Abstract
Free full text

The Role of Cannabis in the Development of Psychosis
ABSTRACT
Cannabis is known to cause psychotic disorders, and the increasing use of cannabis constitutes an important health problem. Growing evidence that cannabis causes the development of psychosis has led to an increase in the number of studies in this field. This review aims to clarify the role of cannabis use in the development of psychosis, discuss the current literature about the underlying neurobiological mechanisms. For this purpose PubMed was searched for the keywords “cannabis use, psychosis, schizophrenia, endocannabinoid system, pathophysiology, neurobiology”; the articles published in the last 10 years were reviewed. Epidemiological studies showed that cannabis use starting at an earlier age is associated with an increased risk of psychosis, this risk is more pronounced in people with genetic predisposition and increases with heavy and high potency cannabis use. Studies showed that the endocannabinoid system, which plays a role in nervous system development and functions as a homeostatic regulator in physiological processes, is affected by cannabis use during critical periods of development like adolescence; cannabis use affects physiological processes such as synaptic pruning due to the effects of this system on neurotransmitters like glutamate and dopamine leading to long-term behavioral and psychological consequences. Additionally, evidence that dysfunctions in the endocannabinoid system play a role in the etiology of schizophrenia suggests that cannabis affects the disease process by worsening existing dysfunctions in this system. Understanding the relationship between cannabis use and the development of psychosis and underlying neurobiological mechanisms will help to identify new treatment targets, and develop appropriate preventive approaches.
INTRODUCTION
Cannabis is the most widely used illicit psychoactive substance in the world today (United Nations Office on Drugs Crime 2019). It is obtained from the flowers, stems, leaves and seeds of Cannabis sativa, also known as Indian hemp. Dried plants are wrapped in tobacco paper and used as cigarettes or inhaled through water pipes called “bucket bong” or “bong”. It can also be used in the form of brewed tea, in various foods, or rectally, sublingually, transdermally and as eye drops. The active ingredient of cannabis and its derivatives is delta-9-tetrahydrocannabinol (Δ9-THC), which exerts its psychoactive effects through substances such as THC and cannabidiol (CBD). Depending on the THC and CBD concentrations, different effects occur at different doses. The main substance responsible for the psychoactive effect is THC. Tetrahydrocannabinol is fat-soluble. After ingestion, it quickly enters the brain and other organs. It exerts its effects through endogenous cannabinoids by binding to cannabinoid receptors on cell membranes in the central nervous system (CNS) and other tissues (Bloomfield et al. 2019). While tetrahydrocannabinol is responsible for the pleasurable and negative effects of cannabis, cannabidiol does not cause euphoria and may exhibit anxiolytic, antiepileptic, anti-inflammatory and analgesic properties (Sideli et al. 2021). Cannabidiol is also thought to be therapeutic in various neuropsychiatric disorders (Marco et al. 2011).
Legalization of cannabis use has been a controversial issue. Canada, Uruguay and some states in the United States have legalized the recreational use of cannabis (O’Grady et al. 2022). In the Netherlands, cannabis consumption in licensed cafes is legal. In addition, the medicinal use of cannabis for indications such as chronic pain has been legalized in different parts of the world, such as Argentina, Brazil, the Czech Republic, Denmark, Finland, Germany, Italy, New Zealand, Norway, Switzerland and the United Kingdom (Kumar et al. 2021). The legalization of the use of cannabis, especially for medicinal purposes, has popularized the belief that it has no harmful effects and may even be beneficial in cases such as anxiety and sleep disorders (Ahmed et al. 2021). It is also thought that legalizing cannabis use may have advantages such as increasing product safety, restricting access by non-adults, reducing interaction with illegal suppliers, facilitating education about cannabis use, and increasing users’ help-seeking behavior (Robertson and Thyne 2021). However, there are studies reporting an increase in cannabis use among young adults in many countries due to the legalization process (O’Grady et al. 2022).
With the increasing prevalence of cannabis use, studies investigating the relationship between cannabis use and mental disorders, especially psychotic disorders, have increased. A study conducted in Germany found that between 2000 and 2018, diagnoses related to cannabinoid use (especially cannabinoid intoxications, harmful use, dependence syndrome, withdrawal state and psychotic disorders) gradually increased in patients hospitalized in psychiatric wards (Gahr et al. 2022). Evidence that cannabis use is associated with mental disorders, especially psychotic disorders, highlights the importance of further studies to better understand the mechanisms involved in this relationship.
The aim of this review is to summarize the clinical effects of cannabis use, discuss the results of studies on its association with psychotic disorders and discuss possible underlying neurobiological mechanisms. For the review, the keywords “cannabis use, psychosis, schizophrenia, endocannabinoid system, pathophysiology, neurobiology” were entered into the “PubMed” search engine and research articles and reviews published in the last 10 years were accessed. Articles published in Turkish or English with full text available were evaluated for the purposes of this review.
CLINICAL EFFECTS OF CANNABIS
The effects of cannabis vary depending on the potency of the cannabis, its administration routes, the experience, expectations and biological predisposition of the users. The effect of cannabis starts within a few minutes after inhalation, reaches a peak in about 30 minutes and ends 2-4 hours later. When taken orally, the effect starts later and lasts longer. Due to the first pass metabolism via the gastrointestinal tract, a smaller amount of THC enters the bloodstream and has less effect (Öztürk Sarıkaya 2019).
Cannabis causes an increase in heart rate, a decrease in blood pressure and muscle relaxation shortly after ingestion (Aldemir 2022). In terms of neuropsychiatric effects, it can lead to euphoria, relaxation, reduction in anxiety, while in some susceptible individuals it can cause anxiety, dysphoria and panic. In addition, increased assertiveness, altered perception of color, sound and time, mystical thoughts, depersonalization, prolonged reaction time, impaired attention, concentration and short-term memory, impaired motor coordination and ability to complete complex tasks requiring divided attention may occur (Aldemir 2022). Hallucinations, temporary grandiosity, paranoia and other psychotic symptoms may also be observed (Aldemir 2022). Clinical signs of cannabis intoxication often improve spontaneously. Due to the absence of the cannabinoid receptor CB1 in the brain stem, life-threatening clinical signs are usually not observed (Aldemir 2022).
Cannabis and its derivatives are also known to be addictive and cause withdrawal symptoms (American Psychiatric Association 2013). The fact that withdrawal symptoms are milder than other substances is due to the longer half-life compared to alcohol, cocaine and heroin. In withdrawal, physical symptoms such as restlessness, anger, depression, sleep disturbances, decreased appetite, abdominal pain, chills, sweating, fever, headache can be observed and these symptoms can last up to three weeks (American Psychiatric Association 2013, Aldemir 2022).
Mood disorders, anxiety disorders, impairment in cognitive functions, social withdrawal, amotivational syndrome, psychotic disorders are common after long-term and heavy cannabis use, and the use that starts in adolescence causes a decrease in academic achievement, and cognitive disorders due to its negative effects on brain development (Volkow et al. 2016). In a study investigating the effect of cannabis on neurocognitive functions, it was found that cannabis users had low performance in working memory, learning, recognition, word naming, planning, problem solving, response rate, perseveration, conceptualizing, abstract thinking, changing sets and visuospatial perception skills (Söyler et al. 2022).
EPIDEMIOLOGICAL STUDIES ON THE RELATIONSHIP BETWEEN CANNABIS AND PSYCHIATRIC DISORDERS
Psychotic disorders affect approximately 4% of the population, and indirectly, many more. (Crocker and Tibbo 2015). Most cases of psychotic disorders begin in early adulthood, when people are developing the life skills and experiences necessary for independent living. Epidemiological studies have shown that an important environmental factor for development of psychotic disorders in this age group is cannabis use (Crocker and Tibbo 2015). In a prospective study, cannabis use between the ages of 15 and 18 was found to be associated with an increased risk of psychosis at age 26 (Arseneault et al. 2022). Pardo et al. (2021) found that the risk of early-onset psychosis was higher in people with lifelong, current and especially daily cannabis use, and that this risk was highest in people who used cannabis before the age of 15. In a systematic review, it was reported that the risk of psychosis increased by 40% in people who had previously used cannabis, and that this risk increased in a dose-dependent manner (Moore et al. 2007). In a meta-analysis, the odds ratio (OR) for schizophrenia and other psychosis-related outcomes was 3.90 (95% CI: 2.84- 5.34) in cannabis users compared to non-users (Marconi et al. 2016). In a study conducted by Binbay et al. (2012) with 4011 individuals representing the general population in Izmir, evaluating the psychosis spectrum in 5 groups as absence of psychosis, subclinical psychotic experiences, low-impact psychotic symptoms, high-impact psychotic symptoms and full-blown clinical psychotic disorder; it was shown that there was a disproportionate increase in risk towards the more severe part of the spectrum with cannabis use, with ORs of 1, 1.6, 9.8, 15.0 and 26.8, respectively. In a recent follow-up study conducted in Turkey, in which 2142 individuals representing the general population were included, a significant relationship was found between cannabis use and the risk of developing clinical psychosis at the end of the 6th year (OR: 24.5, 95% CI: 5.4- 57.2) (Kırlı et al. 2021). Genetic predisposition, early onset of use, younger age, heavy use, use of products with high THC potency are important risk factors for the development of psychosis due to cannabis use (Marconi et al. 2016, Pardo et al. 2021, Wainberg et al. 2021, Livne et al. 2022).
In a self-report-based cohort study examining the relationship between cannabis use and psychotic experiences, the percentage of patients with one of the 4 psychotic experiences (visual hallucination, auditory hallucination, persecutory delusion, delusion of reference) was found to be 4.1% in people who had never used cannabis, 7% in those who have used cannabis at any time, 8.4% in those who have used cannabis at least monthly, 8.8% in those who have used cannabis weekly and 9.6% in those who have used cannabis daily, and persecutory delusion was found to be the psychotic experience most strongly associated with cannabis use (Wainberg et al. 2021). It was also reported that psychotic experiences in cannabis users had an earlier onset and were more distressing (Wainberg et al. 2021). In a study investigating the relationship between the frequency of cannabis use and psychotic experiences in university students, those with higher weekly cannabis use frequency reported increased number of hallucinations and delusions (Wright et al. 2021). In a self-report-based cohort study conducted in the United States, it was found that the prevalence of psychotic disorders increased from 2001-2002 to 2012-2013 and that this was associated with cannabis use (Livne et al. 2022).
Although many studies have reported higher rates of current or previous cannabis use in patients with first episode psychosis or schizophrenia compared to the general population (Ksir and Hart 2016), a study conducted in Turkey found that the rate of cannabis use disorder in schizophrenia patients (2%) was lower than the rates reported in schizophrenia patients in other countries (Akvardar et al. 2004). There are different findings on the role of cannabis use in the development of first episode psychosis and worsening of prodromal symptoms. In a meta-analysis in which prospectively followed patients with clinical high risk (CHR) for psychosis were evaluated, cannabis use did not predict the development of psychotic disorder (Oliver et al. 2020), while in another study, 94% of patients with first episode psychosis reported cannabis use a few years before the episode, and cannabis use at an earlier age was associated with an earlier onset of psychosis (Kline et al. 2022). In a study conducted in patients with first episode psychosis, the group with cannabis use were found to have a higher number of males, younger age, and more positive and manic symptoms (Amoretti et al. 2022). In contrast to some studies which found better cognitive functioning and greater cognitive capacity in first episode psychosis patients with cannabis use compared to those without cannabis use (Maldonado and Torrens 2020), this study found no difference in cognitive capacity between the group with and without cannabis use (Amoretti et al. 2022). In the EUGEI study by Di Forti et al. (2019), which investigated the risk factors that played a role in the development of psychotic disorder, and retrospectively compared 901 first-episode psychosis patients aged 18-65 years with a control group of 1237 healthy individuals in 11 regions of Europe in terms of cannabis use patterns, the odds ratio for psychotic disorder was found to be 3.2 (95% CI: 2.2- 4.1) in daily cannabis users and 4.8 (95% CI: 2.5- 6.3) in daily high-potency cannabis users compared to non-users. Differences in the frequency of daily cannabis use and high potency cannabis use have been shown to contribute to the difference in the prevalence of psychosis between the regions included in the study. Across the three regions with the highest consumption of high-potency cannabis, daily use of high-potency cannabis was associated with the largest increase in odds ratios for psychotic disorder compared to those who had never used it: Paris four times, London five times and Amsterdam more than nine times. The study also estimated that 12.2% of first-episode psychosis cases could have been prevented if high-potential cannabis was never used, assuming a causal relationship between cannabis use and psychotic disorder (Di Forti et al. 2019).
It is suggested that there may be omitted variable bias in studies showing that cannabis is associated with psychotic disorders. This bias is attributed to confounders that cannot be fully measured in combination, such as genetic vulnerabilities, cognitive abilities, fetal exposures and parental rearing practices (van Os et al. 2021). To overcome this bias, a study in which individuals were assessed as their own controls over a 10-year follow-up period was conducted, and found that pre-existing cannabis use was associated with psychotic experiences (aOR: 7.03, 95% CI: 2.39- 20.69), but pre-existing psychotic experiences were not associated with cannabis use (aOR: 0.59, 95% CI: 0.21- 1.71) (van Os et al. 2021). This finding supports a direction of causality from cannabis use to the development of psychosis.
In addition to an increased risk of psychosis, cannabis use is associated with early-onset schizophrenia, increased risk of relapse and poor response to treatment (van Os et al. 2002). In two large-sample studies conducted in Finland and Denmark, it was shown that most of the patients who developed substance-induced psychosis later developed chronic psychotic disorders (Niemi-Pynttari et al. 2013, Kejser Starzer et al. 2018). Another longitudinal study conducted in Denmark reported that the rate of schizophrenia cases associated with cannabis use disorder had increased 3-4 times in the last 20 years (Hjorthoj et al. 2021). A 2-year follow-up study in patients with first episode psychosis found that daily high-potency cannabis use increased the risk of relapse 3 times (OR: 3.28; 95% CI: 1.22- 9.18) (Schoeler et al. 2016). In randomized controlled trials examining the effects of THC in schizophrenia patients, it was reported that THC led to worsening of positive symptoms, impairment in cognitive functions such as verbal learning and recall, and worsening of negative symptoms (D’Souza et al. 2005).
It is known that acute psychotic symptoms can be seen in cannabis intoxication. Cannabis-induced psychosis and persistent psychosis can often be confused with each other. A detailed medical history and clinical follow-up are important in the differential diagnosis. Although there are papers suggesting that the persistence of psychotic symptoms despite cessation of substance use for at least one month proves that psychotic symptoms are “independent” of the substance (Enez Darçın 2021), the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) recommends the use of clinical judgment in the diagnostic process (American Psychiatric Association 2013). In addition, the presence of a family history of psychotic disorder and the persistence of psychotic symptoms in the absence of cannabis use reduce the likelihood of the diagnosis being an acute psychotic disorder due to intoxication (Öztürk Sarıkaya 2019). In the systematic review of Wilson et al. (2018), the distinguishing features were stated as more insight, fewer negative symptoms, and more depression and anxiety symptoms in patients with cannabis-induced psychosis. It has been reported that in psychotic disorders due to cannabis use, auditory hallucinations and blunted affect are less common, and agitation, grandiose delusions and persecutory delusions are more dominant (Volkow et al. 2014). There are various hypotheses about the underlying reasons for the frequent co-occurrence of cannabis use and psychotic disorders. The first of these is that cannabis causes psychotic disorders on the basis of a genetic predisposition (Kendler et al. 2019). Another explanation is that people with primary psychosis are more susceptible to the biological effects of cannabis (D’Souza et al. 2005). In addition, it is thought that reasons such as impaired cognitive functions, low educational level, impaired social functioning and adverse environmental conditions seen in people with psychotic disorders increase the risk of cannabis use (Kolliakou et al. 2011). Another suggested explanation is that patients with psychotic disorders may also use more cannabis to calm themselves, relieve anxiety and alleviate the severity of their symptoms (Khantzian 1997). A common underlying pathogenetic process causing both diseases was also suggested (Sideli et al. 2020). Some Mendelian randomization studies have found evidence that cannabis initiation can be partially explained by common genetic variants associated with schizophrenia, suggesting that the causality between cannabis use and schizophrenia and other psychotic disorders may be from schizophrenia genes to cannabis use (Gage et al. 2017).
The evidence such as a history of cannabis use preceding the development of psychosis and the association of cannabis use with an earlier age of onset of schizophrenia suggest that cannabis use plays a causal role in the onset of schizophrenia. However, given the limitations of epidemiologic studies in determining causal links, a better understanding of possible neurobiological pathways is necessary to determine the extent to which the association between cannabis and schizophrenia reflects underlying causal processes.
NEUROBIOLOGICAL EFFECTS OF CANNABIS
In this section, the endocannabinoid system will be briefly described, then the possible neurotoxic effects of cannabis and how this may lead to psychosis in the clinic, and the neurobiological findings related to this will be discussed.
The Endocannabinoid System
The endocannabinoid system (ECS) involves cannabinoid receptors, endogenous cannabinoid ligands and enzymes responsible for the synthesis and degradation of endocannabinoids (Kucerova et al. 2014). CB1 and CB2 are the endocannabinoid receptors which are G-protein coupled. CB1 receptors are located in presynaptic neurons in CNS regions such as the cerebellum, frontal lobe, hippocampus, substantia nigra and in the peripheral nervous system. In the brain, apart from neurons, it is also found in astrocytes, microglia and oligodendrocytes (Marco et al. 2011). CB1 receptors are also expressed in glutamatergic, GABAergic and dopaminergic nerve terminals (Hurd et al. 2019). CB1 receptors are more abundant in regions of the brain associated with pleasure, memory, thinking, attention, perception, movement and coordination and are responsible for the acute psychotropic and cardiovascular effects of cannabis. CB2 receptors are widely distributed in peripheral immune tissues and are involved in the immune system and inflammatory response.
The discovery of cannabinoid receptors led to the discovery of endogenous cannabinoid ligands. The two most important of these ligands are anandamide and 2-arachidonoylglycerol (2-AG) (Stella et al. 1997). Anandamide, a CB1 receptor full agonist and CB2 receptor partial agonist, is involved in sleep regulation, memory and reward-related functions in the CNS (Fakhoury 2017). 2-AG is a CB1 and CB2 full agonist and is more abundant in brain regions such as the anterior cingulate cortex, hippocampus and prefrontal cortex (PFC) (Fakhoury 2017).
Endocannabinoids are not stored as classical neurotransmitters. They are released from the postsynaptic neuron, diffuse into the synaptic gap and stimulate CB1 receptors in the presynaptic cell (Bossong and Niesink 2010). Stimulation of CB1 receptors inhibits neurotransmitter release from the presynaptic end (Chevaleyre et al. 2006). Inhibition of presynaptic Ca+2 channels by stimulation of CB1 receptors, long-term depression of neurotransmitter release by decreasing cAMP levels, and as shown in a recent study, rapid uptake of synaptic vesicles into the nerve terminal by inhibiting cAMP-dependent phosphorylation of synapsin protein are the mechanisms involved in this suppression (Patzke et al. 2021) (Figure 1). Endocannabinoid system-mediated retrograde inhibition of synaptic transmission in GABAergic and glutamatergic neurons in the brain is thought to be an important mechanism of synaptic regulation. Stimulation of NMDA receptors causes calcium inflow into the cell. Presynaptic release of glutamate should be controlled to prevent excitotoxicity caused by excessive Ca+2 inflow into the cell through postsynaptic ion channels. An important physiological mechanism regulating glutamate homeostasis is thought to be the endocannabinoid system (Bossong and Niesink 2010). Endocannabinoid release occurs in a synapse-specific manner as needed and only affects neurotransmitter release at the relevant presynaptic site.
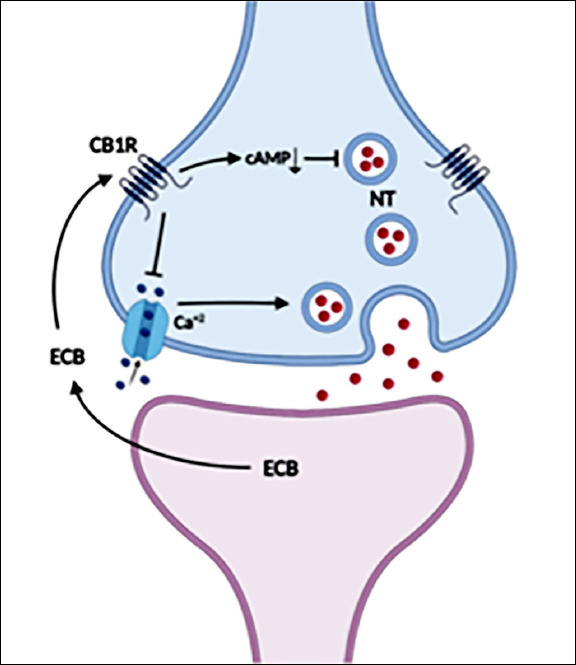
The Mechanism of Action of Endocannabinoids
Endocannabinoids released from the postsynaptic neuron stimulate CB1 receptors in the presynaptic cell. Stimulation of CB1 receptors inhibits neurotransmitter release through both inhibition of presynaptic Ca+2 channels and reduction of cAMP levels.
ECB: endocannabinoids, CB1R: CB1 receptor, NT: neurotransmitter
The endocannabinoid system also has an important role in neuronal development. Endocannabinoid receptors and endogenous cannabinoids are present early in the developing brain and are involved in the proliferation and migration of neurons, neurite outgrowth, new synapse formation and the development of cortical networks (Fernandez-Ruiz et al. 2004, Hurd et al. 2019).
In conclusion, the endocannabinoid system plays an important role during critical periods of development and functions as a homeostatic regulator for various physiological processes. Due to its similarity to endocannabinoids, THC binds to cannabinoid receptors and changes the activity of these receptors. These effects may cause desensitization or down-regulation of the receptors and impair the functioning of the system (Blest-Hopley et al. 2020).
Neurotoxic Effects of Cannabis
It is a controversial issue whether cannabis is a neurotoxic substance. There are different findings in in-vitro studies. Although there are studies showing that cannabis protects neurons against excitotoxicity through CB1 receptors (Shen and Thayer 1998), some studies have shown that THC has toxic effects in cell culture which are prevented by CB1 antagonists (Campbell 2001). In-vivo studies have shown that chronic exposure to THC causes a decrease in the mean volume of neurons and their nuclei, synaptic density, dendritic length and a reduction in neuronal density. Therefore, it is thought to be toxic for hippocampal neurons (Bossong and Niesink 2010). However, in studies with rats, there is not enough evidence that necrosis, edema or injury to brain tissue develops after exposure to THC (Galve-Roperh et al. 2000). In one study, no pathological changes were found in the brain tissue of mice given THC for four days (Breivogel et al. 2020). However, chronic exposure to THC in adolescent rats causes more irreversible effects on behavior than exposure in adult rats, suggesting that age at the time of exposure may be a critical determinant of the outcome of the neurotoxic effect (Scallet 1991). Neurophysiological and functional imaging studies in humans also show that cannabis use during adolescence causes more devastating effects, consistent with preclinical studies (Jager and Ramsey 2008).
Neurobiological Effects of Fetal Exposure to Cannabis
It has been reported that approximately 4% of women in the United States use substances and that the most commonly used substance during pregnancy is cannabis (Shrivastava et al. 2014). Considering that some of the THC crosses the placenta, the question of the effects of cannabis on the developing fetus becomes important. Some studies have reported that prenatal exposure to cannabis impairs fetal growth and has an effect on the maturation of neurotransmitter systems related to mood, motivation and reward (Harkany et al. 2007).
CB1 receptors are expressed in nervous system tissues starting from the early embryonic period. During prenatal development, CB1 receptors are predominantly expressed in mesocorticolimbic brain structures (Hurd et al. 2019). Studies in rodents show that in cortical projection neurons, endogenous cannabinoids coordinate the guidance of axons from both descending efferents and thalamic afferents via CB1 receptors on presynaptic terminals, promote neurite outgrowth, and are tightly controlled during fetal development to prevent ectopic neurite outgrowth and inappropriate synapses (Crocker and Tibbo 2015). Exposure of the fetal brain to THC can impair this physiological process, leading to inappropriate neurite development and long-term physiological, behavioral and cognitive impairments. Longitudinal studies have reported cognitive impairments in adulthood, such as impaired executive functions, in those exposed to cannabis in the fetal period (Fried and Smith 2001). These long-term effects of prenatal cannabis exposure are thought to be the result of impairments in PFC development. In an animal study examining prenatal cannabis exposure, it was found that endocannabinoid-mediated long-term depression (LTD) was impaired in the PFC in male rats, and excitability was increased in PFC pyramidal neurons (Bara et al. 2018).
In fetal studies in humans, dopamine D2 receptor gene expression in mesocorticolimbic structures was found to be altered in those exposed to cannabis (Wang et al. 2004), and changes in dopamine receptors in forebrain regions were found in animal models, similar to findings in human studies (Di Nieri et al. 2011, Hurd et al. 2019). Considering the importance of these regions in motivation, emotion regulation, reward and cognitive functions, fetal exposure to cannabis may play a role in the development of psychiatric disorders through the dopaminergic system. Fetal exposure to cannabis is also thought to alter the function of dopaminergic neurons. In a recent study, Frau et al. (2019) showed that in rats exposed to cannabis in utero, there is an imbalance in the ratio of excitatory and inhibitory inputs to the dopamine neuron, contributing to increased excitability. A hyperdopaminergic state also occurs through epigenetic mechanisms in the amygdala, nucleus accumbens and anterior striatum (Hurd et al. 2019). It has been suggested that these early changes in the dopaminergic system play a role in the development of psychiatric disorders, and that children and adolescents exposed to cannabis during the fetal period are more vulnerable to substance use disorders in adulthood.
Neurobiological Effects of Cannabis Use in Adolescence
In the normal development of the brain, gray matter production, volume increase and new synaptic formations occur during infancy and childhood, while new neuronal connections are formed during adolescence (Blest-Hopley et al. 2020). Adolescence is a developmentally important period in which the endogenous cannabinoid system plays a role in brain development and therefore THC can cause adverse effects. During this period, some dendrites and synapses are preserved, while others are remodeled as a result of a process called “pruning”. During the development of the brain in adolescence, there is a decrease in the volume of gray matter in the brain as a result of synaptic pruning in neurons (Blakemore 2008). Because of these remodeling processes, the brain during adolescence is highly sensitive to external influences such as psychotropic substances. Substances such as cannabis, which target the endocannabinoid system that plays a vital role in neuronal maturation, play a role in the development of psychosis in some individuals by affecting physiological processes such as neurotransmitter systems and synaptic remodeling when used during adolescence. In animal studies, rats exposed to cannabis during adolescence showed early pruning of the spines of prelimbic and PFC pyramidal neurons and atrophy in the distal apical regions (Miller et al. 2019, Rubino et al. 2015).
Cannabis use during adolescence, a developmentally critical period, may cause alterations in glutamate and dopamine neurotransmitter systems, which are thought to play a role in the etiology of psychosis (Blest-Hopley et al. 2020). During postnatal development, when mature neuronal circuits are formed, glutamate plays an important role in strengthening or pruning of synapses. One effect of THC-induced temporary disruption of the endocannabinoid system is the impairment of glutamate release, which leads to impaired synaptic connections. Disorganization of neuronal circuits in the prefrontal cortex has implications for connections with other cortical and subcortical structures, particularly through abnormalities in the transmission of dopamine and GABA.
Brain regions such as the hippocampus and PFC continue to develop throughout adolescence, and CB1 receptors and endocannabinoid levels increase. Preclinical studies show a continuously increasing cortical expression of CB1 receptors in adolescence until adulthood (Blest-Hopley et al. 2020). In contrast, a reverse expression pattern is observed in the striatum (van Waes et al. 2012). A similar pattern is also observed in cortical and striatal levels of dopamine synthesis during adolescence, with dopamine levels increasing in frontal regions and decreasing in the nucleus accumbens and striatum (Andersen et al. 1997). Given the interaction between these signaling systems, exposure to exogenous cannabinoids during adolescence may cause dysregulation in these systems, leading to psychotic disorders.
There are many structural and functional imaging studies on the effects of cannabis use in adolescence. A study using diffusion-weighted magnetic resonance imaging (MRI) and brain connectivity mapping techniques showed that axonal connectivity was impaired in the right fimbriae (fornix) of the hippocampus and splenium of the corpus callosum, which contain abundant cannabinoid receptors, in adolescent cannabis users compared to non-users (Zalesky et al. 2012). Cross-sectional studies in humans have reported a decrease in volume and surface area in frontal and parietal regions (Churchwell et al. 2010, Kumra et al. 2012). In a follow-up study examining the relationship between cannabis use and cortical thickness in adolescents, left and right PFC thickness was found to be negatively correlated with cannabis use and this was dose-dependent (Albaugh et al. 2021). In adolescence, compared to adulthood, significant levels of endocannabinoid receptors have been found in glial cells such as astrocytes and oligodendrocytes, which are responsible for white matter production and maintenance. Therefore, it is hypothesized that cannabis use in early adolescence also interacts with these processes and adversely affects white matter development, ultimately triggering psychosis in individuals vulnerable to the disease (Renard et al. 2014).
Methodological differences such as heterogeneity of samples, different patterns of cannabis use, imaging techniques used, and whether the measurements were performed during rest or performance might be responsible for the different findings in functional imaging studies. However, a meta-analysis with a high level of evidence showed greater activity in two brain regions in cannabis users compared to controls: the inferior parietal gyrus (extending to the superior parietal gyrus and angular gyrus) and the putamen (extending to the striatum and insula) (Blest-Hopley et al. 2018). Some of these brain regions are parts of broader networks such as the salience network (SN) and the default mode network (DMN) (Blest-Hopley et al. 2018). Cannabis use in adolescence may also alter the functioning of SN components such as the insula, which plays a role in the transition between large-scale brain networks (Spechler et al. 2015). Accordingly, cannabis users may have alterations in the functioning of regions such as the angular gyrus that are involved in the integration of multimodal information, the allocation of attentional resources, and the attribution of meaning and importance to information. These alterations may lead to abnormal salience assignments, which are thought to be one of the mechanisms underlying psychotic symptoms (Kapur 2003). In another meta-analysis of functional imaging studies, adolescents who used cannabis showed increased activity in the dorsolateral and ventrolateral prefrontal and posterior parietal cortices, parts of the central executive network involved in higher-order cognitive functions, compared to controls (Blest-Hopley et al. 2019).
Observed functional network changes are thought to result from changes in synaptic pruning, along with the evidence of changes in white matter volume, integrity and connectivity (Blest-Hopley et al. 2020).
ENDOCANNABINOID SYSTEM IN SCHIZOPHRENIA PATIENTS
The endocannabinoid system is thought to be involved in the pathophysiology of schizophrenia. The first evidence for this was obtained from studies with animal models (Seillier et al. 2010). Many studies have reported a decrease in CB1 receptor levels in different parts of the brain in schizophrenia (Fakhoury 2017). In a study conducted in rats given phencyclidine, one of the animal models for schizophrenia, CB1 receptor levels were found to be decreased in the prefrontal cortex, hippocampus, substantia nigra and cerebellum (Vigano et al. 2009). Postmortem autoradiography studies conducted in schizophrenia patients showed that binding of ligands to CB1 receptors was increased in the anterior and posterior cingulate cortex and PFC (Zavitsanou et al. 2004, Newell et al. 2006, Dalton et al. 2011). This increase was found to be negatively correlated with CB1 receptor mRNA levels (Volk et al. 2014). Ranganathan et al. (2016) showed that CB1 receptor densities were decreased in the amygdala, caudate, posterior cingulate cortex, hippocampus, hypothalamus and insula in patients with schizophrenia in a PET study. It has been suggested that the decrease in receptors is associated with impaired GABA-mediated neurotransmission, which is known to play a role in schizophrenia (Fakhoury 2017).
Further studies are needed to fully elucidate the role of cannabinoid receptors in schizophrenia. In addition, future studies should explore whether changes in cannabinoid receptors are directly related to the pathology of schizophrenia or whether they occur in response to the imbalance of neurotransmitters such as glutamate and GABA in the brain (Fakhoury 2017).
Differences have been observed in endocannabinoid levels in schizophrenia patients. Anandamide levels were found to be higher in the cerebrospinal fluid (CSF) and serum of first-episode schizophrenia patients who did not receive antipsychotic treatment compared to healthy controls, while these levels were similar to healthy controls in patients receiving first generation antipsychotic treatment with prominent D2/D3 receptor antagonism (Giuffrida et al. 2004). Cannabis use may affect the course of the disease in schizophrenia patients by worsening or revealing these already existing impairments in the endocannabinoid system.
One of the mechanisms by which endocannabinoid system mediates the pathophysiology of schizophrenia is thought to be neural oscillations. There is ample evidence that neural oscillations, especially gamma (30-80 Hz) and theta (4-7 Hz), are impaired in schizophrenia patients and this is thought to be related to perceptual and cognitive functions (Skosnik et al. 2016). The synchronization of neural oscillations is primarily mediated by GABAergic interneurons, and CB1 receptors are known to regulate GABA release (Skosnik et al. 2016). Preclinical studies show that CB1 receptor agonists decrease the power of neural oscillations (Hajos et al. 2008, Goonawardena et al. 2011). Cannabinoid exposure during critical stages of neural development was shown to disrupt the brain’s ability to generate synchronized neural oscillations in adulthood (Raver et al. 2013). In EEG studies conducted in humans, chronic cannabis users were found to have neural oscillatory changes similar to the pattern observed in schizophrenia patients (Skosnik et al. 2012). This mechanism may also underlie the impairment of cognitive functions such as attention and working memory caused by cannabis (Skosnik et al. 2016).
THE ROLE OF GENE- ENVIRONMENT INTERACTION IN THE CANNABIS-PSYCHOSIS RELATIONSHIP
Studies showing that not all people with cannabis use develop psychosis suggest that genetic predisposition should be considered as an additional factor. The role of genetics is also supported by studies showing that the risk of developing psychosis with cannabis use in first-degree relatives of schizophrenia patients is higher than in the general population. In polygenic disorders with low penetrance and common variance inheritance such as schizophrenia, environmental factors such as cannabis are thought to play a role in the emergence of the disease by making the underlying neurobiological systems susceptible (Collip et al. 2008).
In a study, the risk of developing psychosis with cannabis use was found to be associated with schizophrenia polygenic risk scores (Guloksuz et al. 2019). In another study examining the role of genetics in susceptibility to the harmful effects of cannabis, the association between cannabis use and decreased cortical thickness in adolescents was reported to be strongest in individuals with high schizophrenia polygenic risk scores (French et al. 2015). In a cohort study investigating whether the association between cannabis use and psychotic experiences was stronger in people genetically predisposed to schizophrenia, cannabis use and psychotic experiences were found to be more strongly associated in people with a high genetic risk of schizophrenia (Wainberg et al. 2021).
Single nucleotide polymorphisms (SNPs) in the P2RX7 gene, which is widely expressed in microglia and known to regulate neurotransmitter release and have some immune functions, have been found to play a role in the relationship between regular cannabis use and psychotic experiences (Boks et al. 2020). In addition, in recent studies, it is predicted that being a carrier of the valine158 allele of the catechol-O-methyltransferase (COMT) gene may be a factor that facilitates the emergence of psychosis in people with cannabis use (Vaessen et al. 2018). In the study by Mane et al. (2017), early cannabis use and BDNF Val66Met polymorphism were found to be associated with age of onset of psychosis in patients with first episode psychosis. In another study, CNR1 gene expression in astrocytes was shown to mediate the cognitive effects of cannabis (Han et al. 2012). Exposure to cannabis in adolescence was found to worsen the emotional memory impairment in mice with disrupted DISC1 expression, one of the genes thought to play a role in schizophrenia (Ballinger et al. 2015).
Epigenetics is the alteration of the cell’s gene expression by environmental factors through mechanisms such as DNA methylation and histone modification. It is thought that cannabis may also cause epigenetic changes. In a study comparing DNA samples obtained from the blood of schizophrenia patients with and without cannabis use, methylation was observed in some gene regions involved in various biological pathways such as glutamatergic synapses in cannabis users (Le Hellard et al. 2020). In addition, epigenetic alterations have been reported in cannabis-using adolescents in genes related to synaptic functions and cytoskeleton, such as Pacsin1, Clu and Snap25, which are thought to be involved in schizophrenia and mood disorders, and in genes involved in new neuron formation and synaptic plasticity in PFC, such as KMT2A (Wang et al. 2015, Pouget et al. 2016, Hurd et al. 2019, Miller et al. 2019, Ellis et al. 2021).
CONCLUSION
There is a large body of evidence that cannabis use increases the risk of developing psychosis. Studies have shown that this increased risk is particularly observed in genetically predisposed individuals, and more pronounced in those exposed during critical periods of neural development such as the fetal period or adolescence. Preclinical, neuroimaging and neurophysiological studies provide various evidences that cannabis affects neurotransmitter systems such as glutamate and dopamine via the endocannabinoid system, physiological processes such as synaptic pruning during adolescence, and changes the connectivity between some brain regions.
A better understanding of the relationship between cannabis use and the development of psychosis, and the neurobiological processes involved will help to better understand the disorders and to develop appropriate prevention and treatment strategies. The fact that school age and adolescence are risky periods in terms of initiation of cannabis use and susceptibility to the effects of cannabis has led to the development of preventive approaches especially in these age groups. These approaches include ALERT (Adolescent Learning Experiences Resistance Training) and DARE (Drug Abuse Resistance Education) projects in the United States, and EU-Dap (European Drug Addiction Prevention Trial) in Europe (Kolliakou et al. 2012). In addition, because cannabis is a risk factor for the development of psychosis in clinical high-risk groups, increases the risk of relapse, and worsens the prognosis in people with psychotic disorders, prevention or minimization of cannabis use in these groups is an important preventive approach. Routine questioning of cannabis use in the clinic, informing patients about the harmful effects of cannabis and providing appropriate treatment when necessary, as well as informing the general public will be useful in reducing the harms of cannabis use.
REFERENCES
Articles from Turkish Journal of Psychiatry are provided here courtesy of Turkish Association of Nervous and Mental Health
Citations & impact
This article has not been cited yet.
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/166966495
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Cannabis and psychosis: have we found the missing links?
Asian J Psychiatr, 6(4):281-287, 03 May 2013
Cited by: 12 articles | PMID: 23810133
Review
Adolescent brain maturation, the endogenous cannabinoid system and the neurobiology of cannabis-induced schizophrenia.
Prog Neurobiol, 92(3):370-385, 16 Jul 2010
Cited by: 150 articles | PMID: 20624444
Review
[Cannabis use in subjects at ultra high risk for psychosis].
Presse Med, 48(11 pt 1):1229-1236, 12 Nov 2019
Cited by: 3 articles | PMID: 31732360
Review
Are cannabis-using and non-using patients different groups? Towards understanding the neurobiology of cannabis use in psychotic disorders.
J Psychopharmacol, 32(8):825-849, 29 Mar 2018
Cited by: 14 articles | PMID: 29591635 | PMCID: PMC6058406
Review Free full text in Europe PMC



