscopic treatment, was also collected.
Clinical outcomes
The primary outcome was rebleeding within 30 days and 1 year after the initial colonoscopy. Rebleeding was defined as the presence of a large volume of fresh blood or dark-red stool after the initial colonoscopy15,39. Secondary outcomes included various clinical features, such as blood transfusion, active bleeding, extravasation on CT, length of stay, IVR, surgery, thrombosis, and death within 30 days post-colonoscopy.
Statistical analysis
The association between clinical variables and blood groups was assessed in patients with ALGIB. The analysis included four groups: O, A, B, and AB, and a two-group analysis comparing the O and non-O groups. Categorical data were compared using the χ2 test or Fisher’s exact test. The relationship between blood group and clinical outcomes was analyzed using univariate and multivariate logistic regression models in the two-group comparison (O vs. non-O). The multivariate analysis was adjusted to include clinically significant variables (age ≥
≥ 65, gender, Shock index
65, gender, Shock index >
> 1, active bleeding, and endoscopic ligation) and variables with at least borderline significance (P
1, active bleeding, and endoscopic ligation) and variables with at least borderline significance (P <
< 0.10) in univariate analysis. The cumulative incidence of rebleeding was analyzed using the generalized Wilcoxon test with the Kaplan–Meier method. In the subgroup analysis, we restricted the analysis to patients with CDB and performed similar examinations. This subgroup analysis aimed to elucidate the relationship between the blood group and the risk of rebleeding in CDB, a major cause of ALGIB. Statistical significance was defined as a two-sided P-value of
0.10) in univariate analysis. The cumulative incidence of rebleeding was analyzed using the generalized Wilcoxon test with the Kaplan–Meier method. In the subgroup analysis, we restricted the analysis to patients with CDB and performed similar examinations. This subgroup analysis aimed to elucidate the relationship between the blood group and the risk of rebleeding in CDB, a major cause of ALGIB. Statistical significance was defined as a two-sided P-value of <
< 0.05. Statistical analysis was conducted using JMP® Pro 16.0.0 (SAS Institute, Cary, NC, USA).
0.05. Statistical analysis was conducted using JMP® Pro 16.0.0 (SAS Institute, Cary, NC, USA).
Acknowledgements
The author thanks Yasuji Arimura for assistance with data collection and analysis. We would like to thank Editage (www.editage.jp) for English language editing.
Author contributions
N.N. was the principal investigator of this study. N.N., N.T. and S.S designed and conducted the study and interpreted the data. S.S. mainly wrote the article. T.M, H.K., K.K., A.Y., A.Y., J.O., T.I., T.A., Y.S., T.K., N.I., T.S., M.M., A.T., K.M., K.K., S.F., T.U., M.F., H.S., T.N., J.H., T.F., Y.K., A.M., S.K., T.M., R.G., H.F., Y.F., T.H., Y.T., K.N., N.M., K.N., T.K., Y.S., S.F., K.K., T.M., Y.K., and M.K. collected and interpreted the data. E.S. performed the statistical analysis. N.N., N.T., T. Aoki, and E.S. provided corrections and advice on preparing the article.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to privacy and ethical restrictions but can be requested and reviewed from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Tomonori Aoki and Eiji Sadashima.
Contributor Information
Naoyuki Tominaga, Email: pj.nakiesok@n-aganimot.
Naoyoshi Nagata, Email: pj.oc.oohay@mgcn_atagann.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-64476-9.
References
Articles from Scientific Reports are provided here courtesy of Nature Publishing Group
Citations & impact
This article has not been cited yet.
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/165337637
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Acute lower gastrointestinal bleeding: A population-based five-year follow-up study.
United European Gastroenterol J, 7(10):1330-1336, 08 Jul 2019
Cited by: 1 article | PMID: 31839958 | PMCID: PMC6894003
Long-term Risks of Recurrence After Hospital Discharge for Acute Lower Gastrointestinal Bleeding: A Large Nationwide Cohort Study.
Clin Gastroenterol Hepatol, 21(13):3258-3269.e6, 03 Jun 2023
Cited by: 2 articles | PMID: 37276989
Prevalence and risk factors of acute lower gastrointestinal bleeding in Crohn disease.
Medicine (Baltimore), 94(19):e804, 01 May 2015
Cited by: 8 articles | PMID: 25984665 | PMCID: PMC4602567
Early feeding reduces length of hospital stay in patients with acute lower gastrointestinal bleeding: A large multicentre cohort study.
Colorectal Dis, 25(11):2206-2216, 03 Oct 2023
Cited by: 0 articles | PMID: 37787161
Review

 2
2