Abstract
Background
Vitiligo is an autoimmune disease characterized by loss of skin pigmentation and currently has no effective treatment. This study aimed to investigate the function of SIRT7, being an important desuccinylase mediating multiple disease progression, and its mechanism in vitiligo progression.Methods
Normal human melanocytes (NHM) PIG1 and vitiligo human melanocytes (VHM) PIG3V were utilized in this research. The role of sirtuin 7 (SIRT7) and Ezrin (EZR) on melanin synthesis was investigated by detecting tyrosinase activity, melanin content, α-MSH levels, and the protein levels of melanin-related markers. The function of EZR was identified via rescue experiments, while the underlying mechanism was investigated via bioinformatic analysis, co-immunoprecipitation (co-IP), immunoprecipitation (IP), and Western blot techniques.Results
Results showed that only SIRT7 was highly expressed in vitiligo human melanocytes, where knockingdown SIRT7 translated into increased melanin synthesis in melanocytes. Mechanistically, SIRT7 knockdown promoted the succinylation of EZR at the Lys (K)60 site. Moreover, overexpressing EZR induced higher melanin synthesis in melanocytes, while its knocking down exerted the opposite effect by inhibiting SIRT7 knockdown-induced melanin synthesis.Conclusion
SIRT7 inhibited melanin synthesis in melanocytes by suppressing the succinylation of EZR. These findings are envisaged to provide a novel theoretical basis for vitiligo treatment.Free full text

SIRT7 Inhibits Melanin Synthesis of PIG1 and PIG3V by Suppressing the Succinylation of EZR
Abstract
Background
Vitiligo is an autoimmune disease characterized by loss of skin pigmentation and currently has no effective treatment. This study aimed to investigate the function of SIRT7, being an important desuccinylase mediating multiple disease progression, and its mechanism in vitiligo progression.
Methods
Normal human melanocytes (NHM) PIG1 and vitiligo human melanocytes (VHM) PIG3V were utilized in this research. The role of sirtuin 7 (SIRT7) and Ezrin (EZR) on melanin synthesis was investigated by detecting tyrosinase activity, melanin content, α-MSH levels, and the protein levels of melanin-related markers. The function of EZR was identified via rescue experiments, while the underlying mechanism was investigated via bioinformatic analysis, co-immunoprecipitation (co-IP), immunoprecipitation (IP), and Western blot techniques.
Results
Results showed that only SIRT7 was highly expressed in vitiligo human melanocytes, where knockingdown SIRT7 translated into increased melanin synthesis in melanocytes. Mechanistically, SIRT7 knockdown promoted the succinylation of EZR at the Lys (K)60 site. Moreover, overexpressing EZR induced higher melanin synthesis in melanocytes, while its knocking down exerted the opposite effect by inhibiting SIRT7 knockdown-induced melanin synthesis.
Conclusion
SIRT7 inhibited melanin synthesis in melanocytes by suppressing the succinylation of EZR. These findings are envisaged to provide a novel theoretical basis for vitiligo treatment.
Introduction
Vitiligo is a chronic skin disorder characterized by the selective destruction of melanocytes, leading to depigmentation,1,2 where factors like genetics, melanocyte self-destruction, cytokines, autoimmunity, and oxidative stress are implicated in vitiligo pathogenesis.3,4 It appears as patchy depigmentation of the skin and can appear on any part of the body.5 It is caused by the destruction of epidermal melanocytes, which translates into their failure of melanin synthesis, thereby reducing melanin contents and causing the lack of melanin pigment responsible for skin coloration.6 Vitiligo can result in reduced self-esteem in patients and trigger anxiety, depression, or other emotions that significantly impact the patient’s quality of life.7 Therefore, it is imperative to investigate the vitiligo pathogenesis for developing effective treatment strategies.
Succinylation is the process of modifying proteins with a succinyl group to regulate metabolic pathways involved in the development of various diseases.8 Several enzymes, such as sirtuin 7 (SIRT7), have been demonstrated to participate in succinylation. SIRT7 is a β-NAD + - dependent deacetylase enzyme belonging to the SIRT protein family and is located in nucleoli and regulates the transcription of RNA polymerase I.9 Recently, SIRT7 has been shown to function as a histone desuccinylase associated with chromatin compaction and genome stability,10 in addition to regulating disease progression by participating in multiple cellular processes, like, SIRT7 has been found to selectively deacetylates histone H3 lysine 18 (H3K18Ac) in chromatin, which serves to maintain the cellular transformation ability of human cancer cells and tumor formation,11 and play an important role in aging process.12–15 The SIRT7 knockdown has been found to increase melanin production in melanoma cells, cementing its essential role in regulating melanin synthesis.16 However, whether SIRT7 is involved in the pathogenesis of vitiligo remains unclear.
+ - dependent deacetylase enzyme belonging to the SIRT protein family and is located in nucleoli and regulates the transcription of RNA polymerase I.9 Recently, SIRT7 has been shown to function as a histone desuccinylase associated with chromatin compaction and genome stability,10 in addition to regulating disease progression by participating in multiple cellular processes, like, SIRT7 has been found to selectively deacetylates histone H3 lysine 18 (H3K18Ac) in chromatin, which serves to maintain the cellular transformation ability of human cancer cells and tumor formation,11 and play an important role in aging process.12–15 The SIRT7 knockdown has been found to increase melanin production in melanoma cells, cementing its essential role in regulating melanin synthesis.16 However, whether SIRT7 is involved in the pathogenesis of vitiligo remains unclear.
Ezrin (EZR), a member of the Ezrin-radixin-moesin (ERM) cytoskeletal proteins, has been reported to be involved in multiple biological processes, including adhesion, migration, cytokinesis and myoblast differentiation.17,18 It has also been recognized as a regulator of adhesion signal pathways for maintaining epithelial cell polarity.19 It has lately been proven to regulate the invasion and metastasis of cancers by regulating cellular interactions.20,21 However, the function of EZR in vitiligo remains to be explored.
This study examined the enzyme expression related to succinylation in normal human melanocytes (NHM) PIG1 and vitiligo human melanocytes (VHM) PIG3V. Additionally, it explored the impact of SIRT7 on melanin production and its role in regulating melanin synthesis. The findings of this study are envisaged to offer novel insights into vitiligo treatment.
Methods
Cell Culture
PIG1 and PIG3V were provided by BLUEFBIO (Shanghai, China). The cells were cultured in Dulbecco’s modified eagle medium (DMEM, Gibco, Grand Island, NY, USA) supplement with 10% fetal bovine serum (FBS; Gibco) and maintained at 37°C with 5% CO2.
Cell Transfection
PIG1 and PIG3V cells grown in the logarithmic phase were inoculated into six-well plates (2 × 105 cells/well). Short hairpin RNA targeting SIRT7 (sh-SIRT7), shRNA negative control (shNC), short hairpin RNA targeting EZR (Sh-EZR), EZR overexpressing plasmid (pcDNA3.1-EZR) and empty vector (GenePharma, Shanghai, China) were transfected into cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the guidelines. The transfected cells were harvested after 48 h of transfection.
Quantitative Real-Time PCR (qPCR)
Total RNA was isolated using Trizol reagent (Invitrogen). Then, a 1st Strand cDNA synthesis kit (Takara, Shiga, Japan) was used to synthesize cDNAs. The obtained cDNA samples were used for qPCR by SYBR green master mix (Thermo Scientific, Waltham, MA, USA). Relative mRNA expression was calculated using the 2−ΔΔCt method as normalized to GAPDH. The primers for qPCR are presented in Table 1.
Table 1
The Primers for qPCR
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| CPT1A | TCCAGTTGGCTTATCGTGGTG | TCCAGAGTCCGATTGATTTTTGC |
| KAT2A | GCAAGGCCAATGAAACCTGTA | TCCAAGTGGGATACGTGGTCA |
| KAT3B | AGCCAAGCGGCCTAAACTC | TCACCACCATTGGTTAGTCCC |
| SIRT5 | GCCATAGCCGAGTGTGAGAC | CAACTCCACAAGAGGTACATCG |
| SIRT7 | GACCTGGTAACGGAGCTGC | CGACCAAGTATTTGGCGTTCC |
| EDN1 | AGAGTGTGTCTACTTCTGCCA | CTTCCAAGTCCATACGGAACAA |
| HSP60 | ATGCTTCGGTTACCCACAGTC | AGCCCGAGTGAGATGAGGAG |
| HSP70 | GCTCCCACATCTGCATATTCAT | TTCTGAGACGTTGGAGTCAGT |
| SERP1 | AAATGCCCCCGAAGAGAAGG | TCTGGAAAATTGCAGAACCACA |
| TYR | AACGAGCTGTGCTACAAGGTC | GCGTGGTCGATGAGGAAGA |
| MITF | AGGGAGCTCACAGAGTCTGAA | TGTTAAATCTTCTTCTTCGTTCAATC |
| TRP1 | TCTCTGGGCTGTATCTTCTTCC | GTCTGGGCAACACATACCACT |
| TRP2 | CTTGGGCTGCAAAATCCTGC | CAGCACTCCTTGTTCACTAGG |
| EZR | ACCAATCAATGTCCGAGTTACC | GCCGATAGTCTTTACCACCTGA |
| LAMP1 | TCTCAGTGAACTACGACACCA | AGTGTATGTCCTCTTCCAAAAGC |
| TRPM1 | GTTCACCAACCAGCATATCCC | GCTTTATTGGAATATCCGCCACC |
| PTPN22 | AGGCAGACAAAACCTATCCTACA | TGGGTGGCAATATAAGCCTTG |
| CHOP | AGAACAGCCGTTACTTCAGGA | CCGCTGGTAGGAGGTTTTAGAG |
| IL1R1 | ATGAAATTGATGTTCGTCCCTGT | ACCACGCAATAGTAATGTCCTG |
| IL23A | CTCAGGGACAACAGTCAGTTC | ACAGGGCTATCAGGGAGCA |
| POLH | GCTACTGGACAGGATCGAGTG | CACCACCCTTCCATGATTTGTA |
| PRDX3 | ACAGCCGTTGTCAATGGAGAG | ACGTCGTGAAATTCGTTAGCTT |
| IL1B | ATGATGGCTTATTACAGTGGCAA | GTCGGAGATTCGTAGCTGGA |
Tyrosinase Activity
The tyrosinase activity was assessed using a tyrosinase activity assay kit (Sangon, Shanghai, China). Cells (5×106 cells) were lysed with extracting solution by ultrasonic wave and then centrifuged at 12,000×g for 20 min at 4°C to collect the supernatant. The experiment was completed according to the guidelines. The absorbance was measured using a microplate reader at the wavelength of 475 nm.
Melanin Content
The melanin content was measured using a melanin ELISA kit (mlbio, Shanghai, China). Cells were centrifuged at 1000×g for 10 min to collect supernatant. The experiment was completed according to the guidelines. The absorbance was measured using a microplate reader at the wavelength of 450 nm.
Immunofluorescence (IF) Staining
Cells were seeded into 6-well plates for 12 h incubation. Then, cells were fixed with immunostaining fix solution and permeabilized with 0.2% Triton X-100 in phosphate buffered saline (PBS, Gibco). Next, the cells were washed with PBS and blocked with 3% bovine serum albumin for 1 h. Afterwards, the cells were incubated with anti-α-MSH (1: 4000, Mfcd03454727, Merck, Darmstadt, Germany) for 1 h. Finally, fluorescent secondary antibody (1: 1000, ab150077, Abcam, Cambridge, UK) was incubated with cells for 1 h protected from light. The nuclei were stained with DAPI (1 μg/mL, Beyotime, Beijing, China), and cells were observed under a confocal microscope.
μg/mL, Beyotime, Beijing, China), and cells were observed under a confocal microscope.
Western Blot Assay
Total protein extraction was performed using radio immunoprecipitation assay (RIPA) lysis buffer (Thermo Scientific) and the protein was measured using a BCA kit (Beyotime) after extraction. Protein samples were loaded on 10% SDS-PAGE and transferred to PVDF membranes. The membranes were blocked with 5% skimmed milk for 1 h, and then incubated overnight at 4°C with primary antibodies, including anti-MITF (1/1000, ab303530), anti-TYR (1/1000, ab170905), anti-TRP1 (1/1000, ab235447), anti-TRP2 (1/1000, ab74073), anti-GAPDH (1: 10,000, ab181602) (Abcam, Cambridge, UK) and anti-succinyllysine (1: 2000, PTM-401, PTM BIO, Hangzhou, China). After treatment with secondary antibodies (1: 10,000, ab205718, Abcam), membranes were visualized using the ECL reagent (Yeasen, Shanghai, China).
Co-Immunoprecipitation (Co-IP) and IP
The interaction between SIRT7 and EZR was evaluated using co-IP, and the succinylation levels of EZR were detected by immunoprecipitation before Western blot. The experiments were performed using the Pierce classic magnetic IP/co-IP kit (Thermo Scientific). Briefly, PIG1 and PIG3V cells were lysed on ice for 5 min with a special lysis buffer. The lysate was obtained by centrifugation at 13,000 ×g for 10 min and then incubated overnight with antibodies (anti-Flag, anti-HA, anti-IgG or anti-EZR) at 4°C. Next, the mixture was incubated with Protein A/G magnetic beads at room temperature for 1 h. Finally, the beads were collected and the antigen-antibody mixture was eluted. The levels of proteins were measured using Western blotting.
Bioinformatic Analysis
The succinylation sites in EZR were predicted using the GPSuc database (http://kurata14.bio.kyutech.ac.jp/GPSuc/index.php).
EZR Stability Detection
To evaluate the protein stability of EZR, cells were treated with 10 μM cycloheximide (MKBio, Shanghai, China) and the protein levels were measured after 0, 8, 16 and 24 h of treatment.
Statistical Analysis
All data were analyzed and processed by SPSS 22.0 software, and results were expressed as mean ± standard deviation of at least three replicates. The comparison between two or more groups was performed by Student’s t-test or one-way analysis of variance (ANOVA) followed by Turkey test. P<0.05 was recognized as statistically significant.
Results
SIRT7 is Highly Expressed in PIG3V Cells
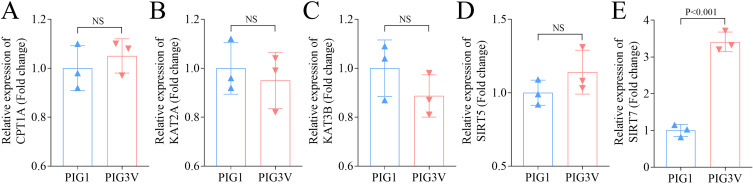
Succinylation plays a role in regulating the processes of numerous diseases,22 but whether succinylation-related enzymes are involved in mediating the vitiligo pathogenesis remains unclear. For this purpose, the expressions of different succinylation-related enzymes in PIG1 (NHM) and PIG3V (VHM) cells were measured, and Results showed insignificantly different expressions for CPT1A, KAT2A, KAT3B and SIRT5 in PIG3V and PIG1 cells (Figures 1A–D), whereas, a significantly higher SIRT7 expression was observed in PIG3V cells (Figure 1E), suggesting SIRT7 might be involved in vitiligo pathogenesis.
Knockdown of SIRT7 Increases the Level of Melanin in Melanocytes
The function of highly expressed SIRT7 in VHM was investigated by transfecting sh-SIRT7 into PIG1 and PIG3V cells to knock down SIRT7, which translated into a significantly decreased SIRT7 expression (Figure 2A). It was proceeded by measuring tyrosinase activity and melanin content in both cell types, and results indicated enhanced tyrosinase activity and melanin content post SIRT7 knockdown (Figure 2B and andC).C). Moreover, IF staining showed that SIRT7 knockdown elevated the content of α-MSH in both cell types (Figure 2D), in addition to increasing the levels of melanin-related proteins MITF, TYR, TRP1 and TRP2 (Figure 2E). These results suggested that knocking down SIRT7 increased the melanin levels in melanocytes, cementing that SIRT7 knockdown promoted melanin synthesis.

SIRT7 knockdown promoted melanin synthesis in PIG1 and PIG3V cells. (A) The expression of SIRT7 in PIG1 and PIG3V cells following sh-NC and sh-SIRT7 transfection. (B) The tyrosinase activity was assessed by a tyrosinase activity assay kit. (C) The melanin content was measured by a melanin content kit. (D) The content of α-MSH was evaluated using IF staining. (E) The levels of melanin-related proteins were measured by Western blot assay.
SIRT7 Inhibits the Succinylation of EZR at the K60 Site
Many genes have been reported to be dysregulated in vitiligo.23 Hence, expressions of various genes and melanogenic-related genes (TYR1 and TYR2)24 in both cell types following SIRT7 knockdown were measured. As shown in heatmaps, the expression of EZR was found to be significantly higher in both cell types with SIRT7 knockdown (Figure 3A). Hence, EZR was selected for subsequent investigations. The knocking down of SIRT7 not only increased the EZR protein but also its succinylation levels in both PIG1 and PIG3V cells (Figure 3B). Similarly, the interaction between SIRT7 and EZR was determined by co-IP, and results are shown in Figure 3C, followed by predicting some potential succinylation sites, which resulted in K60 and K79 being the top most (Figure 3D). To verify the modified site as a function of succinylation, a point mutation technique was employed to mutate those sites into arginine (R), and Western blotting showed that the protein and succinylation levels of EZR were decreased due to the K60 mutation. It was then, finally, proceeded by assessing the stability of EZR in both cell types, and results showed that knocking down SIRT7 accelerated the EZR degradation (Figure 3F and andG).G). All these results suggested that SIRT7 was involved in inhibiting EZR succinylation at the K60 site.
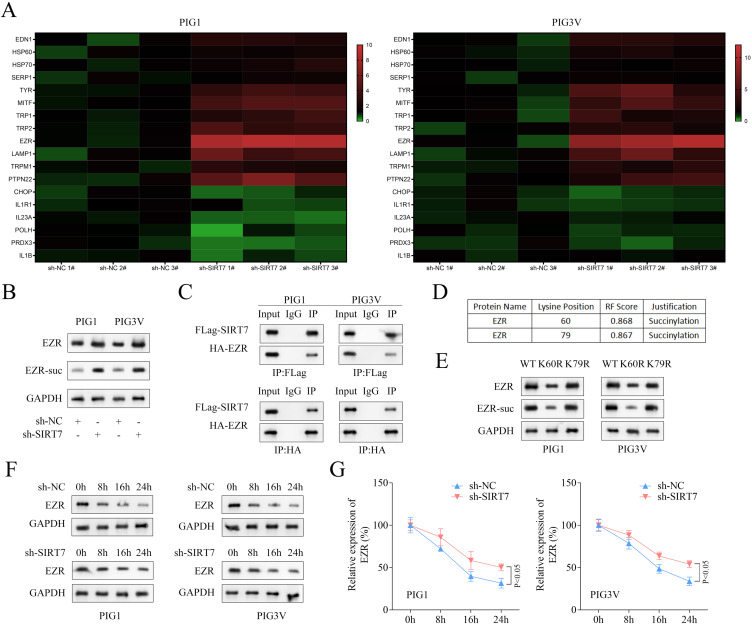
SIRT7 inhibited succinylation of EZR at the K60 site. (A) The expression of genes related to vitiligo and melanogenic was displayed in heatmaps. (B) The levels of EZR succinylation and EZR protein following SIRT7 knockdown were assessed by IP and Western blot. (C) The interaction between SIRT7 and EZR was determined by co-IP. (D) The succinylation sites in EZR were predicted using the GPSuc database. (E) Western blot was used to measure the protein and succinylation levels of EZR after mutating the potential succinylation sites in EZR. (F and G) The stability of the EZR protein was measured using Western blot after treatment with 10 μM cycloheximide for 0, 8, 16, and 24 h.
EZR Overexpression Enhances the Melanin Level in Melanocytes
The role of EZR in regulating melanin levels was evaluated in both cell types transfected with pcDNA3.1-EZR, and results indicated elevated EZR expression following EZR overexpression vector transfection (Figure 4A), which, in turn, significantly increased the tyrosinase activity and melanin content (Figure 4B and andC),C), as well as elevated α-MSH contents (Figure 4D). Moreover, the results of Western blotting for estimating levels of melanin-related proteins MITF, TYR, TRP1 and TRP2 showed upregulated protein levels of melanin-related markers post-EZR overexpression (Figure 4E). These results suggested that EZR overexpression enhanced the melanin level in melanocytes, suggesting that overexpressing EZR promoted melanin synthesis.

EZR overexpression promoted melanin synthesis in PIG1 and PIG3V cells. (A) The expression of EZR in PIG1 and PIG3V cells following pcDNA3.1 and pcDNA3.1-EZR transfection. (B) The tyrosinase activity was assessed by a tyrosinase activity assay kit. (C) The melanin content was measured by a melanin content kit. (D) The content of α-MSH was evaluated using IF staining. (E) The levels of melanin-related proteins were measured by Western blot assay.
EZR Knockdown Reverses the Melanin Level Increased by Silencing SIRT7
The regulating effect of EZR and SIRT7 on melanin levels was subsequently validated by knocking down both EZR and SIRT7, and the results reduced EZR expression in both PIG1 and PIG3V cells (Figure 5A). Similarly, SIRT7 induced higher tyrosinase activity and melanin content in both cell types post-SIRT7 knockdown was significantly inhibited following EZR knockdown (Figure 5B and andC),C), while mixed effects were observed for α-MSH contents, which were found elevated with SIRT7 knockdown, while reduced with EZR knockdown (Figure 5D). Similarly, SIRT7 knockdown was also found to upregulate the melanin-related protein levels, which were downregulated with EZR knockdown (Figure 5E). All these results suggested that EZR knockdown reversed the melanin level increased by silencing of SIRT7.
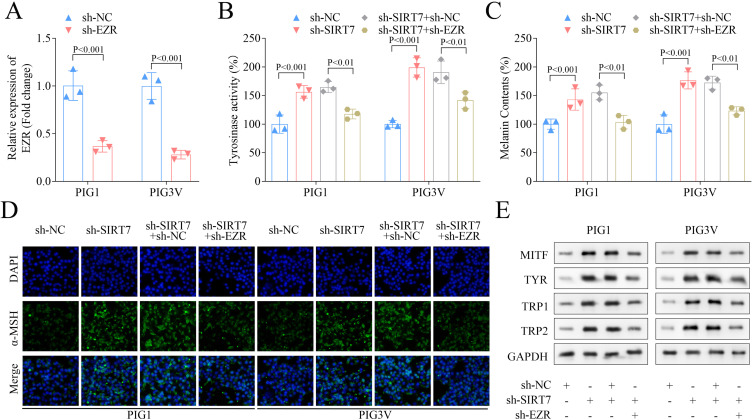
EZR knockdown partially reversed melanin synthesis increased by SIRT7 knockdown. (A) The expression of EZR in PIG1 and PIG3V cells following sh-NC and sh-EZR transfection. (B) The tyrosinase activity was assessed by a tyrosinase activity assay kit. (C) The melanin content was measured by a melanin content kit. (D) The content of α-MSH was evaluated using IF staining. (E) The levels of melanin-related proteins were measured by Western blot assay.
Discussion
SIRT7 is the least explored member of the SIRT family, which has recently been reported to be a histone desuccinylase regulating genome stability.10 It has been found to regulate multiple vital biological processes, including energy metabolism, stress resistance, genomic stability, aging, and tumorigenesis.25,26 However, the role of SIRT7 in the pathogenesis of vitiligo is still unclear.
Vitiligo is an autoimmune depigmentation disease characterized by patchy loss of skin or hair pigmentation due to the loss of epidermal melanocytes,1 since the formation and maturation of melanosomes and the ability to synthesize melanin are key to vitiligo repigmentation.23 It has been demonstrated that SFRP5 inhibits melanin synthesis of melanocytes in vitiligo by suppressing the Wnt/β-catenin signaling.27 Thus, enhancing melanin synthesis ability is envisaged to be an effective strategy for treating vitiligo. Tyrosinase is the primary enzyme responsible for producing pigments in various organisms by catalyzing the initial two steps in melanin synthesis.28 Inhibition of tyrosinase activity and melanin synthesis by salidroside had been found to improve hyperpigmentation in guinea pig skin,29 while activation of tyrosinase activity was found to promote melanin synthesis in vitiligo-affected areas.30 Similarly, α-MSH is considered an accelerator of melanin synthesis, where combinational α-MSH and melanocortin 1 receptor was found to increase tyrosinase activity, thereby promoting melanin synthesis.31 The relationship between SIRT7 and melanin synthesis has been scarcely reported, and only one study reported that knocking down SIRT7 translated into enhanced melanin production in melanoma cells.16 Our data indicated that SIRT7 expression was higher in VHM compared to NHM, where SIRT7 knockdown translated into an increased tyrosinase activity, melanin contents, α-MSH levels, and protein levels of melanin-related markers in both cell types, suggesting SIRT7 inhibited melanin synthesis in melanocytes, thus might be involved in promoting vitiligo development.
Succinylation plays a role in cellular processes in various organisms by regulating protease activity and gene expression, where SIRT7 typically regulates disease progression by desuccinylatiion, eg, SIRT7 had been reported to catalyze desuccinylation of PRMT5 in cells to promote tumor growth and metastasis.32 Moreover, SIRT7 in combination with cccDNA has been reported to catalyze H3K122 desuccinylation to induce silencing of chronic hepatitis B virus transcription.33 Our results suggested an interaction between SIRT7 and EZR, where SIRT7 was found to inhibit the succinylation of EZR at the K60 site. Studies on the EZR family are primarily focused on their functions in tumor invasion and metastasis like it was found to promote pancreatic cancer proliferation and metastasis by activating the FAK/AKT signaling pathway.20 Moreover, silencing of lncRNA EZR-AS1 was found to inhibit proliferation, invasion, and migration of colorectal cancer cells via blocking transforming growth factor β signaling. The EZR has been revealed as a target for vitiligo potential marker miR-184,34 but its role in vitiligo is still unknown. Our data suggested that overexpressing EZR improved melanin synthesis, whereas SIRT7 knockdown-induced elevated melanin synthesis was reversed when EZR was knocked down, suggesting that EZR might be a therapeutic target of vitiligo.
Nevertheless, there were some limitations in this study, like we solely investigated the mechanism of SIRT7 on melanin synthesis using in vitro experiments, which could be further studied in animals in our future research.
In conclusion, our data demonstrated that SIRT7 inhibited melanin synthesis of melanocytes by reducing the succinylation of EZR, which is envisaged to provide a novel therapeutic target for treating vitiligo.
Funding Statement
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
Articles from Clinical, Cosmetic and Investigational Dermatology are provided here courtesy of Dove Press
Full text links
Read article at publisher's site: https://doi.org/10.2147/ccid.s462280
Read article for free, from open access legal sources, via Unpaywall:
https://www.dovepress.com/getfile.php?fileID=100223
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Mechanism of desuccinylation of G6PD mediated by SIRT7 to promote vitiligo disease progression.
Immun Inflamm Dis, 12(8):e1341, 01 Aug 2024
Cited by: 1 article | PMID: 39092715 | PMCID: PMC11295095
SFRP5 inhibits melanin synthesis of melanocytes in vitiligo by suppressing the Wnt/β-catenin signaling.
Genes Dis, 8(5):677-688, 15 Jun 2020
Cited by: 19 articles | PMID: 34291139 | PMCID: PMC8278527
PM2.5 induces apoptosis, oxidative stress injury and melanin metabolic disorder in human melanocytes.
Exp Ther Med, 19(5):3227-3238, 09 Mar 2020
Cited by: 14 articles | PMID: 32269607 | PMCID: PMC7138919
Upregulation of Melanogenesis and Tyrosinase Activity: Potential Agents for Vitiligo.
Molecules, 22(8):E1303, 04 Aug 2017
Cited by: 71 articles | PMID: 28777326 | PMCID: PMC6152334
Review Free full text in Europe PMC