Abstract
Free full text

Fluid Overload-Associated Large B-Cell Lymphoma with Light Chain Restriction Type Plasma Cell Infiltration: A Case Report
Abstract
Patient: Male, 80-year-old
Final Diagnosis: Fluid overload-associated large B-cell lymphoma
Symptoms: Right pleural effusion and short-ness of breath
Clinical Procedure: —
Specialty: Hematology
Objective:
Rare coexistence of disease or pathology
Background:
Fluid overload-associated large B-cell lymphoma (FO-LBCL) is a recently described malignant lymphoma that presents with serous effusions in the pleura, peritoneum, and/or pericardium but without an identifiable lymphoma mass. This report describes the case of an 80-year-old man who presented with a pleural effusion and describes the approach to diagnosis and management of FO-LBCL.
Case Report:
We present a case of an 80-year-old man who presented with right pleural effusion and shortness of breath at work. Initial radiological assessment suggested a pleural effusion on the right side, without an identifiable mass, given the patient’s symptoms and imaging characteristics. Subsequently, he underwent a pleural fluid puncture and biopsy. Based on the initial pathological assessment, malignant lymphoma, a non-epithelial tumor, was considered likely, but differentiation from reactive proliferative cells was difficult, given the patient’s symptoms and cytologic characteristics. Postoperatively, histopathological examination and immunohistochemistry confirmed a diagnosis of FO-LBCL. After 1 year of follow-up, the condition had progressed and the patient died due to recurrence.
Conclusions:
This report has presented a case of FO-LBCL in an elderly man with pleural effusion and described how this rare and recently described lymphoma was diagnosed and managed.
Introduction
In 1989, Sider and Horton first reported a case of human immunodeficiency virus (HIV)-infected lymphoma with clinical presentation of only serous effusion and no solid tumor formation [1]. In 1996, this type of lymphoma was named PEL and was believed to be closely related to human herpes virus 8 (HHV8) infection [2]. In 2001, PEL was officially included in a chapter related to B-cell lymphoma in the WHO classification of hematopoietic and lymphoid tissue tumors [3]. PEL mainly occurs in individuals with immune dysfunction, particularly those infected with HIV, HHV8, or EBV, with HHV8 infection being the most important pathogenic mechanism [3]. The diagnosis of PEL requires comprehensive consideration of clinical imaging, pathological morphology, immunohistochemistry, and virological testing. The prognosis of PEL is extremely poor, with a median survival of only 6 months [3]. Fluid overload-associated large B-cell lymphoma (FO-LBCL) is a new entity described in the Fifth Edition of the World Health Organization Classification of Hematolymphoid Tumors. FO-LBCL refers to malignant lymphoma present with symptoms of serous effusions in body cavities (pleural, peritoneal, and/or pericardial) in the absence of an identifiable tumor mass [4]. It is not associated with the Kaposi sarcoma-associated herpes virus (KSHV)/HHV8. FO-LBCL is a separate tumor entity from PEL, with unique clinical and pathological features and a relatively indolent course [5]. To date, more than 100 cases have been reported [4]. The pathogenesis of FO-LBCL is currently unclear, and it often occurs in elderly patients with underlying diseases, such as liver cirrhosis caused by hepatitis B or C, chronic congestive heart failure, or organ transplantation causing excessive body fluids, which may be related to immune dysfunction [6,7]. Gisriel et al conducted a retrospective multicenter study of 55 cases of non-HHV8-associated PEL-like lymphoma (PEL-LL) and a comprehensive review of 147 cases in the literature to determine the various clinical and pathological features [8]. FO-LBCL is more common in elderly people (median age 70–77 years) and patients with normal immune function. It presents as lymphomatous effusion without solid tumor components, mostly occurring in the pleural cavity (40/55, 73%), followed by the pericardial cavity (17/55, 31%). The detection rate of EBV is only 6% (3/47) [8]. The prognosis of FO-LBCL is favorable in most cases and is largely determined by comorbidities. Most patients are treated with the CHOP therapeutic schedule (including 4 drugs: cyclophosphamide, doxorubicin, vincristine, and prednisone) with or without rituximab, and usually results in a good outcome.
This report describes the case of an 80-year-old man who presented with a pleural effusion and describes the approach to the diagnosis and management of FO-LBCL.
Case Report
An 80-year-old man was admitted to the hospital due to shortness of breath at work and right pleural effusion. He had a history of hypertension, benign prostatic hyperplasia, and atrial fibrillation. He was admitted without cough, fever, or breathing difficulties. A clinical examination showed weakened respiratory sounds and discordant heart sounds on the dorsal side of the right lower lung field, no enlargement of the liver and spleen, no superficial lymph node enlargement, and no pressure edema in the calf. Blood biochemistry revealed a high lactate dehydrogenase (LDH) level of approximately 320 U/L. A serological examination was negative for HIV, hepatitis C, and hepatitis B viruses surface antibodies. Fast-acid staining of pleural effusion was negative and pleural effusion cultures were negative for Mycobacterium tuberculosis. Measurement of the level of soluble interleukin-2 receptor, a tumor marker, revealed a level of 1790 U/mL, which was higher than the reference value. Positron emission tomography-CT revealed pleural effusion on the right side, with mild uneven aggregation observed in the surrounding tissues. The patient’s adenosine deaminase (ADA) level was significantly increased to 176.9 IU/L (normal value: 40–50 IU/L), raising the suspicion of tuberculous pleurisy. No obvious tumor formation was observed in PET-CT, and findings associated with lymphoma, such as lymph node enlargement, were not confirmed (Figure 1). Therefore, a pleural fluid puncture and biopsy were performed.
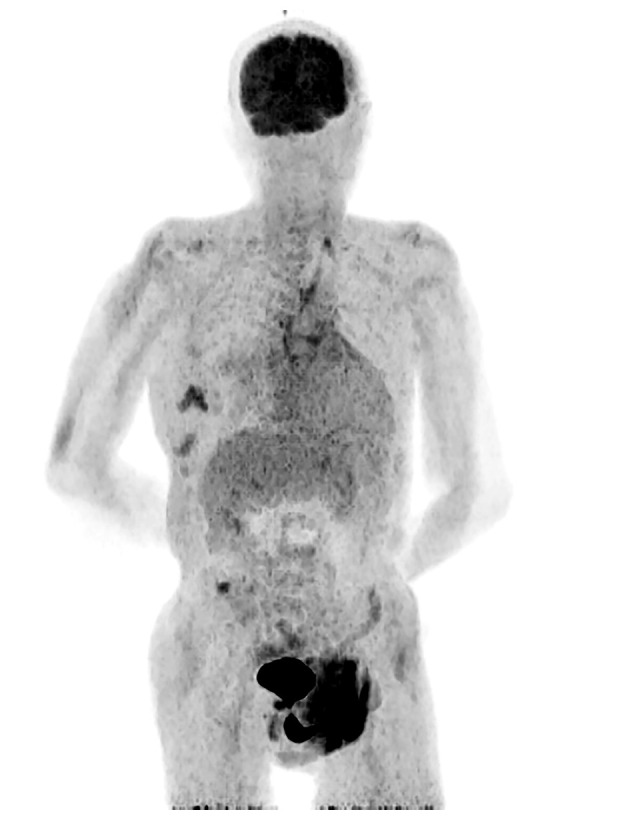
Positron emission tomography. Accumulation in the wound due to right pleural fluid drainage but no obvious tumor formation, lymph node enlargement, or other observations suggesting lymphoma were found. There were accumulations at the neck and right lower abdomen, but it was not known if they were related to lymphoma.
Papanicolaou (PAP) staining of the pleural fluid cytology showed many atypical cells scattered in the background of inflammatory cells and necrotic substances, cells with enlarged nuclei, increased nucleus/cytoplasmic ratio, and irregular nuclear morphology (Figure 2A). Polynuclear and lobulated cells are also observed in places and the cell morphology was equivalent to that of atypical lymphoid-like cells in diffuse large B-cell lymphoma (DLBCL) (Figure 2B). HE staining of the pleural fluid cell block showed scattered isolated atypical cells with nuclear translocation, a larger cell morphology than small lymphocytes, abundant basophilic cytoplasm, and clear nucleosomes (Figure 3A). Polynuclear and lobulated cells are also observed in places (Figure 3B). Therefore, we considered a diagnosis of non-epithelial tumors and suspected lymphomas.
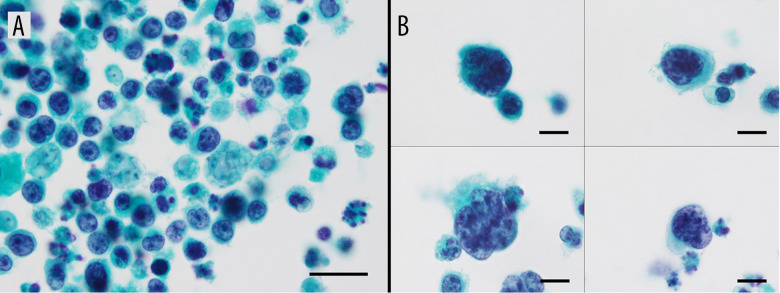
PAP staining of a pleural fluid cytology. (A) Cells with enlarged nuclei, increased nucleus/cytoplasmic ratio, and irregular nuclear morphology were seen (magnification ×1000; scale bar: 20 μm), (B) Polynuclear and lobulated cells are also observed in places. (Cells are presented in a four-part panel, magnification ×1000; scale bar: 10 μm).
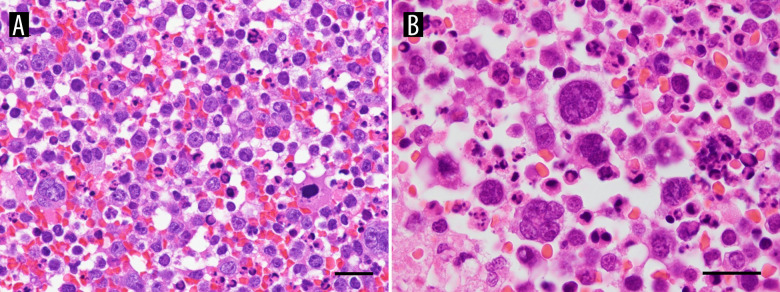
Histopathological features of the pleural fluid cell block. (A, B) Hematoxylin and eosin (H&E). Pleural fluid cell block shows scattered isolated atypical cells with nuclear translocation, larger cell morphology than small lymphocytes, abundant basophilic cytoplasm, and clear nucleosomes. Polynuclear and lobulated cells are also observed in places. (A: magnification ×600; scale bar: 20 μm, B: magnification ×1000; scale bar: 20 μm).
A pleural biopsy showed histological evidence of lymphocyte and plasma cell infiltration into the fat, with occasional areas of atypical large lymphoid cells. Large atypical nuclei and multinucleated atypical cells with high nuclear/cytoplasmic ratios could be observed everywhere. In addition, there were some multinucleated plasma cells, as well as some 3–4 multinucleated plasma cells. An area of coagulation necrosis was visible; however, no caseous granulomas or Langerhans giant cells were observed. Immunohistochemical staining revealed the infiltration of lymphocytes, plasma cells, and adipose tissue cells into the mesothelium and chest cavity, confirming tumor infiltration beyond the mesothelium into the chest cavity. Many large or multinucleated CD20-positive and CD79α-positive atypical cells were observed in most areas. Similar cells were also observed in the cell blocks of pleural fluid cytology specimens – CD20-positive (Figure 4A) and CD79α-positive large atypical lymphocytes, CD3-positive small lymphocytes but negative for large atypical lymphocytes (Figure 4B), CD30-negative large atypical lymphocytes (Figure 4C), Bcl-6 and Bcl-2-positive (Figure 4D, 4E), EBV-negative, and HHV8-negative cells (Figure 4F). Therefore, it was determined to be FO-LBCL.
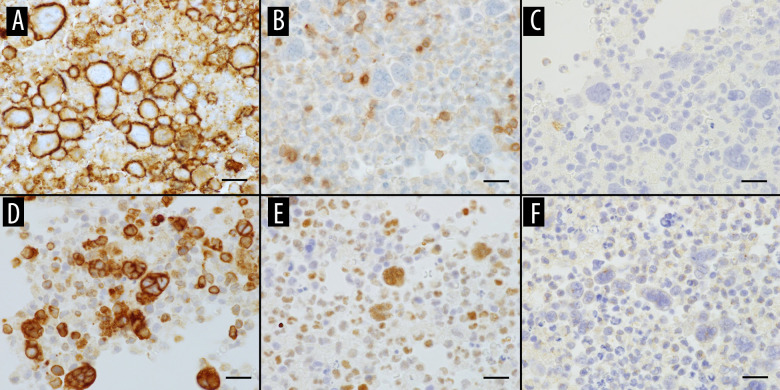
Immunohistochemical profile of FO-LBCL. (A) CD20 positive for large atypical lymphocytes, (B) CD3 negative for large atypical lymphocytes, (C–E) CD30, Bcl-2, and Bcl-6 positive for large atypical lymphocytes, (F) HHV-8 negative for large atypical lymphocytes (magnification ×600; scale bar: 20 μm).
Specimens obtained from pleural biopsy showed a high degree of inflammation extending into the adipose tissue of the chest wall (Figure 5A), and the inflamed areas also showed atypical cells with enlarged nuclei, which are FO-LBCL cells (Figure 5D, red circles in Figure 5A). In another area, the inflamed areas of the chest wall had areas with many plasma cells (Figure 5E, yellow circled area in Figure 5A). With regard to the ISH-κ/λ ratio, both ISH-κ and ISH-λ were stained and there was a difference between the κ-positive plasma cells and the λ-positive plasma cells (Figure 5B, 5C). The number of κ-positive plasma cells was significantly higher than the number of λ-positive plasma cells. In some areas, FO-LBCL cells were mildly positive for ISH-κ (Figure 5F, red circle in Figure 5B), but an increase in ISH-κ positive plasma cells was observed (Figure 5G, yellow circle in Figure 5B). FO-LBCL cells were mildly positive for ISH-λ in some cells but negative in many cells (Figure 5H, red circles in Figure 5C), only a few plasma cells were positive for ISH-λ and most cells were negative (Figure 5I, yellow circles in Figure 5C). The kappa value was highly biased, and we considered that this was due to light chain restriction.
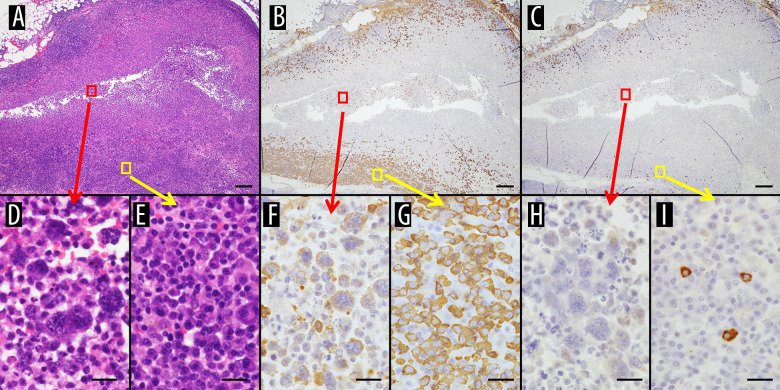
Specimens obtained from pleural biopsy. (A) HE staining. High degree of inflammation extending into the adipose tissue of the chest wall is seen. (B) ISH-κ and (C) ISH-λ. There are areas of predominance of κ-positive cells. (D) HE staining. Inflamed areas show atypical cells with enlarged nuclei, which are FO-LBCL cells (red circles in A). (E) HE staining. Inflamed areas of the chest wall have areas with many plasma cells (yellow circled area in A). (F) ISH-κ. FO-LBCL cells are mildly positive for ISH-κ (red circle in B). (G) ISH-κ. An increase in ISH-κ positive plasma cells is observed (yellow circle in B). (H) ISH-λ. FO-LBCL cells are ISH-λ mildly positive in some cells but negative in many cells (red circles in C). (I) ISH-λ. Only a few plasma cells are ISH-λ positive and most cells are negative (yellow circles in C). (A–C: magnification ×20; scale bar: 500 μm, D–I: magnification ×600; scale bar: 20 μm).
Our patient experienced respiratory depression after receiving the R-THP-COP regimen (rituximab combined with pirarubicin, cyclophosphamide, vincristine, and prednisone) and using rituximab again. However, there were no symptoms after re-administration of rituximab at 50 mL/h. Therefore, the medication was discontinued. The results of blood and pleural fluid extraction showed a decrease in C-reactive protein and LDH levels, as well as a decrease in pleural fluid. This obtained a good therapeutic effect. After 1 year of follow-up, the condition had progressed and the patient died due to recurrence.
Discussion
The present case will deepen pathologists’ understanding of the disease while avoiding misdiagnosis and the misuse of diagnostic terminology. The patient in this case was 80 years of age and presented with right pleural effusion. Since the imaging examination did not reveal any substantial mass or lymph node enlargement, our case coincided with previous literature reports, but there was a light chain restriction. Bahmad recently reported a case of FO-LBCL [5] very similar to our case, including the immunohistochemical expression, but had no detailed description of light chain restriction, which we would like to discuss here. The number of B cells expressing different light chains in normal lymph node kappa/lambda was approximately 2: 1. If there is an absolute advantage of a light chain, it is that it indicates the existence of monoclonal proliferation in plasma cells, which is one of the characteristics of malignant proliferation. In this case, plasma cells surrounding atypical lymphoid cells expressed varying degrees of kappa and lambda, which appeared to be imbalanced. The number of kappa-positive plasma cells was significantly higher than that of lambda-positive plasma cells, which confirmed the presence of malignant proliferation and plasma cell differentiation in the patient and increased the possibility of a lymphoma diagnosis. The morphological expression of FO-LBCL was similar to that of PEL [9,10], with large heterotypic cells present in the form of immune mother cells, central mother cells, or more anaplastic large cells. The nuclei were large, round, or irregularly shaped, with obvious nucleoli and visible binuclear or multinucleated nuclei. The cytoplasm was relatively rich and small vacuoles were observed in some cells. In this case, a large number of inflammatory cells and necrotic substances were observed in the cytological smear, with a scattered distribution of atypical cells, varying cell sizes, round nuclei, increased chromatin and irregular morphology, and clear nucleosomes visible. FO-LBCL expresses B-cell markers such as CD19, CD20, and CD79α, and there was no evidence of HHV8 or HIV infection, which is consistent with the literature.
To avoid a misdiagnosis, FO-LBCL must be differentiated from the following diseases: 1) PEL, both have similar clinical imaging and pathological morphological characteristics, but PEL expresses CD45, CD30, CD38, and CD138, while B-cell markers are often negative, EBV is often positive, and HHV8-positive is an essential diagnostic criterion for PEL [9]. 2) Malignant mesothelioma usually manifests as pleural thickening or a mass, with tumor cells arranged in a patchy, clustered, mulberry-shaped, or papillary shape. Immunohistochemical staining showed positive mesothelial markers, such as Mesothelin, Calretinin, CK5/6, WT1, D2-40. In this case, atypical tumor cells were scattered, and immunohistochemical staining showed CD20 and CD79 α positivity, indicating lymphoma. 3) For DLBCL, the pathological morphology and immune phenotype were consistent with FO-LBCL, but DLBCL usually involves multiple lymph nodes or extranodal organs throughout the body, which could be differentiated from this disease. 4) Regarding fibrin-associated diffuse large B-cell lymphoma, the 2017 WHO Classification of Hematopoietic and Lymphatic Tissue Tumors classifies DLBCL as a special type of chronic inflammatory disease-related DLBCL, similar to FO-LBCL, which does not form a solid mass and occurs in a closed body cavity. Tumor cells are often located in the fibrous material of cysts or hematomas, and they often show a good prognosis [11]. However, unlike FO-LBCL, it is mostly positive for EBV, which is often associated with immune deficiency, whereas FO-LBCL is often EBV-negative and not related to immune deficiency. Our patient was EBV-negative, which could exclude the disease.
Genetic changes in MYC are associated with the pathogenesis of FO-LBCL. Unlike the PEL molecular mechanisms, MYC gene rearrangement occurs in 19% of cases, which is more common than traditional DLBCL [12]. Second-generation sequencing showed that genes related to FO-LBCL have complex chromosomal karyotypes, frequent mutations, copy number amplification, and translocation in the genome [3]. In addition to frequent somatic cell mutations, the most common mutations are HIST1H1E and MYD88, with some individuals exhibiting co-mutations of MYD88 and CD79b [3]. When cases of FO-LBCL showed such “double or triple blow” genetic changes, we believe that following the 2022 ICC classification criteria, it may be more appropriate to classify them as advanced B-cell lymphoma with effusion from the body cavity, as this type of tumor has a poor prognosis and inclusion will increase the heterogeneity of this type of tumor.
Based on previous research data and the relevant literature, it is recommended to use CHOP or a similar regimen for systemic chemotherapy in patients without obvious contraindications or comorbidities. The use of R-CHOP for chemotherapy after fluid drainage may be beneficial for patients with longer survival periods. For patients who cannot tolerate chemotherapy or elderly patients with severe complications who may not benefit from chemotherapy, separate drainage of the pleural effusion may be considered [8]. According to previous reports, the course of FO-LBCL is relatively indolent compared to that of PEL, with a median survival of over 10 months [13] and a 2-year overall survival rate of 87.4% [10]. Zaimoku et al found that patients >70 years of age and with serum LDH ≤500 IU/L had a survival period of >1 year [14]. Kubota et al believed that aging and CD20-positivity were independent prognostic factors for FO-LBCL that were not associated with HHV8 [7].
Conclusions
This report has presented a case of FO-LBCL in an elderly man with pleural effusion and has described how this rare and recently described lymphoma was diagnosed and managed.
Acknowledgments
We would like to thank the staff of the Department of Pathology and Laboratory Medicine, Kanazawa Medical University for their support of our case report.
Footnotes
Publisher’s note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
Articles from The American Journal of Case Reports are provided here courtesy of International Scientific Information, Inc.
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
A rare case of fluid overload-associated large B-cell lymphoma and antigen loss at relapse.
J Hematop, 16(4):235-240, 27 Nov 2023
Cited by: 0 articles | PMID: 38175437
Fluid Overload-Associated Large B-Cell Lymphoma: A Case Report and Review of Literature.
Hematol Rep, 15(3):411-420, 03 Jul 2023
Cited by: 1 article | PMID: 37489372
Anaplastic lymphoma kinase-positive large B-cell lymphoma: a case report with emphasis on the cytological features of the pleural effusion.
Int J Clin Exp Pathol, 6(11):2631-2635, 15 Oct 2013
Cited by: 21 articles | PMID: 24228132 | PMCID: PMC3816839
Serous effusions in malignant lymphomas: a review.
Diagn Cytopathol, 34(5):335-347, 01 May 2006
Cited by: 94 articles | PMID: 16604559
Review

 A
,
B
,
E
,
F
,
1
3
A
,
B
,
E
,
F
,
1
3


