Abstract
Purpose
Schisandrin B (Sch B) is an active monomer of Schisandrin with anti-fibrosis pharmacological action. The study investigated whether Sch B alleviate bleomycin-induced (BLM-Induced) pulmonary fibrosis in mice and attempted to clarify its anti-fibrosis mechanism.Methods
Histopathological examination was performed by H&E staining and immunohistochemistry. The inflammatory cytokines and oxidative stress were determined by ELISA. Western blotting and immunofluorescence were used to investigate the possible molecular mechanism to attenuate pulmonary fibrosis by Sch B.Results
The results indicated that Sch B can significantly attenuate BLM-Induced pulmonary fibrosis, myofibroblast activation, and collagen fibers deposition in mice. In addition, Sch B can inhibit inflammatory response and oxidative stress in early stage. Furthermore, Sch B can inhibit pulmonary fibrosis by promoting autophagy via promoting the dephosphorylation of AKT-mTOR pathway.Conclusions
The results suggest that the anti-fibrotic effect of Sch B is potentially related to the activation of autophagy through AKT-mTOR pathway, and Sch B is a potential agent for the treatment of idiopathic pulmonary fibrosis.Free full text

Schisandrin B attenuates bleomycin-induced pulmonary fibrosis in mice through AKT-mTOR pathway
Associated Data
Abstract
Purpose:
Schisandrin B (Sch B) is an active monomer of Schisandrin with anti-fibrosis pharmacological action. The study investigated whether Sch B alleviate bleomycin-induced (BLM-Induced) pulmonary fibrosis in mice and attempted to clarify its anti-fibrosis mechanism.
Methods:
Histopathological examination was performed by H&E staining and immunohistochemistry. The inflammatory cytokines and oxidative stress were determined by ELISA. Western blotting and immunofluorescence were used to investigate the possible molecular mechanism to attenuate pulmonary fibrosis by Sch B.
Results:
The results indicated that Sch B can significantly attenuate BLM-Induced pulmonary fibrosis, myofibroblast activation, and collagen fibers deposition in mice. In addition, Sch B can inhibit inflammatory response and oxidative stress in early stage. Furthermore, Sch B can inhibit pulmonary fibrosis by promoting autophagy via promoting the dephosphorylation of AKT-mTOR pathway.
Conclusions:
The results suggest that the anti-fibrotic effect of Sch B is potentially related to the activation of autophagy through AKT-mTOR pathway, and Sch B is a potential agent for the treatment of idiopathic pulmonary fibrosis.
Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive, and fatal lung disease characterized by progressive dyspnea, decreased lung function, and death (1). The etiology of IPF is not clear, but several associations have been reported: smoking; exposed to wood and metal dust; chronic viral infections; exposure to certain medications; and genetic factors (2, 3). The occurrence and development of IPF are caused by abnormal damage and activation of alveolar epithelial cells, leading to the secretion of profibrotic, coagulation, and inflammatory cytokines, controlling the proliferation, activation, and differentiation of fibroblasts into myofibroblasts (4, 5). Currently, there is a lack of effective therapy for IPF, as the only approved treatments for IPF (Nintedanib and Pirfenidone) only slow the progression of the disease (6). Hence, there is an urgent need to find new therapies for IPF patients.
Schisandrin B (Sch B) is an active monomer of Schisandrin. Pharmacological studies have shown that Sch B plays an important role in liver protection and neuroprotection by removing free radicals and achieving antioxidant effects (7, 8). In term of cardiac actions, Sch B can inhibit cell apoptosis, downregulate inflammatory cytokines to prevent ischemia-reperfusion injury and doxorubicin induced cardiac toxicity (9, 10). In addition, Sch B plays a certain role in tumor treatment by inducing cell cycle arrest (11). Pharmacological studies have suggested that Sch B has anti-fibrosis effect (12, 13). Sch B can alleviate bleomycin-induced (BLM-Induced) pulmonary fibrosis in mice by inhibiting inflammatory response and oxidative stress (14). In addition, Sch B is associated with autophagy in liver injury mechanism, and autophagy regulation is closely related to the pathogenesis of pulmonary fibrosis. Autophagy regulation involves transforming growth factor-β (TGF-β) and mTOR. TGF- β an important pro-fibrosis factors in the pathogenesis of pulmonary fibrosis. It can activate mTOR to inhibit autophagy in BLM-Induced animals, leading to pulmonary fibrosis (15-17).
Currently, there are rarely reports about the autophagy regulation of Sch B in the process of inhibiting pulmonary fibrosis, so the study aims to investigate the autophagy regulation mechanism of Sch B in BLM-Induced pulmonary fibrosis. We hypothesized that autophagy is involved in the inhibitory effect of Sch B on pulmonary fibrosis. The results may provide a theoretical basis for the clinical development and application of Sch B in the treatment of pulmonary fibrosis.
Methods
Antibodies and reagents
Sch B was obtained from China Institute for the Control of Pharmaceutical and Biological Products (Beijing, China), which was dissolved in normal saline. mTOR antibodies (Affinity AF6308) was provided by Affinity Biosciences (Cincinnati, OH, USA). LC3 antibodies (Proteintech 18725-1-Ig), AKT (Proteintech 18420-1-AP), Beclin-1(Proteintech 11306-I-AP), Actin (Proteintech 66009-1-Ig), α-SMA (Proteintech 23170-1-AP) were purchased from Proteintech (Rosemont, IL). Collagen I antibody (abcam ab34710) was obtained from Abcam (Cambridge, MA). p-mTOR antibodies (CST #5536) was obtained from Cell Signaling Technology (Danvers, MA). The autophagy inhibitor chloroquine, and 3-Methyladenine were provided by Sigma-Aldrich (St Louis, MO, United States).
BLM-induced pulmonary fibrosis model
Adult male C57BL/6 mice with weight from 18 to 22 g were provided by the animal center of Wuhan University, and all experimental protocols were approved by the institutional animal care and use committee. Mice were randomly divided into saline control group, BLM-induced group, Sch B group, Sch B + bleomycin group (Sch B treated group) and Sch B + bleomycin + chloroquine group, with 6 mice in each group. Then, we used bleomycin to induce the early inflammation model and the pulmonary fibrosis model (18). On day 0, C57BL/6 mice were anesthetized by intraperitoneal injection of 10% chloral hydrate (5 ml/kg) and intratracheal injection of 2mg/kg BLM (Kayaku Co., Ltd., Tokyo, Japan). The saline control group was given normal saline according to the same procedure. mice were gavaged with normal saline or Sch B once per day from day 1 to day 7 after bleomycin administration at a dose of 3 mg/kg body weight. In the early inflammation model induced by bleomycin, mice were sacrificed on the day 8 to collect alveolar lavage fluid, serum and lung tissue, and analysis the levels of inflammatory factors and biomarkers of oxidative stress were analyzed. In the bleomycin induced pulmonary fibrosis model, on the day 14, the forced vital capacity (FVC) of mice was recorded by animal lung function tester, and the lung tissue was taken for the determination of hydroxyproline and histopathology (Figure 1A).
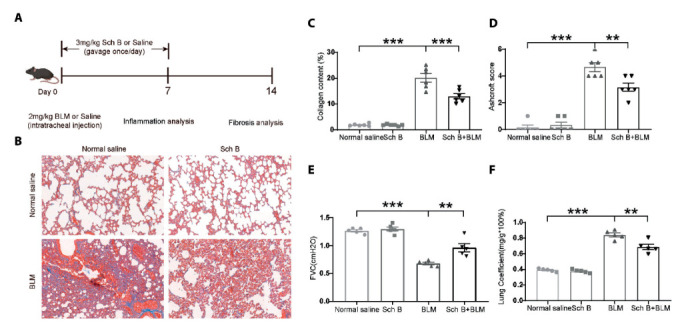
Sch B attenuates BLM-Induced pulmonary fibrosis in mice. A. Informative figure on the different groups of mice and a timeline in treatment and different measurements. B. The representative photographs of the effects of Sch B against BLM-induced pathological changes (Masson staining. Bars, 100 μm). C. The mean optical density (MOD) of positive areas was quantified by Image Pro Plus 6.0 to evaluate the levels of collagen expression. D. Degree of lung fibrosis was quantified by Ashcroft score system. E. The forced vital capacity of the mice. F. The Lung Coefficient of the mice. n = 6. *P < 0.05, **P < 0.01, ***P < 0.001.
Western blotting and quantitative RT-PCR
Lung tissue proteins were lysed and extracted by RIPA (Beyotime Biotechnology, Shanghai, China), and the BCA Protein Assay Kit (Beyotime Biotechnology) were used to determine protein concentration. Then, Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was applied to separate 50µg of protein. The proteins were transferred to a nitrocellulose membrane (Millipore) and incubated with corresponding antibodies. Finally, we used enhanced chemiluminescence system (Affinity Biosciences, Cincinnati, OH, USA) to visualize protein bands. Loading controls were actin. In addition, mRNA RT was performed with the ReverTra Ace kit (Toyobo, Osaka, Japan). The cDNA then served as the template for SYBR real-time PCR. All reactions were run in triplicate on the Real-Time PCR Detection System (Bio-Rad).
Histological and immunohistochemical analyses
After anesthesia, the lung tissue was removed and fixed with 4% paraformaldehyde, dehydrated with gradient ethanol and embedded in paraffin Hindrance. Then, hematoxylin-eosin (H&E) staining and Masson staining were used. The histological severity of fibrosis was quantified by Ashcroft scoring system. The degree of fibrosis was graded from grade 0 (normal lung) to grade 8 (fibrous obstruction) according to the results of microscopic observation (19). The mean score of each microscopic field in the whole lung slice was used as the fibrosis score. After Sirius scarlet staining, it was analyzed by Nikon E600p0L polarized light microscope -Pixerra Penguin 150ES CCD-Image Pro Plus4.5 (Media Cyberneties, US) image analysis system to compare the area ratio of collagen I and collagen III. For immunofluorescence (IF), 2-μm acetone-fixed cryostat sections and 4-μm paraffin sections were cut from the polyoxymethylene-fixed mice lung tissue. The sections were incubated with α-SMA and collagen I antibody (1:100). To detect the primary antibodies, the sections were incubated with fluorescein isothiocyanate (FITC)-conjugated anti-rabbit IgG (1:100, Abcam) for α-SMA and collagen I. The nuclei were stained with DAPI. The sections were visualized using a laser-scanning confocal microscope (Olympus FluoView™ FV1000, Tokyo, Japan). Eight images per slide and six images per treatment group were quantified by Image Pro Plus 6.0 for integral optical density (IOD) and the positive area (Area). The mean optical density (MOD = IOD/Area) was calculated to evaluate the expression of proteins.
Pulmonary function test
After anesthesia, the mice were fixed on the operating table and kept in the supine position. After endotracheal intubation, intubation was fixed with cotton thread. After the mice were transferred to the body description platform, the FVC of the mice was measured.
ELISA for the detection of inflammatory factors
The levels of IL-6, TNF-α in bronchial alveolar lavage fluid (BALF) of BLM-induced inflammation model groups were determined by ELISA kit (Jymbio, Colorful Gene Biological Technology Co. Ltd., Wuhan, China). All samples were measured in triplicate.
Measurement of oxidative stress
The lung homogenates were centrifuged at 4°C, and the supernatant was retained for testing. The levels of superoxide dismutase (SOD), malondialdehyde (MDA) in lung tissue were measured according to the instructions of detection kit (Solarbio).
Statistics
All statistical analyses were performed using GraphPad prism 7.0 (GraphPad Software, Inc., La Jolla, CA, USA). Data are expressed as mean ± standard error or median and interquartile range. Independent sample t-tests and Mann Whitney U tests were used to compare the continuous variables. The estimates of all proportions were made using chi-square analysis for two groups. P < 0.05 was considered to be statistically significant.
Results
Sch B attenuates BLM-Induced pulmonary fibrosis in mice
We first evaluated the histological changes after 14 days of BLM-Induced pulmonary fibrosis in mice by H&E staining and Masson staining. In the BLM-Induced group, there was obvious inflammatory reaction and pulmonary fibrosis. The alveolar wall and airway wall were significantly thickened, and the alveolar structure was disordered. Meanwhile, the lung tissue of mice showed obvious alveolar inflammation and excessive deposition of collagen. However, in the Sch B treated group, the above lesions were alleviated (Figures 1B and andC).C). We used the Ashcroft score to evaluate the histological changes in fibrous lesions. The lung tissue of mice in the Sch B group showed lower Ashcroft score than that in the BLM-Induced treated group (Figure 1D). In addition, we also evaluated the effect of Sch B on the FVC in BLM-Induced pulmonary fibrosis mice. The results showed that the FVC of mice in the Sch B treated group was significantly higher than that in the BLM-Induced group (Figure 1E). In addition, we also measured the Lung Coefficient of the mice, Sch B could inhibit the increase of BLM in lung coefficient (Figure 1F).
Sch B inhibits myofibroblast activation and collagen fibers deposition
The expression of α-SMA and collagen I in the alveolar and interstitium of pulmonary were observed by immunohistochemistry on the 14th day. In the saline control group (normal lung tissue), the immunostaining was exceedingly weakly positive. In the BLM-Induced group (fibrotic lung tissues), high expression of α-SMA and collagen I were observed in the lung interstitium by the large positive immunolocalization area of lung slices (Figures 2A and andC).C). In the Sch B treated group, the expression of α-SMA (Figures 2A and andB)B) and collagen I (Figures 2C and andD)D) were significantly reduced compared with BLM-Induced group.
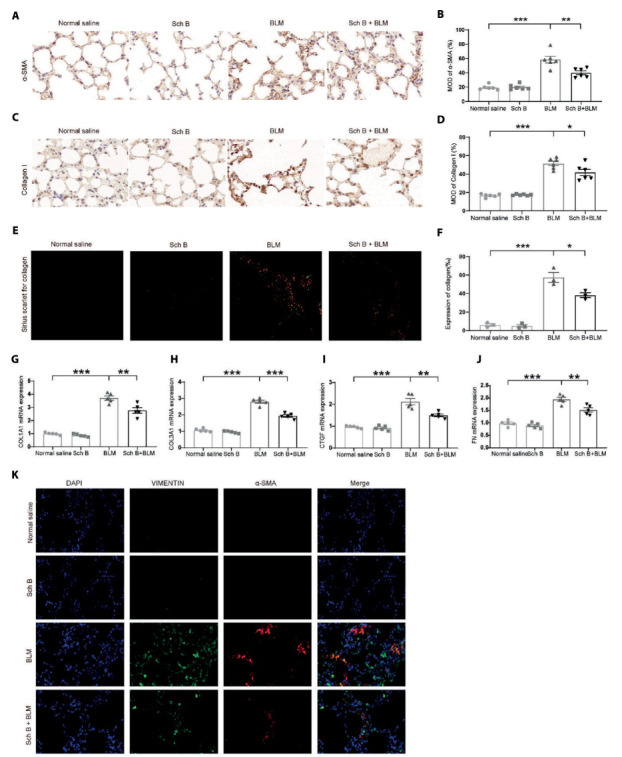
Sch B attenuates myofibroblast activation and collagen fibers deposition. A. Representative images of immunohistochemistry for α-SMA in BLM-induced pulmonary fibrosis in mice. B. The mean optical density (MOD) of positive areas was quantified by Image Pro Plus 6.0 to evaluate the levels of a-SMA expression. C. Representative images of immunohistochemistry for collagen I in BLM-induced pulmonary fibrosis in mice. D. The mean optical density (MOD) of positive areas was quantified by Image Pro Plus 6.0 to evaluate the levels of type I collagen expression. E. The type I collagen content and type III collagen content was measured by using polarized light. F. The mean optical density (MOD) of positive areas was quantified by Image Pro Plus 4.5 to evaluate the levels of type I collagen content and type III collagen content expression. G. COL1A1 mRNA expression. H. COL3A1 mRNA expression. I. CTGF mRNA expression. J. FN mRNA expression. K. Immunofluorescence of VIMENTIN and a-SMA. n = 6. *P < 0.05, **P < 0.01, ***P < 0.001.
The type I and type III collagen fibers were clearly stained with Sirius scarlet staining. We observed that type I collagen fibers were closely arranged and showed strong birefringence by polarized light. It appeared bright red or orange yellow. The type III collagen fibers showed weak birefringence and appeared green (Figure 2E). We observed the type I collagen deposition in Sch B treated group was significantly lower than that in the BLM-Induced group (Figure 2F). Fibrosis related genes were also detected by RT-PCR, and the results showed that BLM could significantly induce mRNA expression of genes such as COL1A1, COL3A1, CTGF, and FN, while Sch B could inhibit the increase of mRNA expression (Figures 2 G-J). In addition, we conducted immunofluorescence staining of VIMENTIN and α-SMA to mark fibroblasts, Sch B could inhibit BLM-Induced increase of fibroblasts (Figure 2K).
Sch B decreases inflammatory response and oxidative stress in early stage
Inflammatory response and oxidative stress in early stage play an important role in the process of pulmonary fibrosis, so we have established bleomycin-induced early inflammation model (Figure 1A). In the early inflammation model induced by bleomycin, inflammatory cytokine IL-6 and TNF-α in BALF and serum of BLM-Induced group was significantly higher than that in the saline control group. In the BALF of BLM-Induced group, the levels of inflammatory cytokines were decreased after Sch B intervention (Figures 3A and andC).C). In the serum of different groups, we found the consistent results (Figures 3B and andD).D). In addition, we also observed a decrease in SOD level and an increase in MDA level in lung tissue homogenate in BLM-Induced group. In contrast, we observed the opposite changes after Sch B intervention (Figures 3E and andF).F). In addition, we conducted immunofluorescence staining of CD45 to represent infiltration of inflammatory cells, Sch B could reduce immune cell infiltration caused by BLM (Figure 3G).
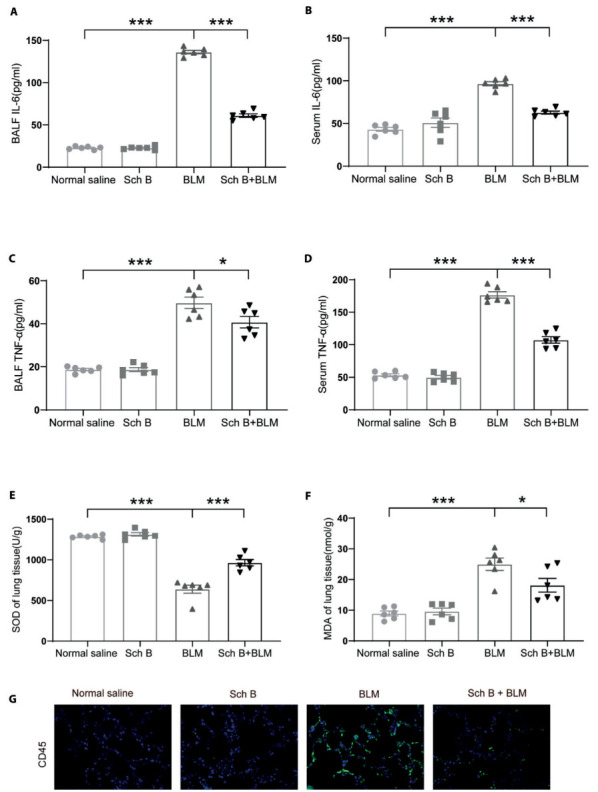
Sch B inhibits inflammatory response and oxidative stress in early stage. A. Effects of Sch B on inflammatory mediators of IL-6 in BALF. B. Effects of Sch B on inflammatory mediators of IL-6 in serum. C. Effects of Sch B on inflammatory mediators of TNF-α in BALF. D. Effects of Sch B on inflammatory mediators of TNF-α in serum. E. Effects of Sch B on oxidative stress markers of SOD in lung tissues of BLM-treated mice. F. Effects of Sch B on oxidative stress markers of MDA in lung tissues of BLM-treated mice. G. Immunofluorescence of CD45. n = 6. *P < 0.05, **P < 0.01, ***P < 0.001.
Sch B inhibits pulmonary fibrosis by promoting autophagy
The autophagic flux is directly related to the pathogenesis of pulmonary fibrosis. We detect the autophagic marker proteins Beclin-1 and LC3 by immunofluorescence staining. In the BLM-Induced group, autophagy is activated to a certain degree, representing the basis of the autophagic flux in fibrosis process. However, we observed that the levels of Beclin-1 and LC3 increased significantly after Sch B administration, which could be blocked to the basis state again by chloroquine (Figure 4A). Western blot analysis showed the sham expression of LC3 and Beclin-1 in different groups. Moreover, western blot analysis showed Sch B could decrease the expression of α-SMA and collagen I to attenuate BLM-Induced pulmonary fibrosis. Furthermore, chloroquine inhibited the autophagic flux increased by Sch B, the expression of fibrosis protein increased again. Therefore, Sch B could inhibit pulmonary fibrosis by promoting autophagy (Figures 4B-F).

Sch B inhibits pulmonary fibrosis by promoting autophagy. A. Effects of Sch B on the expression of Beclin-1 and LC3 in lung tissues of BLM-treated mice by confocal microscope analysis. B. Western blot analyses expression of a-SMA, collagen I, LC3 and Beclin-1 in different groups. C. Quantification of a-SMA expression is achieved using densitometric values. D. Quantification of collagen I LC3-II expression is achieved using densitometric values. E. Quantification of Beclin-1 expression is achieved using densitometric values. F. Quantification of LC3-II expression is achieved using densitometric values. n = 6. *P < 0.05, **P < 0.01, ***P < 0.001.
Sch B promotes autophagy by enhancing the dephosphorylation of AKT-mTOR
To clarify the mechanism of Sch B regulating autophagy, we evaluated the AKT-mTOR pathway by western blot analysis. The results showed that p-AKT could be induced to dephosphorylate by bleomycin to a certain degree. However, the phosphorylation of AKT and mTOR were decreased significantly by Sch B, which could be rescue by 3-Methyladenine. The above results show that Sch B promotes autophagy by promoting the dephosphorylation of AKT-mTOR pathway (Figures 5A-C).

Sch B promotes autophagy by promoting the dephosphorylation of AKT-mTOR pathway. A. Western blot analyses expression of AKT-mTOR pathway in different groups. B. Quantification of p-AKT expression is achieved using densitometric values. C. Quantification of p-mTOR expression is achieved using densitometric values. n = 6. *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
IPF is a chronic progressive interstitial lung disease, which is characterized by aggressive and poor prognosis. IPF has a complex etiology and high mortality (20). So far, lack of effective drugs for IPF is a major difficulty at present. Sch B has a variety of pharmacological activities, it can play a pharmacological role through the regulation of a variety of signal pathways (21). Sch B isolated from Schisandra chinensis is a natural non-enzymatic antioxidant with low toxicity, low cost, and broad application prospects. It has been proven to have multiple pharmacokinetic properties, including antioxidant, anti-inflammatory, cardioprotective, and neuroprotective properties (11). Studies have shown that lignans from Sch can improve clinical symptoms, enhance quality of life, increase exercise tolerance, prolong survival, and partially improve lung function in patients with IPF (11, 22). A study in vitro has shown that Sch B can inhibit epithelial mesenchymal transition, which is closely related to airway remodeling in fibrotic lung disease (23, 24).
In our study, we found that Sch B could improve the lung function of BLM-Induced pulmonary fibrosis in mice. In addition, Sch B could attenuate BLM-Induced pulmonary fibrosis in mice by inhibiting the proliferation and differentiation of myofibroblasts and the deposition of collagen fibers. Results from inflammation models indicate Sch B could reduce lung inflammation and oxidative stress in mice with BLM-induced pulmonary fibrosis in early stage. Moreover, analysis on Belin-1 and LC3 level indicate Sch B could promote dephosphorylation of AKT-mTOR pathway, therefore promoting autophagy to inhibit pulmonary fibrosis. These results should provide a theoretical basis for exploring potential therapeutic drugs for IPF.
Sch B attenuates BLM-induced pulmonary fibrosis via inhibiting myofibroblast activation and lung interstitial ECM deposition. Myofibroblast can inhibit fibroblast differentiation and reduce pulmonary fibrosis (11, 14). α-SMA is a recognized biomarker of myofibroblast activation, and type I collagen is closely associated with ECM deposition in lung mesenchymal tissue (25). Zhou et al found that aucubin could reduce BLM-induced pulmonary fibrosis by decreasing α-SMA expression (26). Our study showed similar effect for Sch B, as the expression of α-SMA and type I collagen decreased after Sch B intervention in the lung tissue of BLM-induced pulmonary fibrosis in mice. These findings support that Sch B attenuates BLM-induced pulmonary fibrosis via inhibiting myofibroblast activation and lung interstitial ECM deposition. As animal models of BLM-induced fibrosis can simulate the clinical pathogenesis of IPF, our findings also suggest a clinical potential of Sch B (21).
Sch B could inhibit inflammatory responses and could inhibit pulmonary fibrosis. The key component of BL-induced pulmonary fibrosis is inflammatory responses, as BLM-induced lung injury lead to inflammatory responses due to the production of excess free radicals (27, 28). The results from the BLM-induced early inflammation model indicated Sch B could significantly decrease inflammatory responses, therefore have the potential for alleviating pulmonary fibrosis. This finding is comparable to previous research. Liu et al. found that AF-1 was able to inhibit TGF-β induced proliferation by reducing the production and release of inflammatory cells and inflammatory factors (29). Moreover, Chitra et al. found that berberine attenuated BLM-induced fibro-proliferation and ECM deposition by improving antioxidant status and blocking the expression and release of inflammatory mediators (30). Collectively, similar results support our findings in the anti-fibrosis effect of Sch B.
Sch B may inhibit pulmonary fibrosis by upregulating autophagy genes. Autophagy is a metabolic process of eukaryotic self-catabolic process (31). Impairment of autophagy is closely related to the promotion of pulmonary fibrosis, and previous research showed that the regulation of autophagy response played an important role in inhibiting ECM accumulation. Taken together, autophagy is considered a key regulator in the pathogenesis of pulmonary fibrosis (32, 33). Recent research has documented that activating autophagy can significantly reduce BLM-induced pulmonary fibrosis and local inflammation in fibrotic tissues. Consequently, insufficient autophagy can lead to cell senescence and myofibroblast (34). Our results indicate BLM can significantly down-regulate autophagy. The decrease in the expression of autophagy protein LC3-II in lung tissues was observed (30), and Sch B increased the expression levels of Beclin-1 and LC3-II in mice with BLM-induced pulmonary fibrosis. This finding strongly suggests that the level of autophagy was up-regulated after the intervention of Sch B. These results suggest that Sch B could attenuate pulmonary fibrosis in mice with BLM-induced pulmonary fibrosis by activating autophagy.
In addition, our study clarified the mechanism of Sch B regulating autophagy. mTOR signaling pathway is an important molecular signaling pathway for the regulation of autophagy. Several studies have reported the relationship between autophagy and the mTOR signaling pathway (35, 36). Recent studies have also shown that TGF-β inhibits autophagy by activating mTOR. Administration of rapamycin (an mTOR inhibitor) in BLM-induced animals increased the expression of autophagy proteins LC3 and Beclin-1, followed by decreased α-SMA and fibronectin, resulting in attenuated pulmonary fibrosis. In addition, the silencing of the autophagy proteins Beclin-1 and LC3 resulted in enhanced levels of α-SMA and fibuconin in response to TGF-β (15). Our western blot analysis focused on the effect of Sch B on autophagy status and examined the effects of Sch B on AKT-mTOR. The results showed that Sch B could induce significant dephosphorylation in p-AKT, which could be rescue by 3-Methyladenine. Our results prove the potential of Sch B in promoting autophagy through the AKT-mTOR pathway, and it is a possible mechanism for attenuating BLM-induced pulmonary fibrosis injury.
Collectively, this study provides evidence for the potential of Sch B as a drug for the treatment of pulmonary fibrosis. However, Sch B has many other pharmacological activities beside its effect in the autophagy mechanism. Whether other mechanisms involve in the inhibition of pulmonary fibrosis remains unclear. In addition, the formation of pulmonary fibrosis involves many pathogenic mechanisms, and mTOR signaling pathway is only one of the important molecular mechanisms of IPF. The mechanism of Sch B in BLM-induced pulmonary fibrosis needs further clarification with clinical studies. In conclusion, our study demonstrated that Sch B exerts anti-fibrotic effects in BLM-induced pulmonary fibrosis. The effect of Sch B may be accomplished by activating autophagy through AKT-mTOR signaling pathway. Therefore, Sch B may be a potential agent for the treatment of IPF.
Statement:
We declare that this work was done by the authors named in this article and all liabilities pertaining to claims relating to the content of this article will be borne by the authors.
Author Contributions:
JY and DZ designed this study; JY, DZ and JH were responsible for the experiments and data collection; JY, DZ, JH and QL were responsible for data analysis; JY, DZ, JH and DY conducted the manuscript writing; YC and QL critically revised the manuscript, QL approved the final version to be published; all authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Availability of Data and Materials:
All data generated or analysis during this study are included in this published article [and its supplementary information files].
Funding Sources:
This work was supported by the Wuhan Municipal Health Commission Project (WZ19Q12).
Ethical Approval:
All animals were treated with humane conditions and institutional guidelines for the care and use of animals were followed.
Conflict of Interest Statement:
The authors declare that they have no competing interests.
References
- Sauleda J, Nunez B, Sala E, Soriano JB. Idiopathic Pulmonary Fibrosis: Epidemiology, Natural History, Phenotypes. Med Sci (Basel) 2018;6(4) [Europe PMC free article] [Abstract] [Google Scholar]
- Aloufi N, Traboulsi H, Ding J, et al. Angiotensin-converting enzyme 2 expression in COPD and IPF fibroblasts: the forgotten cell in COVID-19. Am J Physiol Lung Cell Mol Physiol. 2021;320(1):L152–L7. [Europe PMC free article] [Abstract] [Google Scholar]
- Molyneaux PL, Maher TM. Respiratory microbiome in IPF: cause, effect, or biomarker? Lancet Respir Med. 2014;2(7):511–3. [Abstract] [Google Scholar]
- Samarelli AV, Tonelli R, Heijink I, et al. Dissecting the Role of Mesenchymal Stem Cells in Idiopathic Pulmonary Fibrosis: Cause or Solution. Front Pharmacol. 2021;12:692551. [Europe PMC free article] [Abstract] [Google Scholar]
- Cilli A, Uzer F. Disease progression in idiopathic pulmonary fibrosis under anti-fibrotic treatment. Sarcoidosis Vasc Diffuse Lung Dis. 2023;40(3):e2023034. [Europe PMC free article] [Abstract] [Google Scholar]
- Raghu G, Rochwerg B, Zhang Y, et al. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline: Treatment of Idiopathic Pulmonary Fibrosis. An Update of the 2011 Clinical Practice Guideline. Am J Respir Crit Care Med. 2015;192(2):e3–19. [Abstract] [Google Scholar]
- Jiang EP, Li H, Yu CR, et al. Schisandrin B protects PC12 cells against oxidative stress of neurodegenerative diseases. Neuroreport. 2015;26(6):360–6. [Abstract] [Google Scholar]
- Leong PK, Ko KM. Schisandrin B: A Double-Edged Sword in Nonalcoholic Fatty Liver Disease. Oxid Med Cell Longev. 2016;2016:6171658. [Europe PMC free article] [Abstract] [Google Scholar]
- Li L, Pan Q, Han W, et al. Schisandrin B prevents doxorubicin-induced cardiotoxicity via enhancing glutathione redox cycling. Clin Cancer Res. 2007;13(22 Pt 1):6753–60. [Abstract] [Google Scholar]
- Chiu PY, Leung HY, Siu AH, Poon MK, Ko KM. Schisandrin B decreases the sensitivity of mitochondria to calcium ion-induced permeability transition and protects against ischemia-reperfusion injury in rat hearts. Acta Pharmacol Sin. 2007;28(10):1559–65. [Abstract] [Google Scholar]
- Nasser MI, Zhu S, Chen C, et al. A Comprehensive Review on Schisandrin B and Its Biological Properties. Oxid Med Cell Longev. 2020;2020:2172740. [Europe PMC free article] [Abstract] [Google Scholar]
- Zhu H, Zhang X, Guan J, et al. Pharmacokinetics and tissue distribution study of schisandrin B in rats by ultra-fast liquid chromatography with tandem mass spectrometry. J Pharm Biomed Anal. 2013:78–79. 136–40. [Abstract] [Google Scholar]
- Li WL, Xin HW, Yu AR, Wu XC. In vivo effect of Schisandrin B on cytochrome P450 enzyme activity. Phytomedicine. 2013;20(8-9):760–5. [Abstract] [Google Scholar]
- Wang Y, Dong X, Zhao N, et al. Schisandrin B attenuates bleomycin-induced pulmonary fibrosis in mice through the wingless/integrase-1 signaling pathway. Exp Lung Res. 2020;46(6):185–94. [Abstract] [Google Scholar]
- Patel AS, Lin L, Geyer A, et al. Autophagy in idiopathic pulmonary fibrosis. PLoS One. 2012;7(7):e41394. [Europe PMC free article] [Abstract] [Google Scholar]
- Walters DM, Kleeberger SR. Mouse models of bleomycin-induced pulmonary fibrosis. Curr Protoc Pharmacol. 2008 Chapter 5: Unit 5 46. [Abstract] [Google Scholar]
- Lee JG, Shin JH, Shim HS, et al. Autophagy contributes to the chemo-resistance of non-small cell lung cancer in hypoxic conditions. Respir Res. 2015;16:138. [Europe PMC free article] [Abstract] [Google Scholar]
- Moeller A, Ask K, Warburton D, Gauldie J, Kolb M. The bleomycin animal model: a useful tool to investigate treatment options for idiopathic pulmonary fibrosis? Int J Biochem Cell Biol. 2008;40(3):362–82. [Europe PMC free article] [Abstract] [Google Scholar]
- Ashcroft T, Simpson JM, Timbrell V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol. 1988;41(4):467–70. [Europe PMC free article] [Abstract] [Google Scholar]
- De Andrade J, Luckhardt T, Sonavane S, et al. Evaluating the consistency with guideline recommendations for diagnosis and management of idiopathic pulmonary fibrosis in non-academic settings. Sarcoidosis Vasc Diffuse Lung Dis. 2023;40(1):e2023003. [Europe PMC free article] [Abstract] [Google Scholar]
- Wang K, Zhang T, Lei Y, et al. Identification of ANXA2 (annexin A2) as a specific bleomycin target to induce pulmonary fibrosis by impeding TFEB-mediated autophagic flux. Autophagy. 2018;14(2):269–82. [Europe PMC free article] [Abstract] [Google Scholar]
- Lee S, Chun JN, Lee HJ, et al. Transcriptome Analysis of the Anti-TGFbeta Effect of Schisandra chinensis Fruit Extract and Schisandrin B in A7r5 Vascular Smooth Muscle Cells. Life (Basel) 2021;11(2) [Europe PMC free article] [Abstract] [Google Scholar]
- Valero-Jimenez A, Zuniga J, Cisneros J, et al. Transmembrane protease, serine 4 (TMPRSS4) is upregulated in IPF lungs and increases the fibrotic response in bleomycin-induced lung injury. PLoS One. 2018;13(3):e0192963. [Europe PMC free article] [Abstract] [Google Scholar]
- You S, Qian J, Wu G, et al. Schizandrin B attenuates angiotensin II induced endothelial to mesenchymal transition in vascular endothelium by suppressing NF-kappaB activation. Phytomedicine. 2019;62:152955. [Abstract] [Google Scholar]
- Zhou Y, Li P, Duan JX, et al. Aucubin Alleviates Bleomycin-Induced Pulmonary Fibrosis in a Mouse Model. Inflammation. 2017;40(6):2062–73. [Abstract] [Google Scholar]
- Leask A. Potential therapeutic targets for cardiac fibrosis: TGFbeta, angiotensin, endothelin, CCN2, and PDGF, partners in fibroblast activation. Circ Res. 2010;106(11):1675–80. [Abstract] [Google Scholar]
- Verma R, Kushwah L, Gohel D, et al. Evaluating the Ameliorative Potential of Quercetin against the Bleomycin-Induced Pulmonary Fibrosis in Wistar Rats. Pulm Med. 2013;2013:921724. [Europe PMC free article] [Abstract] [Google Scholar]
- Mouratis MA, Aidinis V. Modeling pulmonary fibrosis with bleomycin. Curr Opin Pulm Med. 2011;17(5):355–61. [Abstract] [Google Scholar]
- Liu W, Wan J, Han JZ, et al. Antiflammin-1 attenuates bleomycin-induced pulmonary fibrosis in mice. Respir Res. 2013;14(1):101. [Europe PMC free article] [Abstract] [Google Scholar]
- Chitra P, Saiprasad G, Manikandan R, Sudhandiran G. Berberine attenuates bleomycin induced pulmonary toxicity and fibrosis via suppressing NF-kappaB dependant TGF-beta activation: a biphasic experimental study. Toxicol Lett. 2013;219(2):178–93. [Abstract] [Google Scholar]
- Haspel JA, Choi AM. Autophagy: a core cellular process with emerging links to pulmonary disease. Am J Respir Crit Care Med. 2011;184(11):1237–46. [Europe PMC free article] [Abstract] [Google Scholar]
- Korfhagen TR, Le Cras TD, Davidson CR, et al. Rapamycin prevents transforming growth factor-alpha-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2009;41(5):562–72. [Europe PMC free article] [Abstract] [Google Scholar]
- Ashford TP, Porter KR. Cytoplasmic components in hepatic cell lysosomes. J Cell Biol. 1962;12(1):198–202. [Europe PMC free article] [Abstract] [Google Scholar]
- Araya J, Kojima J, Takasaka N, et al. Insufficient autophagy in idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2013;304(1):L56–69. [Abstract] [Google Scholar]
- Ching JK, Weihl CC. Rapamycin-induced autophagy aggravates pathology and weakness in a mouse model of VCP-associated myopathy. Autophagy. 2013;9(5):799–800. [Europe PMC free article] [Abstract] [Google Scholar]
- Li X, Wu D, Shen J, Zhou M, Lu Y. Rapamycin induces autophagy in the melanoma cell line M14 via regulation of the expression levels of Bcl-2 and Bax. Oncol Lett. 2013;5(1):167–72. [Europe PMC free article] [Abstract] [Google Scholar]
Articles from Sarcoidosis, Vasculitis, and Diffuse Lung Diseases are provided here courtesy of Mattioli 1885
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Schisandrin B attenuates bleomycin-induced pulmonary fibrosis in mice through the wingless/integrase-1 signaling pathway.
Exp Lung Res, 46(6):185-194, 02 May 2020
Cited by: 7 articles | PMID: 32362157
Nintedanib Ameliorates Bleomycin-Induced Pulmonary Fibrosis, Inflammation, Apoptosis, and Oxidative Stress by Modulating PI3K/Akt/mTOR Pathway in Mice.
Inflammation, 46(4):1531-1542, 09 May 2023
Cited by: 9 articles | PMID: 37160579 | PMCID: PMC10359208
Synergistic protection of Schizandrin B and Glycyrrhizic acid against bleomycin-induced pulmonary fibrosis by inhibiting TGF-β1/Smad2 pathways and overexpression of NOX4.
Int Immunopharmacol, 48:67-75, 03 May 2017
Cited by: 16 articles | PMID: 28476015
Ketogenic diet induces autophagy to alleviate bleomycin-induced pulmonary fibrosis in murine models.
Exp Lung Res, 47(1):26-36, 29 Oct 2020
Cited by: 5 articles | PMID: 33121292

 1
1

