Abstract
Background
People with HIV (PWH) who are coinfected with hepatitis B virus (HBV) have a higher risk of mortality compared with PWH alone. Populations such as people who inject drugs (PWID) and men who have sex with men (MSM) are particularly at high risk for HBV acquisition; yet, limited epidemiological data from these populations exist on HBV prevalence from low- and middle-income country settings (LMICs).Methods
We characterized the prevalence and correlates of HBV serological markers in a sample of PWID and MSM with HIV recruited across 15 Indian cities using hepatitis B surface antigen (HBsAg), hepatitis B core antibody (anti-HBc), and hepatitis B surface antibody (anti-HBs). Testing of stored specimens for the presence of these markers was performed on the Abbott ARCHITECT i1000 as per the manufacturer's instructions. Correlates of ever being infected with HBV (reactive for anti-HBc and/or HBsAg) and chronic HBV (reactive for HBsAg) among those ever infected were assessed using univariable and multivariable multilevel logistic regression models accounting for site-level clustering.Results
A total of 2198 (95%) of the 2314 participants recruited for the trial were screened for HBV markers. The median age among the PWID and MSM participants was 30 and 32 years, respectively. The prevalence of ever being infected with HBV was 75.6% vs 46.9% in PWID vs MSM, respectively (P < .01); prevalence of chronic infection was also higher in PWID vs MSM (14.1% vs 9.5%; P < .01). Correlates of ever being infected with HBV among PWID included unstable housing (adjusted odds ratio [aOR], 5.02) and sharing injection paraphernalia (aOR, 2.70), and among MSM, correlates included history of injection drug use (aOR, 4.87) and gender identity. The prevalence of isolated core (anti-HBc in the absence of anti-HBs) was 34.7% vs 29.4% in PWID vs MSM (P < .05). Vaccination serostatus was <10% in both populations.Conclusions
In this large sample of PWID and MSM with HIV, we observed a high prevalence of serology consistent with HBV infection and low vaccination, highlighting the need for routine screening and catch-up vaccination. The high prevalence of isolated anti-HBc reactivity highlights the need to understand the risk of reactivation with this serological pattern.Free full text

Hepatitis B Virus in People who Inject Drugs and Men who Have Sex With Men With HIV in India: A Cross-sectional Study
Abstract
Background
People with HIV (PWH) who are coinfected with hepatitis B virus (HBV) have a higher risk of mortality compared with PWH alone. Populations such as people who inject drugs (PWID) and men who have sex with men (MSM) are particularly at high risk for HBV acquisition; yet, limited epidemiological data from these populations exist on HBV prevalence from low- and middle-income country settings (LMICs).
Methods
We characterized the prevalence and correlates of HBV serological markers in a sample of PWID and MSM with HIV recruited across 15 Indian cities using hepatitis B surface antigen (HBsAg), hepatitis B core antibody (anti-HBc), and hepatitis B surface antibody (anti-HBs). Testing of stored specimens for the presence of these markers was performed on the Abbott ARCHITECT i1000 as per the manufacturer's instructions. Correlates of ever being infected with HBV (reactive for anti-HBc and/or HBsAg) and chronic HBV (reactive for HBsAg) among those ever infected were assessed using univariable and multivariable multilevel logistic regression models accounting for site-level clustering.
Results
A total of 2198 (95%) of the 2314 participants recruited for the trial were screened for HBV markers. The median age among the PWID and MSM participants was 30 and 32 years, respectively. The prevalence of ever being infected with HBV was 75.6% vs 46.9% in PWID vs MSM, respectively (P < .01); prevalence of chronic infection was also higher in PWID vs MSM (14.1% vs 9.5%; P < .01). Correlates of ever being infected with HBV among PWID included unstable housing (adjusted odds ratio [aOR], 5.02) and sharing injection paraphernalia (aOR, 2.70), and among MSM, correlates included history of injection drug use (aOR, 4.87) and gender identity. The prevalence of isolated core (anti-HBc in the absence of anti-HBs) was 34.7% vs 29.4% in PWID vs MSM (P < .05). Vaccination serostatus was <10% in both populations.
Conclusions
In this large sample of PWID and MSM with HIV, we observed a high prevalence of serology consistent with HBV infection and low vaccination, highlighting the need for routine screening and catch-up vaccination. The high prevalence of isolated anti-HBc reactivity highlights the need to understand the risk of reactivation with this serological pattern.
According to the 2024 World Health Organization Global Hepatitis Report, global mortality from viral hepatitis is increasing; it reached 1.3 million in 2022, which is similar to that of tuberculosis [1]. Hepatitis B virus (HBV) accounts for 83% of viral hepatitis deaths, and India has the second largest burden at 98 305 deaths from HBV in 2022 [1]. The prevalence of HBV differs among risk groups, and one of the highest-risk groups is people with HIV (PWH) due to shared routes of transmission. Approximately 10% of PWH have chronic hepatitis B infection (CHB) and are at 14-fold increased risk for liver-related mortality compared with those with either CHB or HIV alone [2]. There is also a large proportion of PWH who have been infected with HBV in whom the only marker of a past HBV infection is an antibody to hepatitis B core antigen (anti-HBc), also called isolated core antibody positivity [3–6]. These individuals remain at risk for reactivation of HBV resulting in CHB, which is more likely to occur with immunosuppression from HIV. Further, PWH who have isolated core antibody reactivity are at risk for reactivation of HBV with nucelos(t)ide-sparing antiretroviral treatment (ART) regimens, limiting their ability to benefit from novel and emerging long-acting ART regimens [7–9]. Thus, accurate prevalence data of CHB and isolated core antibody reactivity in PWH are essential to the World Health Organization (WHO) goal of HBV elimination, especially in countries like India with a large burden of HIV and global HBV-related mortality.
Recent prevalence data on HBV in PWH from India are limited. In an older multicountry study that recruited PWH from 2005 to 2010 including 249 people from Chennai, India, the prevalence of CHB and isolated core antibody reactivity was 4% and 24%, respectively [10]. In a cross-sectional study of men who have sex with men (MSM) across 12 Indian cities in 2013, the prevalence of CHB was 8%, although there was wide variation across the country, ranging from 0.5% to 18% [10, 11]. However, the study did not address the prevalence of anti-HBc reactivity. It is unknown whether the prevalence of CHB and isolated core antibody positivity in PWH has changed over the past decade and how these numbers may differ between persons who inject drugs (PWID) and MSM, which might have implications for baseline screening and selection of ART regimens as long-acting antiretrovirals (ARVs) continue to evolve.
In this cohort of PWID and MSM with HIV across 15 Indian cities, we determine the prevalence of ever being infected with HBV and correlates of infection. We then further characterize these individuals to determine the prevalence of having a positive hepatitis B surface antigen (HBsAg) using a highly sensitive assay and the prevalence of isolated core antibody reactivity. We also examine correlates for developing either of these HBV serological states to inform public health initiatives to target HIV and HBV treatment and prevention.
METHODS
Study Population and Design
The study population was comprised of 2314 PWH enrolled between October 30, 2017, and October 12, 2018, in the parent cluster randomized trial to evaluate the impact of nonmonetary voucher incentives on improving viral suppression among 1200 PWID and 1114 MSM with HIV across 16 sites in 15 Indian cities (1 city, New Delhi, had both a PWID site and an MSM site) [12]. Participants were referred by study staff at existing integrated care centers (ICCs) that provided HIV prevention, testing, and treatment services as well as other essential services for PWID (eg, harm reduction) and MSM (eg, condoms, sexually transmitted infection management) or by word of mouth from other study participants. Details on the design as well as primary outcomes have been described elsewhere [12, 13].
To be eligible for the trial, MSM and PWID participants had to be ≥18 years of age, have a documented report of HIV infection, be either ART naïve or on ART for ≤1 year, and have no intention to migrate over the next 12 months. Participants were excluded from the trial if they were receiving care in the private sector. There were no exclusion criteria based on sex assigned at birth or gender. Each of the 16 sites (8 MSM and 8 PWID) had a target enrollment of 150 participants, with a restriction that no more than 50% of participants could be ART experienced. MSM participants in India broadly identify with 1 of 3 gender identities: panthi (prefer penetrative anal intercourse), kothi (prefer receptive anal intercourse), and double decker (engage in both penetrative and receptive anal intercourse). In the analyses presented in this manuscript, we utilized the stored baseline specimens of participants enrolled in this cluster randomized trial to quantify HBV burden in a population of MSM and PWID with HIV in India.
Study Procedures
At the baseline visit, all participants provided written informed consent to participate in the cluster randomized trial and consented to future testing of their stored specimens [12]. At baseline, participants completed an interviewer-administered electronic survey and provided a blood sample. The survey captured information on demographics, sexual and substance use practices (including Alcohol Use Disorders Identification Test for alcohol use), ART treatment history, and depression (measured using the Patient Health Questionnaire-9). Collected blood specimens were processed locally and shipped to the YRGCARE central laboratory in Chennai for storage and future testing.
Laboratory Procedures
For this cross-sectional study, we utilized triple panel testing at the baseline visit to identify HBV burden, as recently outlined by the US Centers for Disease Control and Prevention [14, 15]. Testing for hepatitis B core antibody (anti-HBc), HBsAg, and hepatitis B surface antibody (anti-HBs) was performed on the ARCHITECT i1000 fully automated chemiluminescent microparticle immunoassay (CMIA) platform. A 1-time sample load strategy was utilized such that all the 3 assays were carried out using a single plasma specimen loaded into the platform, thereby simplifying sample processing. HBsAg was detected using the ARCHITECT HBsAg Next Qualitative assay (Abbott Diagnostics Division, Sligo, Ireland). The HBsAg Next assay has an analytical sensitivity at a cutoff of 0.005 IU/mL, increasing sensitivity, mutant detection, and detection of occult HBV infection [16, 17]. Anti-HBc was detected using the ARCHITECT Anti-HBc II assay (Abbott GmbH, Wiesbaden, Germany), and anti-HBs was detected using the ARCHITECT Anti-HBs assay (Abbott Diagnostics Division, Sligo, Ireland). All tests were performed as per the manufacturer's instructions, and manufacturer/Food and Drug Administration–approved cutoffs were utilized in the interpretation of the tests.
Outcome Measures
Based on the triple panel testing, we first classified each participant as having ever been infected with HBV, which was defined as reactive for HBsAg or anti-HBc or both. Participants were classified as never infected if they were nonreactive for both HBsAg and anti-HBc. We then classified participants who had ever been infected with HBV as being HBsAg reactive (likely CHB), recovered from HBV infection (anti-HBc and anti-HBs reactive), and isolated anti-HBc reactivity (anti-HBc reactive, HBsAg nonreactive, and anti-HBs nonreactive). Participants with anti-HBs titers >10 mIU/mL in the absence of anti-HBc and HBsAg were classified as having been vaccinated for HBV.
Statistical Analysis
Descriptive statistics were used to examine baseline demographic, risk behavior, and sexual health characteristics and to estimate proportions of PWID and MSM who were ever infected with HBV, had CHB, and were isolated anti-HBc positive. Correlates of ever being infected with HBV and CHB were assessed using univariable and multivariable multilevel logistic regression models with a random intercept to account for site-level clustering. Factors were included in the final multivariable model if they were independently associated with the outcomes, with a significance level of P < .05. All analyses were conducted using Stata, version 17 (StataCorp, College Station, TX, USA). Maps were created using ArcGIS Pro: 3.2.0.
Ethical Clearances
The parent trial including the testing of stored specimens was approved by the Johns Hopkins Medicine and YRGCARE institutional review boards.
RESULTS
Study Population
The study recruited 1200 PWID and 1114 MSM at 16 sites across 15 cities in India; of these, 95% (n = 2198, n = 1126 PWID, and n = 1072 MSM) had results available for HBsAg and anti-HBc testing; these represent the population for this study. A subset of 1325 participants who were reactive for anti-HBc (836 PWID and 489 MSM) also had testing for anti-HBs that could be used to evaluate for isolated core antibody reactivity and recovery from HBV. A subset of 826 (268 PWID and 558 MSM) who were never infected with HBV had testing for anti-HBs to determine vaccination status (Supplementary Figure 1). Serology consistent with HBV vaccination without infection (anti-HBs reactive/[anti-HBc and HBsAg negative]) was detected among 8.9% of PWID and 6.0% of MSM.
Among PWID, the median age (interquartile range [IQR]) was 30 (24–35) years. The majority of PWID participants were male (87%), were heterosexual (95%), had less than a high school education (86%), and were unemployed (38%) or received daily wages (42%). In addition, 11% had experienced unstable housing in the prior 6 months. The median age of first injection drug use (IQR) was 20 (18–25) years, and the median duration (IQR) was 8 (4–12) years. Around 72% of PWID ever shared needles, and ~80% had mild to severe depression. Participants had a median CD4 count (IQR) of 389 (262–506) cells/µL, 27% ever took ART, and 18% exhibited HIV viral suppression (defined as a viral load <150 copies/mL) at baseline (Table 1).
Table 1.
Characteristics of PWID and MSM With HIV Across 15 Indian Cities (2017–2018)
| PWID (n = 1200) | MSM (n = 1114) | |
|---|---|---|
| Sociodemographic | ||
 Age, y Age, y | 30 (24–35) | 32 (26–40) |
| Gender | ||
 Male Male | 1046 (87.2) | 1107 (99.4) |
 Female Female | 154 (12.8) | 6 (0.5) |
 Hijra Hijra | 0 (0) | 1 (0.1) |
| Sexual identitya | ||
 Straight/heterosexual Straight/heterosexual | 1137 (94.8) | 0 (0) |
 Panthi Panthi | 3 (0.3) | 221 (19.8) |
 Kothi Kothi | 2 (0.2) | 279 (25.0) |
 Double decker Double decker | 0 (0) | 312 (28.0) |
 Gay/MSM Gay/MSM | 4 (0.3) | 52 (4.7) |
 Bisexual Bisexual | 53 (4.4) | 248 (22.3) |
 Hijra Hijra | 0 (0) | 2 (0.2) |
| Marital status | ||
 Married Married | 439 (36.6) | 595 (53.4) |
 Never married Never married | 588 (49.0) | 454 (40.8) |
| Widowed/divorced/separated | 173 (14.4) | 65 (5.8) |
| Education | ||
 High school or above High school or above | 162 (13.5) | 307 (27.6) |
 No schooling No schooling | 214 (17.8) | 145 (13.0) |
 Primary/secondary school Primary/secondary school | 824 (68.7) | 662 (59.4) |
| Employment | ||
 Monthly/weekly wages Monthly/weekly wages | 261 (21.8) | 542 (48.6) |
 Daily wages Daily wages | 498 (41.5) | 403 (36.2) |
 Unemployed Unemployed | 441 (37.7) | 168 (15.1) |
| Unstable housing | 126 (10.5) | 14 (1.3) |
| Substance use | ||
| Ever inject drugs | 1200 (100) | 81 (7.3) |
| Age first injected | 20 (18–25) | 22 (18–26) |
| Duration of drug use | 8 (4–12) | 7 (3–12.5) |
| Drugs ever injected | ||
 Heroin Heroin | 831 (69.3) | 44 (54.3) |
 Buprenorphine Buprenorphine | 650 (54.2) | 14 (17.3) |
 Allergy medicine Allergy medicine | 508 (42.3) | 44 (54.3) |
 Otherb Otherb | 409 (34.1) | 27 (2.4) |
| Drugs ever injected, past 6 mo | ||
 Heroin Heroin | 475 (57.2) | 2 (4.6) |
 Buprenorphine Buprenorphine | 481 (74.0) | 5 (35.7) |
 Allergy medicine Allergy medicine | 369 (72.6) | 27 (61.4) |
 Otherb Otherb | 59 (4.9) | 14 (1.3) |
| Ever shared needle/syringe | 868 (72.3) | 64 (5.8) |
| Injection frequency, past 6 mo | ||
 Never Never | 334 (28.5) | 26 (32.1) |
 Daily Daily | 278 (23.7) | 9 (11.1) |
 Less than daily Less than daily | 561 (47.8) | 46 (56.8) |
| Average No. of times injected/d | ||
 Once Once | 274 (31.6) | 14 (25.5) |
 Twice Twice | 245 (28.3) | 33 (60.0) |
 ≥3 times ≥3 times | 347 (40.1) | 8 (14.5) |
| Alcohol dependence | 300 (25.0) | 206 (18.5) |
| Noninjection drug use, past 6 mo | 795 (66.3) | 70 (6.3) |
| Sexual behaviors | ||
 Lifetime sexual partners Lifetime sexual partners | 2 (1–4) | 23 (9–80) |
 Lifetime male sexual partners, among MSM Lifetime male sexual partners, among MSM | … | 20 (5–60) |
 Recent sexual partners, past 6 mo Recent sexual partners, past 6 mo | 1 (0–1) | 4 (2–13) |
 Unprotected sex, past 6 mo (all genders) Unprotected sex, past 6 mo (all genders) | 513 (78.9) | 777 (76.5) |
 Unprotected anal sex, past 6 mo (among MSM) Unprotected anal sex, past 6 mo (among MSM) | … | 669 (72.1) |
| Psychosocial | ||
 Depression severity Depression severity | ||
  None–minimal None–minimal | 243 (20.3) | 448 (40.2) |
  Mild Mild | 502 (41.8) | 315 (28.3) |
  Moderate Moderate | 329 (27.4) | 245 (22.0) |
  Moderately severe Moderately severe | 108 (9.0) | 98 (8.8) |
 Severe Severe | 18 (1.5) | 8 (0.7) |
| HIV | ||
 Duration on ART Duration on ART | ||
  Not on ART at baseline Not on ART at baseline | 872 (72.7) | 779 (69.9) |
  <6 mo <6 mo | 171 (14.3) | 195 (17.5) |
  6 mo to a year 6 mo to a year | 157 (13.1) | 140 (12.6) |
  CD4 count, cells/µL CD4 count, cells/µL | 389 (262–506) | 288 (168–456) |
  HIV viral load suppression HIV viral load suppression | 214 (17.9) | 246 (22.1) |
Abbreviations: ART, antiretroviral therapy; HBV, hepatitis B virus; IQR, interquartile range; MSM, men who have sex with men; PWID, people who inject drugs.
aPanthi (prefer penetrative anal intercourse only); kothi (prefer receptive anal intercourse only); double decker (engage in both penetrative and receptive anal intercourse).
bOther drugs include self-reported use of cocaine, painkillers, sedatives, and other drugs. Response options within the category are not mutually exclusive.
Among MSM, the median age (IQR) was 32 (26–40) years, and gender identities included 20% panthi, 25% kothi, and 28% double decker. Among MSM, 53% were married at baseline, 72% had less than a high school education, and 51% were either unemployed (15%) or receiving daily wages (36%). The median number of male lifetime sexual partners (IQR) was 20 (5–60), and 72% had engaged in unprotected anal sex in the prior 6 months. About 60% experienced mild to severe depression. The median CD4 count (IQR) was 288 (168–456) cells/µL, about a third (30%) were on ART, and 22% had HIV viral suppression at study entry. Injection drug use was low among MSM; 7% (n = 81) self-reported ever injecting (Table 1).
Prevalence and Correlates of HBV Infection
To assess the prevalence of HBV serological markers among the study population, we included all 2198 participants with anti-HBc and HBsAg testing. A greater proportion of PWID (75.6%) than MSM (46.9%) had ever been infected with HBV (P < .01), and, similarly, a greater proportion of PWID vs MSM were HBsAg reactive (14.1% and 9.5%, respectively; P = .001). The prevalence varied by city (Figure 1A–D). To determine if the difference in HBsAg reactivity between PWID and MSM was due to differences in ability to recover once infected or due to differences in exposure, we determined the prevalence of being HBsAg reactive among those ever infected with HBV. We found that a similar percentage of anti-HBc-positive PWID and MSM were HBsAg reactive (163 [19%] of 851 and 102 [20%] of 503, respectively; P = .639), supporting that the ability to control HBV once infected did not differ between the groups (Table 2). Additionally, in this sample, the prevalence of isolated core antibody reactivity was high, at 34.7% and 29.4% of PWID and MSM, respectively (P = .047). The characteristics of PWID and MSM with isolated antibody reactivity are shown in Supplementary Table 1.
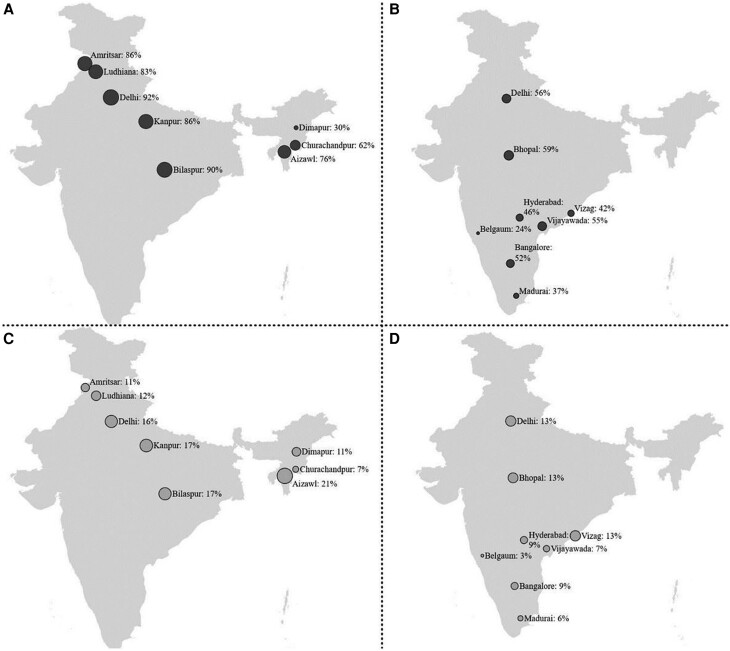
Site-level prevalence of (A) exposure to hepatitis B among PWID, (B) exposure to hepatitis B among MSM, (C) chronic hepatitis B among PWID, and (D) chronic hepatitis B among MSM. The size of the dots is proportional to the percentage of exposure to HBV (A and B) or HBsAg reactive (C and D) in each city. Abbreviations: HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; MSM, men who have sex with men; PWID, people who inject drugs.
Table 2.
Comparison of Hepatitis B Virus Infection Status in PWID and MSM With HIV Across 15 Indian Cities
| PWID | MSM | P Value* | |
|---|---|---|---|
| Ever infected with HBV | 851 (75.6)a | 503 (46.9)a | <.001 |
| HBsAg reactive (study population prevalence) | 163 (14.1)b | 102 (9.5)b | <.001 |
| Outcomes among those ever infected with HBV | … | … | |
 HBsAg reactivec HBsAg reactivec | 163 (19.2) | 102 (20.3) | .639 |
 Isolated core antibody positive Isolated core antibody positive | 295 (34.7) | 148 (29.4) | .047 |
 Anti-HBs and anti-HBc reactive (recovered from HBV)d Anti-HBs and anti-HBc reactive (recovered from HBV)d | 378 (44.4) | 239 (47.5) | .269 |
Abbreviations: HBsAg, hepatitis B surface antigen; MSM, men who have sex with men; PWID, people who inject drugs.
*From the χ2 test.
aTotal tested for anti-HBc: PWID = 1126, MSM = 1072.
bTotal tested for HBsAg: PWID = 1160, MSM = 1077.
cn = 5 PWID and n = 1 MSM were missing HBsAg Nxt results.
dn = 6 PWID and n = 5 MSM were missing required lab results to determine recovery from HBV.
Among PWID, correlates of ever having been infected with HBV in multivariable analysis included those who received daily wages (adjusted odds ratio [aOR], 2.57; 95% CI 1.47–4.52) compared with those receiving monthly/weekly wages, unstable housing in the past 6 months (aOR, 5.02; 95% CI, 1.13–22.42), ever buprenorphine injection (aOR, 2.67; 95% CI, 1.43–4.98), heroin injection in the past 6 months (aOR, 2.39; 95% CI, 1.24–4.58), and ever sharing needles or syringes (aOR, 2.70; 95% CI, 1.63–4.48) (Table 3).
Table 3.
Factors Associated With Ever Being Infected With Hepatitis B Virus Among PWID and MSM With HIV Across 15 Indian Cities
| PWID (n = 1126) | MSM (n = 1072) | |||||||
|---|---|---|---|---|---|---|---|---|
| Not Reactive for Anti-HBc, No. (%)/Median (IQR) | Reactive for Anti-HBc,a No. (%)/Median (IQR) | Odds Ratio (95% CI) | Adjusted Odds Ratio (95% CI) | Not Reactive for Anti-HBc, No. (%)/Median (IQR) | Reactive for Anti-HBc,a No. (%)/Median (IQR) | Odds Ratio (95% CI) | Adjusted Odds Ratio (95% CI) | |
| No. | 275 | 851 | 569 | 503 | ||||
| Sociodemographic | ||||||||
 Age, yb Age, yb | 30 (24–35) | 29 (24–35) | 1.25 (1.01–1.54)* | 1.20 (0.81–1.78) | 30 (26–39) | 30 (25–35) | 1.31 (1.14–1.50)** | 1.34 (1.13–1.60)* |
| Gender | ||||||||
 Male Male | 188 (19.1) | 799 (81.0) | Reference | Reference | 565 (53.1) | 500 (47.0) | … | … |
 Female Female | 87 (62.6) | 52 (37.4) | 0.52 (0.32–0.84)** | 0.72 (0.38–1.36) | 4 (66.7) | 2 (33.3) | … | … |
 Hijra Hijra | … | … | … | … | … | 1 (100) | … | … |
| Sexual identityc | ||||||||
 Straight/heterosexual Straight/heterosexual | 266 (25.0) | 798 (75.0) | … | … | … | … | … | … |
 Panthi Panthi | 0 | 3 (100) | … | … | 143 (66.2) | 73 (33.8) | Reference | Reference |
 Kothi Kothi | 0 | 2 (100) | … | … | 122 (45.5) | 146 (54.5) | 1.88 (1.26–2.79)** | 2.01 (1.31–3.08)* |
 Double decker Double decker | … | … | … | … | 154 (51.7) | 144 (48.3) | 1.37 (0.92–2.04) | 1.33 (0.88–2.00) |
 Gay/MSM Gay/MSM | 1 (25.0) | 3 (75.0) | … | … | 28 (54.9) | 23 (45.1) | 0.84 (0.41–1.71) | 0.80 (0.38–1.70) |
 Bisexual Bisexual | 8 (15.4) | 44 (84.6) | … | … | 121 (51.1) | 116 (49.0) | 1.21 (0.76–1.93) | 1.34 (0.83–2.19) |
 Hijra Hijra | … | … | … | … | 1 (50.0) | 1 (50.0) | … | |
| Marital status | ||||||||
 Married Married | 137 (33.2) | 276 (66.8) | Reference | Reference | 307 (53.1) | 271 (46.9) | Reference | Reference |
 Never married Never married | 103 (18.6) | 451 (81.4) | 1.14 (0.80–1.61) | 1.47 (0.91–2.37) | 226 (52.6) | 204 (47.4) | 0.90 (0.69–1.17) | 1.16 (0.82–1.65) |
| Widowed/divorced/separated | 35 (22.0) | 124 (78.0) | 1.56 (0.97–2.51) | 1.60 (0.89–2.86) | 36 (56.3) | 28 (43.8) | 0.75 (0.44–1.28) | 0.61 (0.35–1.07) |
| Education | ||||||||
 High school or above High school or above | 46 (30.1) | 107 (69.9) | Reference | Reference | 186 (62.8) | 110 (37.2) | Reference | Reference |
 No schooling No schooling | 44 (22.2) | 154 (77.8) | 1.28 (0.73–2.24) | 0.66 (0.32–1.35) | 54 (39.4) | 83 (60.6) | 2.87 (1.85–4.45)** | 2.33 (1.43–3.78)* |
 Primary school/secondary school Primary school/secondary school | 185 (23.9) | 590 (76.1) | 1.27 (0.83–1.95) | 0.79 (0.47–1.33) | 329 (51.5) | 310 (48.5) | 1.81 (1.35–2.44)** | 1.63 (1.16–2.29)* |
| Employment | ||||||||
 Monthly/weekly wages Monthly/weekly wages | 71 (29.1) | 173 (70.9) | Reference | Reference | 281 (54.1) | 238 (45.9) | Reference | Reference |
 Daily wages Daily wages | 51 (10.7) | 425 (89.3) | 2.25 (1.43–3.52)** | 2.57 (1.47–4.52)** | 194 (50.1) | 193 (49.9) | 1.18 (0.89–1.56) | 0.83 (0.61–1.12) |
 Unemployed Unemployed | 153 (37.7) | 253 (62.3) | 1.14 (0.75–1.74) | 1.32 (0.79–2.18) | 93 (56.4) | 72 (43.6) | 0.92 (0.64–1.33) | 0.92 (0.62–1.37) |
| Unstable housing | 7 (6.0) | 109 (94.0) | 2.56 (1.04–6.33)* | 5.02 (1.13–22.42)* | 4 (33.3) | 8 (66.7) | … | … |
| Substance use | ||||||||
 Ever inject drugs Ever inject drugs | 275 (24.4) | 851 (75.6) | … | … | 17 (21.5) | 62 (78.5) | 4.24 (2.29–7.83)** | 4.87 (2.58–9.21)** |
 Age first injected, y Age first injected, y | 20 (18–25) | 20 (17–25) | 1.01 (0.98–1.04) | 21 (19–26) | 22 (18–25) | 1.00 (0.92–1.09) | … | |
 Duration of drug use, y Duration of drug use, y | 7 (4–13) | 7 (4–12) | 1.03 (1.00–1.05)* | 1.01 (0.97–1.06) | 4 (3–8) | 7 (3–13) | 1.05 (0.96–1.16) | … |
| Drugs ever injected | ||||||||
 Heroin Heroin | 185 (23.5) | 601 (76.5) | 0.96 (0.61–1.52) | … | 7 (16.7) | 35 (83.3) | … | … |
 Buprenorphine Buprenorphine | 68 (11.1) | 545 (88.9) | 2.97 (1.71–5.16)** | 2.67 (1.43–4.98)** | 2 (15.5) | 11 (84.6) | … | … |
 Allergy medicine Allergy medicine | 55 (11.4) | 428 (88.6) | 1.95 (1.01–3.77)* | 0.96 (0.51–1.80) | 9 (20.9) | 34 (79.1) | … | … |
 Otherd Otherd | 118 (31.0) | 263 (69.0) | 2.50 (1.55–4.03)** | 1.37 (0.89–2.12) | 6 (22.2) | 21 (77.8) | … | … |
| Drugs ever injected, past 6 mo | ||||||||
 Heroin Heroin | 77 (17.0) | 375 (83.0) | 2.06 (1.39–3.05)** | 2.39 (1.24–4.58)** | 0 (0) | 2 (100) | … | … |
 Buprenorphine Buprenorphine | 50 (11.0) | 403 (89.0) | 1.02 (0.58–1.81) | … | 1 (25.0) | 3 (75.0) | … | … |
 Allergy medicine Allergy medicine | 35 (10.1) | 313 (89.9) | 1.79 (0.86–3.74) | … | 6 (23.1) | 20 (76.9) | … | … |
 Otherd Otherd | 7 (13.2) | 46 (86.8) | 1.76 (0.71–4.37) | … | 4 (28.6) | 10 (71.4) | … | … |
| Ever shared needle/syringe | 125 (15.4) | 686 (84.6) | 2.39 (1.55–3.70)** | 2.70 (1.63–4.48)** | 12 (19.4) | 50 (80.7) | … | … |
| Injection frequency, past 6 mo | ||||||||
 Never Never | 146 (47.6) | 161 (52.4) | Reference | Reference | 7 (26.9) | 19 (73.1) | … | … |
 Daily Daily | 21 (12.1) | 232 (87.9) | 2.88 (1.66–5.01)** | 1.07 (0.47–2.42) | 0 (0) | 9 (100) | … | … |
 Less than daily Less than daily | 97 (18.4) | 431 (81.6) | 1.69 (1.11–2.58)* | 0.70 (0.33–1.48) | 10 (22.7) | 34 (77.3) | … | … |
| Average No. of times injected/d | ||||||||
 Once Once | 44 (17.0) | 215 (83.0) | Reference | … | 2 (14.3) | 12 (85.7) | … | … |
 Twice Twice | 33 (14.5) | 194 (85.5) | 0.57 (0.10–3.18) | … | 7 (22.6) | 24 (77.4) | … | … |
 ≥3 times ≥3 times | 52 (15.6) | 281 (84.4) | 1.17 (0.09–15.32) | … | 1 (12.5) | 7 (87.5) | … | … |
 Alcohol dependence Alcohol dependence | 77 (27.4) | 204 (72.6) | 0.91 (0.64–1.30) | … | 104 (52.8) | 93 (47.2) | 1.06 (0.77–1.46) | … |
 Noninjection drug use, past 6 mo Noninjection drug use, past 6 mo | 160 (21.3) | 592 (78.7) | 1.10 (0.78–1.55) | … | 30 (46.2) | 35 (53.9) | 0.98 (0.56–1.69) | … |
| Sexual behaviors | ||||||||
 Lifetime sexual partnerse Lifetime sexual partnerse | 2 (1–3) | 2 (1–5) | 1.04 (0.92–1.17) | … | 20 (7–50) | 30 (13–150) | 1.01 (1.00–1.01)* | 1.01 (1.00–1.01) |
 Lifetime male sexual partners, among MSMe Lifetime male sexual partners, among MSMe | … | … | … | … | 15 (4–50) | 25 (9–100) | 1.01 (1.00–1.01)* | … |
 Recent sexual partners, past 6 mo Recent sexual partners, past 6 mo | 1 (0–1) | 1 (0–1) | … | … | 4 (2–11) | 5 (2–16) | 1.00 (1.00–1.00) | … |
 Unprotected sex, past 6 mo (all genders) Unprotected sex, past 6 mo (all genders) | 160 (33.0) | 325 (67.0) | 0.99 (0.59–1.69) | … | 413 (54.9) | 340 (45.2) | 0.74 (0.53–1.05) | … |
 Unprotected anal sex, past 6 mo (among MSM) Unprotected anal sex, past 6 mo (among MSM) | … | … | … | … | 352 (54.2) | 297 (45.8) | 0.77 (0.53–1.12) | … |
| Psychosocial | ||||||||
 Depression severity Depression severity | … | … | … | … | … | … | … | … |
  None–minimal None–minimal | 71 (32.1) | 150 (67.9) | Reference | Reference | 257 (59.5) | 175 (40.5) | Reference | Reference |
  Mild Mild | 129 (27.0) | 348 (73.0) | 1.43 (0.95–2.16) | 1.49 (0.86–2.57) | 167 (54.9) | 137 (45.1) | 1.06 (0.77–1.46) | 0.99 (0.71–1.38) |
  Moderate Moderate | 60 (19.7) | 245 (80.3) | 1.79 (1.11–2.88)* | 1.53 (0.84–2.76) | 96 (40.9) | 139 (59.2) | 1.79 (1.26–2.55)* | 1.73 (1.20–2.49)* |
  Moderately severe Moderately severe | 14 (13.3) | 91 (86.7) | 1.94 (0.95–3.98) | 1.30 (0.57–2.95) | 45 (48.4) | 48 (51.6) | 1.29 (0.80–2.07) | 1.24 (0.75–2.03) |
  Severe Severe | 1 (5.6) | 17 (94.4) | 6.08 (0.76–48.75) | … | 4 (50) | 4 (50) | 1.20 (0.29–4.97) | 1.34 (0.31–5.80) |
| HIV | ||||||||
 Duration on ART Duration on ART | … | … | … | … | … | … | … | … |
  Not on ART at baseline Not on ART at baseline | 179 (21.8) | 642 (78.2) | Reference | … | 407 (54.0) | 347 (46.0) | Reference | … |
  <6 mo <6 mo | 52 (32.9) | 106 (67.1) | 0.86 (0.57–1.32) | … | 90 (48.1) | 97 (51.9) | 1.30 (0.94–1.81) | … |
  6 mo to a year 6 mo to a year | 44 (29.9) | 103 (70.1) | 1.23 (0.78–1.93) | … | 72 (55.0) | 59 (45.0) | 0.95 (0.65–1.41) | … |
 CD4 count,f cells/µL CD4 count,f cells/µL | 370 (216–497) | 390 (279–511) | 1.00 (0.99–1.01) | … | 262 (144–416) | 311 (187–496) | 1.00 (1.00–1.01) | … |
 HIV viral load suppression HIV viral load suppression | 72 (37.1) | 122 (63.0) | 0.88 (0.60–1.29) | … | 125 (53.9) | 107 (46.1) | 0.99 (0.97–1.02) | … |
Abbreviations: anti-HBc, hepatitis B core antibody; ART, antiretroviral therapy; IQR, interquartile range; MSM, men who have sex with men; PWID, people who inject drugs.
* P < .05; **P < .01.
aIncludes anyone ever infected with HBV.
bScaled per 10 y.
cPanthi (prefer penetrative anal intercourse only); kothi (prefer receptive anal intercourse only); double decker (engage in both penetrative and receptive anal intercourse).
dOther drugs include self-reported use of cocaine, painkillers, sedatives, and other drugs. Response options within the category are not mutually exclusive.
eScaled per 10 partners.
fScaled per 10 cells/µL.
Among MSM, correlates of ever having been infected with HBV in multivariable analysis included older age (aOR per 10 years, 1.34; 95% CI, 1.13–1.60), sexual identity (aOR for MSM who identified as being “kothi” vs “panthi,” 2.01; 95% CI, 1.31–3.08), higher educational attainment (vs no education for primary school; aOR, 2.33; 95% CI, 1.43–3.78; and secondary school: aOR, 1.63; 95% CI, 1.16–2.29), history of injection drug use (aOR, 4.87; 95% CI, 2.58–9.21), and depressive symptoms (aOR for moderate vs minimal symptoms, 1.73; 95% CI, 1.20–2.49) (Table 3).
Prevalence and Correlates of Being HBsAg Reactive Among Those Ever Infected With HBV (Anti-HBc Reactive)
The overall prevalence of being HBsAg reactive among PWID was 14.1% (Table 2). Of the 851 PWID who were ever infected with HBV, 163 (19%) were HBsAg reactive (Table 4). Among the 163 PWID with CHB, the median age (IQR) was 29 (24–33) years, and 89% were male. The median age of first injection (IQR) was 20 (18–26) years, 77% self-reported ever sharing a needle or syringe, and 43% reported injecting less than daily. In addition, 20% had alcohol dependence. In multivariable analysis, a shorter duration of drug use was associated with having CHB (aOR, 0.94 per year of drug use; 95% CI, 0.90–0.98).
Table 4.
Factors Associated With Being HBsAg Reactive Among PWID and MSM With HIV Across 15 Indian Cities
| PWID (n = 846) | MSM (n = 502) | |||||||
|---|---|---|---|---|---|---|---|---|
| HBsAg Nonreactive, No. %/Median (IQR) | HBsAg Reactive, No. %/Median (IQR) | Odds Ratio (95% CI) | Adjusted Odds Ratio (95% CI) | HBsAg Nonreactive, No. %/Median (IQR) | HBsAg Reactive, No. %/Median (IQR) | Odds Ratio (95% CI) | Adjusted Odds Ratio (95% CI) | |
| No. | 683 | 163 | … | … | 400 | 102 | … | … |
| Sociodemographic | ||||||||
 Age, ya Age, ya | 30 (24–35) | 29 (24–33) | 0.85 (0.67–1.08) | 1.19 (0.83–1.70) | 34 (27–40) | 31 (27–38) | 0.84 (0.66–1.08) | 0.71 (0.52–0.97)* |
| Gender | ||||||||
 Male Male | 649 (81.7) | 145 (18.3) | Reference | Reference | 397 (79.6) | 102 (20.4) | … | … |
 Female Female | 34 (65.4) | 18 (34.6) | 2.20 (1.14–4.25)* | 2.10 (0.77–5.73) | 2 (100) | 0 (0) | … | … |
 Hijra Hijra | 0 (0) | 0 (0) | … | … | 1 (100) | 0 (0) | … | … |
| Sexual identityb | ||||||||
 Straight/heterosexual Straight/heterosexual | 640 (80.7) | 153 (19.3) | … | … | 0 (0) | 0 (0) | … | … |
 Panthi Panthi | 3 (100) | 0 (0) | … | … | 54 (74.0) | 19 (26.0) | Reference | Reference |
 Kothi Kothi | 0 (0) | 2 (100) | … | … | 120 (82.2) | 26 (17.8) | 0.61 (0.31–1.21) | 0.58 (0.28–1.18) |
 Double decker Double decker | 0 (0) | 0 (0) | … | … | 122 (84.7) | 22 (15.3) | 0.51 (0.25–1.04) | 0.49 (0.24–1.01) |
 Gay/MSM Gay/MSM | 3 (100) | 0 (0) | … | … | 16 (69.6) | 7 (30.4) | 1.25 (0.44–3.52) | 1.15 (0.38–3.51) |
 Bisexual Bisexual | 37 (84.1) | 7 (15.9) | … | … | 87 (75.7) | 28 (24.3) | 0.91 (0.46–1.80) | 0.79 (0.39–1.61) |
 Hijra Hijra | 0 (0) | 1 (100) | … | … | 1 (100) | 0 (0) | … | … |
| Marital status | ||||||||
 Married Married | 218 (79.6) | 56 (20.4) | Reference | Reference | 217 (80.1) | 54 (19.9) | Reference | Reference |
 Never married Never married | 361 (80.6) | 87 (19.4) | 1.00 (0.68–1.48) | 1.05 (0.65–1.71) | 163 (80.3) | 40 (19.7) | 0.98 (0.62–1.56) | 0.74 (0.41–1.34) |
| Widowed/divorced/separated | 104 (83.9) | 20 (16.1) | 0.71 (0.40–1.27) | 0.92 (0.47–1.77) | 20 (71.4) | 8 (28.6) | 1.71 (0.70–4.15) | 2.17 (0.85–5.54) |
| Education | ||||||||
 High school or above High school or above | 84 (78.5) | 23 (21.5) | Reference | Reference | 87 (79.8) | 22 (20.2) | Reference | Reference |
 No schooling No schooling | 124 (82.1) | 27 (17.9) | 0.85 (0.44–1.64) | 0.71 (0.34–1.48) | 61 (73.5) | 22 (26.5) | 1.38 (0.69–2.76) | 1.71 (0.78–3.75) |
 Primary/secondary school Primary/secondary school | 475 (80.8) | 113 (19.2) | 0.94 (0.56–1.59) | 0.83 (0.46–1.49) | 252 (81.3) | 58 (18.7) | 0.90 (0.52–1.57) | 0.95 (0.50–1.80) |
| Employment | ||||||||
 Monthly/weekly wages Monthly/weekly wages | 141 (82.0) | 31 (18.0) | Reference | Reference | 188 (79.0) | 50 (21.0) | Reference | Reference |
 Daily wages Daily wages | 350 (82.7) | 73 (17.3) | 0.94 (0.58–1.51) | 0.93 (0.54–1.58) | 155 (80.3) | 38 (19.7) | 0.88 (0.53–1.44) | 0.85 (0.50–1.45) |
 Unemployed Unemployed | 192 (76.5) | 59 (23.5) | 1.29 (0.76–2.18) | 1.06 (0.58–1.96) | 57 (80.3) | 14 (19.7) | 0.92 (0.47–1.82) | 0.89 (0.44–1.81) |
 Unstable housing Unstable housing | 89 (82.4) | 19 (17.6) | 0.81 (0.44–1.52) | … | 6 (75.0) | 2 (25.0) | … | … |
| Substance use | ||||||||
 Ever inject drugs Ever inject drugs | 683 (100) | 163 (19.3) | … | … | 47 (75.8) | 15 (24.2) | 1.30 (0.66–2.56) | … |
 Age first injected, y Age first injected, y | 20 (17–25) | 20 (18–26) | 1.02 (0.99–1.05) | … | 21 (17–25) | 22 (18–26) | 1.04 (0.94–1.14) | … |
 Duration of drug use, y Duration of drug use, y | 8 (4–13) | 6 (3–11) | 0.95 (0.92–0.98)** | 0.94 (0.90–0.98)** | 8.5 (4–15) | 6 (2–7) | 0.92 (0.82–1.03) | … |
| Drugs ever injected | ||||||||
 Heroin Heroin | 483 (80.9) | 114 (19.1) | 1.00 (0.63–1.58) | … | 27 (77.1) | 8 (22.9) | … | … |
 Buprenorphine Buprenorphine | 445 (82.1) | 97 (17.9) | 0.84 (0.53–1.35) | … | 10 (90.9) | 1 (9.1) | … | … |
 Allergy medicine Allergy medicine | 346 (81.2) | 80 (18.8) | 0.92 (0.57–1.47) | … | 23 (67.7) | 11 (32.3) | … | … |
 Otherc Otherc | 210 (79.9) | 53 (20.1) | 0.73 (0.44–1.21) | … | 18 (85.7) | 3 (14.3) | … | … |
| Drugs ever injected, past 6 mo | ||||||||
 Heroin Heroin | 302 (81.0) | 71 (19.0) | 0.99 (0.64–1.53) | … | 2 (100) | 0 (0) | … | … |
 Buprenorphine Buprenorphine | 325 (81.3) | 75 (18.8) | 1.26 (0.75–2.12) | … | 3 (100) | 0 (0) | … | … |
 Allergy medicine Allergy medicine | 249 (80.1) | 62 (19.9) | 1.34 (0.76–2.38) | … | 13 (65.0) | 7 (35.0) | … | … |
 Otherc Otherc | 38 (82.6) | 8 (17.4) | 0.85 (0.38–1.90) | … | 8 (80.0) | 2 (20.0) | … | … |
| Ever shared needle/syringe | 556 (81.6) | 125 (18.4) | 0.81 (0.50–1.30) | … | 37 (74.0) | 13 (26.0) | … | … |
| Injection frequency, past 6 mo | ||||||||
  Never Never | 129 (80.6) | 31 (19.4) | Reference | … | 15 (78.9) | 4 (21.1) | … | … |
  Daily Daily | 170 (73.6) | 61 (26.4) | 1.82 (0.98–3.39) | … | 5 (55.6) | 4 (44.4) | … | … |
  Less than daily Less than daily | 359 (83.9) | 69 (16.1) | 0.96 (0.54–1.70) | … | 27 (79.4) | 7 (20.6) | … | … |
| Average No. of times injected/d | ||||||||
 Once Once | 180 (84.1) | 34 (15.9) | Reference | Reference | 11 (91.7) | 1 (8.3) | … | … |
 Twice Twice | 159 (82.8) | 33 (17.2) | 1.10 (0.65–1.86) | 1.09 (0.64–1.85) | 16 (66.7) | 8 (33.3) | … | … |
 ≥3 times ≥3 times | 215 (76.8) | 65 (23.2) | 1.60 (1.01–2.53)* | 1.60 (0.99–2.59) | 5 (71.4) | 2 (28.6) | … | … |
 Alcohol dependence Alcohol dependence | 171 (84.2) | 32 (15.8) | 0.71 (0.46–1.10) | … | 80 (86.0) | 13 (14.0) | 0.58 (0.31–1.10) | … |
 Noninjection drug use, past 6 mo Noninjection drug use, past 6 mo | 479 (81.5) | 109 (18.5) | 0.91 (0.61–1.35) | … | 24 (68.6) | 11 (31.4) | 1.86 (0.86–4.04) | … |
| Sexual behaviors | ||||||||
 Lifetime sexual partnersd Lifetime sexual partnersd | 2 (1–4) | 2 (1–5) | 0.99 (0.93–1.06) | … | 33 (14–150) | 21 (5–60) | 0.99 (0.98–1.00) | … |
 Lifetime male sexual partners, among MSMd Lifetime male sexual partners, among MSMd | … | … | … | … | 30 (10–100) | 15.5 (4–60) | 0.99 (0.98–1.00) | … |
 Recent sexual partners, past 6 mo Recent sexual partners, past 6 mo | 1 (0–1) | 0 (0–1) | 1.04 (0.95–1.14) | … | 5 (2–20) | 3 (1–10) | 1.00 (0.99–1.00) | … |
 Unprotected sex, past 6 mo (all genders) Unprotected sex, past 6 mo (all genders) | 256 (79.5) | 66 (20.5) | 1.50 (0.81–2.77) | … | 273 (80.3) | 67 (19.7) | 0.83 (0.48–1.43) | … |
 Unprotected anal sex, past 6 mo (among MSM) Unprotected anal sex, past 6 mo (among MSM) | … | … | … | … | 242 (81.5) | 55 (18.5) | 0.87 (0.51–1.46) | … |
| Psychosocial | ||||||||
 Depression severity Depression severity | … | … | … | … | … | … | … | … |
  None–minimal None–minimal | 115 (77.2) | 34 (22.8) | Reference | … | 145 (82.9) | 30 (17.1) | Reference | Reference |
  Mild Mild | 280 (80.9) | 66 (19.1) | 0.81 (0.50–1.31) | … | 98 (72.1) | 38 (27.9) | 1.85 (1.06–3.24)* | 1.85 (1.05–3.25)* |
  Moderate Moderate | 201 (82.4) | 43 (17.6) | 0.71 (0.41–1.20) | … | 115 (82.7) | 24 (17.3) | 1.00 (0.55–1.82) | 0.98 (0.52–1.83) |
  Moderately severe Moderately severe | 76 (84.4) | 14 (15.6) | 0.60 (0.29–1.24) | … | 38 (79.2) | 10 (20.8) | 1.27 (0.57–2.83) | 1.23 (0.53–2.84) |
  Severe Severe | 11 (64.7) | 6 (35.3) | 1.99 (0.66–5.96) | … | 4 (100) | 0 (0) | … | … |
| HIV | ||||||||
 Duration on ART Duration on ART | … | … | … | … | … | … | … | … |
  Not on ART at baseline Not on ART at baseline | 518 (81.3) | 119 (18.7) | Reference | Reference | 273 (78.9) | 73 (21.1) | Reference | Reference |
  <6 mo <6 mo | 80 (75.5) | 26 (24.5) | 1.43 (0.87–2.34) | 1.37 (0.76–2.47) | 79 (81.4) | 18 (18.6) | 0.86 (0.48–1.53) | 0.91 (0.50–1.67) |
  6 mo to a year 6 mo to a year | 85 (82.5) | 18 (17.5) | 0.85 (0.48–1.51) | 0.65 (0.29–1.47) | 48 (81.4) | 11 (18.6) | 0.82 (0.40–1.69) | 0.76 (0.37–1.57) |
 CD4 count,e cells/µL CD4 count,e cells/µL | 388 (273–506) | 400 (301–531) | 1.01 (1.00–1.01) | … | 307 (184–494) | 323 (199–497) | 1.00 (0.99–1.01) | … |
 HIV viral load suppression HIV viral load suppression | 99 (81.2) | 23 (18.8) | 0.91 (0.55–1.52) | … | 86 (80.4) | 21 (19.6) | 0.96 (0.56–1.64) | … |
Abbreviations: ART, antiretroviral therapy; HBsAg, hepatitis B surface antigen; HBV hepatitis B virus; IQR, interquartile range; MSM, men who have sex with men; PWID, people who inject drugs.
* P < .05; **P < .01.
aScaled per 10 y.
bPanthi (prefer penetrative anal intercourse only); kothi (prefer receptive anal intercourse only); double decker (engage in both penetrative and receptive anal intercourse).
cOther drugs include self-reported use of cocaine, painkillers, sedatives, and other drugs. Response options within the category are not mutually exclusive.
dScaled per 10 partners.
eScaled per 10 cells/µL.
The overall prevalence of being HBsAg reactive among MSM was 9.5% (Table 2). Of the 503 MSM ever infected with HBV, 102 (20.3%) were HBsAg reactive; their median age (IQR) was 31 (27–38) years, 53% were married, 57% had a primary or secondary school education, and 49% were on monthly or weekly wages. Their median number of recent sexual partners (IQR) was 3 (1–10), and 68% had had unprotected anal sex in the prior 6 months. Of the HIV parameters, 28% were on ART at baseline and 21% were HIV virally suppressed.
In the multivariable analysis for being HBsAg reactive among MSM, age was associated with lower likelihood (aOR per 10 years, 0.71; 95% CI, 0.52–0.97). In addition, MSM experiencing mild depression had increased odds of being HBsAg reactive compared with those without or with minimal depression (aOR, 1.85; 95% CI, 1.05–3.25) (Table 4).
DISCUSSION
In this investigation, we discovered a high burden of being HBsAg reactive (representing CHB) among MSM and PWID with HIV throughout India and low rates of vaccination. As HBV infection is a preventable and treatable cause of significant global mortality, this finding underscores the importance of testing PWH for HBV, vaccinating those who are susceptible, and treating both HBV and HIV in those dually infected. Moreover, the finding that nearly two-thirds of PWH in this sample have been infected with HBV raises important questions about the safety of ARVs that are not active against HBV, such as long-acting formulations, even though PWID might otherwise benefit from that option. Additionally, the low rates of vaccination highlight the importance of routine infant vaccination and catch-up vaccination in adults.
Given limited recent epidemiological data on HBV among PWH, particularly key populations from LMICs, these findings address a gap in the literature. Other studies have also found a high prevalence of CHB in PWH [2, 18–21]. One meta-analysis considered studies published from 2002 to 2018 and estimated an overall prevalence of HBsAg in PWH of 7.6% or a total of 2.7 million globally [21]. An estimated 69% of cases were in Sub-Saharan Africa, and the average prevalence of HBsAg was also higher in PWH who injected drugs (~11% compared with 14.1% in this study). Other studies have provided regional and risk group–specific estimates of relevance to the present study. We previously characterized the prevalence of CHB in MSM with HIV across 12 Indian cities from 2012 to 2013 [11]. The prevalence in this prior study was 8%, comparable to this study (9.5%), and again, there were significant regional variations detected. The studies differed in that the prior sample was recruited from the community using respondent-driven sampling in 2012–2013, while this study recruited participants with a known HIV status from a clinic or referral by a friend between 2017 and 2018. Also, the prior study only characterized CHB.
Another study also found a high prevalence (9.7%) of CHB in 2292 PWID in India [22]. The prevalence was especially high (26%) in PWID with HIV but not those with hepatitis C (HCV). This prevalence compares with our overall prevalence of CHB, but we did not have HCV testing in the present study to confirm its absence. In contrast, the prevalence of CHB is much lower in the general population in India. For example, a study from Punjab reported a prevalence of 1.4%, while the overall prevalence for India has been estimated to be 3% [23, 24].
In this study, we also found a high prevalence of persons with anti-HBc, but not HBsAg. This finding is consistent with the expectation that most people who acquire HBV as adults will clear HBsAg. However, only about 80% of those ever infected cleared HBsAg, which is lower than the 95% that would be expected in the immunocompetent population. Although those who clear HBsAg do not (by definition) have CHB, many remain latently infected, have low levels of plasma HBV DNA, and are capable of reactivation to CHB [17, 25]. This is especially common when the person has anti-HBc but not HBsAg or anti-HBs, a situation called occult hepatitis B [3–6]. Unfortunately, we did not quantify HBV DNA in this study. Recently, there have been reports of PWH developing CHB following a change in ART to regimens that are not active against HBV, such as injectable cabotegravir and rilpivirine [7, 8, 25]. Because of this concern, PWH with anti-HBc are generally excluded from clinical trials of cabotegravir and rilpivirine, which restricts this potentially important therapeutic option from the many PWH in regions of the world where they might particularly benefit.
The high rates of HBV infection in this study support the 2017 WHO recommendation for catch-up vaccination in PWH, PWID, and MSM [26]. However, we found low serologic evidence of HBV vaccination, which emphasizes the structural barriers that need to be addressed to reach these populations and the need for public health campaigns to educate these populations regarding HBV vaccination. Our multivariable analysis supports initially focusing campaigns on PWID, especially those with the most difficult social situations such as unstable housing and unstable employment.
This study has some limitations. This study was cross-sectional. Therefore, we cannot be confident about the temporal sequence of key events, such as HIV and HBV infections and the potential exposures, and cannot infer causality to associations, such as the link between depression among MSM and CHB. In addition, of those positive for HBsAg, we were not able to perform testing 6 months later to confirm CHB as they started HBV-active ART after their baseline visit. Differential survival of a subset of PWH could also affect associations, possibly masking some risk assessments and introducing bias in our estimates such as the association between suppressive ART and CHB where duration of ART could be associated with clearance. We also did not quantify HBV DNA or anti-HCV, which has been shown to impact the transition between HBV disease states.
Nonetheless, this investigation underscores the high burden of HBV in PWH in India and the importance of testing PWH for HBV, vaccinating those who are susceptible, and treating both HBV and HIV in those dually infected. Our findings also highlight the importance of future work to address the optimal clinical management of PWH with anti-HBc reactivity, particularly with the use of long-acting antiretrovirals, which represent the majority in India and Sub-Saharan Africa, and thus globally.
Acknowledgments
We would like to thank our participants, without whom this research would not have been possible.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Patient consent. The parent trial, including the testing of stored specimens, was approved by the Johns Hopkins Medicine and YRGCARE institutional review boards, and participants' written consent was obtained.
Financial support. This trial was supported by the National Institute on Drug Abuse of the National Institutes of Health (grant number: R01DA041034) and the Johns Hopkins Center for AIDS Research (grant number: P30 AI094189). C.L.T. is supported by R56AI138810. Abbott Laboratories donated kits for the characterization of HBV in this study as part of the Abbott Pandemic Defense Coalition.
Contributor Information
Talia A Loeb, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Mihili P Gunaratne, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Syed Iqbal, YR Gaitonde Centre for AIDS Research and Education, Chennai, India.
Mark Anderson, Abbott Laboratories, Abbott Park, Illinois, USA.
Allison M McFall, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Pradeep Amrose, YR Gaitonde Centre for AIDS Research and Education, Chennai, India.
Mary A Rodgers, Abbott Laboratories, Abbott Park, Illinois, USA.
Aylur K Srikrishnan, YR Gaitonde Centre for AIDS Research and Education, Chennai, India.
Ashwin Balagopal, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Gregory M Lucas, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Shruti H Mehta, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
David L Thomas, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Gavin Cloherty, Abbott Laboratories, Abbott Park, Illinois, USA.
Chloe L Thio, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Sunil S Solomon, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
Articles from Open Forum Infectious Diseases are provided here courtesy of Oxford University Press
Full text links
Read article at publisher's site: https://doi.org/10.1093/ofid/ofae350
Read article for free, from open access legal sources, via Unpaywall:
https://academic.oup.com/ofid/article-pdf/11/7/ofae350/58626728/ofae350.pdf
Citations & impact
This article has not been cited yet.
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/165272792
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Respondent-driven sampling for identification of HIV- and HCV-infected people who inject drugs and men who have sex with men in India: A cross-sectional, community-based analysis.
PLoS Med, 14(11):e1002460, 28 Nov 2017
Cited by: 30 articles | PMID: 29182638 | PMCID: PMC5705124
Association between universal hepatitis B prison vaccination, vaccine uptake and hepatitis B infection among people who inject drugs.
Addiction, 113(1):80-90, 21 Aug 2017
Cited by: 13 articles | PMID: 28710874
Prevalence and risk factors associated with HIV/hepatitis B and HIV/hepatitis C co-infections among people who inject drugs in Mozambique.
BMC Public Health, 20(1):851, 03 Jun 2020
Cited by: 11 articles | PMID: 32493347 | PMCID: PMC7271460
Hepatitis B/C in the countries of the EU/EEA: a systematic review of the prevalence among at-risk groups.
BMC Infect Dis, 18(1):79, 12 Feb 2018
Cited by: 51 articles | PMID: 29433454 | PMCID: PMC5809955
Review Free full text in Europe PMC
Funding
Funders who supported this work.
Johns Hopkins Center for AIDS Research (1)
Grant ID: P30 AI094189
NIAID NIH HHS (2)
Grant ID: P30 AI094189
Grant ID: R56 AI138810
NIDA NIH HHS (1)
Grant ID: R01 DA041034
National Institutes of Health (1)
Grant ID: R01DA041034





