Abstract
Introduction
Central venous access devices (CVADs) are commonly used for the treatment of paediatric cancer patients. Catheter locking is a routine intervention that prevents CVAD-associated adverse events, such as infection, occlusion and thrombosis. While laboratory and clinical data are promising, tetra-EDTA (T-EDTA) has yet to be rigorously evaluated or introduced in cancer care as a catheter lock.Methods and analysis
This is a protocol for a two-arm, superiority type 1 hybrid effectiveness-implementation randomised controlled trial conducted at seven hospitals across Australia and New Zealand. Randomisation will be in a 3:2 ratio between the saline (heparinised saline and normal saline) and T-EDTA groups, with randomly varied blocks of size 10 or 20 and stratification by (1) healthcare facility; (2) CVAD type and (3) duration of dwell since insertion. Within the saline group, there will be a random allocation between normal and heparin saline. Participants can be re-recruited and randomised on insertion of a new CVAD. Primary outcome for effectiveness will be a composite of CVAD-associated bloodstream infections (CABSI), CVAD-associated thrombosis or CVAD occlusion during CVAD dwell or at removal. Secondary outcomes will include CABSI, CVAD-associated-thrombosis, CVAD failure, incidental asymptomatic CVAD-associated-thrombosis, other adverse events, health-related quality of life, healthcare costs and mortality. To achieve 90% power (alpha=0.05) for the primary outcome, data from 720 recruitments are required. A mixed-methods approach will be employed to explore implementation contexts from the perspective of clinicians and healthcare purchasers.Ethics and dissemination
Ethics approval has been provided by Children's Health Queensland Hospital and Health Service Human Research Ethics Committee (HREC) (HREC/22/QCHQ/81744) and the University of Queensland HREC (2022/HE000196) with subsequent governance approval at all sites. Informed consent is required from the substitute decision-maker or legal guardian prior to participation. In addition, consent may also be obtained from mature minors, depending on the legislative requirements of the study site. The primary trial and substudies will be written by the investigators and published in peer-reviewed journals. The findings will also be disseminated through local health and clinical trial networks by investigators and presented at conferences.Trial registration number
ACTRN12622000499785.Free full text

Preventing adverse events during paediatric cancer treatment: protocol for a multi-site hybrid randomised controlled trial of catheter lock solutions (the CLOCK trial)
Abstract
Introduction
Central venous access devices (CVADs) are commonly used for the treatment of paediatric cancer patients. Catheter locking is a routine intervention that prevents CVAD-associated adverse events, such as infection, occlusion and thrombosis. While laboratory and clinical data are promising, tetra-EDTA (T-EDTA) has yet to be rigorously evaluated or introduced in cancer care as a catheter lock.
Methods and analysis
This is a protocol for a two-arm, superiority type 1 hybrid effectiveness-implementation randomised controlled trial conducted at seven hospitals across Australia and New Zealand. Randomisation will be in a 3:2 ratio between the saline (heparinised saline and normal saline) and T-EDTA groups, with randomly varied blocks of size 10 or 20 and stratification by (1) healthcare facility; (2) CVAD type and (3) duration of dwell since insertion. Within the saline group, there will be a random allocation between normal and heparin saline. Participants can be re-recruited and randomised on insertion of a new CVAD. Primary outcome for effectiveness will be a composite of CVAD-associated bloodstream infections (CABSI), CVAD-associated thrombosis or CVAD occlusion during CVAD dwell or at removal. Secondary outcomes will include CABSI, CVAD-associated-thrombosis, CVAD failure, incidental asymptomatic CVAD-associated-thrombosis, other adverse events, health-related quality of life, healthcare costs and mortality. To achieve 90% power (alpha=0.05) for the primary outcome, data from 720 recruitments are required. A mixed-methods approach will be employed to explore implementation contexts from the perspective of clinicians and healthcare purchasers.
Ethics and dissemination
Ethics approval has been provided by Children’s Health Queensland Hospital and Health Service Human Research Ethics Committee (HREC) (HREC/22/QCHQ/81744) and the University of Queensland HREC (2022/HE000196) with subsequent governance approval at all sites. Informed consent is required from the substitute decision-maker or legal guardian prior to participation. In addition, consent may also be obtained from mature minors, depending on the legislative requirements of the study site. The primary trial and substudies will be written by the investigators and published in peer-reviewed journals. The findings will also be disseminated through local health and clinical trial networks by investigators and presented at conferences.
Trial registration number
ACTRN12622000499785.
Introduction
Central venous access devices (CVADs) are commonly used for the treatment of paediatric cancer patients who require frequent and prolonged intravenous therapy. CVADs most commonly used in cancer care within the participating sites include peripherally inserted central catheters (PICCs), tunnelled-cuffed CVADs (eg, Hickman catheters) and totally implanted CVADs (eg, Port-a-cath). These devices have contributed to improved paediatric cancer care outcomes by providing reliable access to the central vascular system for the administration of chemotherapy, immunotherapy and supportive therapies across cancer care. Most children with cancer will require a CVAD during their treatment.1 However, one in three CVADs becomes infected, thrombosed or blocked during treatment.2 These events disrupt and complicate treatment delivery and the recovery trajectory, potentially impacting morbidity and mortality.3,5
Common and significant complications impacting CVADs during cancer treatment include CVAD-associated bloodstream infections (CABSI), thrombosis (including deep-vein thrombosis (DVT)) and occlusion. CABSI is commonly caused by intraluminal catheter colonisation by bacteria and fungi. CVAD-associated DVT accounts for over 80% of all childhood DVT.2 6 Complete catheter occlusion, where the catheter cannot be aspirated or injected into, affects 14%–36% of children undergoing cancer treatment with long-term CVAD devices,7 with an estimated incidence rate of 1.4 per 1000 catheter days.8 Partial catheter occlusion—either aspirate occlusion (difficulty or inability to aspirate) or injection occlusion (difficulty or inability to inject into the catheter) occurs secondary to fibrin development, medication precipitate, catheter tip thrombus or tip malposition.2 These complications can lead to treatment delays and catheter rupture if excessive flushing force is applied by clinicians using the catheter.2 Children with cancer who have an occluded CVAD have a significantly increased risk of vessel thrombosis4 9 10 and local or systemic infection.11 These complications may result in CVAD failure, treatment delays and device replacement.12
Catheter locking is the instillation of a solution into a CVAD used to maintain patency in between CVAD use and/or reduce the risk of catheter-related complications.13 Solutions dwell in the CVAD tubing between therapy administration (between 6 hours and 8+ weeks), providing a fluid-based ‘lock’ to prevent backflow of blood through the tubing during normal movement (eg, thoracic pressure changes).13 Internationally, both normal saline and varied concentrations of heparinised saline are used as routine CVAD lock solutions in paediatric cancer care and beyond.14 15 There are two lock types: primary and secondary prophylaxis lock solutions.14 15 Primary prophylaxis lock solutions are used across CVAD types and populations prior to the development of infective and thrombotic complications.15 Secondary prophylactic lock solutions are in response to the patient having a history of infective or thrombotic complication or because of the perceived high risk of developing these conditions due to underlying diagnosis (eg, cancer) or CVAD characteristics (eg, chronic CVAD dependency).14 16
hours and 8+ weeks), providing a fluid-based ‘lock’ to prevent backflow of blood through the tubing during normal movement (eg, thoracic pressure changes).13 Internationally, both normal saline and varied concentrations of heparinised saline are used as routine CVAD lock solutions in paediatric cancer care and beyond.14 15 There are two lock types: primary and secondary prophylaxis lock solutions.14 15 Primary prophylaxis lock solutions are used across CVAD types and populations prior to the development of infective and thrombotic complications.15 Secondary prophylactic lock solutions are in response to the patient having a history of infective or thrombotic complication or because of the perceived high risk of developing these conditions due to underlying diagnosis (eg, cancer) or CVAD characteristics (eg, chronic CVAD dependency).14 16
The two most common prophylaxis lock solutions are heparin and normal saline locks. Historically, low-dose heparin (10–100 units/mL) has been used as a locking solution because of its anticoagulant properties.17 However, the effectiveness of heparin in preventing thrombotic occlusion over extended time periods is questionable, given its short half-life (60–90 min).18 Additionally, there is a risk of bleeding due to inadvertent administration of or exposure to heparin during the locking and aspiration procedures or overdose due to incorrect dilution or excess volume administration.17 Comparatively, normal saline (0.9% sodium chloride) relies on the turbulent movement of the solution through the catheter to physically clear blood or fibrin build‐up. To date, research in adult cancer populations has shown promise for using normal saline as a lock solution to prevent potential complications associated with heparin use, including the risk of heparin-induced thrombocytopaenia and accidental heparin administration.19 However, a recent Cochrane Systematic Review found there was insufficient evidence to establish the superiority of either normal or heparinised saline to prevent complications of CVADs in paediatric cancer care.15
min).18 Additionally, there is a risk of bleeding due to inadvertent administration of or exposure to heparin during the locking and aspiration procedures or overdose due to incorrect dilution or excess volume administration.17 Comparatively, normal saline (0.9% sodium chloride) relies on the turbulent movement of the solution through the catheter to physically clear blood or fibrin build‐up. To date, research in adult cancer populations has shown promise for using normal saline as a lock solution to prevent potential complications associated with heparin use, including the risk of heparin-induced thrombocytopaenia and accidental heparin administration.19 However, a recent Cochrane Systematic Review found there was insufficient evidence to establish the superiority of either normal or heparinised saline to prevent complications of CVADs in paediatric cancer care.15
Other lock solutions include anti-infective, taurolidine, citrate and ethanol locks. Taurolidine lock, derived from the amino acid taurine, exhibits broad-spectrum antimicrobial properties, including effectiveness against antibiotic-resistant bacteria while citrate lock functions as an anticoagulant agent by chelating ionised calcium while possessing antimicrobial activity in higher concentrations.20 21 However, the combined solution of taurolidine citrate was observed to be effective in reducing infectious complications but did not show efficacy against thrombotic complications.16 22 To promote antibiotic stewardship, therapeutic anti-infective lock solutions, such as antibiotic locks, are reserved for the treatment of patients with known infections. Ethanol locks have been used as treatment and secondary prophylaxis for CABSI in high-risk populations. However, recent randomised controlled trials (RCTs) in children with cancer or haematological disorders found that the treatment failure was similar to ethanol lock therapy and placebo (N=94, relative risk (RR) 1.0; 95% CI 0.6 to 1.6), and they were associated with an increased risk of catheter occlusion in comparison to heparinised saline (RR 1.8 (95% CI 1.1 to 2.9)).14 In a recent network meta-analysis of 29 studies, chelating agents and antibiotic locks given as prevention were associated with lower odds (OR 0.11; 95%
CI 0.6 to 1.6), and they were associated with an increased risk of catheter occlusion in comparison to heparinised saline (RR 1.8 (95% CI 1.1 to 2.9)).14 In a recent network meta-analysis of 29 studies, chelating agents and antibiotic locks given as prevention were associated with lower odds (OR 0.11; 95% CI 0.02 to 0.67; moderate quality; OR 0.19; 95%
CI 0.02 to 0.67; moderate quality; OR 0.19; 95% CI 0.05 to 0.79, high quality, respectively) of CVAD-associated BSI compared with heparinised saline.23 Preventative thrombolytic agents demonstrated reduced odds (OR 0.64, 95%
CI 0.05 to 0.79, high quality, respectively) of CVAD-associated BSI compared with heparinised saline.23 Preventative thrombolytic agents demonstrated reduced odds (OR 0.64, 95% CI 0.44 to 0.93; low quality) of CVAD occlusion, while ethanol had higher odds (OR 2.84, 95%
CI 0.44 to 0.93; low quality) of CVAD occlusion, while ethanol had higher odds (OR 2.84, 95% CI 1.31 to 6.16; high quality) compared with heparinised saline.23 No lock solution had effects on thrombosis prevention or treatment, CVAD failure, CVAD-associated BSI treatment failure or mortality.23
CI 1.31 to 6.16; high quality) compared with heparinised saline.23 No lock solution had effects on thrombosis prevention or treatment, CVAD failure, CVAD-associated BSI treatment failure or mortality.23
EDTA is a calcium and iron chelator with anticoagulant activity.24 Reconstituted as tetrasodium-EDTA (T-EDTA), the 4% solution has been proposed as an alternative primary prophylactic lock solution against CABSI, thrombosis and occlusion.24,26 During in vitro testing, T-EDTA reduced the count of most Gram-positive biofilms, and exposure time of >6 hour was associated with a count reduction close to the limits of detection. Similar results were also seen in Gram-negative bacteria and fungal species.25,27 In the clinical setting, an RCT in haemodialysis catheters (N=117) found a reduction in catheter colonisation/1000 catheter days (T-EDTA 0.14 vs heparin 1.08; incident rate ratio (IRR) 0.13; 95%
hour was associated with a count reduction close to the limits of detection. Similar results were also seen in Gram-negative bacteria and fungal species.25,27 In the clinical setting, an RCT in haemodialysis catheters (N=117) found a reduction in catheter colonisation/1000 catheter days (T-EDTA 0.14 vs heparin 1.08; incident rate ratio (IRR) 0.13; 95% CI 0.003 to 0.94), but it was underpowered for CABSI/1000 catheter days (T-EDTA 0.28 vs heparin 0.68; IRR 0.4; 95%
CI 0.003 to 0.94), but it was underpowered for CABSI/1000 catheter days (T-EDTA 0.28 vs heparin 0.68; IRR 0.4; 95% CI 0.08 to 2.09) in haemodialysis patients.28 A small pre/post implementation study for parenteral nutrition (PN)-dependent patients (N=22) found T-EDTA to be associated with a reduction of CABSI/1000 catheter days (pre 1.9 vs post 0.6) and eradication of CVAD occlusions, without significant adverse events.24 These results were replicated in a paediatric PN pre–post evaluation study (N=20; CABSI/1000 pre: 2.7 vs post: 0.0; p=0.002; occlusion median/1000 pre: 0 (0–5) vs post: 0 (0–2)).29 While these laboratory and clinical data are promising, T-EDTA has yet to be rigorously evaluated or introduced in cancer care as a catheter lock solution.
CI 0.08 to 2.09) in haemodialysis patients.28 A small pre/post implementation study for parenteral nutrition (PN)-dependent patients (N=22) found T-EDTA to be associated with a reduction of CABSI/1000 catheter days (pre 1.9 vs post 0.6) and eradication of CVAD occlusions, without significant adverse events.24 These results were replicated in a paediatric PN pre–post evaluation study (N=20; CABSI/1000 pre: 2.7 vs post: 0.0; p=0.002; occlusion median/1000 pre: 0 (0–5) vs post: 0 (0–2)).29 While these laboratory and clinical data are promising, T-EDTA has yet to be rigorously evaluated or introduced in cancer care as a catheter lock solution.
This study will aim to prevent adverse events associated with CVAD use in paediatric cancer care and has four main objectives:
Evaluation of the clinical effectiveness of T-EDTA, in comparison to normal or heparinised saline locks, to reduce CVAD complications (CABSI, thrombosis, occlusion) for children with cancer.
Evaluation of the effectiveness of normal and heparinised saline locks to reduce CVAD complications (CABSI, thrombosis, occlusion) for children with cancer.
Evaluation of the cost-effectiveness and perceived value of T-EDTA CVAD lock solutions in paediatric cancer care to reduce CVAD complications.
Evaluating the contexts for implementing T-EDTA as a CVAD lock solution in paediatric cancer treatment.
Methods and analysis
Approach
An effectiveness RCT employing a two-arm superiority design, integrating process evaluation and implementation (type 1 hybrid trial), will compare the clinical effectiveness of two CVAD lock solutions (T-EDTA vs saline) in preventing complications associated with CVAD (CABSI, thrombosis, occlusions). A secondary comparison will be between the two types of saline solutions: heparinised and normal saline. Simultaneously, a mixed-methods evaluation will assess the implementation contexts of CVAD maintenance in paediatric cancer care to prepare for the translation of study findings into practice.
Setting
The hybrid RCT will recruit and follow-up participants from tertiary and quaternary units in metropolitan and regional centres, including three Queensland public paediatric cancer care centres (Queensland Children’s (Brisbane), Gold Coast and Sunshine Coast Hospitals) and four other Australian and New Zealand hospitals: Monash Children’s Hospital (Melbourne), Royal Children’s Hospital (Melbourne), Sydney Children’s Hospital (Sydney) and Starship Children’s Hospital (Auckland).
Effectiveness RCT
Hypotheses
The study aims will be fulfilled through testing of the following hypotheses:
Primary hypothesis
Children treated for cancer who have their CVADs locked with T-EDTA will have significantly fewer CVAD complications (CABSI, thrombosis and occlusion) compared with children who have their CVADs locked with saline.
Secondary hypotheses
Children treated for cancer who have their CVADs locked with heparinised saline will have significantly fewer CVAD complications (CABSI, thrombosis and occlusion) compared with children who have their CVADs locked with normal saline.
Children treated for cancer who have their CVADs locked with T-EDTA will have a significantly reduced risk of CVAD failure compared with children who have their CVADs locked with saline.
The administration of T-EDTA lock solutions for children treated for cancer will not be associated with an increase in adverse events.
There will be significant cost savings to the Australian health system with T-EDTA in comparison to usual care (either normal or heparinised saline).
Interventions
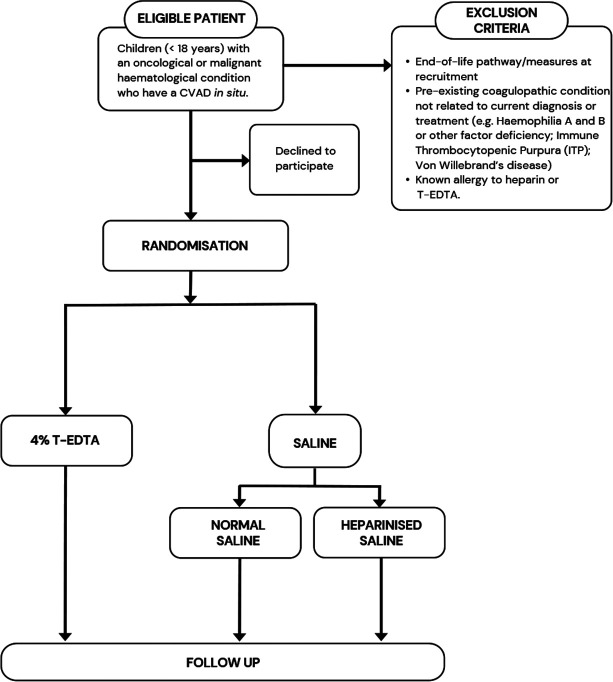
Participants will be randomly allocated to have their CVAD routinely locked (ie, when the device is not planned to be used for >6 hours or during routine CVAD care; with a maximum of 1 administration/per lumen/per 24 hours) (table 1, figure 1) with T-EDTA or Saline. To ensure feasibility, safety and generalisability, the solution strength (heparinised saline) and volume of the intervention products will be titrated to CVAD type, based on local/international clinical practice guidelines,13 30 our Cochrane Review15 and prior clinical evaluations.24 29 The allocated lock solution will be administered by clinicians based on clinical requirements (eg, if the CVAD is accessed, needleless connector changes, totally implanted device needling) with a minimum locking frequency between 1 and 8+ weeks (dependent on catheter type and organisational policy), and a maximum of one administration per lumen every 24 hours for a 3-month intervention period.
hours or during routine CVAD care; with a maximum of 1 administration/per lumen/per 24 hours) (table 1, figure 1) with T-EDTA or Saline. To ensure feasibility, safety and generalisability, the solution strength (heparinised saline) and volume of the intervention products will be titrated to CVAD type, based on local/international clinical practice guidelines,13 30 our Cochrane Review15 and prior clinical evaluations.24 29 The allocated lock solution will be administered by clinicians based on clinical requirements (eg, if the CVAD is accessed, needleless connector changes, totally implanted device needling) with a minimum locking frequency between 1 and 8+ weeks (dependent on catheter type and organisational policy), and a maximum of one administration per lumen every 24 hours for a 3-month intervention period.
Table 1
| Intervention arm | Catheter lock solution | Volume |
| (a) Normal saline | 0.9% sodium chloride | 10 mL mL |
| (b) Heparinised saline | ||
 PICC and tunnelled CVADs PICC and tunnelled CVADs | Sodium heparin 50 units/5 mL mL | 1–2 mL per catheter lumen mL per catheter lumen |
 Totally implanted CVADs Totally implanted CVADs | Sodium heparin 50 units/5 mL or 100 units/mL* mL or 100 units/mL* | 2 mL per catheter lumen mL per catheter lumen |
| (c) T-EDTA | ||
 PICC and tunnelled CVAD PICC and tunnelled CVAD | 4% T-EDTA | 1–2 mL per catheter lumen mL per catheter lumen |
 Totally implanted CVADs Totally implanted CVADs | 4% T-EDTA | 2 mL per catheter lumen mL per catheter lumen |
CVADscentral venous access devicesPICCsperipherally inserted central cathetersT-EDTAtetrasodium-EDTA
Sample population
Children (<18 years) with an oncological or malignant haematological condition who have a CVAD in situ (including PICCs, tunnelled (cuffed or non-cuffed) (eg, Hickman; Becton Dickinson, USA) and totally implanted (eg, PORT-A-CATH; Smith Medical, USA)) will be eligible for inclusion. Exclusion criteria include end-of-life pathway/measures at recruitment, pre-existing coagulopathic condition not related to current diagnosis or treatment (eg, haemophilia A and B or other factor deficiency; immune thrombocytopenic purpura; von Willebrand’s disease) and known allergy to heparin or T-EDTA. Participants will be prospectively and sequentially recruited at each of the study sites to allow follow-up for 3 months or until CVAD removal (whichever occurs first). Participants may be re-recruited to the study on the insertion of a new CVAD. Participants who are re-recruited will be randomised on each occasion. Including re-recruited participants gives asymptotically unbiased estimates of the treatment effect and correct type I error as long as follow-up for the previous recruitment period is complete prior to re-randomisation; randomisations for the same participant are performed independently and the treatment effect is consistent across all randomisations.31
Outcome measures and definitions
Primary outcome
The primary outcome is a serious CVAD complication. This is defined as a composite of CABSI, CVAD-associated thrombosis or CVAD occlusion during CVAD dwell:
CABSI: A laboratory-confirmed BSI where an eligible organism is identified in a CVAD that has been in place for >2 consecutive calendar days on the day of the first positive blood culture (day of CVAD placement being day 1) and the CVAD is in place on the date of the event or the day before (see Centers for Disease Control and Prevention Device (CDC)-associated Module BSI for full criteria), unrelated to infection at another site.32 Single positive blood cultures (SPBC) for common commensals may be considered CABSI in the presence of clinical evidence of systemic infection and where there is a clinical intent to treat as CABSI (ie, commencement of antibiotics).33 Adjudication of CABSI is confirmed by a masked infectious disease specialist.
CVAD-associated thrombosis: A symptomatic thrombosed CVAD vessel, or fibrin sheath occluding the vessel lumen, diagnosed via ultrasound or venography. The outcome is to be confirmed by masked radiologist and haematologist.34 ‘Symptomatic’ is defined by pain and/or swelling of the area drained by the veins where the device is placed, an increasing clinically obvious collateral network superficially in the region of the catheter or occluded CVAD.
CVAD occlusion: Complete injection and/or aspiration catheter occlusion of at least 1 CVAD lumen (assessed using the Catheter Injection and Aspiration classification system), administration of a thrombolytic agent and/or visible split in the CVAD structure related to occlusion event.35
Secondary outcomes
CABSI: Defined above, proportionally and per 1000 catheter days.
CDC-National Healthcare Safety Network CABSI only: Excluding SPBC for common commensals,32 proportionally and per 1000 catheter days.
CVAD-associated thrombosis: Defined above, proportionally and per 1000 catheter days.
CVAD occlusion: Defined above, proportionally and per 1000 catheter days.
CVAD failure: Cessation of CVAD function prior to completion of treatment, resulting in CVAD removal and replacement IV access.2 34
Asymptomatic CVAD-associated thrombosis: An asymptomatic non-occlusive filling defect or occluded CVAD vessel diagnosed via ultrasound, CT scan, MRI or venography, which was performed for other reasons, not related to clinical suspicion of CVAD-associated thrombosis or occlusion.36
Adverse events: Infusion reactions (eg, allergy), accidental administration of lock, thrombocytopaenia (<10×109/L), hypocalcaemia (<1.8
 mmol/L) and mortality.15 24
mmol/L) and mortality.15 24Health-related quality of life: Using the validated EuroQol Five Dimension-Y (EQ5D-Y) multiattribute utility instrument.37,39
Healthcare costs: Estimate of direct product costs, healthcare resource utilisation and complication-associated resource use (described below).
Sample size and study power
Our international and local data demonstrate the current CVAD complication rate for children with cancer is 33%,234 40,42 and a reduction to 22% is clinically important (to reduce treatment disruptions).3 This reduction appears feasible based on laboratory25 26 and clinical data.24 29 For the primary analysis, we will compare T-EDTA with a combined saline group with an allocation 3:2 (saline: T-EDTA) ratio. Participants allocated to receive saline will be randomly assigned either normal or heparin saline in a 1:1 ratio. The allocation ratio was chosen with regard to optimising sample size for the primary outcome, as well as the secondary comparison within the saline group (normal vs heparin). With 90% power, we will require primary outcome data from 720 recruitments (two-tailed alpha=0.05). This sample size is feasible to recruit over 3 years across the seven sites.
Recruitment, randomisation, allocation concealment and masking
Hospital-based Clinical Research Nurses (ReNs) based at each site will screen potential patients and assess whether patients meet all eligibility criteria and no exclusion criteria. After consultation with clinicians, eligible patients and their parents/caregivers will be approached regarding the project and given an information and consent form (online supplemental material 1). The ReNs explain the nature of the study, its purpose, procedures, expected duration and the potential benefits, risks and inconveniences in participation. A video explaining the trial requirements can also be used to aid the consent process at the discretion of the ReNs. Informed consent will be obtained, with a paper or electronic information and consent form used and documented. ReNs will be responsible for randomisation, educating clinical staff, patients and families, monitoring protocol compliance and collecting twice-weekly data on patients ‘on trial’ with outcome data for patients who have reached the 3-month follow-up period or had their CVAD removed. Patients will be randomised via a central web-based service (Griffith University). Randomisation will be in a 3:2 ratio between the saline: T-EDTA groups, with randomly varied blocks of size 10 or 20 and stratification by (1) healthcare facility; (2) CVAD type (PICC, tunnelled CVAD, totally implanted CVAD) and (3) duration of dwell since insertion (≥8 weeks). Within the saline groups, participants will be randomly allocated to either normal or heparinised saline in a 1:1 ratio. All interdisciplinary clinicians will be provided with extensive education regarding the use of the intervention product with additional resources available to support practice. Intervention fidelity will be assured via an electronic medical record-facilitated prescription data set where available, to ensure only the randomised product is prescribed for each study participant. Interventions will not be masked to participants, parents/caregivers and clinicians locking the CVAD. Infectious disease physicians and radiologists will be masked when determining infection and thrombosis outcomes and standardised definitions will be used for these outcomes, and the biostatistician will be masked to allocation when provided the dataset.
Insertion and care of CVAD
Other than the CVAD lock solution, all other CVAD insertion, care and management will be in accordance with local clinical practice guidelines (at all study sites).30 Variations in such care will be noted but not considered as protocol violations. Administration of a non-randomised routine CVAD lock solution or failure to use the randomised lock solution in the protocol defined time frame will be classified as a protocol deviation.
Data collection
Data will be directly collected by the ReNs from the patient and EMR and will be inputted into a purpose-built digital case research form in Research Electronic Data Capture, (Vanderbilt), hosted by the University of Queensland on password-protected devices.43 ReNs will follow participants biweekly for a maximum of 3 months (censored at that point) or until CVAD removal if this is earlier. Discharged patients will be followed up by phone, a method that has previously been demonstrated as reliable.44 45 Other data (including screening logs and signed consent forms) will be kept password protected or in locked filing cabinets in locked premises on-site at the healthcare facility or approved archiving facility.
At enrolment, the ReNs will obtain informed consent and demographic data on the patient (including First Nations status, language spoken at home, country of birth and cultural background), and CVAD provider and device factors that may potentiate CVAD-associated complications and failure.46 These factors will include patient (eg, critical illness factors, coagulopathy, diagnosis, neutropenia, a history of CABSI or venous thrombosis); device (device type, material, lumens, device size, vessel size) and provider (eg, inserter discipline, care during insertion, care during access).
Twice-weekly, the ReNs will visit or contact participants to assess and collect microbiological and clinical data for infectious, thrombotic and occlusive outcomes, device utilisation and any clinical, CVAD or treatment factors that have changed since recruitment.
Within 24 hours of CVAD removal notification or at 3 months, ReNs will collect data on the reason for removal, including presence and type of complication, if present and infusates.
hours of CVAD removal notification or at 3 months, ReNs will collect data on the reason for removal, including presence and type of complication, if present and infusates.
Within 1 week of CVAD removal or at 3 months (maximum data collection), ReNs will collect and finalise microbiological and clinical data for infectious, thrombotic and occlusive outcomes.
week of CVAD removal or at 3 months (maximum data collection), ReNs will collect and finalise microbiological and clinical data for infectious, thrombotic and occlusive outcomes.
Data analysis
Baseline characteristics for each group will be described using frequencies and percentages for categorical variables and mean and SDs (or median and IQR if appropriate) for continuous variables. The primary analysis will compare between-group CVAD complications using mixed effects logistic regression with a group (T-EDTA/saline) included as the fixed effect and child included as a random effect to account for re-recruitments of some participants. Effect estimates will be presented as both OR and absolute risk difference with corresponding 95% CIs. The key secondary analysis will examine CVAD complications between heparinised and normal saline. Secondary outcomes assessed using an interval scale will be analysed using mixed-effect linear regression models, dichotomous outcomes will be analysed using missed-effects logistic regression models, count outcomes will be analysed using mixed-effects Poisson regression, and time-to-event outcomes will be analysed using Cox proportional hazards regression with robust variance estimators. Sensitivity analyses to investigate the effect of re-recruitment will be conducted. The cause of any missing data will be assessed, and sensitivity analyses to investigate the potential impact of missingness will be undertaken using multiple imputation techniques, if appropriate.
Analysis will be ‘intention-to-treat’ with CVAD as the unit of measurement. Subgroup analyses of the primary outcome will compare totally implanted CVAD versus other CVAD; participant age (neonates/infants vs child/adolescent); previous CABSI versus no previous CABSI; first CVAD versus subsequent CVAD and newly inserted CVAD vs insertion >1 month prior to enrolment. A per-protocol analysis will assess the effect of protocol violations (ie, administration of non-randomised catheter lock solution). For the primary outcome, p values ≤0.05
month prior to enrolment. A per-protocol analysis will assess the effect of protocol violations (ie, administration of non-randomised catheter lock solution). For the primary outcome, p values ≤0.05 will be considered significant. No interim analyses or stopping criteria for efficacy, safety or futility assessments are planned for this study. A detailed statistical analysis plan will be finalised prior to the completion of data cleaning and will be published online.
will be considered significant. No interim analyses or stopping criteria for efficacy, safety or futility assessments are planned for this study. A detailed statistical analysis plan will be finalised prior to the completion of data cleaning and will be published online.
Estimating cost-effectiveness
A probabilistic decision model will be constructed to simulate the clinical pathways associated with the three interventions. We hypothesise significantly reduced costs over control from a direct hospital perspective for the episode of care and either no statistically significant difference or an increase in health-related quality-adjusted life-years. We will quantify additional costs, cost offsets and net monetary benefit, considering CVAD complications, catheter removal, reinsertion and treatment costs for complications per group. Costs associated with staff time for troubleshooting, replacement, consultation and equipment used will be estimated based on previously published estimates and localised expert opinion on the time taken and cost based on direct and indirect salary estimates of those staff involved. Regression analysis of the total cost per patient as the dependent variable will be conducted using a generalised linear model with trial group allocation as the predictive variable and controlling possible confounders. A gamma family, log-link model has been assumed given the typically skewed nature of health cost data but will be further tested for appropriate model specification. To explore potential differences in health-related QoL and patient experience, participants will be asked to complete both the EQ5D-Y multiattribute utility instrument at recruitment and study completion37 38 and the Australian Hospital Patient Experience Question Set,39 at study completion. Quality-adjusted life-years will be estimated using an area under the curve approach based on utility index values derived from responses to the EQ5D-Y. Cost–utility analysis will be carried out, and either the incremental cost per quality-adjusted life-year gained or net monetary benefit will be calculated. Deterministic and probabilistic sensitivity analysis will be used to characterise the uncertainty of the economic evaluation. Particular consideration will be given to the potential for cost-effectiveness to vary by patient, treatment and other characteristics.
Implementation mixed methods
Design and theoretical framework
Embedded in the type 1 hybrid trial approach is a mixed-methods, formal process evaluation. This will run parallel to the effectiveness RCT to identify potential/actual influences for implementation across Australian and New Zealand paediatric cancer care.47 It will comprise interviews, focus groups, surveys and a discrete choice experiment of children, caregivers, clinicians, purchasers and the public. Informed consent will be obtained from participants by a member of the study team prior to any study activities taking place.
Population and sample
Using purposive maximum variation sampling, participants will be selected with the intention to recruit approximately 45 children and parents (RCT participants and/or oncology families), approximately 50 interdisciplinary cancer care clinicians (including oncologists, haematologists, paediatricians, nurses, pharmacists) and approximately 5 purchasers/decision-makers from a range of metropolitan and regional settings (study sites) during the final years of the project. Following this, a sample of approximately 200 children and parents/caregivers (RCT participants) and 400 general population (recruited via a commercial survey panel provider) will be recruited to complete a discrete choice experiment online.
Data analysis
Transcribed interviews will be analysed using a deductive qualitative content analysis technique recommended for Consolidated Framework for Implementation Research (CFIR).48 The analysis will be performed by two researchers independently using the CFIR NVivo template, prepopulated with construct codes. The postinterview surveys will be summarised using descriptive statistics, with inferential analysis conducted to test for association as appropriate. Data from the DCE will be analysed using a multinomial logistic regression model to estimate mean estimates and a mixed multinomial logit model to explore the heterogeneity of preferences.49 Differences in preferences from the public and parents/caregivers will be explicitly explored. Patient values for a CVAD-associated adverse event avoided derived from the DCE will be included within sensitivity analyses of the economic evaluation.
Monitoring and safety
As recommended by the National Health and Medical Research Council, a risk-based approach to management and safety monitoring will be used.50 Central remote monitoring by the PMs of data quality and patient safety will be conducted ongoing. Onsite monitoring will be conducted every 6–12 months in response to recruitment numbers, data quality and identified safety issues as per the trial monitoring plan.
An independent Data and Safety Monitoring Board (DSMB) will be established to safeguard the interests of the trial participants, potential participants and investigators and to assess the safety and integrity of the trial’s interventions. The DSMB will periodically monitor safety, study conduct and progress. The DSMB will consider the relevant background and emerging evidence about the interventions or patient population under study. The DSMB will make recommendations to a separate governance committee concerning the continuation, modification or termination of the trial. The DSMB will comprise a paediatric haematologist, clinical trial professional (chair) and biostatistician. Prior to study commencement, the DSMB meeting schedule will be devised prior to the commencement of patient recruitment, once 10% of participants are recruited and then annually for the duration of the trial unless an earlier review is required. Following each review, the DSMB will advise the steering committee (SC) as to whether the trial should continue without change, be modified or be terminated.
Safety data, including serious adverse events, will be monitored and reported annually to the HRECs and local governance officers. If important protocol amendments are made (eg, changes to eligibility criteria), the chief investigator will update all investigators, HRECs, patient information and consent forms and the trial registry. Clinical trial insurance will be held by the university.
The SC will be chaired by an independent content expert and vice-chaired by the consumer representative. This group will consist of key investigators who supervise other research governance activities, including ethical approval, financial management and general adherence to good clinical practice. The SC will consider recommendations made by the DSMB and enact the appropriate course of action. The SC and chief investigator will have final responsibility and decision-making for the trial. The SC will meet every 6 months unless required earlier.
Patient and public involvement
During the pilot studies, participants and their parents/caregivers were asked for feedback on the acceptability of the intervention products, which helped shape the development of this study. A consumer representative (parent/caregiver of a child who has received cancer treatment) is an investigator on the research team, including the SC. On study completion, a plain language summary and associated peer-reviewed articles will be disseminated to the email addresses provided by study participants, conveying the overall findings.
Ethics and dissemination
This study has been prospectively registered with the Australian and New Zealand Clinical Trial Registry (online supplemental table 1) and is reported in accordance with the Consolidated Standards of Reporting Trials (CONSORT) statement.51 The study is supported by the Australian and New Zealand Children’s Haematology/Oncology Group (ANZCHOG). Sponsorship is provided by UQ, with insurance as per Australian legislative requirements. Ethics approval was provided by the Children’s Health Queensland Hospital and Health Service Human Research Ethics Committee (HREC) (HREC/22/QCHQ/81744) and the University of Queensland HREC (2022/HE000196) with additional HRECs added as required. The study is carried out in accordance with the Declaration of Helsinki, ICH E6(R2) for Good Clinical Practice (annotated with TGA comments), the National Statement on Ethical Conduct in Human Research,National Statement on Ethical Conduct in Human Research (2023)52 and the applicable local regulations. Any amendments to the study protocol will be rigorously documented and submitted for approval to the primary HREC and the research governance office at each site. On approval, these amendments will be promptly updated in the SC/DSMC documentation, included in the study’s newsletter for broader dissemination among stakeholders and reflected in the trial registry entries. For the RCT, informed consent is required from the substitute decision-maker or legal guardian, prior to participation. In addition, consent may also be obtained from mature minors, depending on the legislative requirements of the study site. A translator or independent witness will be used for families with diverse literacy and language requirements. Informed consent includes the participant data to be used in ancillary studies. Informed consent will also be obtained from participants who participate in the planned interviews, focus groups and discrete choice experiments.
The trial and substudies will be written by the investigators and published in peer-reviewed journals using appropriate reporting formats (ie, EQUATOR) with authorship consistent with the International Committee of Medical Journal Editors Guidelines. The findings will also be disseminated through local health and clinical trial networks by investigators and presented at conferences. All participants may elect to receive a language summary of the results. Deidentified data will be held at the University of Queensland.
Trial status
The trial began recruitment in September 2022. At the time of manuscript submission (15 June 2024), 165 patients had been recruited, with a 3-year recruitment period per study site planned.
supplementary material
online supplemental file 1
online supplemental table 1
Acknowledgements
We gratefully acknowledge John Roy and Rachel Edwards for their initial contributions and insights that helped shape the early stages of this work.
Footnotes
Funding: We would like to thank our funders, the Cancer Council Queensland (ACCR-118) and the University of Queensland.
Prepublication history and additional supplemental material for this paper are available online. To view these files, please visit the journal online (https://doi.org/10.1136/bmjopen-2024-085637).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Consent obtained from parent(s)/guardian(s).
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Data availability: Data generated and/or analysed during this trial are not yet publicly available as the trial is ongoing. When the trial is complete, data sets will be available from the Chief Investigator (AU) on reasonable request and after appropriate ethics approval is obtained.
References
Review Process File
Articles from BMJ Open are provided here courtesy of BMJ Publishing Group
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Control of Line Complications with KiteLock (CLiCK) in the critical care unit: study protocol for a multi-center, cluster-randomized, double-blinded, crossover trial investigating the effect of a novel locking fluid on central line complications in the critical care population.
Trials, 23(1):719, 30 Aug 2022
Cited by: 2 articles | PMID: 36042488 | PMCID: PMC9425798
Central venous Access device SeCurement And Dressing Effectiveness (CASCADE) in paediatrics: protocol for pilot randomised controlled trials.
BMJ Open, 6(6):e011197, 03 Jun 2016
Cited by: 4 articles | PMID: 27259529 | PMCID: PMC4893865
Routine Catheter Lock Solutions in Pediatric Cancer Care: A Pilot Randomized Controlled Trial of Heparin vs Saline.
Cancer Nurs, 45(6):438-446, 05 Feb 2022
Cited by: 1 article | PMID: 35131974 | PMCID: PMC9584054
Frequency of dressing changes for central venous access devices on catheter-related infections.
Cochrane Database Syst Rev, 2:CD009213, 01 Feb 2016
Cited by: 13 articles | PMID: 26827714 | PMCID: PMC8765739
Review Free full text in Europe PMC
Funding
Funders who supported this work.
Cancer Council Queensland (1)
Grant ID: ACCR-118

 1
1