Abstract
Free full text

Oxygen regulation in Salmonella typhimurium.
Abstract
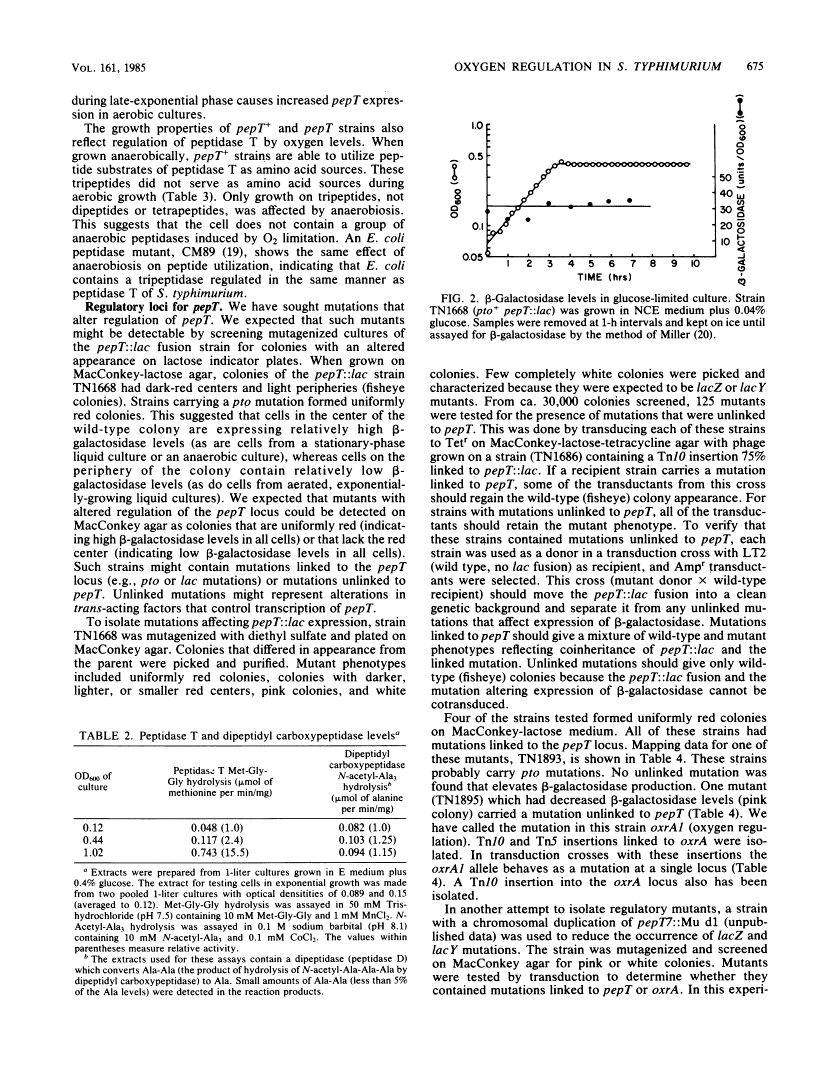
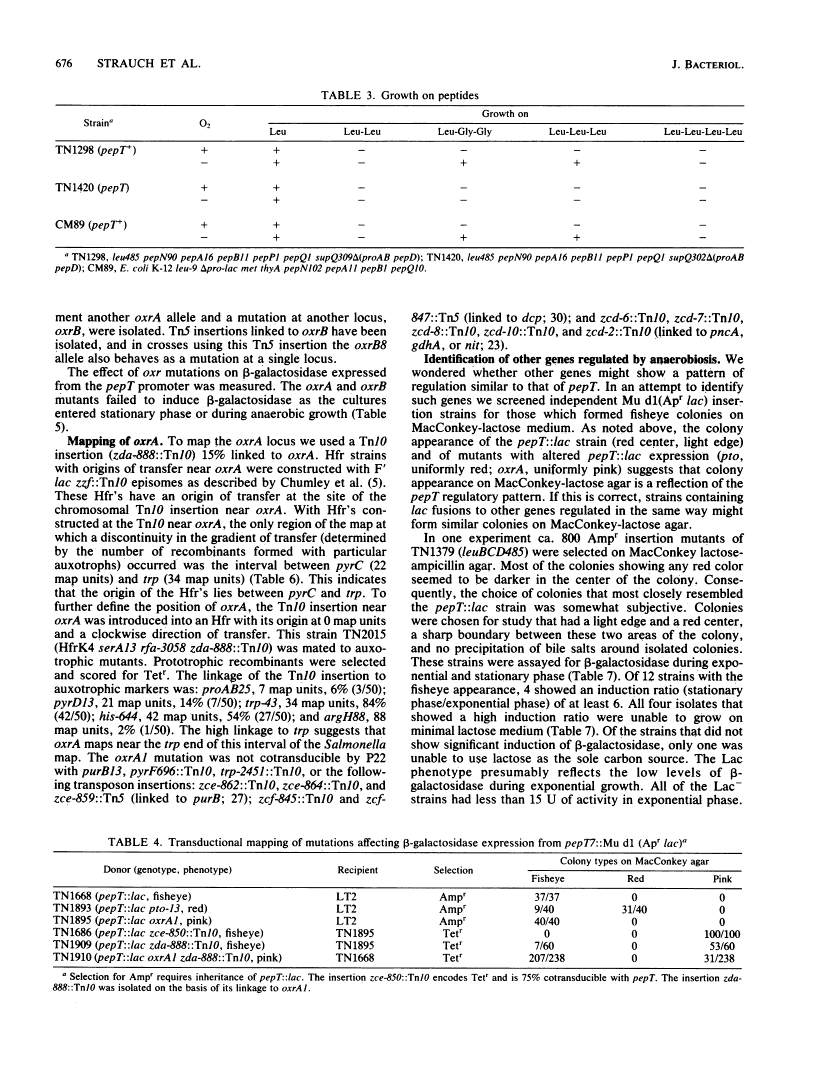
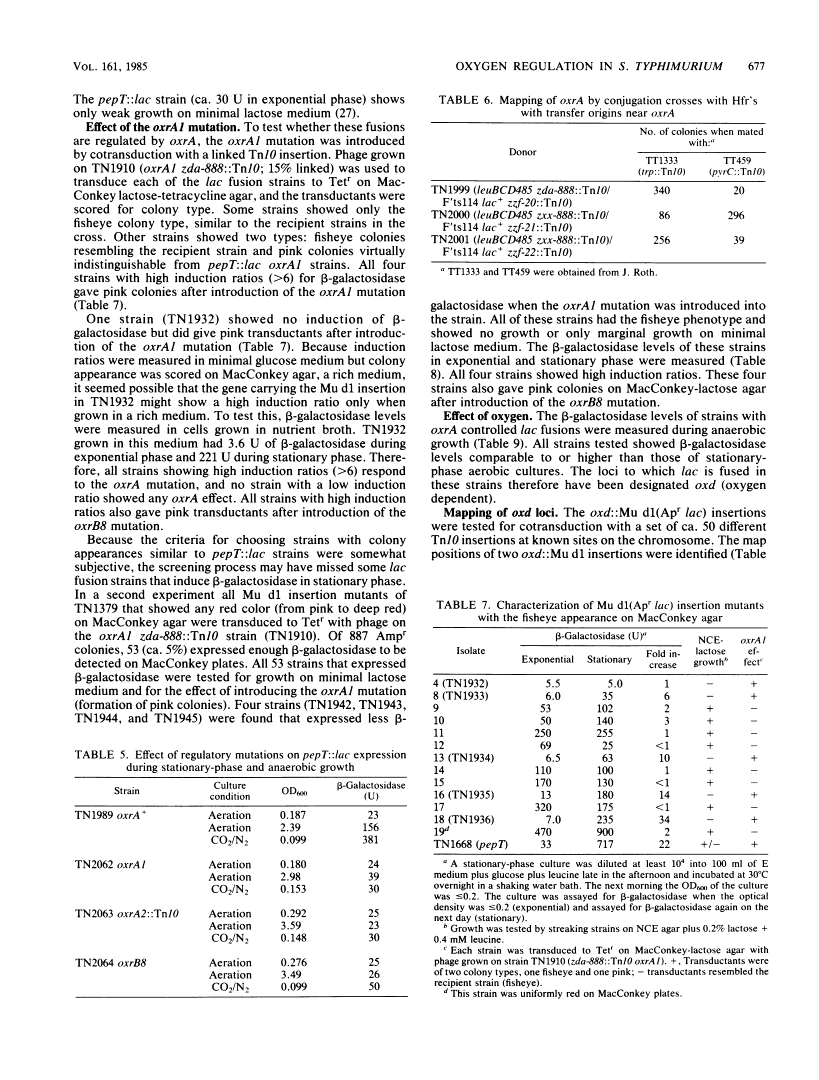
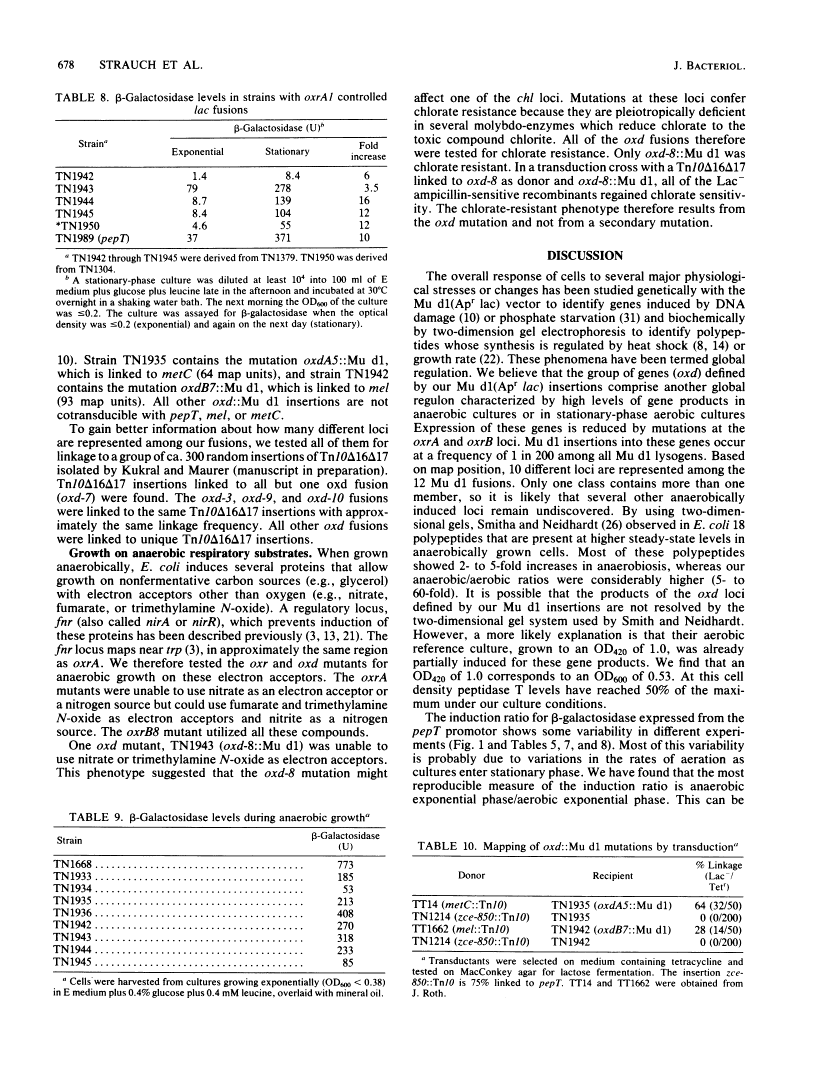
Regulation by oxygen of the peptidase T (pepT) locus of Salmonella typhimurium was studied by measuring beta-galactosidase levels in strains containing a pepT::Mu d1(Apr lac) operon fusion. beta-Galactosidase was induced in anaerobic cultures and late-exponential and stationary-phase aerated cultures. Peptidase T activity also was induced under these growth conditions. pepT+ but not pepT strains will utilize as amino acid sources the tripeptides Leu-Leu-Leu and Leu-Gly-Gly only when grown anaerobically. Mutations at two loci, oxrA and oxrB (oxygen regulation) prevent induction of the pepT locus. The oxrA locus is homologous to the fnr locus of Escherichia coli. We have isolated 12 independent Mu d1 insertions (oxd::Mu d1, oxygen dependent) that show induction of beta-galactosidase in anaerobic cultures and stationary-phase aerated cultures. These insertions fall into nine classes based on map location. All of the oxd::Mu d1 insertions are regulated by oxrA and oxrB and therefore define a global regulon that responds to oxygen limitation.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.5M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bukhari AI. Reversal of mutator phage Mu integration. J Mol Biol. 1975 Jul 25;96(1):87–99. [Abstract] [Google Scholar]
- Casadaban MJ, Cohen SN. Lactose genes fused to exogenous promoters in one step using a Mu-lac bacteriophage: in vivo probe for transcriptional control sequences. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4530–4533. [Europe PMC free article] [Abstract] [Google Scholar]
- Chippaux M, Bonnefoy-Orth V, Ratouchniak J, Pascal MC. Operon fusions in the nitrate reductase operon and study of the control gene nir R in Escherichia coli. Mol Gen Genet. 1981;182(3):477–479. [Abstract] [Google Scholar]
- Chippaux M, Giudici D, Abou-Jaoudé A, Casse F, Pascal MC. Laboratoire de Chimie Bactérienne C.N.R.S., Marsielle, France. Mol Gen Genet. 1978 Apr 6;160(2):225–229. [Abstract] [Google Scholar]
- Chumley FG, Menzel R, Roth JR. Hfr formation directed by tn10. Genetics. 1979 Apr;91(4):639–655. [Europe PMC free article] [Abstract] [Google Scholar]
- Csonka LN, Howe MM, Ingraham JL, Pierson LS, 3rd, Turnbough CL., Jr Infection of Salmonella typhimurium with coliphage Mu d1 (Apr lac): construction of pyr::lac gene fusions. J Bacteriol. 1981 Jan;145(1):299–305. [Europe PMC free article] [Abstract] [Google Scholar]
- Foster TJ, Davis MA, Roberts DE, Takeshita K, Kleckner N. Genetic organization of transposon Tn10. Cell. 1981 Jan;23(1):201–213. [Abstract] [Google Scholar]
- Herendeen SL, VanBogelen RA, Neidhardt FC. Levels of major proteins of Escherichia coli during growth at different temperatures. J Bacteriol. 1979 Jul;139(1):185–194. [Europe PMC free article] [Abstract] [Google Scholar]
- Hermsdorf CL. Tripeptide-specific aminopeptidase from Escherichia coli AJ005. Biochemistry. 1978 Aug 8;17(16):3370–3376. [Abstract] [Google Scholar]
- Jamieson DJ, Higgins CF. Anaerobic and leucine-dependent expression of a peptide transport gene in Salmonella typhimurium. J Bacteriol. 1984 Oct;160(1):131–136. [Europe PMC free article] [Abstract] [Google Scholar]
- Kenyon CJ, Walker GC. Expression of the E. coli uvrA gene is inducible. Nature. 1981 Feb 26;289(5800):808–810. [Abstract] [Google Scholar]
- Kranz RG, Gennis RB. Isoelectric focusing and crossed immunoelectrophoresis of heme proteins in the Escherichia coli cytoplasmic membrane. J Bacteriol. 1982 Apr;150(1):36–45. [Europe PMC free article] [Abstract] [Google Scholar]
- Kwan HS, Barrett EL. Roles for menaquinone and the two trimethylamine oxide (TMAO) reductases in TMAO respiration in Salmonella typhimurium: Mu d(Apr lac) insertion mutations in men and tor. J Bacteriol. 1983 Sep;155(3):1147–1155. [Europe PMC free article] [Abstract] [Google Scholar]
- Lambden PR, Guest JR. Mutants of Escherichia coli K12 unable to use fumarate as an anaerobic electron acceptor. J Gen Microbiol. 1976 Dec;97(2):145–160. [Abstract] [Google Scholar]
- Lemaux PG, Herendeen SL, Bloch PL, Neidhardt FC. Transient rates of synthesis of individual polypeptides in E. coli following temperature shifts. Cell. 1978 Mar;13(3):427–434. [Abstract] [Google Scholar]
- LOWRY OH, ROSEBROUGH NJ, FARR AL, RANDALL RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [Abstract] [Google Scholar]
- Maurer R, Osmond BC, Shekhtman E, Wong A, Botstein D. Functional interchangeability of DNA replication genes in Salmonella typhimurium and Escherichia coli demonstrated by a general complementation procedure. Genetics. 1984 Sep;108(1):1–23. [Europe PMC free article] [Abstract] [Google Scholar]
- McHugh GL, Miller CG. Isolation and characterization of proline peptidase mutants of Salmonella typhimurium. J Bacteriol. 1974 Oct;120(1):364–371. [Europe PMC free article] [Abstract] [Google Scholar]
- Miller CG, Mackinnon K. Peptidase mutants of Salmonella typhimurium. J Bacteriol. 1974 Oct;120(1):355–363. [Europe PMC free article] [Abstract] [Google Scholar]
- Miller CG, Schwartz G. Peptidase-deficient mutants of Escherichia coli. J Bacteriol. 1978 Aug;135(2):603–611. [Europe PMC free article] [Abstract] [Google Scholar]
- Newman BM, Cole JA. The chromosomal location and pleiotropic effects of mutations of the nirA+ gene of Escherichia coli K12: the essential role of nirA+ in nitrite reduction and in other anaerobic redox reactions. J Gen Microbiol. 1978 May;106(1):1–12. [Abstract] [Google Scholar]
- Pedersen S, Bloch PL, Reeh S, Neidhardt FC. Patterns of protein synthesis in E. coli: a catalog of the amount of 140 individual proteins at different growth rates. Cell. 1978 May;14(1):179–190. [Abstract] [Google Scholar]
- Sanderson KE, Ross H, Ziegler L, Mäkelä PH. F + , Hfr, and F' strains of Salmonella typhimurium and Salmonella abony. Bacteriol Rev. 1972 Dec;36(4):608–637. [Europe PMC free article] [Abstract] [Google Scholar]
- Smith MW, Neidhardt FC. Proteins induced by anaerobiosis in Escherichia coli. J Bacteriol. 1983 Apr;154(1):336–343. [Europe PMC free article] [Abstract] [Google Scholar]
- Strauch KL, Carter TH, Miller CG. Overproduction of Salmonella typhimurium peptidase T. J Bacteriol. 1983 Nov;156(2):743–751. [Europe PMC free article] [Abstract] [Google Scholar]
- Strauch KL, Miller CG. Isolation and characterization Salmonella typhimurium mutants lacking a tripeptidase (peptidase T). J Bacteriol. 1983 May;154(2):763–771. [Europe PMC free article] [Abstract] [Google Scholar]
- Vimr ER, Green L, Miller CG. Oligopeptidase-deficient mutants of Salmonella typhimurium. J Bacteriol. 1983 Mar;153(3):1259–1265. [Europe PMC free article] [Abstract] [Google Scholar]
- Vimr ER, Miller CG. Dipeptidyl carboxypeptidase-deficient mutants of Salmonella typhimurium. J Bacteriol. 1983 Mar;153(3):1252–1258. [Europe PMC free article] [Abstract] [Google Scholar]
- Wanner BL, McSharry R. Phosphate-controlled gene expression in Escherichia coli K12 using Mudl-directed lacZ fusions. J Mol Biol. 1982 Jul 5;158(3):347–363. [Abstract] [Google Scholar]
Associated Data
Articles from Journal of Bacteriology are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/jb.161.2.673-680.1985
Read article for free, from open access legal sources, via Unpaywall:
https://jb.asm.org/content/jb/161/2/673.full.pdf
Free to read at jb.asm.org
http://jb.asm.org/cgi/content/abstract/161/2/673
Free after 4 months at jb.asm.org
http://jb.asm.org/cgi/reprint/161/2/673
Citations & impact
Impact metrics
Article citations
Proteomic Analysis of FNR-Regulated Anaerobiosis in Salmonella Typhimurium.
J Am Soc Mass Spectrom, 30(6):1001-1012, 22 Mar 2019
Cited by: 3 articles | PMID: 30903387
Engineering the perfect (bacterial) cancer therapy.
Nat Rev Cancer, 10(11):785-794, 14 Oct 2010
Cited by: 347 articles | PMID: 20944664 | PMCID: PMC3756932
Review Free full text in Europe PMC
Proteomic analysis of the adaptive response of Salmonella enterica serovar Typhimurium to growth under anaerobic conditions.
Microbiology (Reading), 155(pt 7):2429-2441, 23 Apr 2009
Cited by: 19 articles | PMID: 19389776
Regulatory and structural differences in the Cu,Zn-superoxide dismutases of Salmonella enterica and their significance for virulence.
J Biol Chem, 283(20):13688-13699, 24 Mar 2008
Cited by: 37 articles | PMID: 18362154 | PMCID: PMC2376220
Transcription modulation of Salmonella enterica serovar Typhimurium promoters by sub-MIC levels of rifampin.
J Bacteriol, 188(22):7988-7991, 15 Sep 2006
Cited by: 46 articles | PMID: 16980465 | PMCID: PMC1636311
Go to all (73) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Regulation of the Salmonella typhimurium pepT gene by cyclic AMP receptor protein (CRP) and FNR acting at a hybrid CRP-FNR site.
J Bacteriol, 179(6):1909-1917, 01 Mar 1997
Cited by: 9 articles | PMID: 9068635 | PMCID: PMC178913
Overproduction of Salmonella typhimurium peptidase T.
J Bacteriol, 156(2):743-751, 01 Nov 1983
Cited by: 9 articles | PMID: 6313618 | PMCID: PMC217891
Oxygen-regulated stimulons of Salmonella typhimurium identified by Mu d(Ap lac) operon fusions.
J Bacteriol, 165(3):780-786, 01 Mar 1986
Cited by: 31 articles | PMID: 3512523 | PMCID: PMC214496
Cloning and nucleotide sequence of the anaerobically regulated pepT gene of Salmonella typhimurium.
J Bacteriol, 173(11):3554-3558, 01 Jun 1991
Cited by: 11 articles | PMID: 1904438 | PMCID: PMC207971