Abstract
Background
Patients with pathologic complete response (pCR) to neoadjuvant chemotherapy for invasive breast cancer (BC) have better outcomes, potentially warranting less extensive surgical and systemic treatments. Early prediction of treatment response could aid in adapting therapies.Methods
On-treatment biopsies from 297 patients with invasive BC in three randomized, prospective neoadjuvant trials were assessed (GeparQuattro, GeparQuinto, GeparSixto). BC quantity, tumor-infiltrating lymphocytes (TILs), and the proliferation marker Ki-67 were compared to pre-treatment samples. The study investigated the correlation between residual cancer, changes in Ki-67 and TILs, and their impact on pathologic complete response (pCR) and disease-free survival (DFS).Results
Among the 297 samples, 138 (46%) were hormone receptor-positive (HR+)/human epidermal growth factor 2-negative (HER2-), 87 (29%) were triple-negative (TNBC), and 72 (24%) were HER2+. Invasive tumor cells were found in 70% of on-treatment biopsies, with varying rates across subtypes (HR+/HER2-: 84%, TNBC: 62%, HER2+: 51%; p < 0.001). Patients with residual tumor on-treatment had an 8% pCR rate post-treatment (HR+/HER2-: 3%, TNBC: 19%, HER2+: 11%), while those without any invasive tumor had a 50% pCR rate (HR+/HER2-: 27%; TNBC: 48%, HER2+: 66%). Sensitivity for predicting residual disease was 0.81, with positive and negative predictive values of 0.92 and 0.50, respectively. Increasing TILs from baseline to on-treatment biopsy (if residual tumor was present) were linked to higher pCR likelihood in the overall cohort (OR 1.034, 95% CI 1.013-1.056 per % increase; p = 0.001) and with a longer DFS in TNBC (HR 0.980, 95% CI 0.963-0.997 per % increase; p = 0.026). Persisting or increased Ki-67 was associated with with lower pCR probability in the overall cohort (OR 0.957, 95% CI 0.928-0.986; p = 0.004) and shorter DFS in TNBC (HR 1.023, 95% CI 1.001-1.047; p = 0.04).Conclusion
On-treatment biopsies can predict patients unlikely to achieve pCR post-therapy. This could facilitate therapy adjustments for TNBC or HER2 + BC. They also might offer insights into therapy resistance mechanisms. Future research should explore whether standardized or expanded sampling enhances the accuracy of on-treatment biopsy procedures. Trial registration GeparQuattro (EudraCT 2005-001546-17), GeparQuinto (EudraCT 2006-005834-19) and GeparSixto (EudraCT 2011-000553-23).Free full text

On-treatment biopsies to predict response to neoadjuvant chemotherapy for breast cancer
Abstract
Background
Patients with pathologic complete response (pCR) to neoadjuvant chemotherapy for invasive breast cancer (BC) have better outcomes, potentially warranting less extensive surgical and systemic treatments. Early prediction of treatment response could aid in adapting therapies.
Methods
On-treatment biopsies from 297 patients with invasive BC in three randomized, prospective neoadjuvant trials were assessed (GeparQuattro, GeparQuinto, GeparSixto). BC quantity, tumor-infiltrating lymphocytes (TILs), and the proliferation marker Ki-67 were compared to pre-treatment samples. The study investigated the correlation between residual cancer, changes in Ki-67 and TILs, and their impact on pathologic complete response (pCR) and disease-free survival (DFS).
Results
Among the 297 samples, 138 (46%) were hormone receptor-positive (HR+)/human epidermal growth factor 2-negative (HER2−), 87 (29%) were triple-negative (TNBC), and 72 (24%) were HER2+. Invasive tumor cells were found in 70% of on-treatment biopsies, with varying rates across subtypes (HR+/HER2−: 84%, TNBC: 62%, HER2+: 51%; p <
< 0.001). Patients with residual tumor on-treatment had an 8% pCR rate post-treatment (HR+/HER2−: 3%, TNBC: 19%, HER2+: 11%), while those without any invasive tumor had a 50% pCR rate (HR+/HER2−: 27%; TNBC: 48%, HER2+: 66%). Sensitivity for predicting residual disease was 0.81, with positive and negative predictive values of 0.92 and 0.50, respectively. Increasing TILs from baseline to on-treatment biopsy (if residual tumor was present) were linked to higher pCR likelihood in the overall cohort (OR 1.034, 95% CI 1.013–1.056 per % increase; p
0.001). Patients with residual tumor on-treatment had an 8% pCR rate post-treatment (HR+/HER2−: 3%, TNBC: 19%, HER2+: 11%), while those without any invasive tumor had a 50% pCR rate (HR+/HER2−: 27%; TNBC: 48%, HER2+: 66%). Sensitivity for predicting residual disease was 0.81, with positive and negative predictive values of 0.92 and 0.50, respectively. Increasing TILs from baseline to on-treatment biopsy (if residual tumor was present) were linked to higher pCR likelihood in the overall cohort (OR 1.034, 95% CI 1.013–1.056 per % increase; p =
= 0.001) and with a longer DFS in TNBC (HR 0.980, 95% CI 0.963–0.997 per % increase; p
0.001) and with a longer DFS in TNBC (HR 0.980, 95% CI 0.963–0.997 per % increase; p =
= 0.026). Persisting or increased Ki-67 was associated with with lower pCR probability in the overall cohort (OR 0.957, 95% CI 0.928–0.986; p
0.026). Persisting or increased Ki-67 was associated with with lower pCR probability in the overall cohort (OR 0.957, 95% CI 0.928–0.986; p =
= 0.004) and shorter DFS in TNBC (HR 1.023, 95% CI 1.001–1.047; p
0.004) and shorter DFS in TNBC (HR 1.023, 95% CI 1.001–1.047; p =
= 0.04).
0.04).
Conclusion
On-treatment biopsies can predict patients unlikely to achieve pCR post-therapy. This could facilitate therapy adjustments for TNBC or HER2 +
+ BC. They also might offer insights into therapy resistance mechanisms. Future research should explore whether standardized or expanded sampling enhances the accuracy of on-treatment biopsy procedures.
BC. They also might offer insights into therapy resistance mechanisms. Future research should explore whether standardized or expanded sampling enhances the accuracy of on-treatment biopsy procedures.
Trial registration GeparQuattro (EudraCT 2005-001546-17), GeparQuinto (EudraCT 2006-005834-19) and GeparSixto (EudraCT 2011-000553-23).
Supplementary Information
The online version contains supplementary material available at 10.1186/s13058-024-01883-w.
Introduction
Retrospective analyses of prospective breast cancer (BC) trials have shown comparable efficacy between chemotherapy administered in the adjuvant versus neoadjuvant settings [1]. Excellent response, defined as achieving pathologic complete response (pCR) to neoadjuvant chemotherapy (NACT), varies across breast cancer (BC) subtypes and is strongly influenced by the treatment regimen used. During the analyzed trials, pCR rates were approximately 50% and 30% for patients with triple-negative and HER2-positive disease, respectively [2]. In recent developments, incorporating immune checkpoint inhibitors into NACT for TNBC resulted in pCR rates of 64.8% [3], while employing dual anti-HER2 blockade yielded pCR rates of 66.2% in HER2-positive disease, depending on hormone receptor status [4]. Excellent response serves as a prognostic indicator for patient survival, especially in triple-negative and HER2-positive disease [5], and neoadjuvant therapy can serve as an in vivo assay for chemotherapy response.
Prediction of therapy response is of clinical interest offering the potential to customize treatment approaches and enhance response rates. With the emergence of new therapies and refined treatment protocols, there arises the question of whether de-escalating local and/or systemic treatment is viable for patients with a strong likelihood of achieving pCR [6]. For example, PET-based imaging can be used to predict pCR in patients with HER2-positive BC during neoadjuvant treatment with dual anti-HER2 treatment [7].
The conventional method for identifying response markers involves correlating genomic measurements from pre-therapeutic samples with clinical outcomes [8]. On-treatment tissue samples offer the opportunity for microscopic confirmation of response, biomarker examination, and tissue provision for translational research. However, the reliability of biopsy procedures and histopathological assessment for predicting on-treatment pCR remains uncertain. The RESPONDER trial examines if vacuum-assisted biopsies can be used to predict response in the breast with a false negative rate below 10% [9].
In neoadjuvant aromatase inhibition for HR +
+ BC, gene expression analysis of on-treatment samples has been shown to predict treatment response and patient survival [10]. Ki-67 immunohistochemistry can indicate the need to switch to neoadjuvant chemotherapy if Ki-67 levels remain elevated during endocrine treatment alone [11, 12]. In the context of neoadjuvant chemotherapy (NACT), we assessed on-treatment response using ultrasound in the GeparTrio (G3) [13] and GeparQuinto (G5) [14] trials. In G3, we could demonstrate that response-guided switch of chemotherapy regimens can improve patient outcome.
BC, gene expression analysis of on-treatment samples has been shown to predict treatment response and patient survival [10]. Ki-67 immunohistochemistry can indicate the need to switch to neoadjuvant chemotherapy if Ki-67 levels remain elevated during endocrine treatment alone [11, 12]. In the context of neoadjuvant chemotherapy (NACT), we assessed on-treatment response using ultrasound in the GeparTrio (G3) [13] and GeparQuinto (G5) [14] trials. In G3, we could demonstrate that response-guided switch of chemotherapy regimens can improve patient outcome.
During neoadjuvant chemotherapy (NACT), on-treatment samples can be utilized to uncover molecular mechanisms linked to therapy response, such as immune and proliferation signatures [15] and to pinpoint potential markers of resistance/response through comparative gene expression analysis between responders and non-responders [16].
Aim of this retrospective-prospective biomarker study was to evaluate the frequency of residual cancer cells in on-treatment samples from neoadjuvant clinical chemotherapy trials for BC, and to correlate their presence with response to treatment.
We also included the evaluation of two biomarkers: tumor-infiltrating lymphocytes and the proliferation marker Ki-67. Pre- and post-treatment levels of tumor-infiltrating lymphocytes (TILs) can predict response to chemotherapy and survival [17, 18] and chemotherapy may trigger or amplify a cytotoxic immune response [19]. High levels of the proliferation marker Ki-67 can predict a better response to neoadjuvant chemotherapy but also a poorer long-term prognosis due to more aggressive tumor biology [20]. During neoadjuvant aromatase inhibition, on-treatment Ki-67 evaluation also predicts patient outcomes [12]. We hypothesized that an increase in TILs or a decrease in Ki-67 could predict patient outcome after completion of chemotherapy.
Methods
Patients and samples
Patients were treated within the randomized, multi-center neoadjuvant clinical trials GeparQuattro (G4) [21–23], GeparQuinto (G5) [14, 24, 25] and GeparSixto (G6) [2]. Details on the study designs and outcomes are available in the original publications. In brief, G4 was a phase III study comparing the simultaneous or sequential use of capecitabine with epirubicin, cyclophosphamide and docetaxel (EC-T) with concomitant trastuzumab in HER2 +
+ disease. G5 was a phase III study to evaluate EC-T with or without bevacizumab (B) in HER2-negative BC (setting I), to compare pCR rates of patients treated with paclitaxel with or without everolimus with HER2-negative BC without sonographic response after four cycles EC
disease. G5 was a phase III study to evaluate EC-T with or without bevacizumab (B) in HER2-negative BC (setting I), to compare pCR rates of patients treated with paclitaxel with or without everolimus with HER2-negative BC without sonographic response after four cycles EC ±
± B (setting II) and to compare pCR rates in patients treated with EC-T followed by trastuzumab or lapatinib in HER2-positive disease (setting III). G6 was a phase II trial to evaluate the addition of carboplatin to neoadjuvant treatment for patients with triple-negative or HER2-positive BC. Biopsies were obtained at the time of diagnosis and during chemotherapy: in G4 and G5 after 4 of 8 cycles and in G6 after 2 of 6 cycles. Ultrasound was used to guide sampling on-treatment. Only biopsies from primary breast tumors were included in this study, not those from lymph nodes. Detailed information on sampling procedures (tumour size on ultrasound, number of biopsies, needle size) was not available for data analysis in these older clinical trials. All patients with available material in the central GBG tumor bank were eligible for this retrospective biomarker analysis. Of the 1495, 1948 and 588 patients in the G4, G5, and G6 trials, respectively, 106, 145 and 61 matched pre-therapeutic and on-treatment biopsies were available in the biobank and included in the study, resulting in 312 matched pairs. 15 samples had to be excluded due to insufficient pre-treatment material, resulting in a total of 297 matched samples. Table Table11 details the baseline patient characteristics.
B (setting II) and to compare pCR rates in patients treated with EC-T followed by trastuzumab or lapatinib in HER2-positive disease (setting III). G6 was a phase II trial to evaluate the addition of carboplatin to neoadjuvant treatment for patients with triple-negative or HER2-positive BC. Biopsies were obtained at the time of diagnosis and during chemotherapy: in G4 and G5 after 4 of 8 cycles and in G6 after 2 of 6 cycles. Ultrasound was used to guide sampling on-treatment. Only biopsies from primary breast tumors were included in this study, not those from lymph nodes. Detailed information on sampling procedures (tumour size on ultrasound, number of biopsies, needle size) was not available for data analysis in these older clinical trials. All patients with available material in the central GBG tumor bank were eligible for this retrospective biomarker analysis. Of the 1495, 1948 and 588 patients in the G4, G5, and G6 trials, respectively, 106, 145 and 61 matched pre-therapeutic and on-treatment biopsies were available in the biobank and included in the study, resulting in 312 matched pairs. 15 samples had to be excluded due to insufficient pre-treatment material, resulting in a total of 297 matched samples. Table Table11 details the baseline patient characteristics.
Table 1
Baseline characteristics of the study cohort
| All | G4 | G5 | G6 | ||
|---|---|---|---|---|---|
| Subtype | HR−/HER2− | 87 (29.3%) | 19 (19%) | 43 (30.9%) | 25 (43.1%) |
| HR+/HER2− | 138 (46.5%) | 53 (53%) | 85 (61.2%) | 0 (0%) | |
| HER2+ | 72 (24.2%) | 28 (28%) | 11 (7.9%) | 33 (56.9%) | |
| Response | no pCR | 235 (79.1%) | 81 (81%) | 125 (89.9%) | 29 (50%) |
| pCR | 62 (20.9%) | 19 (19%) | 14 (10.1%) | 29 (50%) | |
| On-treatment biopsy | tu+ | 207 (69.7%) | 69 (69%) | 112 (80.6%) | 26 (44.8%) |
| tu− | 90 (30.3%) | 31 (31%) | 27 (19.4%) | 32 (55.2%) | |
| cT stage | T1 | 25 (8.4%) | 0 (0%) | 11 (7.9%) | 14 (24.1%) |
| T2 | 181 (60.9%) | 68 (68%) | 81 (58.3%) | 32 (55.2%) | |
| T3 | 42 (14.1%) | 16 (16%) | 17 (12.2%) | 9 (15.5%) | |
| T4 | 49 (16.5%) | 16 (16%) | 30 (21.6%) | 3 (5.2%) | |
| cN stage | N0 | 125 (42.1%) | 43 (43%) | 51 (36.7%) | 31 (53.4%) |
| N1-3 | 171 (57.6%) | 57 (57%) | 88 (63.3%) | 26 (44.8%) | |
| NA | 1 (0.3%) | 0 (0%) | 0 (0%) | 1 (1.7%) | |
| Grading | G1-2 | 156 (52.5%) | 55 (55%) | 76 (54.7%) | 25 (43.1%) |
| G3 | 136 (45.8%) | 40 (40%) | 63 (45.3%) | 33 (56.9%) | |
| NA | 5 (1.7%) | 5 (5%) | 0 (0%) | 0 (0%) | |
| Histology | NST | 268 (90.2%) | 89 (89%) | 122 (87.8%) | 57 (98.3%) |
| Lobular | 23 (7.7%) | 9 (9%) | 14 (10.1%) | 0 (0%) | |
| Other | 6 (2%) | 2 (2%) | 3 (2.2%) | 1 (1.7%) | |
TILs Median | < ≥ | 267 (89.9%) 30 (10.1%) | 91 (91.0%) 9 (9.0%) | 129 (92.8%) 10 (7.2%) | 47 (81.0%) 11 (19.0%) |
Ki-67 Median | ≥ < NA | 149 (50.2%) 142 (47.8%) 6 (2.0%) | 55 (55.0%) 41 (41.0%) 4 (4.0%) | 72 (51.8%) 65 (46.8%) 2 (1.4%) | 15 (25.9%) 43 (74.1%) 0 (0%) |
All patients provided written informed consent for participation in the study and the utilization of biomaterials for translational research purposes. The study protocol received approval from the relevant ethics committee and national competent authority.
Biomarker analysis
An experienced pathologist (BVS, CD) reassessed the biopsies on an H&E-stained slide to identify the presence of invasive breast cancer (BC) at the German Breast Group’s central histopathology laboratory. On-treatment biopsies were categorized as positive for invasive tumor (tu+) if residual invasive cancer cells were observed. Ductal carcinoma in situ or other precursor lesions were not included in this classification.
The presence and quantity of tumor-infiltrating lymphocytes (TILs) in the stromal compartment were documented following the guidelines of the international TIL working group. This involved comparing the H&E-stained slide under review to standardized reference images. [26].
Immunostaining for Ki-67 was performed on a Ventana Discovery XT instrument (Ventana, Tucson, AZ) using the MIB-1 clone (diluted 1:50). Quantification of stained tumor cells was performed using a digital software solution (VMScope, Berlin, Germany) according to recommendation of the Ki-67 in BC working group [27]. For each case, three areas were chosen and counted, and the mean value of the different areas was used for analysis.
Statistical considerations
Pathologic complete response (pCR) was defined as the absence of invasive or non-invasive BC in the breast and lymph nodes after completion of neoadjuvant treatment (ypT0 ypN0). Disease-free survival (DFS) was defined as the time from study entry to distant or local relapse or death from any cause.
Statistical analyses were computed in R 4.0.3 (R Project for Statistical Computing, RRID:SCR_001905). The change of TILs (ΔTILs) and Ki-67 (ΔKi-67) was calculated as the difference between on-treatment and pre-treatment as a continuous parameter. To test the association of positive on-treatment biopsies (tu+) with tumor characteristics and pCR, chi-squared test was used. The Kaplan Meier method with log rank test was used to illustrate the association of response parameters with DFS. Uni- and bivariate Cox proportional hazard regression models were fit to examine the association of biomarkers with DFS. Logistic regression models were fit to examine the association of biomarkers with pCR.
Results
Frequencies of residual cancer cells and their association with patient and tumor characteristics (Fig. 1)
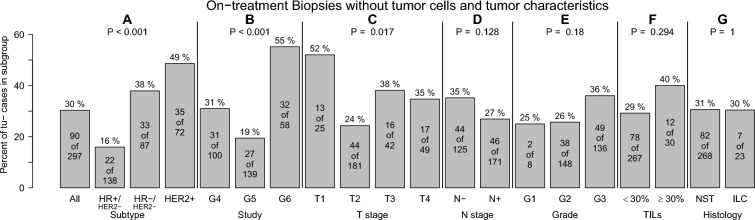
Frequency of on-treatment biopsies without tumor cells according to tumor characteristics. On-treatment biopsies without tumor cells were more frequent in triple-negative and HER2-positive breast cancers (A), in biopsies from G6 trial patients (B), and in smaller tumors (C). There was no statistical association with lymph node status (D), histological grading (E), tumor-infiltrating lymphocytes (F) or histology (G)
Residual cancer cells were present in biopsies of 207 patients (tu+; 70%) after 4 of 8 cycles (G4, G5) and 2 of 6 cycles chemotherapy (G6), respectively. 90 biopsies showed no residual disease (tu−; 30%). The highest frequencies of tu− biopsies were observed in patients with HER2+ (49%) and TNBC (38%) BC (Fig. 1). The highest frequency of tu− patients was observed among patients of the G6 trial (all patients had triple-negative or HER2-positive disease). The frequency of tu− patients was also higher in patients with small tumors. There was no statistically significant association with lymph node status, histological grading, TILs or histologic subtype (Fig. 1).
Frequencies of residual cancer cells and their association with response to treatment (Figs. 2, ,33)
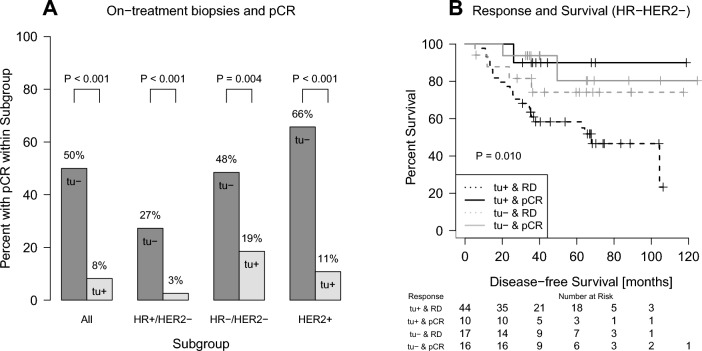
Association of cancer cells in on-treatment samples with pathological complete response after completion of chemotherapy (pCR, A) and disease-free survival (B). The rate of pCR was 8% in patients with evidence of cancer cells on-treatment (tu+), but 50% when the biopsy was negative for cancer (tu−) (A). In the Kaplan–Meier analysis of patients with HR−/HER2− BC, residual cancer on-treatment (tu+) and residual disease (RD) after completion of chemotherapy were associated with shorter survival (B)
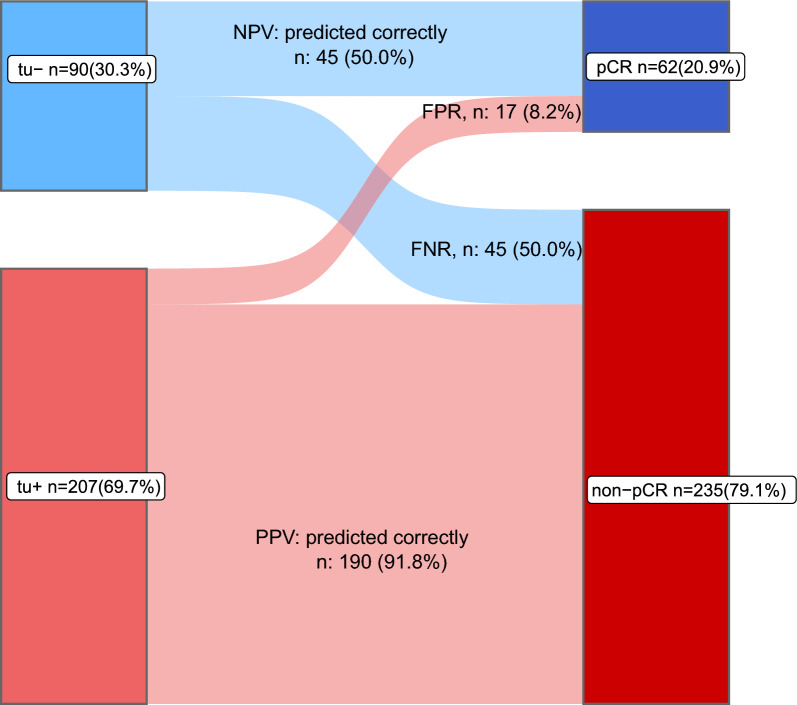
Distribution of patients with pCR or non-pCR after completion of chemotherapy according to on-treatment biopsies with (tu+) or without (tu−) residual cancer cells. Of the 90 on-treatment samples without residual cancer cells (blue), 50% had a pCR at the end of treatment and 50% had a non-pCR. Of the 207 on-treatment samples with residual cancer (red), 8.2% had a pCR and 91.8% had a non-pCR. NPV =
= negative predictive value; PPV
negative predictive value; PPV =
= positive predictive value; FPR
positive predictive value; FPR =
= false positive rate; FNR
false positive rate; FNR =
= false negative rate
false negative rate
In tu− patients a pCR was observed in 50% (45/90) (Fig. 2A). In contrast, only 17 of 207 (8%) patients with positive biopsies (tu+) had a pCR after completion of the full treatment course, and 92% had residual diseases (190/207). A similar association could be observed in the different BC subtypes (Fig. 2A). The distribution of patients with pCR or non-pCR after completion of chemotherapy according to on-treatment biopsies with (tu+) or without (tu−) residual cancer cells is demonstrated in a Sankey plot (Fig. 3) in detail. Sensitivity to predict residual disease was 0.81 (specificity 0.72). The positive and negative predictive values were 0.92 and 0.50, respectively.
In univariate Cox regression analyses, the absence of tumor cells in on-treatment biopsies was associated with a lower risk of relapse in patients with triple-negative disease (Table 2). However, the effect was not statistically significant when adjusted for pCR in a bivariate model (Table 3). The relationship between the presence of residual disease during and/or after chemotherapy and patient survival was demonstrated in a Kaplan–Meier analysis (Fig. 2B). Patients with residual cancer cells during chemotherapy (tu+) and residual disease (RD) after completion of the full course show the highest risk of relapse.
Table 2
Univariate Cox regression models to predict disease-free survival according to residual cancer in on-treatment biopsies during neoadjuvant chemotherapy
| Subtype | Covariate | Hazard ratio (95% CI) | P |
|---|---|---|---|
| HR−/HER2− | tu− vs. tu+ | 0.402 (0.163–0.987) | 0.047 |
| HR+/HER2− | tu− vs. tu+ | 1.039 (0.428–2.525) | 0.933 |
| HER2+ | tu− vs. tu+ | 1.77 (0.577–5.43) | 0.318 |
Table 3
Bivariate Cox regression models to predict disease-free survival according to residual cancer in on-treatment biopsies during neoadjuvant chemotherapy and after treatment
| Subtype | Covariate | Hazard ratio (95% CI) | P |
|---|---|---|---|
| HR−/HER2− | tu− vs. tu+ | 0.544 (0.217–1.369) | 0.196 |
| pCR vs. no pCR | 0.282 (0.083–0.964) | 0.043 | |
| HR+/HER2− | tu− vs. tu+ | 1.14 (0.442–2.939) | 0.787 |
| pCR vs. no pCR | 0.689 (0.149–3.18) | 0.633 | |
| HER2+ | tu− vs. tu+ | 2.114 (0.589–7.584) | 0.251 |
| pCR vs. no pCR | 0.704 (0.197–2.516) | 0.589 |
Patients with false negative predictions, i.e. those without residual tumor cells on-treatment but residual disease after completion of treatment were analyzed (Fig. S1). A higher frequency of false negative predictions was observed in HR+/HER2− disease. There was no statistically significant association with tumor stage, nodal status, grade or TILs. Patients with false positive predictions, i.e. those with residual cancer cells on-treatment, but with pCR after chemotherapy were demonstrated in Fig. S2. This was more frequently observed in HR−/HER2− cases, in the GeparSixto trial, in smaller tumors and in patients with negative clinical lymph node status.
Dynamic change of TILs and Ki-67 and its association with patient outcome (Fig. 4)
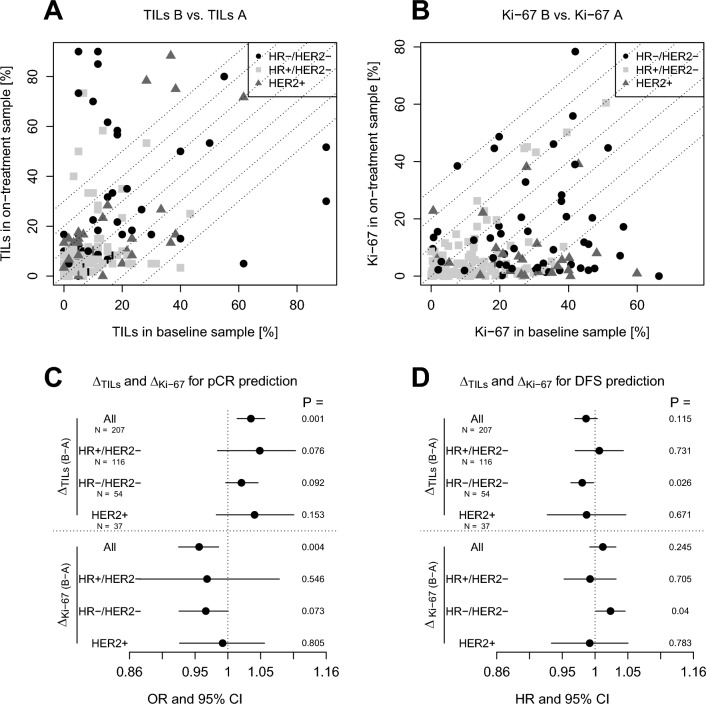
The number of tumor-infiltrating lymphocytes TILs (A) and Ki67 levels (B) in the on-treatment biopsies are plotted against the baseline sample. Only samples with residual cancer in the on-treatment biopsies are shown. The association of dynamic changes in TILs (ΔTILs) and Ki-67 (ΔKi-67) with pathological complete response (pCR) and disease-free survival (DFS) was evaluated using logistic regression and Cox regression analyses, respectively (C, D). An increase in TILs was associated with the likelihood of pCR across tumor subtypes (C) and with a lower risk of relapse in patients with triple-negative disease (D). No decrease in Ki-67 was associated with a lower likelihood of pCR across subtypes and a higher risk of relapse in patients with triple-negative disease. HR =
= hazard ratio, OR
hazard ratio, OR =
= odds ratio, CI
odds ratio, CI =
= confidence interval
confidence interval
Paired TIL data was available for 207 patients (all samples with residual cancer on-treatment), paired Ki-67 data was available for 196 patients. There was no association between the dynamic change in Ki-67 and dynamic change of TILs (Fig. S3).
In patients with residual invasive cancer cells in the on-treatment biopsy an increase of TILs in a subset of patients was observed, while only a few patients showed a decrease (Fig. 4A). An increase of TILs was associated with a higher probability of pCR in the overall study cohort, but not within the BC subtypes (Fig. 4C). It was associated with a lower risk of relapse in patients with triple negative disease.
The proliferation index as measured by Ki-67 immunohistochemistry decreased in most patients during chemotherapy (Fig. 4B). The lack of decrease in Ki-67 was associated with a low probability of pCR in all patients and was associated with a higher risk of relapse in patients with triple negative disease (Fig. 4C).
In bivariate Cox regression analyses in patients with triple-negative disease adjusted for pCR, the dynamic change of TILs was statistically significantly associated with DFS. The change of Ki-67 was not significantly associated with relapse-free survival in a bivariate model adjusted for pCR (Table 4).
Table 4
Bivariate Cox regression models to predict disease-free survival according to pCR and dynamic change in TILs or Ki-67, respectively
| Covariate | Hazard ratio (95% CI) | P | |
|---|---|---|---|
| HR−/HER2− | Delta TILs | 0.979 (0.959–1.000) | 0.048 |
| pCR vs. no pCR | 0.190 (0.025–1.418) | 0.105 | |
| HR−/HER2− | Delta Ki-67 | 1.019 (0.996–1.043) | 0.098 |
| pCR vs. no pCR | 0.211 (0.028–1.600) | 0.132 |
Discussion
In this retrospective research study, we analyzed on-treatment biopsies obtained during neoadjuvant chemotherapy (NACT) for breast cancer (BC).
30% of on-treatment biopsies showed no cancer cells. However, if residual cancer cells were detected, achieving a pathologic complete response (pCR) post-treatment was less likely. This suggests the potential for early treatment adjustment, including alternative chemotherapy agents or targeted therapies, to enhance response rates, particularly within the framework of clinical trials. In the GeparTrio and GeparQuinto neoadjuvant trials [13, 14], treatment was adjusted according to evaluation of on-treatment response using ultrasound and led to improved patient’s survival in GeparTrio. Biopsy procedures might be an additional tool to identify tumors prone to treatment failure early on treatment in future trials.
If no cancer cells were detected during treatment, this information could not reliably predict chemotherapy outcome, as the rate of pCR was 50% in this group in the current study. This suggests that sampling error of the residual disease may have led to a false negative on-treatment sample. False negative prediction was more frequent in HR+/HER2− disease, reflecting a tumor biology with a lower a priori probability for pCR. False positive prediction (cancer cells on-treatment but pCR after therapy) was more frequent in HR−/HER2− tumors, in the GeparSixto trial and in smaller tumors, reflecting patients with a higher a priori probability of pCR.
As our study collected on-treatment samples for translational research rather than for predicting pCR or guiding treatment decisions, its comparability to studies focused on on-treatment pCR prediction may be limited (reviewed in [6]). The reported negative predictive values of these studies vary, ranging from 71% for core needle biopsy (CNB) to as high as 95% for vacuum-assisted biopsy, which offers a larger specimen for analysis. However, with its false negative rates reaching 49.3%, presents a considerable margin for error [28, 29]. However, these investigations aimed to evaluate pathologic complete response (pCR) after completion of neoadjuvant chemotherapy (NACT) before surgery, utilizing minimally invasive methods to identify patients potentially eligible for surgery omission. These trials did not assess the capability to predict pCR early during treatment.
In a report of 40 patients from the ISPY2 trial, the presence of cancer cells in mid-treatment biopsies was associated with a 20% pCR rate, while the pCR rate in patients without invasive cancer cells was 90% [30].
Regarding survival, patients with triple-negative tumors and residual disease both on- and post-treatment had the highest risk of relapse. Patients without the evidence of cancer cells on-treatment but residual disease after the completion of the full course of treatment had a lower risk. This observation could be explained by the fact that the biopsy procedure is more likely to miss smaller tumors or tumors with only a minimal amount of residual disease.
Pre-treatment levels of TILs can be used to predict response to chemotherapy and patient’s survival in BC [17]. Chemotherapy might induce or augment a cytotoxic immune response [19] and an influx of TILs during treatment is associated with better response [31]. Moreover, their presence in surgical specimens is associated with better outcome [18] and a gene signature for prediction of TILs in post-treatment samples is predictive of survival [32].
In this study, we observed an increase of TILs in a subset of patients and only a few cases showed a decrease. An increase of TILs was associated with a higher probability of pCR in all patients and with a lower risk of relapse in patients with TNBC reflecting their known predictive value in triple-negative disease. This observation is particularly interesting, as it can identify patients early on treatment that still harbor invasive tumor residuals, where continuing standard therapy might be the option that is superior to a change in treatment plan. Further investigation in this group of patients could help to refine adapting tumor response into treatment plans and could ultimately allow to move the risk assessment after NACT to an earlier timepoint.
The marker of tumor cell proliferation Ki-67 can be used to predict response to NACT and patient’s survival [20, 33]. High levels are typically associated with better response to cytotoxic treatment but shorter long-term outcome due to more aggressive tumor biology. In the context of neoadjuvant aromatase inhibition, on-treatment evaluation of Ki-67 can predict patient outcome [11, 12].
In this study, most patients showed a decrease in Ki-67 index and this was associated with a higher probability of response in triple-negative disease. It was also associated with a lower risk of relapse in triple negative disease, but not in other subtypes. Bivariate survival analyses demonstrated that these effects were probably due to the association with pCR and its strong association with survival in this subtype.
From a translational research perspective, these observations suggest that on-treatment biopsies could be valuable for studying mechanisms of therapy resistance and predicting failure to achieve pathologic complete response (pCR) after neoadjuvant chemotherapy (NACT). However, they are not suitable for identifying markers of chemotherapy sensitivity, as highly sensitive tumors would not contain residual cancer cells in on-treatment biopsies. To address this, further analyses should focus on revisiting naive biopsies from patients who achieved early pCR. On a molecular level, NACT induces global changes in gene expression [34]. Examples of alterations are genes involved in proliferation, epithelial-mesenchymal transition and metabolic processes [35]. Analysis of serial biopsy samples during chemotherapy allows the characterization of mechanisms of early response and adaption to therapy. In such a study, a decreased expression of genes related to immune response and proliferation could be observed and the downregulation of cell-cycle inhibitors was associated with worse response [15]. The use of on-treatment biopsies for patient stratification should be further explored in the context to clinical trials and should be a part of the study protocol.
Several limitations of the study warrant consideration. Firstly, it's important to note that the biopsy procedure was not strictly standardized according to the study protocol. Detailed records of the sampling procedure were not available in the database of these older clinical trials. This limits the interpretation of the results, as bias due to patient selection (e.g. due to different or absent tumor visibility on ultrasound) or different sampling procedures (e.g. different number and/or diameter of biopsies) cannot be ruled out. At the time of the clinical trials used in this study, no clips were placed in the primary tumor area prior to treatment. The accuracy of the biopsy procedure may be limited. Additionally, variations in the timing of the biopsy procedure across the three trials were inevitable due to differences in study design. In GeparQuattro and GeparQuinto, on-treatment samples were obtained after 4 of 8 cycles, in GeparSixto after 2 of 6 cycles. Moreover, GeparQuattro and GeparQuinto switched from anthracycline to taxane therapy following the biopsy, whereas GeparSixto continued with the same regimen (concurrent taxane/anthracycline). The collection of on-treatment biopsies was not a mandatory part of the study protocol with a potential selection bias and comparably small samples sizes in subgroup analyses. The study was primarily designed to collect material for translational research purposes. It must be considered that patients within this trial had been treated before 2012 which possibly limits accuracy of on-treatment biopsy due to less experienced examiners and less refined examination instruments. Regimen not matching current standards thereby limiting the chances of achieving a pCR, are also able to influence false negative rates and predictive values of on treatment biopsies. Immune checkpoint inhibitors were not part of neoadjuvant chemotherapy in patients with triple-negative breast cancer, and patients with HER2-positive disease did not receive dual blockade.
In summary, our findings show that on-treatment biopsies can effectively predict non-pCR across breast cancer (BC) subtypes when residual cancer is present. This discovery presents potential avenues for tailoring therapy concepts in future clinical trials, such as implementing de-escalation strategies for responders and exploring experimental treatments for non-responders.
A reliable method for predicting chemotherapy response during treatment could greatly impact patient management and improve treatment strategies. By monitoring response, treatment plans can be tailored to individual patients, and if a patient is unlikely to respond well to the initial regimen, adjustments can be made to optimize therapeutic outcomes by switching to more effective drugs or modifying the dose. Early prediction of non-response allows for discontinuing ineffective treatments, avoiding unnecessary toxicity and complications, and better allocation of healthcare resources.
For example, ISPY2 trial is evaluating a combination of mid-treatment MRI and core biopsies to predict response and guide de-escalation of neoadjuvant treatment [36].
Predicting response helps plan the extent of surgery more accurately, potentially reducing the need for extensive procedures like mastectomy. Early prediction of treatment success can improve overall prognostication and support informed decision-making for post-neoadjuvant treatment and observation strategies.
Moreover, analyzing sequential biopsies could be instrumental in identifying molecular markers of therapy resistance to enhance our understanding of tumor biology and its adaptation to therapy. These advancements can ultimately improve overall treatment paradigms, improving the precision and effectiveness, leading to better patient outcomes. Further research is warranted to determine whether more standardized or extensive sampling procedures, or their combination with additional clinicopathological features, could enhance the sensitivity of on-treatment biopsy procedures.
Acknowledgements
We would like to thank all patients, investigators and study personnel who supported the trials.
Abbreviations
| B | Bevacizumab |
| CI | Confidence interval |
| DFS | Disease-free survival |
| EC-T | Epirubicin, cyclophosphamide and docetaxel |
| G4 | GeparQuattro |
| G5 | GeparQuinto |
| G6 | GeparSixto |
| HER2 | Human epidermal growth factor receptor |
| HR | Hazard ratio |
| NACT | Neoadjuvant chemotherapy |
| pCR | Pathological complete response |
| RD | Residual disease |
| TILs | Tumor-infiltrating lymphocytes |
| TNBC | Triple negative breast cancer |
| tu+ | Residual tumor |
| tu− | No residual tumor |
Author contributions
The analysis was designed by the members of the translational research subcommittee of the German Breast Group (BS, TK, MvM, FM, CS, ES, PAF, VM, SL and CD). BS and VN has analysed the data. BS and VN had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors interpreted the data. The first draft of the manuscript was written by BS. The decision to submit the manuscript for publication was made by all authors. All authors contributed to the review of the manuscript. No persons other than the listed authors contributed to the writing of the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. This analysis was funded by GBG. The GeparQuattro trial received funding support from Roche and Sanofi-Aventis. The GeparQuinto trial received funding support from GlaxoSmithKline, Novartis, Roche and Sanofi Aventis. The GeparSixto trial received funding support from Cephalon, GlaxoSmithKline and Roche. The funders had no access to the study database and were not involved in the analysis and interpretation of the results. No grant number applicable.
Availability of data and materials
All data needed for this analysis and results generation is included in this published article.
Declarations
The study was performed in accordance with good clinical practice guidelines, national laws and the Declaration of Helsinki. Informed written consent for analysis was ontained from all patients. The GeparQuattro (EudraCT 2005-001546-17) and GeparQuinto (EudraCT 2006-005834-19) studies were approved by the Ethics Committee of the special field ‘Medicine’ at the Johann Wolfgang Goethe University Frankfurt, Theodor-Stern-Kai 7, 60590 Frankfurt (ethical vote number 110/05 for GeparQuattro and 44/07 for Gepar Quinto). The GeparSixto study (EudraCT 2011-000553-23) was approved by the Ethics Committee of the Medical Association (Aerztekammer) Nordrhein, Tersteegenstr.9, 40474 Düsseldorf (ethical vote number 2011154.
Not applicable.
B. Sinn is an employee of BioNTech SE, has received consultancy honoraria from Sanofi and holds a pending patent WO2020109570A1. V. Nekljudova declares to be GBG Forschungs GmbH employee. GBG Forschungs GmbH received funding for research grants from Abbvie, Amgen, AstraZeneca, BMS, Daiichi-Sankyo, Gilead, Molecular Health, Novartis, Pfizer and Roche (paid to the institution); other (non-financial/medical writing) from Daiichi-Sankyo, Gilead, Novartis, Pfizer, Roche and Seagen (paid to the institution). GBG Forschungs GmbH has licensing fees from VMscope GmbH. In addition, GBG Forschungs GmbH has a patent EP21152186.9 pending, a patent EP19808852.8 pending, and a patent EP14153692.0 pending. T. Karn reports a patent for EP18209672 pending. V. Mueller reports speaker honoraria from Astra Zeneca, Daiichi-Sankyo, Eisai, Pfizer, MSD, Medac, Novartis, Roche, Seagen, Onkowissen, high5 Oncology, Medscape, Gilead, Pierre Fabre and iMED Institut; receives consultancy honoraria from Roche, Pierre Fabre, PINK, ClinSol, Novartis, MSD, Daiichi-Sankyo, Eisai, Lilly, Seagen, Gilead and Stemline; he also declares to receive institutional research support from Novartis, Roche, Seagen, Genentech and Astra Zeneca; and reports travel grants from Astra Zeneca, Roche, Pfizer, Daiichi Sankyo and Gilead. C. Schem declares honoraria from Roche, Lilly, AstraZeneca, MSD Oncology, Exact Sciences and Novartis; operate in a consulting or advisory role for Novartis, AstraZeneca and Roche; reports honoraria for speakers’ bureau from Roche, AstraZeneca and Novartis; CS also reports to receive research funding from Roche (Inst.), Daiichi Sankyo Europe GmbH (Inst.), AstraZeneca (Inst.), GlaxoSmithKline (Inst.), Novartis (Inst.) and Lilly (Inst.); and to receive travel accommodations and expenses from Pfizer, Roche, AstraZeneca, Novartis and Gilead Sciences. M. Untch reports honoraria from AstraZeneca, Art tempi, Amgen, Daiji Sankyo, Lilly, Roche, Pfizer, MSD Oncology, Pierre Fabre, Sanofi-Aventis, Myriad, Seagen, Gilead, Novartis and Stemline; to act in a consulting or advisory Role for Amgen, Lilly, Roche, Pfizer, Lilly, Pierre Fabre, Novartis, MSD Oncology, Roche, Agendia, Seagen, Gilead, Lily, Stemline, Genzyme and Onkowissen.de. All honoraria and fees to the employer/institution. J. Huober declares to receive honoraria from Lilly, Novartis, Roche, Pfizer, AstraZeneca, Seagen, Gilead and Daiichi; to have a consulting or advisory relationship with Lilly, Novartis, Roche, Pfizer, AstraZeneca, Gilead and Daiichi; and to receive honoraria for travel expenses from Roche, Novartis, Daiichi and Gilead. J. Holtschmidt reports personal fees and non-financial support from Daiichi Sankyo, non-financial support from Hologic, personal fees from MSD Oncology, Novartis, Palleos Health Care, Pfizer, Roche Pharma and Seagen, outside the submitted work; he also declares to be GBG Forschungs GmbH employee. GBG Forschungs GmbH received funding for research grants from Abbvie, Amgen, AstraZeneca, BMS, Daiichi-Sankyo, Gilead, Molecular Health, Novartis, Pfizer and Roche (paid to the institution); other (non-financial/medical writing) from Daiichi-Sankyo, Gilead, Novartis, Pfizer, Roche and Seagen (paid to the institution). GBG Forschungs GmbH has licensing fees from VMscope GmbH. In addition, GBG Forschungs GmbH has a patent EP21152186.9 pending, a patent EP19808852.8 pending, and a patent EP14153692.0 pending. M. van Mackelenbergh reiceived personal fees, honoraria or travel grants from Amgen, AstraZeneca, Daiichi Sankyo, Gilead, GSK, Lilly, Molecular Health, Mylan, MSD, Novartis, Pfizer, PierreFabre, Roche and Seagen. S. Loibl declares to be GBG Forschungs GmbH employee (CEO);The company receives grants from AbbVie, AstraZeneca, Celgene, Daiichi-Sankyo, Immunomedics/Gilead, Molecular Health, Novartis, Pfizer and Roche; honoraria for Advisory board from Abbvie, Amgen, AstraZeneca, BMS, Celgene, DSI, EirGenix, Gilead, GSK, Lilly, Merck, Novartis, Olema, Pfizer, Pierre Fabre, Relay Therapeutics, Roche, Sanofi and Seagen; honoraria as invented speaker from AstraZeneca, DSI, Gilead, Novartis, Pfizer, Roche, Seage and Medscape. S. Loibl reports non-financial interest as advisory role in AGO Kommission Mamma, PI Aphinity (principal investigator) as member in AGO, ASCO, DKG, ESMO and other non-financial interest from AstraZeneca, Daiichi-Sankyo, Gilead, Novartis, Pfizer, Roche and Seagen. GBG Forschungs GmbH has following /patents pending: EP14153692.0, EP21152186.9, EP19808852.8 and receives licensing fees from VM Scope GmbH. C.D. reports grants from European Commission H2020, grants from German Cancer Aid Translational Oncology, grants from German Breast Group, grants from BMBF, to the institution during the conduct of the study; personal fees from Novartis, personal fees from Roche, personal fees from MSD Oncology, personal fees from Daiichi Sankyo, personal fees from AstraZeneca, from Molecular Health, grants from Myriad, personal fees from Merck, other from Sividon diagnostics, outside the submitted work; In addition, Dr. Denkert has a patent VMScope digital pathology software with royalties paid, a patent WO2020109570A1—cancer immunotherapy pending, and a patent WO2015114146A1 and WO2010076322A1- therapy response issued. No other potential conflict of interest relevant to this article was reported.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Articles from Breast Cancer Research : BCR are provided here courtesy of BMC
Citations & impact
This article has not been cited yet.
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/168772515
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
European Clinical Trials (3)
- (1 citation) EU Clinical Trials Register - 2011-000553-23
- (1 citation) EU Clinical Trials Register - 2005-001546-17
- (1 citation) EU Clinical Trials Register - 2006-005834-19
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy.
Lancet Oncol, 19(1):40-50, 07 Dec 2017
Cited by: 1017 articles | PMID: 29233559
Predictive and prognostic value of stromal tumour-infiltrating lymphocytes before and after neoadjuvant therapy in triple negative and HER2-positive breast cancer.
Eur J Cancer, 118:41-48, 11 Jul 2019
Cited by: 47 articles | PMID: 31302586
Predictive and prognostic role of tumour-infiltrating lymphocytes in breast cancer patients with different molecular subtypes: a meta-analysis.
BMC Cancer, 20(1):1150, 25 Nov 2020
Cited by: 57 articles | PMID: 33238978 | PMCID: PMC7690150
Funding
Funders who supported this work.

 1
1 

