Abstract
Free full text

Advancements and challenges in developing in vivo CAR T cell therapies for cancer treatment
Summary
The Chimeric Antigen Receptor (CAR) T cell therapy has emerged as a ground-breaking immunotherapeutic approach in cancer treatment. To overcome the complexity and high manufacturing cost associated with current ex vivo CAR T cell therapy products, alternative strategies to produce CAR T cells directly in the body have been developed in recent years. These strategies involve the direct infusion of CAR genes via engineered nanocarriers or viral vectors to generate CAR T cells in situ. This review offers a comprehensive overview of recent advancements in the development of T cell-targeted CAR generation in situ. Additionally, it identifies the challenges associated with in vivo CAR T method and potential strategies to overcome these issues.
Introduction
Despite considerable research efforts, cancer persists as a significant global health challenge which impacts millions worldwide with a substantial burden on healthcare systems.1 While conventional cancer treatments like surgery, radiation and chemotherapy have made advancements, their effectiveness remains limited, particularly in cases of advanced or resistant cancers.2 Recently, several immunotherapy strategies have emerged as a novel strategy for cancer treatment. These include check point inhibitors, chimeric antigen receptor (CAR) T cell therapies, monoclonal antibodies, cancer vaccines and cytokines.3 Among these strategies, the CAR T therapy demonstrates promising therapeutic outcomes by modifying T cells to induce anti-tumour immune responses, either through direct triggering of cancer cell death or by altering the tumour microenvironment.4 This pioneering method involves inducing CAR expression in a patient’s T cells either ex vivo or in vivo, allowing them to accurately identify and eliminate cancer cells.5 Although advancements in ex vivo T cell strategy have demonstrated success in addressing different types of blood cancers,6 with potential applications towards eliminating solid tumours,6, 7, 8 it also faces significant challenges in manufacturing processes and costs, limiting its accessibility to a broad range of patients. To address these issues, the in vivo CAR T cell method has been explored and developed in recent years. In this review, we firstly introduce the basics of CAR T therapy, highlighting the current challenges of the ex vivo CAR T method. We then discuss the advancements in vivo CAR T strategies, including preclinical studies on the development of T cell-targeted delivery systems, progress in industrial development and the technical challenges in developing in vivo CAR T therapy. We also emphasise the regulatory considerations and the clinical approval process, providing a clinical perspective on in vivo CAR T therapy.
Technological features of CAR T therapies
CAR structure
CARs are modular synthetic cellular receptors with four main regions: an extracellular domain, a hinge region, a transmembrane domain, and intracellular domains; each of which has distinct functions8 (Fig. 1). First, the extracellular domain, is essential for the targeted recognition and binding to cancer cells. This domain, often derived from a monoclonal antibody, forms a single-chain variable fragment (scFv), which enables CAR T cells to specifically recognise cancer cell surface antigens and trigger T cell activation.9 Besides scFvs, the extracellular domain can include natural ligands or mutated ligands which are modified to enhance binding specificity and affinity for cancer cell antigens, reducing off-target effects and increasing therapeutic efficacy.10 It can also include short peptides designed to target specific epitopes on cancer cells, improving stability and binding characteristics as an alternative to traditional scFvs.11
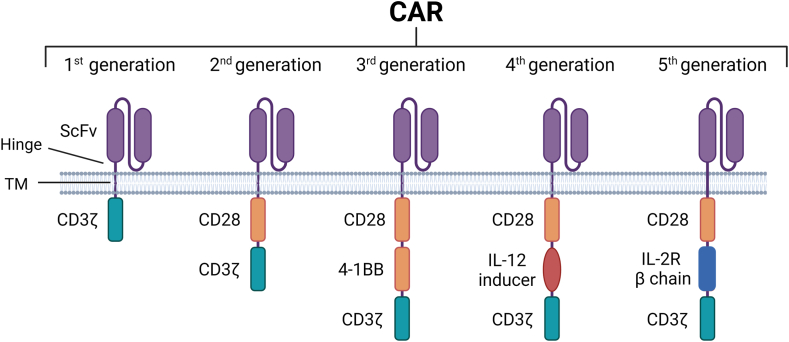
Overview of CAR structure. All five generations of CAR constructs share common structures with 4 domains: an extracellular domain targeting tumour-specific antigens (ScFV), a hinge region, transmembrane domain (TM), and finally an intracellular domain. As demonstrated, the structure of CAR intracellular domain indicates CAR generation as well as its functional activity. For instance, the CD3ζ domain initiates essential signal transduction pathways necessary for T-cell activation, proliferation, cytokine production, and cytotoxicity. Meanwhile, the CD28 and 4-1BB domains function as co-stimulatory signals, augmenting T-cell activation, persistence, and functionality. The IL-12 inducer domain is employed to prompt cytokine release within the tumour microenvironment, and the IL-2R beta chain mimics IL-2 signalling, enhancing CAR-T cell survival, proliferation, and persistence.
The hinge region, the second structure of CAR, is a common connector between the extracellular domain and the transmembrane domain of CAR.9,12 The hinge region is a common connector between the extracellular domain and the transmembrane domain of CAR.12 The transmembrane domain anchors the CAR construct through the T cell membrane.13 Finally, the intracellular domain contains different signalling transduction components similar to those in T cell receptors such as CD3ζ, CD28, IL-2 and 4-1BB.14,15 This is the domain responsible for triggering the intracellular signal transduction for T cell activation and proliferation, leading to the generation of a new pool of CAR T cells that specifically target and kill cancer cells.12 Mechanistically, when CAR T cells detect cancer cells, the interaction between the two cell types activates the CAR intracellular domain, which results in the release of cytotoxic molecules by CAR T cells and the initiation of cell death mechanisms in the cancer cells.16
To date, CARs have evolved through five generations with different anti-tumour capabilities based on the structure of their intracellular domains (Fig. 1). The first-generation CARs mainly contain the CD3ζ intracellular domain which is responsible for T cell activation with low cytotoxicity and anti-tumour efficacy.16,17 The second-generation CARs contain CD3ζ and co-stimulatory domain such as CD28, which enhances T cell proliferation.18,19 The third and fourth CAR generations expanded upon the second CAR generation by incorporating additional signalling domains such as 4-1BB or interleukin 12 (IL-12) cytokines.20 These constructs have been employed to generate a new cell type known as T cell redirected for antigen-unrestricted cytokine-initiated killing (TRUCKs), which exerts both cytotoxic and cytokine-releasing effects on the targeted cancer cells.21 The fourth CAR generations also feature dual targeting and logic-gated designs to improve specificity and reduce off-target effects, holding promise for more effective and safer CAR T cell therapy.22,23 Finally, the fifth-generation CARs possess a structure resembling the second generation but incorporate an additional truncated cytoplasmic IL-2 receptor β-chain domain.21 This domain allows drug-dependent switches to control CAR T cell functions, thus potentially optimising therapeutic outcomes.24 However, challenges needs to be overcome for the fifth-generation CARs are off-target and low tumour specificity.22
Gene editing tools for CAR T cell generation
Various gene editing systems are employed to induce stable CAR expression in transduced primary T cells. They include transposons, zinc finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs) and the Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) system. Transposon systems, such as Sleeping Beauty and PiggyBac, show potential for introducing CAR constructs into T cells.25,26 The Sleeping Beauty DNA plasmid system exhibits stable transgene expression with minimal disruption to crucial genes and low genotoxicity.27 Meanwhile, the PiggyBac plasmid system can generate CD19 CAR T cells, exhibiting potent anti-tumour activity against acute lymphoblastic leukaemia cells within the central nervous system.28 However, despite their benefits such as cost-effectiveness and reduced toxicity, challenges with transposon systems remain, including stability issues and the risk of off-target effects.29,30
In addition to transposons, ZFN has been employed to examine CAR T therapeutic effects in different animal cancer models as well as in clinical studies.31 For instance, ZFN has been employed to create HIV-resistant CD4+ T cells via simultaneous deletion of both CCR5 and CXCR4 co-receptors.32,33 Despite its potential for effective and specific gene editing, optimising the targeting protein molecules in ZFNs can be time-consuming and technically challenging.34 TALEN, another gene editing tool similar to ZFN, are also being used for CAR production.35 In clinical trials, TALEN has been employed to introduces CAR constructs specific for certain types of blood cancer, such as acute myeloid leukaemia, advanced lymphoid malignancy, refractory B-ALL, multiple myeloma and B cell acute lymphoblastic leukaemia.36 However, TALEN has major limitations, including limited space for gene editing tools and the need for extensive nuclease modification for enhanced gene editing outcomes.35,36 Last but not least, the newly emerged CRISPR/Cas9 offers precise genetic modifications of T cells while improving their effectiveness against cancer cells.37 The use of CRISPR in generating CAR T cell therapy are currently at early stages of clinical investigation.38 It is worth noting that, among these four editing tools, the transposon, ZFN and CRISPR systems are suitable for CAR T generation in both in vivo and ex vivo settings, thus holding promise for novel CAR T-based cancer therapies.39, 40, 41
Limitations of current ex vivo CAR T therapies
Presently, most CAR T cells have been generated outside the body where genetic modification of T cells is performed. This strategy, also known as the ex vivo CAR T method, involves harvesting patient- or donor-derived T cells from a blood sample. Next, the isolated T cells are genetically modified to express CAR construct, thus creating a pool of CAR T cells. After that, these modified CAR T cells are expanded in the laboratory before being delivered into the patient’s bloodstream via infusion where the therapeutic cells will attack the target cells which present tumour-specific antigens (Fig. 2). This therapy has revolutionised the clinical applications of novel cancer therapies, with several approved products now available to patients with blood cancer and more trials underway for patients with solid tumour (Table 1).
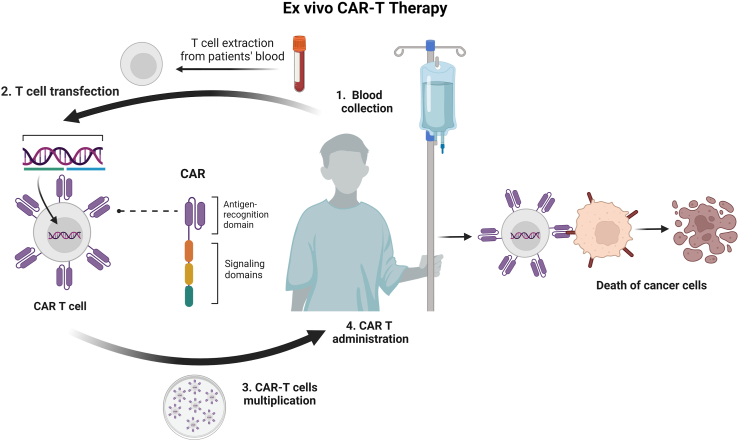
Process of ex vivo CAR T therapies.Ex vivo CAR T-cell therapy involves isolating a patient’s or healthy donor’s T-cells via leukapheresis, genetically modifying them with a CAR construct designed for specific cancer antigens. These modified cells are then cultured and expanded. The expanded and modified T-cells are reintroduced into the patient via infusion. Activated CAR T-cells recognise and destroy cancer cells expressing the targeted antigen, potentially providing long-term immunity against cancer recurrence.
Table 1
FDA approved ex vivo CAR T products for blood cancer and other representative trials for solid tumour treatment.a
| Target | Diseases | Delivery vehicle | Cargo | CAR construct | Approved/Trial phase | Ref |
|---|---|---|---|---|---|---|
| CD19 | Relapsed and refractory B cell lymphoma | Lentivirus | Single-stranded RNA genome | AntiCD19-CD8-α-4-1BB- CD3ζ | Tisagenlecleucel | 42 |
| CD19 | B cell lymphoma | Lentivirus | Single-stranded RNA genome | AntiCD19-CD28-CD3ζ | Axicabtagene ciloleucel | 43 |
| CD19 | B cell lymphoma | Lentivirus | Single-stranded RNA genome | AntiCD19- IgG4-CD28-4-1BB- CD3ζ | Lisocabtagene maraleucel | 44 |
| CD19 | Relapsed and refractory B cell lymphoma | Lentivirus | Single-stranded RNA genome | AntiCD19-CD28- CD3ζ | Brexucabtagene autoleucel | 45 |
| BMCA | Multiple myeloma | Lentivirus | Single-stranded RNA genome | AntiBMCA-CD8α-4-1BB- CD3ζ | Idecabtagene vicleucel | 45 |
| BMCA | Multiple myeloma | Lentivirus | Single-stranded RNA genome | Dual AntiBMCA- CD8α-4-1BB- CD3ζ | Ciltacabtagene autoleucel | 46 |
| AJMUC-1 | Breast Cancer | Lentivirus | Single-stranded RNA genome (CRISPR/Cas9 editing tool) | AntiMUC1-CD28-CD3ζ | Phase 2 | 47 |
| MUC1 | TnMUC positive solid tumours (triple negative breast cancer, epithelial ovarian cancer, pancreatic cancer and non-small cell lung cancer) | Lentivirus | Single-stranded RNA genome | AntiMUC1-CD8a-CD2- CD3ζ | Phase 1 | 48 |
| Mesothelin | Triple negative breast cancer | Lentivirus | Single-stranded RNA genome | Antimesothelin-4-1BB- CD3ζ | Phase 1 | 49,50 |
| EGFR | Glioblastoma | Lentivirus | Single-stranded RNA genome | AntiEGFRvIII-4-1BB- CD3ζ | Phase 1 | 51 |
| EGFR | Lung, liver and stomach cancer | Lentivirus | Single-stranded RNA genome | AntiPD-1-EGFR-CAR | Phase 2 | 52,53 |
| CEA | colorectal cancer (CRC), pancreatic cancer (PANC), non-small cell lung cancer (NSCLC) | Lentivirus | Single-stranded RNA genome | AntiCEA-CD8α-CD28-4-1BB-CD CD3ζ | Phase 1/2 | 54 |
| CLDN18 | Advanced gastric/gastroesophageal junction adenocarcinoma Pancreatic cancer | Lentivirus | Single-stranded RNA genome | Humanised anti-CLDN18.2-CD8α-CD28- CD3ζ | Phase 2 | 55,56 |
| GD2 | Neuroblastoma | Retrovirus | Single-stranded RNA genome | AntiGD2- CD28- CD3ζ | Phase 2 | 57,58 |
| CLDN18 | Gastrointestinal cancer | Lentivirus | Single-stranded RNA genome | AntiCLDN18-CD8-CD28- CD3ζ | Phase 1 | 56 |
| B7H3 | Ovarian cancer | Retrovirus | Single-stranded RNA genome | AntiB7H3-CD8α-CD28-CD3ζ | Phase 1, 2 | 59,60 |
| PSCA | Prostate cancer | Lentivirus | Single-stranded RNA genome | aPSCA-4-1BB/TCRzeta-CD19t | Phase 1 | 61, 62, 63 |
| GPC-3 | Hepatocellular carcinoma | Lentivirus | Single-stranded RNA genome | AntiGPC3-CD8-CD28- CD3ζ | Phase 1 | 64, 65, 66, 67, 68 |
| CD70 | Relapsed or refractory renal cell carcinoma | Lentivirus | Single-stranded RNA genome | AntiCD70-TRC-Costimulatory domain- CD3ζ | Phase 1 | 69, 70, 71 |
| NKG2D | Unresectable metastatic colorectal cancer | Retrovirus | Single-stranded RNA genome | NKG2DR-CD3ζ | Phase 1 | 72 |
| CLDN18 | Gastrointestinal cancer | Lentivirus | Single-stranded RNA genome | AntiCLDN18-CD8-CD28- CD3ζ | Phase 1 | 56 |
There are two distinct therapeutic strategies for ex vivo CAR T therapy: autologous and allogeneic. The autologous approach uses a patient’s own T cells to recognise tumour-specific antigens on cancer cell surfaces, allowing for a targeted attack on the tumours.17,20 Some of the common tumour-specific antigens are CD19, B cell maturation antigen (BCMA), CD22, CD20 and EGFR which present in B cell malignancies, acute myelogenous leukaemia (AML), lung and breast cancer.73 The allogeneic strategy employs T cells from healthy donors, avoiding the need for patient-derived cells by using modified donor T cells.74 In certain types of blood cancers, there is a risk of CAR T cell fratricide and depletion due to the presence of common antigens between the leukemic and CAR T cells such as CD7 or CD5.75,76 To cope with this challenge, CD7-specific CAR T cells can be engineered into universal CAR T cells by deleting both CD7 and α chain of T cell receptors using CRISPR system.77 This method exhibited a fratricide-resistant therapeutic potentials for relapsed and refractory T cell acute lymphoblastic leukaemia (T-ALL).77 A similar approach has been applied in a phase I study in which CRISPR base editing system was employed to modify T cells derived from healthy donors for expressing a CAR with specificity for CD7 (CAR7).78 These modifications were intended to delete CD7 and CD52 receptors, along with the β chain of T cell receptors, preventing graft-versus-host disease and T-cell fratricide and enhancing resistance to alemtuzumab-induced depletion.78 Additionally further modifications such as permanent deletion of the intrinsic α and β chain of T cell receptors by using ZFN technology can be considered.79
Although the ex vivo CAR T cell approach has shown promising potential as an anti-cancer therapy, many challenges limit its therapeutic efficacy and safety. First, this therapeutic method carries potential side effects, including cytokine release syndrome and neurotoxicity from the immune response.50 It requires more thorough clinical oversight and adverse event management.80,81 Second, tumour cells may develop mechanisms to evade CAR T cell recognition and destruction over time, leading to disease relapse.8 Third, if the ex vivo CAR T therapy involves using donor cells (allogeneic cells), the risk of allogeneic graft rejection becomes a concern.82 This risk arises when the recipient’s immune system to recognise the donor cells as foreign and mount an immune response against them, leading to graft rejection.83 Last but not least, despite promising outcomes in specific blood cancers, the efficacy of the ex vivo CAR T-cell therapy in solid tumours remains limited, thus posing a challenge to its extension across diverse cancer types.8
In addition to the main obstacles in therapeutic development, the ex vivo CAR T therapies also face significant challenges in industrial development and manufacturing processes. First, the procedure involving isolation, modification, and expansion of T-cells outside the body before re-administration is time-consuming and intricate, spanning several weeks.84 This prolonged process may not suit patients with rapidly advancing cancers who need urgent treatment.8 Moreover, this manufacturing process also poses logistical and safety challenges where transport of patient’s cells to and from specialised manufacturing facilities is required. It is also technically demanding and costly, thus potentially restricting broad accessibility to this therapy.85
In vivo CAR T strategies for cancer treatment
Technical challenges associated with the ex vivo CAR T production require the invention of alternative treatment methods that can avoid the complex manufacturing process and reduce costs. Recent advancements have led to the in vivo CAR T therapeutic strategy, where T cells are directly engineered into CAR T cells within the patient by using delivery vehicles incorporating gene editing tools (Fig. 3).86 Compared to the ex vivo CAR T therapy, the in vivo CAR T method has the potential to reduce manufacturing costs, achieve faster turnaround times and offer greater convenience for patients.87 In this section, we introduce delivery strategies used for the in vivo CAR T method and discuss recent advancements in this field.
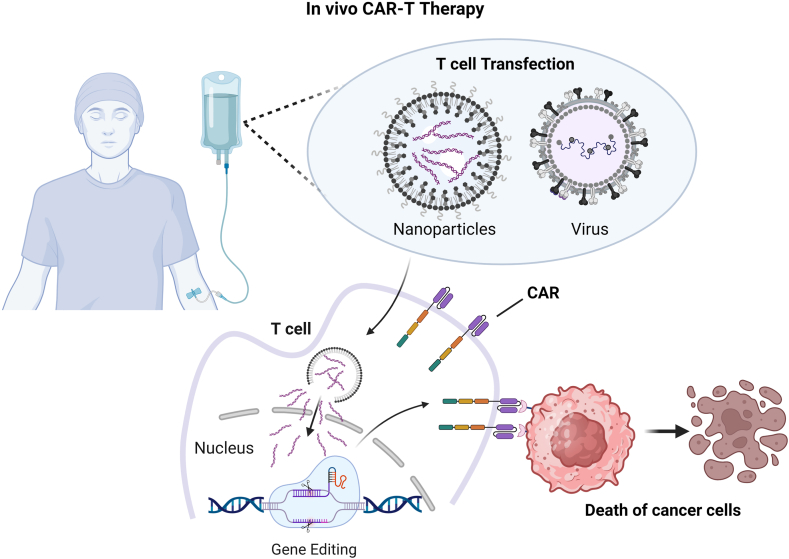
Schematic explanation of the in vivo CAR T therapy for cancer treatment. The in vivo CAR T therapy simplifies ex vivo CAR T method by systemic administration of the CAR gene editing construct enveloped in viral vectors or nanoparticles. These carriers specifically target T cells to unload gene editing cargo, thus inducing the expression of the CAR construct on the T cell surface. The resulting CAR T cells can then specifically detect cancer cells, thus activating themselves and expanding to effectively eliminate cancer cells in the bloodstream or malignant tumours.
Delivery systems for in vivo CAR T generation
CAR delivery in vivo requires meeting certain criteria including precise T cell targeting, high gene editing efficiency and low toxicity.88 Currently viral vectors and nanocarriers are widely explored and utilised for in vivo delivery of CAR constructs (Fig. 4).17
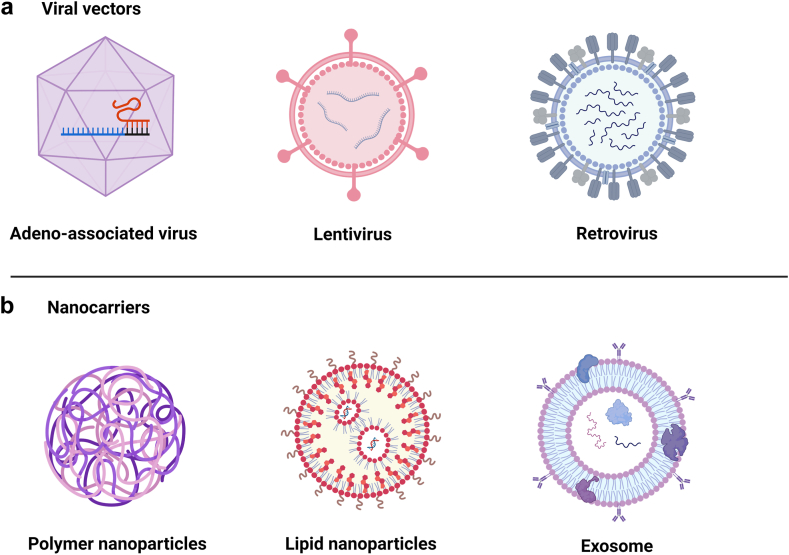
Delivery vehicles for in vivo CAR T generation. a. Viral vectors: Adeno-associated virus, lentivirus and retrovirus; b. Nanocarriers: Polymer nanocarriers, lipid nanoparticles and exosome.
The viral vectors used for CAR T cell therapy are generally produced by transiently transfecting source plasmids into HEK-293T or viral production cell lines to clone the CAR gene into viral vectors. For in vivo CAR T cell generation, these viral vectors can be further engineered to specifically target T cells by incorporating T cell-targeting ligands. These ligands can either be genetically fused to the viral envelope protein or attached to the viral vector through chemical conjugation. These provided references offer more detailed methods for viral vectors used in vivo CAR T cell generation.89,90
The major types of viruses used in vivo CAR T cell generation include lentivirus, retrovirus and adeno-associated virus (AAV). Pfeiffer et al. was the first to demonstrate CAR T cells could be generated directly in vivo by injection of lentiviral vectors (LV) targeting CD8+ T cells and delivering the CD19-CAR.91 Following the injection of LV into mice transplanted with human blood cells, CAR T cells were successfully detected in the blood and lymphoid organs. These CAR T cells expanded upon recognizing antigens and effectively eliminated CD19-positive cells. Recently Michels et al. developed anti-CD3 scFv modified LV for in vivo generating anti-CD19 CAR in CD3+ T cells, exhibiting selective expansion in rapamycin and achieving B-cell malignancies elimination in xenograft mouse model.92 Agarwalla et al. developed an implantable and multifunctional scaffold to simultaneously load patient-derived T cells and CD19-encoding retroviral particles for in vivo CAR T cell manufacturing, condensing the entire process to a single day.93 This scaffold facilitates in vivo activation and expansion of T cells through αCD3-and αCD28-mediated cell activation and interleukin-mediated proliferation upon subcutaneous implantation. It sustainably releases fully functional CAR T cells over 5 days and achieves equivalent efficacy in controlling tumour growth as the ex vivo CAR T method, while offering better cell expansion and presence in post-tumour clearance.
AAV has also been applied for in vivo generation of CAR T cells for human T-cell leukaemia regression in a mouse model.94 In this work, authors utilised AAV-DJ, a chimera of type 2, type 8 and type 9 AAVs, to deliver a plasmid encoding CD4 CAR gene to T cells in a humanised leukaemic mouse model. However, AAV lacks a membrane envelope for directed delivery to the target sites, thus posing critical technical challenges. Therefore, in vivo gene delivery by AAV vectors requires precise selection of AAV serotypes for tissue-specific delivery or modification of AAV capsid proteins with T cell targeting constructs.88 For instance, the designed ankyrin repeat proteins (DARPins) can be inserted into AAV2 capsid protein to specifically target murine CD8 T cells, resulting in a 20-fold increase in mouse splenocyte CAR gene delivery.95 This group further engineered bispecific DAPRins-AAV2 to dual-target CD4 and CD32a, enhancing T cell targeting and CAR gene generation in vivo.96 Despite the promising outcomes shown in the preclinical stage, viral vectors raise safety concerns due to their potential to trigger immune responses, causing tissue inflammation.97
Nanocarriers have also shown promise for delivering genetic material such as mRNA, plasmid and protein to introduce CAR construct into T cells in vivo.98 The general synthesis methods for these nanocarriers are summarised in several reviews.99, 100, 101 For example, microfluidics has been used to prepare lipid nanoparticles for gene delivery including CAR T constructs.102 This technique allows precise control over preparation parameters (such as flow rates, components concentrations and mixing ratios), facilitating scalable production for industrial development.103 Compared with viral vectors, the nanocarriers provide minimal off-target toxicity and immunogenicity98,104 and they can be manufactured in a large scale.105 Furthermore, nanocarriers can be custom designed by conjugating with different targeting biomaterials to achieve targeting capability.104 In addition, they can be chemically conjugated and lyophilised for extended stability.99
Currently polymers, lipids and exosomes are the most used nanocarriers for in vivo CAR T generation.99 Cationic polymer nanoparticles can form complexes with negative nucleic acids via electrostatic condensation. The positive surface charge of the complexes also enhances cellular uptake through electrostatic binding to the negatively charged cell membrane.106 These complexes are more stable due to their ability to avoid enzymatic degradation and escape endosomes thanks to their proton sponge effect.107 Smith et al. developed polymer nanocarriers for the in vivo CAR T therapy, achieving leukaemia regression through targeted T cell programming with minimal side effects.108 This system, later adapted for CAR-mRNA and T cell receptor (TCR)-mRNA delivery, effectively reprogrammed T cells in situ in mouse models of leukaemia, prostate cancer and hepatitis B induced-hepatocellular carcinoma.109 However, the high toxicity of cationic polymers requires further engineering, including lipid co-complexation, to mitigate toxicity and enhance functionality.110, 111, 112, 113 Lipid nanoparticles and exosomes have also shown promise in facilitating T cell reprogramming in vivo.73,103 Fan et al. developed a CAR T inspiration platform via tumour-antigen stimulated dendritic cell-derived exosomes (tDC-Exo) modified with anti-CD3 and anti-epidermal growth factor receptors (EGFR) antibodies, allowing simultaneously targeting endogenous T cells and cancer cells.73 However, the small size of exosomes makes it difficult to effectively package large gene editing tools such as CRISPR/Cas9 RNP. To tackle this challenge, scientists have explored various strategies, including exosome engineering114,115 and hybrid delivery systems.116
Progress of T cell-targeted delivery of CAR construct
As stated above, precise targeting of T cells is a critical factor affecting both the efficacy and safety of the in vivo CAR T cell therapy. In this section, we mainly explore recent strategies for developing T cell-targeted CAR delivery by using viral vectors and nanocarriers, with more studies on the in vivo T cell therapy listed in Table 2.
Table 2
Preclinical research on in vivo CAR T for cancer treatment.
| Disease model | Delivery vector | Cargo | T cell target | CAR construct | Outcomes | Ref |
|---|---|---|---|---|---|---|
| B cell leukaemia | Lentiviral | Single-stranded RNA genome | CD8 | AntiCD8-LVCD19CAR | Successful production of in vivo CAR T cells Clearance of CD19+ B malignant cells in 7/10 animals Cytokine release syndrome detected | 91 |
| B cell leukaemia | Lentiviral | Single-stranded RNA genome | CD8 | AntiCD8-CD19-CAR | CD8-LV successfully deliver CD19-CAR Tumour reduction 2 weeks post treatment CAR-high expression in spleen and blood | 86 |
| B cell leukaemia | Lentiviral | Single-stranded RNA genome | CD4 | AntiCD4-CD19-hCD28-hCD3z-CAR | CD4-LV leads 40–60% CAR-positive cells among CD4+ T cells CAR + T cells shows Th1/Th2 phenotype and eliminate CD19+ B cells Tumour cell lysis faster after CD4-LV treatment compared to CD8-LV or CD4/CD8-LV. | 117 |
| B cell leukaemia | Lentiviral | Single-stranded RNA genome | CD3 | AntiCD3-CD19-CD28-CD3z-myc-CAR | CD3+ T cells are efficiently and exclusively transduced. CAR-T generation can be observed Human CD19+ B cells eliminated 20 days post-injection | 118 |
| B cell leukaemia | Lentiviral | Single-stranded RNA genome | CD3 | AntiCD3-CD19-CD8-CD28-CD3z-CAR (SINV-CAR) | Increased CAR T cell count after 24 days of treatment Reduced tumour growth in spleen Prolonged survival time | 119 |
| B cell leukaemia | Lentiviral | Single-stranded RNA genome | CD3 | AntiCD3-CD19-FMC63-CD3z-4-1BB-CAR (VivoVec) | Dose-dependent CAR T cell transduction Tumour elimination in mice treated with VivoVec Intranodal injection in canines successfully transduced immune cells, exhibiting favourable biodistribution characteristics | 92 |
| T cell leukaemia | AAV | Single-stranded DNA genome | CD4 | AntiCD4-CD28-4-1BB-CD3z-CAR | Successful in vivo CAR T cell generation Tumour regression from day 10 post-treatment Complete tumour regression in 4/6 mice | 94 |
| T cell leukaemia | Poly (β-amino ester) polymer nanoparticle | Plasmid DNA | CD3 | AntiCD3-CD194-1BB-CD3z-CAR | DNA-carrying nanoparticles effectively bring CAR-gene into T cell nuclei Tumour regression from day 12 post-treatment | 108 |
| Acute lymphobastic leukaemia | DSPE-PEG-CD3 antibody-targeted lipid nanoparticles | IL6 shRNA, CAR gene (Double-stranded DNA) | CD3 | AntiCD3-LNP/CAR19+shIL6 | In vivo transfection efficiency of around 8% in AntiCD3-LNP/CAR19+shIL6-treated animals CAR T cell production peaking at day 21 with sustained expression towards day 90 after injection. | 103 |
Researchers have developed viral vectors to improve the targeting capabilities and maximise in vivo T cell editing. For instance, one study reported that LV modified with the T-cell receptor-CD3 complex (CD3-LVs) can specifically identify and transfect circulating T cells to produce CAR gene.118 This study has demonstrated that CD3-LVs successfully delivered CD19-specific CAR transgenes into T lymphocytes in vivo in humanised NSG mice, leading to the elimination of human CD19+ cells from the bloodstream.118 In another investigation, bispecific LV platform was engineered through the modification of azide group-modified LV with anti-CD3 antibody.120 These LVs were intravenously administered into humanised NOD-scid-IL2Rγnull (huNSG) mice engrafted with Nalm6-luc cells. The results demonstrated targeted delivery of CD19-CAR genes to T cells both in vitro and in vivo, with ongoing exploration of their efficiency in tumour elimination.
Research has also shown significant interest in delivering CAR constructs by using targeted nanocarriers. In a recent work, Zhou et al. developed CD3-targeted lipid nanocarriers loaded with plasmids containing interleukin 6 short hairpin RNA (IL-6 shRNA) and CD19-CAR combination genes (AntiCD3-LNP/CAR19 + shIL6).103 Following tail vein injection into leukaemic nude mice, the nanocarriers were observed to selectively target circulating T cells. This resulted in increased CAR expression and extended anti-tumour effects of CAR T cells in leukaemic mice for up to 41 days post-administration, comparable to ex vivo T cell therapy. Additionally, the co-administration of IL6-shRNA for IL-6 knockdown reduced the risk of cytokine release syndrome. In another study, researchers utilised CD3-targeted biodegradable poly (β-amino ester)-based nanoparticles to deliver leukaemia-specific CAR genes to T cells in a leukaemic mouse model.108 After 4 h of tail vein injection, these T cell-targeted polymer nanoparticles have been found to successfully transfect CAR genes to circulating T cells. This resulted in tumour regression and enhanced survival by 5 days during a 120-day follow-up period. In a follow-up study, similar polymer nanoparticles were used to transport CAR mRNA to the targeted T cells in animal models of lymphoma, prostate cancer, and HBV-induced hepatocellular carcinoma.109 This study has shown that these CD3-and CD8-targeted nanoparticles could elevate CAR T cell count, leading to tumour elimination and increased survival rates. More importantly, this therapeutic strategy demonstrated efficiency in immunocompetent leukaemic mice as well as immunodeficient mice carrying solid tumours. However, the lack of evaluation regarding CAR transgene delivery in both studies poses a challenge in quantifying the extent of CAR production via these nanocarrier platforms. Additionally, the poor biodegradability and instability nature of cationic polymers within host could reduce CAR production capacity and compromise the therapeutic effects of the in vivo CAR T therapies.121
Industrial development of in vivo CAR T therapy
The prospects of the viral-based in vivo CAR T therapies within the industrial landscape are evolving, thanks to the progress in surface-engineered lentiviral vectors that enable the targeted delivery of CARs to specific T cell subsets. For example, EXUMA Biotech claims to have developed a lentiviral vector encoding a CD19 CAR to target and activate CD3+ T cells in the humanised NSG-SGM3 mouse model.122 Their preclinical data show that in vivo editing the CD3+ T cells can produce functional CAR T cells and eliminate pre-existing B cells at the same time.122 In addition to addressing haematological disorders, this company has developed CCT303-406 as a new therapeutic strategy against HER2-positive relapsed or refractory stage IV metastatic solid tumours. This study is currently under a phase I clinical investigation.119 In a parallel venture to EXUMA Biotech, Umoja Biopharma introduces VivoVec™, an innovative surface-engineered lentiviral vectors designed for in vivo CAR-T cell generation.123 This system consists of lentiviral particles modified with a multi-domain fusion protein on their surface and carries a CD19-targeted CAR transgene. After a single dose of VivoVec™ particles to immune-competent non-human primates, this platform could induce potent and specific generation of functional CAR T cells, peaking between day 7 and day 42, with another peak observed at day 51. More importantly, this treatment leads to persistent B cell aplasia up to 76 days and is well-tolerated, showing no signs of toxicity in the treated primates. This proof-of-concept study suggests the potential for an off-the-shelf therapy to address the limitations of current ex vivo CAR T therapies.
Along with viral vectors, industrial advancements in the nanoparticle-based in vivo CAR T therapies also present significant progress. For instance, Ensoma has developed virus-like-particle Engenious™ platform to deliver gene materials up to 35 kilobases of packaged DNA, which is more than seven times the limit of AAV vector.124 Capstan Therapeutics has successfully generated in vivo CD5-specific CAR T cells in a mouse model of heart disease by using mRNA-lipid nanoparticle platform.125 This company employed CD5-targeting ionisable lipid nanoparticles (CD5/LNP) to encapsulate CAR mRNA molecules targeting fibroblast activation protein during in vivo CAR T generation. At 48 h following LNP injection, a new subset of CAR T cells emerged accounting for 20% of the total T cell population.126 This new CAR T cell population was comprised of 87% CD4+ T cells and 9–10% CD8+ T cells, a ratio indicated a high efficacy of CAR-based treatment.
Challenges of in vivo CAR T therapy development
Despite the promising data, the in vivo CAR T therapy encounters its own technical challenges. One major issue is efficiently delivering CAR transgenes to the targeted T cells within the body.127 Viral vectors boast high gene transduction efficiency due to their inherent viral properties that facilitate cellular uptake and nucleus penetration.128 However, directly infusing patients with viral vectors carrying CAR constructs is associated with limited targeting capability.129 Moreover, this method also poses the risk of off-target effects, where other cell types may be inadvertently transfected instead of the targeted T cells.8 To address this issue, researchers have focused on the engineering of viral vectors specifically for targeted delivery to T cells. For example, Jesse Green et al. developed T cell-targeted viral vector by engineering fusogen with a novel single-chain variable fragment targeting human CD8+ T cells.130 These engineered vectors exhibited the capability to selectively target and transduce non-activated CD8+ T cells that can eliminate CD19-expressing tumours both in vitro and in vivo settings. Given the risks and high production costs associated with viral vector-based therapies, nanocarrier platforms appear to be more suitable for targeting T cells in vivo. Their surface can be conjugated with ligands to target the receptors highly expressed by T cells.131 To date, the frequently selected receptors include general T cell markers such as CD3,108 CD4,132 CD7133 and CD8,134 which could be taken into consideration when designing T cell targeting strategies. Moreover, additional research is necessary to enhance the stability and biocompatibility of these nanoparticles within biological environments. This is crucial to prevent degradation and potential adverse reactions in the body before they can effectively reach and targeted T cells.
Another major issue associated with nanocarriers is their limited transfection efficiency, which becomes more apparent when they are used for in vivo transfection of genes including CAR constructs. Even with lipid nanoparticles which have been used in clinical settings, they exhibit a very limited ability to escape from the endo/lysosomal compartments before entering the cytoplasm, which can result in enzymatic degradation of genetic cargo (such as mRNA) within the lysosomes.135 It is important to note that an additional barrier exists for plasmid DNA cargo, which must be transported into the nucleus for transcription and subsequent translation into proteins in the cytosol.136 To tackle this problem, various strategies have been developed including use of pH-responsive nanocarriers and incorporation of components that trigger escape mechanisms, such as pore-forming proteins or peptides.137,138
Achieving sustained functionality and persistence of in vivo CAR T cells presents another critical issue in optimising therapeutic outcomes for patients with cancer.139 Researchers are exploring various strategies to overcome this challenge. One approach involves refining CAR design by incorporating co-stimulatory domains that enhance T cell longevity and activity.140 Modulating intracellular signalling pathways within CAR T cells using co-stimulatory domains can prolong their functionality.141 Strategies to prevent T cell exhaustion, such as checkpoint inhibition or cytokine support, are also investigated.142 Moreover, efforts have been made to promote the formation of memory CAR T cells, enabling lasting anti-tumour responses of in vivo CAR T cells in both haematological and solid malignancies.143
Similar to the ex vivo CAR T therapy, the in vivo CART cell therapy for solid tumours is much more challenging compared to haematological malignancies. Solid tumours commonly exhibit immunosuppressive tumour microenvironment which can inhibit T cell activities, thus reducing the efficacy of CAR T therapies. One strategy to enhance the efficacy for solid tumours involves improving the homing of CAR T cells to tumour sites by remodelling tumour vasculature with agents such as Bevacizumab,144 combretastatin A-4 phosphate145 or blood–brain barrier permeabilizer NEO100.60 Moreover, the heterogeneity of antigens in solid tumours poses challenges for CAR-T cells, preventing them from effectively detecting cancer cells and significantly limiting their antitumour function.146 This issue is linked to the inadequate production of CAR-T cells in vivo that target various tumour-associated antigens, thus resulting in the limited efficacy of CAR-T therapy for solid tumours and the occurrence of disease relapse.147 To address this challenge, several methods have been explored such as co-expression of several CARs on a single T cell and expression of a chimeric receptor including two or more antigen recognition domains.148
In addition to the above mentioned challenges, considering the clinical relevance of the animal models utilised in preclinical studies is also crucial for successful therapy development.149 Presently, the evaluation of antitumour activity of the in vivo CAR T cell therapy primarily relies on xenograft model on immunodeficient mice engrafted with human tumour cell lines.150 However, these approaches hinder in-depth understanding of cancer progression mechanisms as observed in patients, thereby limiting treatment options. To address this limitation, researchers have adopted patient-derived xenograft (PDX) models, where patient-specific cancer cells are engrafted into immunodeficient mice.151 This approach enables the assessment of immune responses to primary cancer cells, enhancing the relevance to clinical applications.151 Nonetheless, the major drawback of the PDX model lies in their use of immunodeficient mouse strains like NOG/NSG, which might evoke different immune responses compared to those potentially detected in patients with cancer, thus restricting the efficacy evaluation of therapeutic strategies.152 An alternative advancement in preclinical study design involves employing humanised mouse models where nude mice are transplanted with human immune cells to develop fully functional human haematopoietic and immune systems.150 These models facilitate the examination of safety and efficacy of the in vivo CAR T therapy in immunocompetent hosts, providing insights on how human CAR T constructs interact with human tumours while circumventing allogeneic and anti-mouse xeno-antigens.149 For instance, findings from humanised mouse models suggest that the VivoVec™ consistently exhibits low toxicity and robust generation of functional CAR T cells in vivo in non-human primates.123 This implies its potential safety and efficacy for further clinical investigations.
Regulatory consideration and approval of the in vivo CAR T therapy
Similar to the ex vivo CAR T therapy, transitioning in vivo CAR T products from preclinical success to the clinical settings requires overcoming regulatory and translational hurdles, ensuring theirs safety and efficacy in the clinical setting.153 Regulatory bodies such as the Food and Drug Administration (FDA) and European Medicines Agency (EMA) require viral and nanocarrier vectors to adhere to stringent quality, safety, and efficacy standards prior to approval.154,155 For instance, toxicology evaluations are crucial for ensuring lipid nanocarrier compliance with regulations, as the accumulation of lipid nanocarriers in healthy tissues can lead to cyto- and/or geno-toxicity.156 This may be due to the cationic lipid components used in lipid formulations.157 Meanwhile, viral vectors used in the clinic for CAR gene delivery are also required to carefully test their purity, safety, stability, and functionality.128,156 Certain viral vectors used to deliver CARs have been implicated to occasional instances of cancer in other gene therapy applications. This is because they carry substantial risks of oncogenic insertional mutagenesis or triggering unwanted inflammatory responses in the modified T cells.139,158
Another regulatory concern regarding the in vivo CAR T therapeutic strategies is the safety profile of gene editing tools, such as the CRISPR/Cas9 system.159 For example, it may result in unwanted gene editing events with the introduction of variable length insertion/deletion (indel) at off-target sites within the targeted T cells.160 These off-target effects can promote functional gene disruption and epigenetic modifications, thus potentially leading to low therapeutic efficacy or even hazardous consequences such as excessive cytokine releases or adverse immune responses within the hosts.161 To minimise unintended off-target effects of CRISPR/Cas9 editing tool during T cell modification both in vivo and ex vivo, high-fidelity Cas9 variants could be considered.160
In addition to the regulatory considerations, the approval process for the in vivo CAR T therapy also pose the challenges during setup of clinical trials.162 First, establishing suitable patient eligibility criteria for clinical trials is a crucial step. This involves accurately identifying individuals who are most likely to benefit from the treatment with minimal potential risks.163 Additionally, regulatory agencies demand compelling evidence of efficacy of the in vivo CAR T therapy in treating the targeted cancer.162 This often requires well-designed clinical trials with appropriate dose level, analysis of safety outcomes such as neurotoxicity, cytokine release syndrome events and dose-limiting toxicity alongside with assessment on clinical benefits.162 Moreover, long-term follow-up data are indispensable for evaluating the safety and efficacy of the therapy over time.164 This includes monitoring patients for delayed adverse events and assessing treatment response durability.162 Lastly, determining the appropriate regulatory pathway for in vivo CAR T therapy can be complex and time-consuming. Depending on the therapy’s classification, it may require approval through various regulatory channels, such as the FDA’s Biologics License Application (BLA) or the EMA’s Marketing Authorization Application (MAA).165
Outstanding questions
In preclinical studies, in vivo CAR gene delivery using nanocarriers or viral vectors has produced therapeutic CAR T cells comparable to those generated by the ex vivo method. These advancements have shown promise in enhancing cancer treatment strategies. Moving forward, current research efforts are attempting to address outstanding questions and limitations, including how to precisely target T cells in vivo while improving the transfection efficiency of nanocarriers. Researchers are also exploring methods to enhance the long-term effectiveness of CAR T cells and refine the components of genetic cargo to minimise unintended off-target effects. Furthermore, nonhuman primate models will be crucial for evaluating the safety and pharmacokinetic profiles of the in vivo CAR T cell products. Additionally, it is also essential to address manufacturing practices, quality assurance and regulatory considerations to advance this treatment into clinical applications.
Conclusions
While existing ex vivo CAR T products are effective, their complexity and high cost limit accessibility and benefit to all patients. As a feasible off-the-shelf alternative, the in vivo CAR therapy may address the significant concerns associated with ex vivo CAR T therapy. It has the potential to enhance safety and efficacy, reduce treatment waiting times, eliminate the need for lymphodepleting chemotherapy and offer greater convenience to patients. Although some questions and challenges remain, ongoing research efforts and industry investments are essential for realising the full potential of the in vivo CAR T cell therapy in revolutionising cancer medicine and benefiting numerous patients.
Contributors
T. Bui and H. Mei conducted the literature search, drafted the manuscript and designed figures. R. Sang drafted the section on delivery systems. D. Ortega contributed to editing the manuscript. W. Deng contributed to conceptualisation of the study and manuscript review and editing. All authors read and approved the final version of the manuscript.
Declaration of interests
The authors report no conflicts of interest in this work.
Acknowledgements
This work was financially supported by the funding (GNT1181889) from the Australian National Health and Medical Research Council and fellowship award (2019/CDF1013) from Cancer Institute NSW, Australia. The funders had no role in paper design, data collection, data analysis, interpretation, writing of the paper.
References
Articles from eBioMedicine are provided here courtesy of Elsevier
Citations & impact
This article has not been cited yet.
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/166261823
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Use of Cell and Genome Modification Technologies to Generate Improved "Off-the-Shelf" CAR T and CAR NK Cells.
Front Immunol, 11:1965, 07 Aug 2020
Cited by: 73 articles | PMID: 32903482 | PMCID: PMC7438733
Review Free full text in Europe PMC
Engineering strategies to safely drive CAR T-cells into the future.
Front Immunol, 15:1411393, 19 Jun 2024
Cited by: 2 articles | PMID: 38962002 | PMCID: PMC11219585
Review Free full text in Europe PMC
CAR-T lymphocyte-based cell therapies; mechanistic substantiation, applications and biosafety enhancement with suicide genes: new opportunities to melt side effects.
Front Immunol, 15:1333150, 18 Jul 2024
Cited by: 0 articles | PMID: 39091493 | PMCID: PMC11291200
Review Free full text in Europe PMC
Immune Cell Hacking: Challenges and Clinical Approaches to Create Smarter Generations of Chimeric Antigen Receptor T Cells.
Front Immunol, 9:1717, 31 Jul 2018
Cited by: 32 articles | PMID: 30108584 | PMCID: PMC6080612
Review Free full text in Europe PMC
Funding
Funders who supported this work.
Cancer Institute NSW
National Health and Medical Research Council (1)
Grant ID: 2019/CDF1013




