Abstract
Free full text

RNA m6A modification in ferroptosis: implications for advancing tumor immunotherapy
Abstract
The pursuit of innovative therapeutic strategies in oncology remains imperative, given the persistent global impact of cancer as a leading cause of mortality. Immunotherapy is regarded as one of the most promising techniques for systemic cancer therapies among the several therapeutic options available. Nevertheless, limited immune response rates and immune resistance urge us on an augmentation for therapeutic efficacy rather than sticking to conventional approaches. Ferroptosis, a novel reprogrammed cell death, is tightly correlated with the tumor immune environment and interferes with cancer progression. Highly mutant or metastasis-prone tumor cells are more susceptible to iron-dependent nonapoptotic cell death. Consequently, ferroptosis-induction therapies hold the promise of overcoming resistance to conventional treatments. The most prevalent post-transcriptional modification, RNA m6A modification, regulates the metabolic processes of targeted RNAs and is involved in numerous physiological and pathological processes. Aberrant m6A modification influences cell susceptibility to ferroptosis, as well as the expression of immune checkpoints. Clarifying the regulation of m6A modification on ferroptosis and its significance in tumor cell response will provide a distinct method for finding potential targets to enhance the effectiveness of immunotherapy. In this review, we comprehensively summarized regulatory characteristics of RNA m6A modification on ferroptosis and discussed the role of RNA m6A-mediated ferroptosis on immunotherapy, aiming to enhance the effectiveness of ferroptosis-sensitive immunotherapy as a treatment for immune-resistant malignancies.
Introduction
The occurrence of various genetic mutations and structural abnormalities during cancer growth is attributed to the process of malignant transformation and metastasis [1, 2]. Consequently, the mutated genes contribute to the emergent tumor antigen, which can be recognized as foreign substances for immune elimination [3]. Upon capturing and identifying the tumor antigen, the innate and adaptive immune systems are triggered, resulting in the suppression of tumor growth [4]. Innate immune cells, including natural killer (NK) cells, eosinophils, neutrophils, and macrophages, contribute to tumor suppression either by directly eliminating tumor cells or by activating adaptive immunological responses [5, 6]. Effective immune responses have the potential to either eliminate cancerous cells or hinder their metastatic ability [7]. Nevertheless, reducing immunogenicity and producing an immune-suppressive tumor microenvironment (TME) pose significant obstacles to immune surveillance and ultimately lead to immune evasion [8]. In this case, immunotherapy is utilized for overcoming the two major barriers described above by enhancing immune defense and effectively removing malignant cells.
The anti-tumor effectiveness of immune therapy is achieved via immune checkpoint inhibitors (ICIs) [9], dendric cell vaccination [10], chimeric antigen receptor T cells (CAR-T cells), and cytokine therapies [11, 12]. Although immunotherapy has made breakthroughs over the past few years, limited response rate and confinement of particular tumor types are the difficulties that cannot be ignored, and further investigations are required to explore the underlying mechanism of immunotherapy [9, 13]. It was believed that immunotherapy-activated CD8+ T cells induce tumor cell death mainly through the perforin-granzyme pathway and the Fas–Fas ligand (FASL) pathway [14]. However, multiple studies have proved that CD8+ T cells can trigger ferroptosis in tumor cells via inhibiting SLC7A11 expression and cystine uptake through the JAK-STAT1 pathway by releasing interferon gamma (IFNγ), indicating the participation of this novel non-apoptotic type of regulated cell death (RCD) in immunotherapy [15, 16]. Another study has documented that in clear cell renal cell carcinoma (CRCC), there is an increase in the expression of PD-L1, as well as an upregulation of ferroptosis regulators such as ACSL4, CARS, NCOA4, and other targets. This finding also suggests an underlying correlation between ferroptosis and immune checkpoints [17]. Ferroptosis, a distinctive iron-dependent form of regulated cell death induced by the excessive accumulation of lipid peroxides on cellular membranes, is proved to have a dual role in cancer, leading to tumor cell proliferation or elimination [18]. Understanding the regulation of this iron-dependent RCD, as well as the mechanisms by which cancer cells evolve to avoid ferroptosis, can provide us with potential targets for ferroptosis-based therapy.
N6-methyladenine (m6A) modification is regarded as the most prevalent post-transcriptional modification in mammalian mRNA, which plays critical functions in regulating mRNA stability, splicing, and translation via controlling the activity of m6A writers, erasers, and readers [19–21]. Numerous studies focusing on RNA m6A modification have demonstrated its involvement in several significant physiological processes, including adipogenesis [22], bone marrow development [23], as well as the regulation of the central nervous system [24], reproductive system [25], and hematopoietic system [26]. Recent advances respectively underscore the pivotal role of N6-methyladenosine (m6A) epigenetic modification in tumorigenesis [27], ferroptosis, and immune checkpoints [28–31]. Nevertheless, there is limited discussion regarding the relationship between m6A-regulated ferroptosis, tumor immune response, and the efficacy of immunotherapy. Thus, we summarized the recent advances concerning m6A modification on ferroptosis and its potential significance in immunotherapy, aiming to provide more clues for clinical application.
Regulatory mechanism of ferroptosis
Unlike other programmed cell death pathways, which are activated by specific proteins and signaling cascades (such as caspases in apoptosis, MLKL in necroptosis, and gasdermin proteins in pyroptosis) [32, 33], ferroptosis is triggered by iron accumulation and upregulation of reactive oxidation species (ROS) and lipid peroxidation [34]. Cells experiencing ferroptosis will display mitochondrial shrinkage, elevated mitochondrial membrane density, and diminished mitochondrial cristae. Here, we mainly focus on the regulation of ferroptosis from three basic perspectives: iron accumulation, lipid peroxidation, and disruption of the antioxidant system, as shown in Fig. 1.
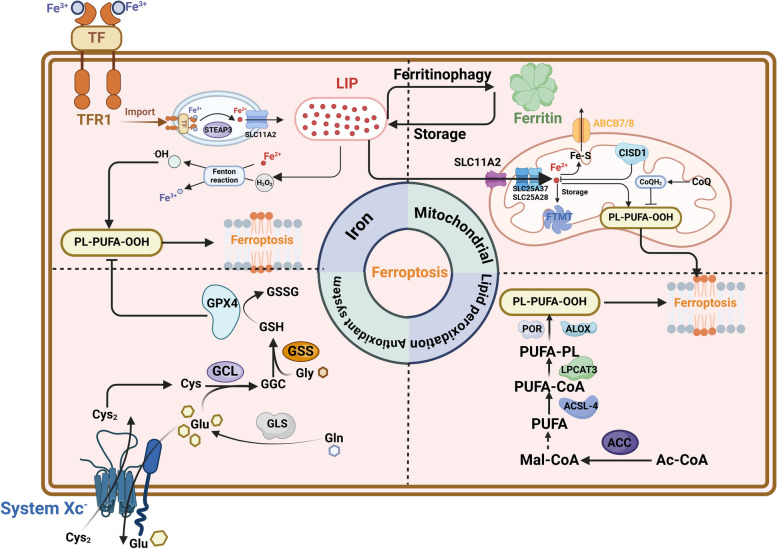
Molecular regulating mechanisms of ferroptosis. Ferroptosis is mainly driven by PUFL-PLs synthesis and features abnormal iron accumulation, diminished mitochondrial cristae, and rupture of the cell membrane. The synthesis of PUFL-PLs attributes to three perspectives: iron toxicity through the Fenton reaction; transduction of polyunsaturated fatty acids (PUFAs); iron metabolism and ROS metabolism in the mitochondrion. Meanwhile, ferroptosis occurs when oxidation-promoting activities surpass the detoxification capabilities or the antioxidant system is impaired. TF transferrin, TFR1 transferrin receptor, ABCB7 ATP binding cassette subfamily B member 7, LPCAT3 lysophosphatidylcholine acyltransferase 3, ACC acetyl-CoA carboxylase, ALOX lipoxygenase, CISD1 CDGSH iron sulfur domain 1, CoQ coenzyme Q, Cys cysteine, Cys2 cystine, FTMT ferritin mitochondrial, GCL glutamate-cysteine ligase, GSH glutathione, GSSG oxidized glutathione, LIP labile iron pool, POR cytochrome P450 oxidoreductase, SLC25A37, solute carrier family 25 member37, SLC25A28 solute carrier family 25 member 28, ACSL-4, Acyl-CoA synthetase long-chain family member 4. This figure was created with BioRender.com
Iron accumulation
Iron is an essential mineral that is maintained by orchestrated regulation and subsequently impacts the sensitivity of ferroptosis. The ferric ions (Fe3+) in the bloodstream are transported to cells by binding to transferrin (TF) and then undergoing endocytosis assisted by the transferrin receptor 1 (TFR1), and finally they are localized in the endosomes [35, 36]. Then the six-transmembrane epithelial antigen of the prostate 3 (STEAP3) converts ferric iron in endosomes to ferrous ion (Fe2+) [37]. Endocytosis takes up ferrous iron, which is then released into the cytoplasm by solute carrier family 11 member 2 (SLC11A2), leading to the creation of the labile iron pool (LIP). The LIP could catalyze the synthesis of hydroxyl radicals that are highly reactive forms of activated oxygen and induce the peroxidation of unsaturated or saturated fatty acids [38]. Studies have proven a sequential regulating pathway resulting in ferroptosis through the production of reactive oxygen species (ROS) via the Fenton reaction [39, 40]. Notably, non-enzymatic lipid peroxidation of PUFA-PLs is driven by the Fenton reaction, with iron serving as a catalyst [41, 42]. In addition, several enzymes that are crucial for the process of lipid peroxidation, such as ALOXs and POR, are iron-dependent. Furthermore, unbound Fe (II) not only enhances the spread of peroxides during lipid peroxidation, but also contributes to the development of extensive ferroptosis [41, 43, 44]. Hence, interventions that regulate the transportation, retention, and release of iron within the cytoplasm play a role in enhancing vulnerability to ferroptosis.
Lipid peroxidation
Previous studies suggest that polyunsaturated fatty acids (PUFAs) are substrates for lipid peroxidation, which is oxidized by the ACSL4-LPCAT3-arachidonic acid lipoxygenase (ALOX) axis [45]. Among all the PUFAs, AA (20:4) and AdA (22:4) are the main PUFAs that undergo lipid peroxidation in the process of ferroptosis [46]. For instance, ACSL4 facilitates the union of unbound AA (20:4) and CoA to produce a CoA-AA (20:4) intermediate. This intermediate is further esterified by LPCAT3 to make PEs, resulting in the formation of AA (20:4)-PE (PE-AA), which are essential for the occurrence of ferroptosis [47, 48]. Regularly, the production of malonyl-CoA through the carboxylation of acetyl-CoA by acetyl-CoA carboxylase (ACC) is essential for the creation of certain PUFAs and is hence required for ferroptosis [49]. It has been proposed that cytochrome P450 oxidoreductase (POR) accelerates the circulation of Fe (II) and Fe (III) in the heme fractions of cytochrome P450 enzymes (CYPs), thereby promoting lipid peroxidation [50, 51]. In addition, NADPH oxidase (NOX) utilizes NADPH as a substrate to transfer electrons to oxygen, resulting in the production of superoxide radicals, which can promote lipid peroxidation and subsequently induce ferroptosis [52]. The downregulation of peroxidized PUFA-PLs within cancer cells has been associated with the evasion of ferroptosis and the progression of the tumor. For example, iPLA2β, an enzyme that degrades and neutralizes peroxidized lipids to prevent ferroptosis, is highly expressed in certain types of human malignancies and is involved in the inhibition of p53-mediated ferroptosis and tumor suppression [53]. Therefore, ferroptosis is induced when the peroxidation of phospholipid-PUFAs exceeds the scavenging capacity of the cell antioxidant system, and malignant cancer cells obtain the capability of ferroptotic evasion when production of PUFAs is inhibited [54].
Disruption of the antioxidant system
Tumor cells demonstrate an increased antioxidant capacity through the stabilization and overexpression of anti-ferroptotic systems, which are essential mechanisms that tumor cells have developed to prevent ferroptosis and promote tumor progression [55]. The Xc− system consists of a heavy chain (SLC3A2) and a light chain (SLC7A11), which is responsible for cysteine transportation and facilitates the use of glutathione [56]. Previous studies have already indicated that system Xc– is the determinant element towards ferroptosis resistance [57–59]. Studies have proved that inhibition of GPX4 and SLC7A11 by corresponding inhibitors can trigger ferroptosis, while the interaction between p53 and SLC7A11 is weakened with a p53 deficiency or mutation [60]. Nuclear factor erythroid factor 2-related factor 2 (NRF2) is a transcription factor that promotes the transcription of SLC7A11 under oxidative stress by binding to the promotor region of antioxidant response elements [61, 62]. Furthermore, p53 directly targets and promotes the expression of SAT1, the rate-limiting enzyme directing polyamine catabolism, which induces lipid peroxidation and ferroptosis by boosting ALOX15 levels during ROS stress [63]. SLC7A11, in particular, is considerably increased in a variety of cancers and is one of the most frequently investigated strategies for evading ferroptosis. In addition, oncogenic KRAS activation has been demonstrated to upregulate SLC7A11 expression, leading to ferroptosis resistance [64–66]. Glutamate is a crucial substrate necessary for the production of GHS, and its absorption mostly relies on SLC38A1 and SLC1A5 [67]. GCLC, also known as the glutamate-cysteine ligase catalytic subunit, facilitates the initial stage of glutathione production by connecting cysteine and glutamate. Nevertheless, in cases of cysteine deficiency, GCLC facilitates the production of γ-glutamyl peptides (γ-Glu-AAs) [68], which leads to the removal of glutamate and serves as a preventive measure against ferroptosis. GPX4 is a selenoprotein that functions as an essential cofactor by utilizing GSH to reduce hydroperoxide in the cell membrane [69]. The enzymatic activity of GPX4 is directly influenced by cysteine concentration, glutamine-cysteine synthase activity, and GSH feedback inhibition, which in turn regulate GSH synthesis [70]. Ferroptosis inhibitor protein 1 (FSP1), previously referred to as apoptosis-inducing factor mitochondrial-related 2 (AIFM2), has been found as a substance that inhibits ferroptosis. Recent research has also discovered that FSP1-CoQ functions as an antioxidant system that operates alongside the GPX4 pathway and specifically targets cells that are lacking GPX4. FSP1 is brought to the plasma membrane by N-terminal myristoylation as an oxidoreductase. It decreases the amount of ubiquinone (CoQ10), which is a byproduct of mevalonate metabolism, and converts it into the lipophilic free radical scavenger panthenol (CoQ10H2), which helps prevent the buildup of lipid ROS [69, 71, 72].
system consists of a heavy chain (SLC3A2) and a light chain (SLC7A11), which is responsible for cysteine transportation and facilitates the use of glutathione [56]. Previous studies have already indicated that system Xc– is the determinant element towards ferroptosis resistance [57–59]. Studies have proved that inhibition of GPX4 and SLC7A11 by corresponding inhibitors can trigger ferroptosis, while the interaction between p53 and SLC7A11 is weakened with a p53 deficiency or mutation [60]. Nuclear factor erythroid factor 2-related factor 2 (NRF2) is a transcription factor that promotes the transcription of SLC7A11 under oxidative stress by binding to the promotor region of antioxidant response elements [61, 62]. Furthermore, p53 directly targets and promotes the expression of SAT1, the rate-limiting enzyme directing polyamine catabolism, which induces lipid peroxidation and ferroptosis by boosting ALOX15 levels during ROS stress [63]. SLC7A11, in particular, is considerably increased in a variety of cancers and is one of the most frequently investigated strategies for evading ferroptosis. In addition, oncogenic KRAS activation has been demonstrated to upregulate SLC7A11 expression, leading to ferroptosis resistance [64–66]. Glutamate is a crucial substrate necessary for the production of GHS, and its absorption mostly relies on SLC38A1 and SLC1A5 [67]. GCLC, also known as the glutamate-cysteine ligase catalytic subunit, facilitates the initial stage of glutathione production by connecting cysteine and glutamate. Nevertheless, in cases of cysteine deficiency, GCLC facilitates the production of γ-glutamyl peptides (γ-Glu-AAs) [68], which leads to the removal of glutamate and serves as a preventive measure against ferroptosis. GPX4 is a selenoprotein that functions as an essential cofactor by utilizing GSH to reduce hydroperoxide in the cell membrane [69]. The enzymatic activity of GPX4 is directly influenced by cysteine concentration, glutamine-cysteine synthase activity, and GSH feedback inhibition, which in turn regulate GSH synthesis [70]. Ferroptosis inhibitor protein 1 (FSP1), previously referred to as apoptosis-inducing factor mitochondrial-related 2 (AIFM2), has been found as a substance that inhibits ferroptosis. Recent research has also discovered that FSP1-CoQ functions as an antioxidant system that operates alongside the GPX4 pathway and specifically targets cells that are lacking GPX4. FSP1 is brought to the plasma membrane by N-terminal myristoylation as an oxidoreductase. It decreases the amount of ubiquinone (CoQ10), which is a byproduct of mevalonate metabolism, and converts it into the lipophilic free radical scavenger panthenol (CoQ10H2), which helps prevent the buildup of lipid ROS [69, 71, 72].
Mitochondrial metabolism is the primary origin of cellular reactive oxygen species (ROS), and the mitochondrial tricarboxylic acid (TCA) cycle can control ferroptosis by supplying α-ketoglutarate (α-KG) [73]. Research has shown that ferroptosis is characterized by abnormal synthesis of ROS by the mitochondria. In details, inhibition of the mitochondrial TCA cycle and electron transfer chain (ETC) reduces lipid peroxide accumulation and ferroptosis [74]. Specifically, when there is a deficiency of cysteine due to the inhibition of GPX4, the metabolism in the mitochondria leads to a quick decrease in glutathione levels and the subsequent formation of lipid ROS, resulting in ferroptosis [74].
Iron consumption is crucial for controlling redox-active processes and ferroptosis in addition to the TCA cycle in mitochondria [75, 76]. To reach the mitochondria, iron must traverse both the outer and inner mitochondrial membranes to enter the matrix through SLC11A2, and solute carrier family 25 member 37 (SLC25A37) or solute carrier family 25 member 28 (SLC25A28), respectively [77, 78]. Recent studies highlighted the key role of CDGSH iron sulfur domain 1 (CISD1) in regulating iron homeostasis in mitochondria [79]. CISD1 knockdown dramatically increases the content of erastin-induced mitochondrial ferrous irons, promoting mitochondrial lipid peroxidation and ferroptosis [80]. Ferritin mitochondrial (FTMT) is the iron-storage protein in mitochondria, which inhibits ferroptosis by lowering total and chelatable iron levels [81, 82]. ABCB8, a member of the ATP-binding cassette subfamily B, has a role in transporting iron from mitochondria to the cytosol and can aid in exporting iron from mitochondria. Increased expression of ABCB8 decreases the amount of iron in the mitochondria and provides protection against cardiomyopathy associated with ferroptosis [83].
Regulation of RNA m6A-modified ferroptosis and its roles in cancers
The double-edged role of ferroptosis in tumor development
The current array of cancer therapies is unable to effectively target tumor cells due to their therapeutic resistance and high mutation burden. Nevertheless, an increasing amount of evidence indicates that the occurrence of lymphatic metastasis in cancer and the advancement of tumors, which are driven by cancer stem cells (CSCs), are strongly associated with the suppression of ferroptosis [84]. In addition, ferroptosis has the capacity to limit the functionality of immunosuppressive cells, such as TAMs and Treg cells within tumors. This process can transform the immunosuppressive TME into an inflammatory TME, which is rich in antitumor immune cells [85, 86]. Consequently, selectively inducing ferroptosis could be a new approach to the efficient elimination of cancer cells. However, it should be noted that many immune cells are also sensitive to ferroptosis, including CD8+ T cells, NK cells, and DC cells. Furthermore, stimulation of ferroptosis can reduce the function of antigen processing and tumor elimination of immune cells [87, 88]. Collectively, ferroptosis induction can be regarded as a double-edged role, given that it occurs on different types of cells [89], as shown in Fig. 2.
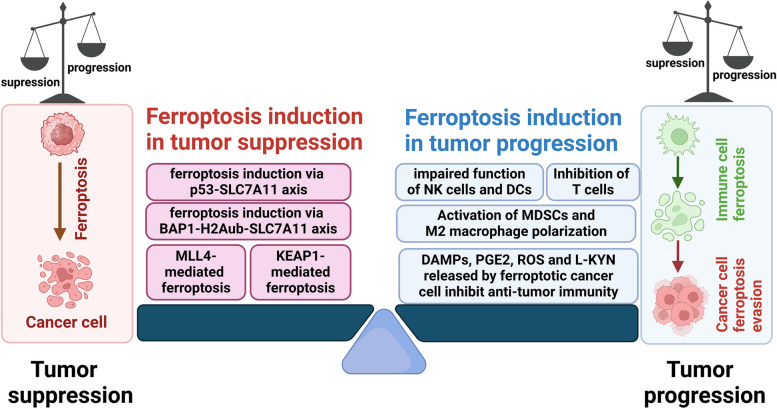
The roles of ferroptosis in the tumor environment. The tumor microenvironment (TME) is a dynamic and complex ecosystem comprising cancer cells, stromal cells, diverse subpopulations of immune cells, the blood and lymphatic vasculature, and various metabolic components. Ferroptosis in cancer cells can be triggered by not only the tumor suppressor gene p53, BRCA1-associated protein 1 (BAP1), and Kelch-like ECH-associated protein 1 (KEAP1), but also epigenetic regulator MLL4, which consequently suppress tumor progression. However, it has been demonstrated that some immune cells are also susceptible to ferroptosis. When ferroptosis occurs in immune cells, impaired function of NK cells and DCs, inhibition of T cells, activation of MDSCs, and M2 macrophage polarization are frequently observed, which eventually induce tumor progression in various manners. In conclusion, whether tumor progression is promoted or inhibited depends on the specific cell type in which ferroptosis occurs and its location within the tumor environment. This figure was created with BioRender.com
Ferroptosis induction in tumor suppression
Accumulating evidence indicates that ferroptosis acts as a tumor suppressor and mediates anti-cancer capability associated with tumor suppressor genes. Tumor suppressors such as p53, BRCA1-associated protein 1 (BAP1), and Kelch-like ECH-associated protein 1 (KEAP1) have been shown to exert their tumor-suppressive functions via inducing ferroptosis.
P53 is regarded as the most critical barrier for cancer development by its cell-cycle surveillance function. A mechanical study demonstrated that inhibiting p53 will result in insensitivity to ferroptosis by increasing the expression of the epigenetic marker H2Bub1, hence enhancing the capacity of SLC7A11 [90]. ALOX12 is a crucial lipoxygenase that has been demonstrated to play a pivotal role in the p53-mediated ferroptosis pathway. The deletion of ALOX12 significantly reduces the level of ferroptosis, and the inhibitory effect of ferroptosis in ALOX12-knockout cells is lost. Furthermore, ALOX12 interacts with SLC7A11, which specifically binds to and inhibits the enzymatic activity of ALOX12. It has been demonstrated that p53 can indirectly promote the function of ALOX12 by downregulating the expression of SLC7A11, thereby regulating the p53-mediated ferroptosis pathway [91].
BAP1 has been demonstrated to be accountable for the removal of ubiquitin from histone 2A and frequently displays inactivating mutations and deletions in various sporadic cancers [92]. Remarkably, BAP1 suppresses tumorigenesis partly through ferroptosis by repressing SLC7A11 via reducing histone 2A ubiquitination (H2Aub) occupancy on the SLC7A11 promoter. Deletions and mutations of BAP1 lead to the impairment of its capacity to suppress SLC7A11, allowing tumor cells to avoid ferroptosis and facilitating tumor development [93].
KEAP1, a ubiquitinated enzyme, is commonly mutated or inactivated in lung cancers [94]. Loss of KEAP1 function leads to increased tumor burden and accelerates tumor growth because its mutants or deficiency in lung cancers upregulate the expression of FSP1 by stabilizing NRF2 proteins, resulting in ferroptosis resistance [95, 96]. Moreover, KEAP1 knockdown protects glioma cells from ferroptosis and promotes their proliferation by upregulating NRF2-mediated expression of SLC7A11 [61]. These findings indicate that the ferroptosis-promoting role of KEAP1 potentially at least partly accounts for its tumor-suppressive function.
Recent investigations have unveiled the role of epigenetic regulator MLL4, which is frequently mutated in human cancers, in ferroptosis-mediated tumor suppression. MLL4 deficiency results in the development of precancerous neoplasms and resistance to ferroptosis, which is accompanied by downregulation of pro-ferroptosis genes ALOXs (ALOX12, ALOX12B, and ALOXE3) and the upregulation of anti-ferroptosis genes (GPX4 and SLC7A11) [97]. This molecular reprogramming demonstrates the enhancement of ferroptosis resistance, thereby enhancing neoplastic development.
Ferroptosis induction in tumor progression
The destruction of pancreatic cells through ferroptosis triggers the release of 8-OHG, a biomarker for oxidative DNA damage that also functions as a damage-associated molecular pattern (DAMP). 8-OHG, when produced, triggers the STING-dependent DNA sensor pathway, which promotes the infiltration and M2 polarization of macrophages and consequently facilitates pancreatic carcinogenesis [98, 99]. It has been demonstrated that ferroptosis in cancer cells is linked to increased expression of post-transcriptional gene silencing (PTGS2) and the release of PGE2 [100]. The released PGE2 promotes the recruitment and activation of MDSCs and M2 macrophages and inhibits the antitumor function of NK cells, DCs, and cytotoxic T cells. Mechanically, in myeloid cells, PGE2 can activate DNA methyltransferase 3A (DNMT3A), leading to DNA methylation and suppressing immunogenic gene expression [101]. Besides being considered a major immunosuppressive mediator, PGE2 directly suppressed cytotoxic T cell activity [102] and compromised DCs directly by reducing the amount of chemokine receptors that induce the recruitment into tumors [100]. In bladder cancer, during chemotherapy cycles, decreased GPX4 activity may result in the release of greater quantities of PGE2. It is therefore tempting to speculate that tumors that are intrinsically sensitive to ferroptosis will release PGE2 in order to achieve an immunosuppressive environment [103].
Ferroptosis will also trigger a high level of ROS, which will inhibit the activation and proliferation of T cells and suppress the formation of TCR and MHC antigen complexes in T cells, thus inhibiting immune responses [104]. ROS are also involved in the activation of macrophage signaling. Lin X et al. demonstrated that ROS may stimulate an invasive phenotype in TAMs derived from melanoma through the secretion of TNFα [105]. Studies indicate that ferroptosis obtains a double-sided effect on the regulation of tumor immune tolerance. Meanwhile, oxidative stress and peroxidation caused by ferroptosis will also lead to impaired function of NK cells and DCs. In a study by Poznanski and colleagues, it was demonstrated that oxidative stress, which is associated with lipid peroxidation, inhibits glucose metabolism in natural killer (NK) cells, leading to their dysfunction [88]. L-kynurenine (L-KYN), a tryptophan metabolite in gastric cancer TME, has been reported to induce lipid peroxidation and ferroptosis in NK cells, thereby promoting tumor growth in vivo [106]. Similarly, dendritic cells (DCs) that are associated with tumors usually exhibit reduced antigen-presentation capacity due to elevated lipid levels, which is associated with ferroptosis susceptibility [107]. Recent evidence suggests that pro-ferroptotic regulators can impair the anti-tumor function of tumor-infiltrating DCs. Noxious molecules, such as ROS and the lipid peroxidation byproduct 4-HNE, which is a marker of ferroptosis, are observed to accumulate in DCs associated with ovarian cancer [108]. Interestingly, GPX4 inhibitors could induce ferroptosis in DCs in a PPARG-dependent manner. In turn, PPARG downregulation significantly restored the impaired anti-tumor activities of ferroptotic DCs in vivo [109]. However, the direct correlation and underlying mechanism between ferroptosis and the dysfunction of NK cells and DCs still need further investigation.
Ferroptosis can also occur in T cells, specifically in cytotoxic T lymphocyte subset 9 (Tc9) cells through the IL-9/STAT3/fatty acid oxidation (FAO) pathway, leading to impairment of anti-tumor immunity [110, 111]. The inhibition of GPX4 activity has been demonstrated to stimulate ferroptosis, which in turn reduces the specific killing function of these immune cells. CD36 expression on the cell surface has been reported to be crucial for fatty acid or oxidized lipid-induced ferroptosis. Significant lipid peroxidation activity has been observed in CD36-positive CD8+ T cells, which results in ferroptosis induced by GPX4 inhibitors, leading to reduced release of IFNγ and thus inducing immunosuppression [87, 110]. Furthermore, TFH cells, a subpopulation of CD4+ T cells that support antitumor immunity, are also susceptible to ferroptosis, along with Treg cells. Therefore, GPX4 expression has been shown to be essential for their survival and functionality [112]. Moreover, in a hepatocellular tumorigenic model, GPX4 deletion induces ferroptosis, resulting in the release of large amounts of HMGB1, boosting the recruitment of immunosuppressive MDSCs and HCC growth [113]. As for the immunosuppressive cell, Gpx4-deficient activated Tregs are prone to ferroptosis and exhibit increased production of the proinflammatory cytokine IL-1β, leading to the promotion of T helper cell 17 (Th17) function and enhancement of immune responses [86]. Therefore, targeting ferroptosis by inhibiting GPX4 in intratumoral Tregs appears to be a promising strategy for reprogramming the TME and cancer treatment.
Meanwhile, via pro-ferroptotic stimuli, TAMs can be reprogrammed to an anti-tumorigenic M1 phenotype through multiple pathways during ferroptosis, thereby inhibiting tumor progression. For example, inhibition of apolipoprotein C1 (APOC1) or SLC7A11 promotes ferroptosis in TAMs. These pro-ferroptosis modifications by APOC1 and SLC7A11 further increase the expression of CD86 of the M1 phenotype and decrease the expression of CD206, CD163, and ARG1 of the M2 phenotype in TAMs, thus inhibiting the pro-tumoral M2 polarization and the development of HCC [114, 115].
Collectively, these advances suggest the elements in TME are complex and heterogeneous, and ferroptosis will impact tumor progression via multiple cells and downstream targets. Consequently, whether tumor progression is promoted or inhibited is dependent on the cell type in which ferroptosis occurs. However, further investigations are still needed to decipher the underlying mechanism of ferroptosis occurrence and dominant factors on tumor progression brought by ferroptosis in TME.
The double-edged role of RNA m6A modification in tumor progression
The RNA m6A modification process is dynamically and reversibly regulated by three types of enzymes or proteins: m6A methyltransferases (writers), m6A demethylases (erasers), and m6A binding proteins (readers) [116]. RNA m6A methyltransferases, commonly referred to as m6A writers, function by transferring a methyl group to the nitrogen atom at the 6th position of adenine through complex formation. Prominent m6A writers include METTL3, METTL14, METTL16, Wilms tumor 1-associated protein (WTAP), RNA binding motif proteins RBM15 and RBM15B, vir-like m6A methyltransferase associated (VIRMA), and zinc finger CCCH-type containing 13 (ZC3H13). In contrast, m6A demethylases act as erasers by removing m6A modifications from RNAs. Significant demethylases include fat mass and obesity-associated protein (FTO), AlkB homolog 5 (ALKBH5), and ALKBH3. Reader proteins recognize the m6A modification on RNAs and modulate the metabolic processes of these RNAs. A comprehensive array of m6A readers has been identified, including YTH domain-containing proteins YTHDC1 and YTHDC2, YTH domain family members YTHDF1, YTHDF2, and YTHDF3, insulin-like growth factor 2 mRNA binding proteins IGF2BP1, IGF2BP2, and IGF2BP3, heterogeneous nuclear ribonucleoproteins HNRNPA2B1 and HNRNPC, and eukaryotic initiation factor 3 (eIF3) [117]. Therefore, the collective action of these three components functions as a unified system, facilitating the reversible and dynamic process of m6A modification.
Numerous studies discussing RNA m6A modifications have shown its involvement in essential physiological processes, including adipogenesis [22], bone marrow formation [23], and the control of the central neurological [24], reproductive, and hematological systems [25, 26]. Adipogenesis is closely related to FTO-mediated autophagy [118], METTL3-mediated fatty acid metabolism [119], and YTHDF1-mediated preadipocyte maturation [120]. In details, depletion of METTL3 alleviated lipid accumulation and improved insulin sensitivity [121], while overexpression of FTO contributed to adipogenesis and fat accumulation [26]. Additionally, METTL3 is considered to have a central role in osteogenesis as its pivotal function in regeneration and differentiation on bone marrow stem cells (BMSCs) [122]. Therefore, METTL3 deficiency leads to impaired bone formation by activation of the JAK1/STAT5/C/EBPβ pathway and elimination of SOX4 mRNA [123, 124]. In CNS development, emerging studies have indicated that learning and memory consolidation are maintained by the involvement of METTL3-mediated Immediate-Early Gene (IEG) translation [125], FTO-modulated brain-derived neurotrophic factor (BDNF) expression, and YTHDF1-regulated synaptic function [126, 127]. The silencing of METTL14 decreased myelin production within CNS [128], while the inhibition of YTHDF1 results in hippocampal neuronal dysfunction, leading to impaired spatial memory and learning abilities [129]. As for the reproductive system, hypermethylated mRNAs recognized by YTHDF2 were mainly associated with controlling oocyte meiotic maturation [130], and METTL3 deficiency disrupts gamete maturation and reduces fertility in female mice [131, 132]. Similarly, L1 mRNA degradation mediated by the METTL3-YTHDF2 pathway maintains the capacity of male fertility [133]. Recent research has demonstrated that METTL3 and METTL16 are responsible for the regulation of the differentiation and proliferation of hematopoietic stem cells, and deficiency of these two m6A writers contributes to the inhibition of endothelial-to-hematopoietic transition (EHT) and hematopoietic failure [26, 134].
In recent years, researchers have also become increasingly interested in studying the role of RNA m6A modification in tumor progression, as our understanding of the regulatory mechanism of this change has advanced. The m6A gene has the ability to selectively target and control oncogenes or tumor suppressor genes through methylation or demethylation, hence either encouraging or impeding tumor progression by the recognition of m6A readers, as shown in Fig. 3. Thus, it is the downstream m6A regulators, rather than the levels of m6A modification, that directly exert control over the progressive or suppressive effect in tumor progression. The m6A regulators are the primary mechanism responsible for implementing m6A modifications to control tumor development in various tumor-specific, cellular-level, or environmental settings. However, it is worth noting that the emergence of contentious situations coincided with the release of new findings, such as the implication of YTHDF2 in the advancement of HCC. Considering the current complex information about m6A regulators, an in-depth investigation of each m6A regulator implicated in tumor development is necessary to advance our understanding of the intricate molecular pathways involved.
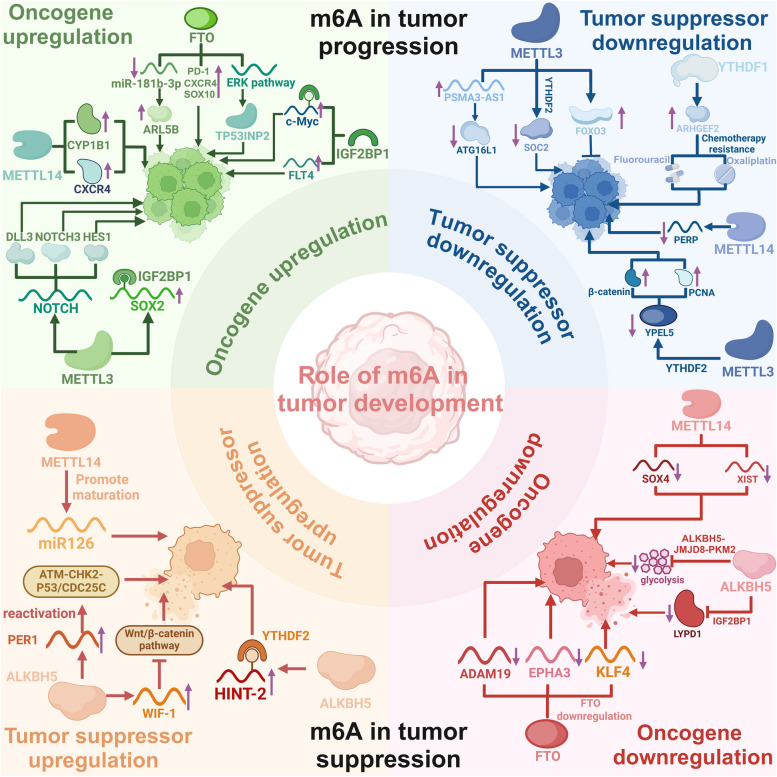
The double-edged role of RNA m6A modification in tumor progression. Two primary mechanisms regulate the progression of tumors: modulation of the expression levels of oncogenes and tumor suppressor genes. RNA m6A regulators promote tumor cell proliferation by activating the NOTCH signaling pathway and targeting downstream oncogenes, such as c-Myc, CXCR4, and SOX2. Alternatively, the activation of tumor suppressor genes, including P53, HINT-2, and PERP, or the reduction of oncogene expression in tumor cells can impede the development of tumors. Consequently, the m6A regulators are the primary mechanism responsible for the implementation of m6A modifications that regulate tumor growth in a variety of tumor-specific, cellular, or environmental contexts. This figure was created with BioRender.com
RNA m6A regulators function as tumor promoters
RNA m6A regulators upregulate oncogenes
SOX2 is recognized as a significant marker for CSCs, which facilitates the initiation and spread of tumors by controlling downstream MYC genes. METTL3 facilitated the progression of colorectal cancer (CRC) by enhancing the stability of SOX2 mRNA through the METTL3-IGF2BP2 pathway [135]. In glioblastoma (GBM), METTL3 activates the NOTCH pathway and facilitates the formation of gliomas by controlling the transcriptional expression of delta-like ligand 3 (DLL3), neurogenic locus notch homolog protein 3 (NOTCH3), and hairy and enhancer of split 1 (HES1) [136]. METTL14 promoted the proliferation and progression of breast cancer (BC) by increasing the expression of CXCR4 and CYP1B1 in an m6A-dependent manner [137].
Furthermore, FTO suppresses miR-181b-3p, leading to increased expression of the cancer-promoting gene ARL5B. This, in turn, promotes the movement and invasion of breast cancer cells [138]. In acute myeloid leukemia (AML), ALKBH5 stimulates the growth of AML cells by improving the stability of TACC3 mRNA and ITPA mRNA, which has pro-carcinogenic effects through demethylation of targeted mRNAs [139, 140]. Elevated levels of FTO in AML with mutations in nucleophosmin 1 (NPM1) stimulate the PDGFRβ/extracellular signal-regulated kinase (ERK) signaling pathway and promote the production of the tumor protein p53 inducible nuclear protein 2 (TP53INP2). This, in turn, fosters the growth and regeneration of leukemia cells without NPM1 [141, 142]. In melanoma, FTO demethylates pivotal melanoma-promoting genes, such as PD-1, CXCR4, and SOX10, resulting in their augmented expression and the advancement of melanoma [143].
IGF2BP1 is recruited by hypoxia-inducible lncRNA kb-1980e3 and sustains self-renewal and tumorigenesis of breast cancer stem cells by stabilizing c-Myc mRNA [144]. Through m6A modification, IGF2BP2 improves the RNA stability of Fms Related Tyrosine Kinase 4 (FLT4) in lung adenocarcinoma (LUDA). Consequently, this stimulates the PI3K-AKT signaling pathway, hence promoting angiogenesis and metastasis of LUDA [145].
YTHDF1 facilitates the translation of the Wnt receptor frizzled 7 (FZD7) in an m6A-dependent manner. This, in turn, results in the overstimulation of the Wnt/β-catenin pathway and the subsequent progression of gastric cancer [146]. OCT4 is a pivotal pluripotency factor that plays a crucial role in sustaining the phenotype of hepatocellular carcinoma stem cells. YTHDF2 promotes OCT4 translation and expression by enhancing m6A modification of OCT4 mRNA, which in turn facilitates the development of HCC progression and metastasis [147]. However, the absence of YTHDF2 has been demonstrated to stabilize the mRNAs of the inflammatory and angiogenic factors IL-11 and SERPINE2, thereby facilitating the proliferation of liver tumors and the emergence of vascular abnormalities [148]. Moreover, YTHDF3 promotes the increase in YAP, an important mediator of the Hippo pathway, which greatly contributes to the progression of colorectal cancer by enhancing the degradation of long non-coding RNA GAS5 through m6A modification [149].
RNA m6A regulators downregulate tumor suppressor genes
In AML, METTL3 stabilizes long-chain non-coding RNA PSMA3-AS1 by upregulating its methylation level. PSMA3-AS1 has been shown to promote the progression of FLT3-ITD+ AML by competitively binding to miR-20a-5p, thereby inhibiting its expression of the anti-tumor gene ATG16L1 [150]. Furthermore, METTL3 downregulates the expression of the tumor suppressor gene SOCS2 through an m6A-YTHDF2-dependent mechanism, leading to HCC oncogenesis [151], while inhibition of METTL3 conferred sorafenib resistance in HCC by decreasing the expression of FOXO3 in a YTHDF1-dependent manner [152].
In PDAC, METTL14 increases the methylation of PERP mRNA and enhances its degradation through m6A modification, thereby promoting pancreatic cancer growth and metastasis [153]. In CRC, the proliferation and dissemination of CRC cells are facilitated by YTHDF1 by enhancing the translation of ARHGEF2, therefore conferring resistance to chemotherapy medications such as fluorouracil and oxaliplatin [154, 155]. The METTL3/YTDHF2 axis has been identified to induce β-catenin and PCNA upregulation by inhibiting the expression of YPEL5, which enhances tumorigenicity and metastasis in CRC [156].
RNA m6A regulators function as tumor suppressors
RNA m6A regulators downregulate oncogenes
In CRC, SOX4 and long non-coding RNA XIST can both promote tumor progression by regulating the epithelial-mesenchymal transition (EMT) process. However, METTL14 has been shown to inhibit the motility, invasion, and metastasis of CRC cells through the regulation of m6A modification on SOX4 mRNA and lncRNA XIST, leading to their destruction and tumor suppression [157, 158]. In addition, the expression of ALKBH5 effectively inhibits the progression of CRC by obstructing glycolysis through the ALKBH5/JMJD8/PKM2 pathway [159].
In GBM, FTO overexpression interacts with miR-27a-3p, which is a pro-carcinogenic agent, to hinder the proliferation, migration, and invasion of glioma cells in hypoxic settings [160]. While FTO downregulation decreases the production of oncogenic ADAM19, EPHA3, and KLF4 mRNA, which in turn lowers the proliferation of glioblastoma stem cells [160, 161].
In HCC, ALKBH5 repressed and demethylated the oncogene LYPD1, which IGF2BP1 then recognized at the post-transcriptional level, thereby interfering with tumor progression [162]. After an inadequate radiofrequency ablation (IRFA) treatment of HCC cells, it was observed that the sublethal heat stress caused by IRFA increased the expression of YTHDF1. This, in turn, accelerated the m6A modification and translation of EGFR mRNAs, which promoted the survival and spread of HCC cells. In light of these discoveries, a potential approach to preventing HCC metastasis following IRFA may involve targeting the m6A-YTHDF1-EGFR axis in conjunction with EGFR inhibitors [163].
RNA m6A regulators upregulate tumor suppressor genes
In HCC, METTL14 overexpression promotes m6A modification of precursor microRNA-126 and the production of mature miR126, which inhibits tumor cell metastasis [164]. In PDAC, ALKBH5 upregulates PER1 and WIF-1 mRNA expression, thereby mediating reactivation of the ATM-CHK2-P53/CDC25C and inhibition of the Wnt/β-catenin signaling pathway, which ultimately inhibits pancreatic cancer cell growth [165, 166]. In ocular melanoma, enhanced ALKBH5 expression facilitated the translation of histidine triad nucleotide-binding protein 2 (HINT-2) in an m6A-YTHDF1-dependent manner, a tumor suppressor in ocular melanoma [167].
RNA m6A regulators impact tumor formation and progression through various mechanisms. However, even among the same type of human cancer, different researchers have obtained contradictory results, possibly due to the specific cellular environment or variations in the expression levels of m6A-targeted genes [161, 168, 169]. To address this issue, further studies should be conducted from several angles and across multiple physiological routes, thereby deducing the combinational effects of one specific m6A regulator in the same tumor type or cellular context.
RNA m6A modification is involved in regulation of ferroptosis
Multiple investigations have established a connection between m6A and programmed cell death, specifically ferroptosis [30, 31], as shown in Fig. 4. The Xc− system is considered a crucial target for modulating the sensitivity to ferroptosis. A mechanic study has discovered that the expression of SLC3A2 can be regulated by m6A reader YTHDC2, which disrupted the stability of Homeobox A13 (HOXA13) mRNA in an m6A-dependent manner, ultimately leading to the induction of ferroptosis in lung adenocarcinoma cells via the YTHDC2-HOXA13-SLC3A2 pathway [170]. SLC7A11 is one more important target for controlling m6A-mediated ferroptosis, whose mRNA can be modified by METTL14 at the 5'-UTR region and subsequently be degraded by m6A reader YTHDF2 [171]. Moreover, IGF2BP1 recognizes the m6A modification site on SLC7A11 mRNA, while its competitive binding blocks the recruitment of the BTG2/CCR4-NOT complex, thereby inhibiting SLC7A11 mRNA deadenylation. Therefore, METTL3/IGF2BP1-mediated m6A alteration of SLC7A11 mRNA could enhance the RNA stability of SLC7A11 by inhibiting the deadenylation process in an m6A-dependent manner, thus enhancing tumor ferroptosis resistance and consequently promoting tumor growth [172]. In NSCLC, ALKBH5 is identified to function as the tumor suppressor via m6A-mediated SLC7A11 mRNA and ferroptosis induction [173]. FTO has been documented to control the death of papillary thyroid carcinoma cells by facilitating m6A demethylation of SLC7A11 RNA, thus preventing the progression of thyroid cancer [174]. In contrast, elevated FTO expression induced SLC7A11 and GPX4 expression through an m6A-YTHDF2-dependent mechanism to resist ferroptosis in CRC cells [175]. NF-κB activating protein (NKAP) is an RNA-binding protein that acts as an inhibitor of ferroptosis. In glioblastoma, NKAP functions as an m6A reader and binds to SLC7A11 mRNA with a high m6A content, recruiting the splicing factor SFPQ and promoting mRNA maturation. Ultimately, NKAP leads to ferroptosis evasion of glioblastoma [176]. Fibroblast growth factor receptor 4 (FGFR4) is found to be upregulated in breast cancer due to m6A-hypomethylation, which controls the phosphorylation of FGFR4 and subsequently activates GSK-3β and initiates the β-catenin/TCF4 signaling pathway, leading to the development of resistance against HER2. However, FGFR4 inhibition triggers ferroptosis via the β-catenin/TCF4-SLC7A11/FPN1 axis, indicating a novel clue for breast cancer therapy [177].
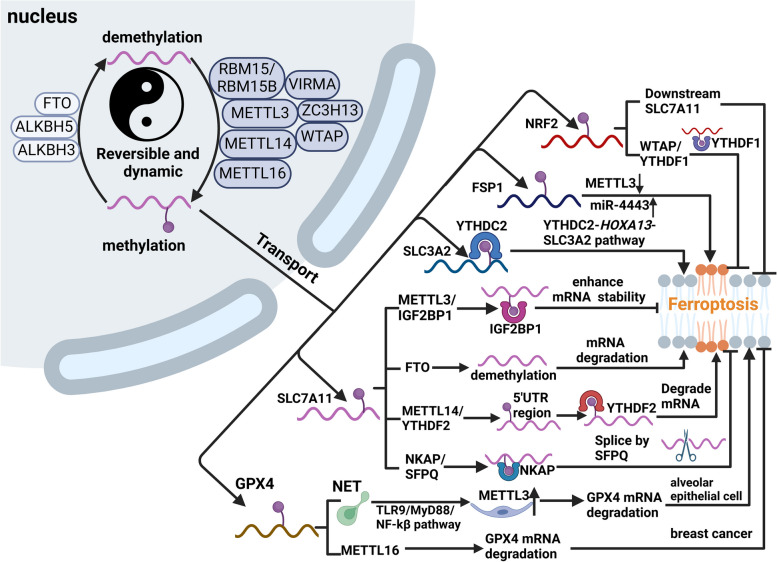
Regulation of RNA m6A modification on ferroptosis. m6A modification is a reversible and dynamic process mediated by methyltransferases (writers), demethylases (erasers), and m6A binding proteins (readers). All of these three components are implicated in the splicing, translation, and stability of mRNA. RNA m6A modification can induce or inhibit ferroptosis via regulating the expression of ferroptosis-related targets. For instance, WTAP/YTHDF1 can increase the stability of NRF2, which can activate the transcriptions of SLC7A11 and consequently inhibit ferroptosis. miR-4443 inhibited cisplatin-induced ferroptosis of tumor cells by decreasing the expression level of METTL3 and increasing the level of FSP1. YTHDC2 can promote the degradation of SLC3A2 mRNA and induce ferroptosis. METTL3/IGF2BP1 pathway can stabilize SLC7A11 mRNA and NKAP/SFPQ pathway can promote its maturation, both of which inhibit ferroptosis. The FTO and METTL14/YTHDF2 axes accelerate its degradation, thereby inducing ferroptosis. Moreover, NETs-induced upregulation of METTL3 acts through the TLR9/MyD88/NF-κB signaling pathway in alveolar epithelial cells, which in turn induces ferroptosis in alveolar epithelial cells. In breast cancer, decreased levels of METTL16 expression lead to decreased levels of m6A methylation of GPX4 RNA and ferroptosis. This figure was created with BioRender.com
FSP1 is another key regulatory target of the m6A alteration. Previous studies identified FSP1 as a powerful ferroptosis-resistance factor and established that FSP1 functions parallel to the glutathione-dependent lipid hydroperoxidase GPX4 in inhibiting ferroptosis [71, 72], while inhibition of FSP1 via 3-phenylquinazolinones could induce ferroptosis and impair tumor growth [178]. miR-4443 can influence m6A modification and FSP1 expression by targeting METTL3, resulting in FSP1-mediated ferroptosis [179]. Mechanically, miR-4443 expression was considerably enhanced in the tumor environment and mediated FSP1 upregulation and the generation of intracellular superoxide, ROS, and ferrous iron, which ultimately contribute to ferroptosis inhibition [179]. Moreover, the upregulation of METTL3 induced by fear stress stabilizes FSP1 mRNA through m6A modification, which leads to glioma progression by inhibiting ferroptosis [180].
Similarly, m6A alteration controls the target molecule GPX4. The m6A modification of GPX4 caused by METTL3 is necessary for the induction of ferroptosis [181], whereas METTL16 promotes the growth of breast cancer by increasing the m6A modification-mediated GPX4 expression and anti-ferroptosis effect [182]. Additionally, METTL16 interacts with IGF2BP2 and enhances the stability of SENP3 mRNA, thereby inhibiting the lactoferrin degradation. Consequently, elevated lactoferrin expression contributes to the ferric chelation, thereby increasing the resistance of HCC cells to ferroptosis [183]. In alveolar epithelial cells, the neutrophil extracellular trap (NET) induces alterations in GPX4 mRNA through the activation of METTL3-mediated m6A modification, which in turn affects ferroptosis in alveolar epithelial cells [181].
NRF2 is another target modified by RNA m6A methylation. WTAP enhances m6A modification at the 3'-UTR region of the endogenous antioxidant factor NRF2 mRNA and increases its stability by interacting with the m6A reader YTHDF1 [184]. On the one hand, SLC7A11 is one of the downstream target genes of NRF2 that has the ability to directly bind to the promoter region of SLC7A11 and stimulate the expression of SLC7A11, hence regulating ferroptosis [185]. The upregulation of NRF2 expression has been demonstrated in various types of cancer, where it is considered to be the main factor driving cancer development and metastasis. This is achieved through the regulation of SLC7A11, GPX4, and FSP1, which help protect against ferroptosis and contribute to resistance against therapy [62, 186, 187].
Additionally, studies have shown that IGF2BP3 is highly expressed in lung adenocarcinoma and can prevent ferroptosis by binding to m6A-methylated mRNAs that code for anti-ferroptotic factors such as GPX4, SLC3A2, acyl-CoA synthetase long-chain family member 3 (ACSL3), and ferritin heavy chain 1 (FTH1) [188]. Furthermore, Lu et al. propose that IGF2BP3 identifies the m6A alteration of NRF2 mRNA and stabilizes it. They also find that IGF2BP3 knockdown markedly increases the ferroptosis of hepatocellular carcinoma cells upon sorafenib treatment [189]. Similarly, METTL14 reduces FTH1 mRNA stability through m6A methylation, thereby enhancing sorafenib-induced ferroptosis, which contributes to suppressing cervical cancer progression via the PI3K/Akt signaling pathway [190]. In CRC, the lnc RNA ABHD11-AS1 functions as a mediator to facilitate the interaction between IGF2BP2 and the E3 ubiquitin ligase TRIM21, thereby inhibiting ferroptosis and enhancing the stability of the transcription factor FOXM1, which in turn promotes tumor cell proliferation [191]. It is noteworthy that a reduction in mitochondrial RNA methylation levels results in mitochondrial dysfunction and a decline in cellular antioxidant capacity, which in turn gives rise to ferroptosis [192]. Taken together, m6A modification could mediate the occurrence of ferroptosis via regulating the expression of ferroptosis-related targets in an m6A-dependent manner; however, the outcome of m6A-mediated ferroptosis in tumor progression is different, as shown in Table 1.
Table 1
The roles of RNA m6A-regulated ferroptosis in various tumors
| Types | Genes | Functions | Targets | Molecular regulatory mechanism | Tumor types | References |
|---|---|---|---|---|---|---|
| Writer | METTL14 | Promote | SLC7A11 | In hypoxic conditions, METTL14 inhibits ferroptosis in hepatocellular carcinoma cells through m6A-YTHDF2-mediated degradation of SLC7A11 mRNA at the 5'UTR, which in turn promotes tumor development | Hepatocellular carcinoma (HCC) | [171] |
| Writer | METTL14 | Promote | FTH1 | METTL14 reduces FTH1 mRNA stability through m6A methylation, thereby enhancing sorafenib-induced ferroptosis, which contributes to suppressing cervical cancer progression via the PI3K/Akt signaling pathway | Cervical cancer | [190] |
| Writer | METTL3 | Inhibit | SLC7A11 | In HB, METTL3-IGF2BP1 mediated m6A modification promotes the inhibition of CCR4-NOT complex-mediated adenylate deadenylation, which enhances SLC7A11 mRNA stability and expression and inhibits tumor ferroptosis | Hepatoblastoma (HB) | [172] |
| Writer | METTL3 | Inhibit | FSP1 | In NSCLC, miR-4443 inhibits cisplatin-induced ferroptosis by negatively regulating the level of METTL3-induced FSP1 m6A methylation, thereby conferring cisplatin resistance in NSCLC cells | Non-small cell lung cancer (NSCLC) | [179] |
| Writer | METTL3 | Inhibit | FSP1 | In glioma, the induction of fear stress resulted in an upregulation of METTL3, which increases the methylation level of FSP1 and stabilizes FSP1 mRNA and ultimately inhibits ferroptosis | Glioma | [180] |
| Writer | WTAP | Inhibit | NRF2 | In bladder cancer, WTAP/YTHDF1 promotes cell viability of bladder cancer and inhibits erastin-induced ferroptosis by promoting the levels of the antioxidant factor NRF2 mRNA | Bladder cancer | [184] |
| Writer | METTL16 | Inhibit | GPX4 | In breast cancer, METTL16 promotes GPX4 expression through m6A modification, which inhibits cancer cell ferroptosis and promotes breast cancer progression | Breast cancer | [182] |
| Writer | METTL16 | Inhibit | SENP3 | METTL16 interacts with IGF2BP2 and enhances the stability of SENP3 mRNA, thereby inhibiting the lactoferrin degradation. Consequently, elevated lactoferrin expression contributes to the ferric chelation, thereby increasing the resistance of HCC cells to ferroptosis | Hepatocellular carcinoma (HCC) | [183] |
| Eraser | ALKBH5 | Promote | SLC7A11 | In NSCLC, ALKBH5 is identified to function as the tumor suppressor via m6A-mediated SLC7A11 mRNA and ferroptosis induction | Non-small cell lung cancer (NSCLC) | [173] |
| Eraser | FTO | Promote | SLC7A11 | In PTC, FTO regulates PTC cell ferroptosis by mediating the m6A methylation of SLC7A11, which in turn promotes the degradation of SLC7A11 mRNA, thereby inducing ferroptosis and attenuating tumor migration and invasion | Papillary thyroid cancer (PTC) | [174] |
| Eraser | FTO | Inhibit | SLC7A11 and GPX4 | Elevated FTO expression induced SLC7A11 and GPX4 expression through an m6A-YTHDF2-dependent mechanism to resist ferroptosis in CRC cells | Colorectal cancer (CRC) | [175] |
| Reader | YTHDC2 | Promote | SLC3A2 | YTHDC2 induces ferroptosis by inhibiting SLC7A11 and SLC3A2. YTHDC2-induced ferroptosis occurs via m6A-dependent mRNA degradation of HOXA13, which results in a subsequent reduction in SLC3A2 expression | Lung adenocarcinoma cells | [170] |
| Reader | NKAP | Inhibit | SLC7A11 | In glioblastoma, NKAP inhibits tumor cell ferroptosis by recognizing methylation sites and recruiting the splicing factor SFPQ for the processing of SLC7A11 mRNA, thereby promoting mRNA maturation | Glioblastoma | [176] |
| Reader | IGF2BP3 | Inhibit | GPX4, SLC3A2, ACSL3, and FTH1 | In LUAD, the overexpression of IGF2BP3 inhibits ferroptosis by stabilizing the mRNAs of ferroptosis-resistant factors, such as GPX4, SLC3A2, ACSL3, and FTH1 | Lung adenocarcinoma (LUAD) | [188] |
| Reader | IGF2BP3 | Inhibit | NRF2 | In HCC, IGF2BP3 inhibits sorafenib-induced ferroptosis by promoting NRF2 mRNA stability | Hepatocellular carcinoma (HCC) | [189] |
| Reader | IGF2BP2 | Inhibit | FOXM1 | The lnc RNA ABHD11-AS1 functions as a mediator to facilitate the interaction between IGF2BP2 and the E3 ubiquitin ligase TRIM21, thereby inhibiting ferroptosis and enhancing the stability of the transcription factor FOXM1, which in turn promotes tumor cell proliferation | Colorectal cancer (CRC) | [191] |
Improving the efficacy of immunotherapy via m6A-mediated ferroptosis
The primary obstacle of conducting effective immunotherapy is the immunosuppressive tumor microenvironment (TME). This situation can arise from the accumulation of cells with negative regulatory immune activity, such as regulatory T cells (Tregs), inhibitory B cells, myeloid-derived suppressor cells (MDSCs), or M2-polarized tumor-associated macrophages (TAMs). Lymphocytes in the TME exhibit elevated expression of co-inhibitory signals, such as immune checkpoint ligands and receptors. Additionally, there are elevated amounts of tolerogenic enzymes, such as indoleamine 2,3-dioxygenase-1 (IDO) or arginase-1, and a reduction in immunoglobulin-mediated opsonization. Moreover, the immune cells are subjected to an unfavorable metabolic environment [193]. Nevertheless, the endorsement of immune checkpoint inhibitors (ICIs) for various types of cancer has brought about a significant transformation in cancer treatment. This is particularly true for metastatic malignancies, where certain patients who were previously deemed untreatable can now experience prolonged periods of remission and survival. Recent breakthroughs have highlighted the involvement of m6A alteration and ferroptosis in immunotherapy. Here, we provide a concise overview of the latest discoveries and explore the approach of utilizing m6A-mediated ferroptosis to enhance the effectiveness of immunotherapy.
Immunotherapy enhancement via ferroptosis
On the one hand, IFNγ produced by CD8+ T lymphocytes stimulates the JAK/STAT1 pathway to decrease the expression of SLC7A11 and SLC3A2, thereby enhancing the susceptibility of tumor cells to ferroptosis [16, 194]; On the other hand, IFNγ can transcriptionally stimulate ACSL4 expression, ultimately inducing ferroptosis in tumor cells [195]. Additionally, a growing amount of evidence has demonstrated that combining ICIs and ferroptosis-relating agents synergistically inhibits tumor growth in vitro and in vivo [196]. For instance, the combined treatment of GPX4 inhibitors and anti-PD-1 blockade significantly suppressed tumor growth and induced a pronounced immune response with increased proportions of activated CD8+
T lymphocytes stimulates the JAK/STAT1 pathway to decrease the expression of SLC7A11 and SLC3A2, thereby enhancing the susceptibility of tumor cells to ferroptosis [16, 194]; On the other hand, IFNγ can transcriptionally stimulate ACSL4 expression, ultimately inducing ferroptosis in tumor cells [195]. Additionally, a growing amount of evidence has demonstrated that combining ICIs and ferroptosis-relating agents synergistically inhibits tumor growth in vitro and in vivo [196]. For instance, the combined treatment of GPX4 inhibitors and anti-PD-1 blockade significantly suppressed tumor growth and induced a pronounced immune response with increased proportions of activated CD8+ T cells in tumor-bearing immunocompetent mice [196]. Similarly, SLC7A11 deficiency renders tumors more responsive to anti-PD-L1 therapy or a combination of anti-PD-L1 therapy [15]. Furthermore, the resistance of tumor cells to ferroptosis is associated with unresponsiveness to ICIs. Restoring sensitivity to ferroptosis could enhance the efficacy of immunotherapy. Tumors with high TYRO3 expression, which are resistant to ICIs, can be re-sensitized to anti-PD1 therapy by restoring ferroptosis via the inhibition of the TYRO3-mediated AKT/NRF2 pathway [197].
T cells in tumor-bearing immunocompetent mice [196]. Similarly, SLC7A11 deficiency renders tumors more responsive to anti-PD-L1 therapy or a combination of anti-PD-L1 therapy [15]. Furthermore, the resistance of tumor cells to ferroptosis is associated with unresponsiveness to ICIs. Restoring sensitivity to ferroptosis could enhance the efficacy of immunotherapy. Tumors with high TYRO3 expression, which are resistant to ICIs, can be re-sensitized to anti-PD1 therapy by restoring ferroptosis via the inhibition of the TYRO3-mediated AKT/NRF2 pathway [197].
The majority of preclinical experiments employed FINs that specifically target the cystine transport mediated by SLC7A11, such as IKE, sulfasalazine, and cyst(e)inase. In HCC, there are multiple components, including immune checkpoint regulation, immune cell filtration, and ferroptosis induction, that are involved in consideration for ferroptosis-mediated immunotherapy. It has been proven that inhibition of GPX4 induced ferroptosis, which in turn increased the infiltration of CD8+ T cells. However, this effect can be eliminated by PD-L1 upregulation on tumor cells and immunosuppressive MDSC infiltration through increased release of high-mobility group box 1 (HMGB1) from hepatocytes [113]. Therefore, the combination of pharmacological FINs, checkpoint blockade, and MDSC reduction has been shown to successfully inhibit primary liver tumors and liver metastasis [113]. Meanwhile, recent preclinical studies have shown that GPX4-targeting FINs can make tumors more responsive to immunotherapy. When GPX4 inhibitors are combined with anti-PD-1/PD-L1 treatment, it enhances the antitumor immune response and tumor suppression [198]. IL-1β plays a role in maintaining Fe-S cluster stability, which in turn represses iron accumulation and ferroptosis. The combination of IL-1β blockade and anti-PD-1 antibody has been demonstrated to result in enhanced tumor inhibition compared to monotherapy. However, this effect has been shown to be reversible by liproxstatin-1, indicating the involvement of ferroptosis [197].
T cells. However, this effect can be eliminated by PD-L1 upregulation on tumor cells and immunosuppressive MDSC infiltration through increased release of high-mobility group box 1 (HMGB1) from hepatocytes [113]. Therefore, the combination of pharmacological FINs, checkpoint blockade, and MDSC reduction has been shown to successfully inhibit primary liver tumors and liver metastasis [113]. Meanwhile, recent preclinical studies have shown that GPX4-targeting FINs can make tumors more responsive to immunotherapy. When GPX4 inhibitors are combined with anti-PD-1/PD-L1 treatment, it enhances the antitumor immune response and tumor suppression [198]. IL-1β plays a role in maintaining Fe-S cluster stability, which in turn represses iron accumulation and ferroptosis. The combination of IL-1β blockade and anti-PD-1 antibody has been demonstrated to result in enhanced tumor inhibition compared to monotherapy. However, this effect has been shown to be reversible by liproxstatin-1, indicating the involvement of ferroptosis [197].
Collectively, these findings underscore the potential of enhancing the effectiveness of immunotherapy by targeting ferroptosis, providing compelling evidence for the combination of immunotherapy and ferroptosis-inducing agents in cancer treatment. Besides, broadening the application of targeting ferroptosis in immunotherapy extends beyond conventional FINs and encompasses various therapeutic approaches that can induce ferroptosis in cancer cells.
Immunotherapy enhancement via RNA m6A modification
RNA m6A modification on immune checkpoints
Immune checkpoint inhibitors (ICIs) are immunotherapies that selectively target programmed cell death/ligand 1 (PD-1/PD-L1) and cytotoxic T lymphocyte antigen 4 (CTLA-4), which are closely associated with the ability of cancer cells to evade the immune system [199]. These ICIs have demonstrated effectiveness in treating various types of malignancies [200].
Mounting evidence has shown that m6A modification is consistently controlled in different types of tumors, and the expression of m6A regulatory factors is strongly associated with the expression of PD-1 and PD-L1. Yang et al. revealed that FTO knockdown increased m6A methylation of PD-1 through the m6A reader YTHDF2, leading to the promotion of melanoma cell growth and proliferation [143]. The IGF2BP family, which serves as a regulatory component of m6A readers, exhibits a positive association with PD-1 expression [201]. In non-small cell lung cancer (NSCLC), METTL3 mediates m6A modification of the circIGF2BP3 gene in an m6A-dependent manner, and upregulates the expression of PKP3. PKP3 stabilizes the PD-L1 protein, which ultimately leads to immune evasion by NSCLC cells [202]. In breast cancer, there exists a positive correlation between PD-L1 and METTL3 expression. Knocking down METTL3 reduces PD-L1 expression and boosts antitumor immunity by activating and infiltrating T cells [203]. In contrast, the lack of METTL3 leads to the stabilization of signal transducer and activator of transcription 1 (STAT1) by YTHDF2, which in turn enhances the immunological responses to anti-PD-1 treatment [204]. In cholangiocarcinoma (CCA), METTL14 binds the mRNA of SIAH2 in the 3′-UTR region and promotes its degradation via m6A modification. Therefore, it increases the stability of PD-L1 protein and inhibits T cell expansion and anti-tumor activity [205]. Furthermore, lipopolysaccharide (LPS) modifies MIR155HG by METTL14-mediated m6A methylation, which in turn upregulates PD-L1 expression through the miR-223/STAT1 axis. Therefore, LPS promotes PD-L1 expression in HCC and contributes to immune escape [206].
FTO and ALKBH5 are two main m6A erasers and regarded as the most promising targets for the regulation of immune checkpoints. To date, over ten FTO inhibitors have been discovered, and their treatment efficiency has been verified in different models [207]. Su et al. screened two highly effective and selective FTO inhibitors, CS1 and CS2, showing a better effect in suppressing leukemia cell activity by decreasing the expression of the immune checkpoint gene LILRB4 through suspending immune escape [208]. In addition to METTL3/14, ALKBH5 also regulates the TME and increases PD-L1 expression. In contrast to METTL3, ALKBH5 suppression in ICC enhances the m6A modification of PD-L1 mRNA, thereby facilitating the degradation of PD-L1 in a YTHDF2-dependent manner [209]. ALKBH5 has also been demonstrated to orchestrate an immunosuppressive tumor microenvironment. In hypoxic conditions, increased expression of ALKBH5 stabilizes the long non-coding RNA nuclear paraspeckle assembly transcript1 (NEAT1), resulting in higher production of CXCL8/IL8, which is essential for recruiting tumor-associated macrophages (TAMs) [210]. Furthermore, ALK-04, a selective inhibitor of ALKBH5, can expedite anti-PD-1 treatment in melanoma while greatly reducing tumor growth [211].
Researchers specifically focusing on m6A modifications also methodically developed a scoring system that correlates the m6A score with immune response in AML. Increased expression of immune regulators PD-L1, PD-L2, MRP1, and MRP2 was linked to elevated tumor mutation and infiltration rates in patients with low m6A grades. Patients with elevated m6A scores not only maintained a higher 5-year survival rate but also showed greater benefits in clinical therapy [212–214]. Yang et al. utilized m6A regulators to develop a predictive model for AML resistance to cytarabine, which presents a potential approach for adjuvant therapy of AML resistance [215].
RNA m6A modification on immune cells in tumor immune microenvironment
It is widely known that intrinsic m6A modification regulates tumor cell fate by targeting specific genes in different cancers [117, 216]. In addition, immune cells infiltrated in the microenvironment are also regarded as having an essential role in immune surveillance and preventing immune escape [217]. However, few studies have focused on how the m6A modification controls immune cell anti-tumor capabilities, and here we summarize current discoveries, as shown in Table 2.
Table 2
The functions and mechanisms of various ferroptotic cells in the TIME
| Cell Types | Targets | Ferroptotic consequences | Mechanisms | Tumor types | References |
|---|---|---|---|---|---|
| Pancreatic tumor cells | GPX4 | Infiltration and activation of M2 macrophages to promote pancreatic tumorigenesis | Ferroptotic pancreatic cells result in the release of 8-OHG, a component of damage-associated molecular pattern (DAMP), which promotes the infiltration and M2 polarization of macrophages via the STING pathway | Pancreatic tumor | [98, 99] |
| Bladder cancer cells | GPX4 | Promotion of the proliferation, migration, and invasion of tumor cells via Prostaglandin E2 (PGE2) released by ferroptotic cancer cells | Following chemotherapy, tumor cells express lower levels of GPX4. This reduction in GPX4 expression is accompanied by the release of PGE2, a prostaglandin that stimulates the proliferation of cancer stem cells (CSCs), allowing them to repopulate the tumor during the period between chemotherapy cycles | Bladder cancer | [103] |
| Natural killer cells (NK cells) | GPX4 | Decreased capability of natural killer (NK) cells to eliminate tumor cells | L-Cysteine (L-KYN) has been demonstrated to impair natural killer (NK) cell survival in the tumor microenvironment (TME) by inducing cellular ferroptosis in an aromatic hydrocarbon receptor (AHR)-independent manner | Gastric cancer | [106] |
| Dendritic cells (DCs) | GPX4 | Reduced cytokine production capacity, impaired promotion of MCH I expression, and impaired T-cell activation | The RSL3-GPX4-induced ferroptosis observed in DC cells is manifested by lipid peroxidation, the production of oxidized polyunsaturated fatty acids, and the release of HMGB1. Ferroptotic DCs are unable to secrete pro-inflammatory cytokines or express MHC I molecules in response to lipopolysaccharide maturation signals. They are unable to induce CD8 + + T cells to produce IFNγ T cells to produce IFNγ | Pancreatic ductal adenocarcinoma | [108] |
CD8+ T cells T cells | CD36 | Decreased production of cytotoxic cytokines and anti-tumor capacity | CD36 expression is upregulated in tumor-infiltrating CD8+ T cells, which is accompanied by an increase in cholesterol in the TME and induces CD8+ T cells to undergo ferroptosis. This process results in a reduction in the production of cytotoxic cytokines as well as the anti-tumor capacity of CD8+ T cells | Melanoma | [87, 110] |
| Tc9 cells | STAT3 | Inhibition of the specific killing function | The activation of STAT3 by IL-9 derived from Tc9 cells resulted in the upregulation of fatty acid oxidation and mitochondrial activity, as well as the reduction of lipid peroxidation and resistance to tumor- or ROS-induced ferroptosis. Deficiency in IL-9/STAT3 signaling ultimately leads to impaired longevity and antitumor ability of Tc9 cells | Melanoma | [110, 111] |
| Treg cells | GPX4 | Inhibition of tumor growth and enhances anti-tumor immunity | Gpx4-deficient Treg cells undergo aberrant accumulation of lipid peroxides and ferroptosis in response to T cell receptor (TCR) and co-stimulatory signaling | Colorectal cancer, Melanoma | [86] |
| TAMs | APOC1 | Improvement of the efficacy of anti-PD1 immunotherapy | Inhibition of APOC1 promotes the conversion of M2 macrophages to M1 macrophages via the ferroptosis pathway, thereby altering the tumor immune microenvironment | Hepatocellular carcinoma (HCC) | [114] |
| TAMs | SLC7A11 | Improvement of the anti-tumor effect of anti-PD-L1 therapy | Downregulating SLC7A11 regulates macrophage phenotypes by inducing ferroptosis, which in turn activates the SOCS3-STAT6-PPARγ signaling pathway, consequently affecting tumor progression and metastasis | Hepatocellular carcinoma (HCC) | [115] |
Dendritic cells
Dendritic cells (DCs) are regarded as the most significant antigen-processing cells (APC) and operate as the bridge for adaptive immune response through major histocompatibility complex (MHC) class II ( MHC-II) molecules [218, 219]. Recently, METTL3-mediated m6A modification was found to promote DC activation and maturation, causing them to present new antigens to and thereby activate T cells. Regarding this process, METTL3 amplifies the translation of CD40, CD80, and Tirap transcripts in DCs. Simultaneously, METTL3 enhances the activation of T cells by facilitating the production of cytokines [220]. Han et al. reported that the m6A reader YTHDF1 negatively regulates the anti-tumor immune responses of DCs by enhancing the translation of lysosomal proteases. Without YTHDF1, the translation of lysosomal proteases was diminished, favoring antigen cross-presentation and promoting more CTL responses against tumors [221].
Macrophages
Macrophages are phagocytic cells of the innate immune system and mainly involved in the recognition, phagocytosis, and degradation of pathogens and tumor cells, and are highly involved in tumor progression [222]. Specifically, tumor-associated macrophages (TAMs) in the tumor-associated environment are very plastic, being able to switch their functions to inhibit or promote tumor progression in response to different environmental stimuli [223]. The main type of macrophage is divided into anti-tumor TAMs (M1 type) or pro-tumor TAMs (M2 type) [224]. Recently, Yin et al. found that ablating the METTL3 expression in macrophages promoted tumor growth and lung metastasis, suggesting a correlation between m6A modification in macrophages and tumor progression. Furthermore, METTL3 reduction in macrophages impaired the efficiency of programmed cell death protein 1 (PD-1) blocking therapy, indicating an immune-relevant function for macrophages [224]. Tong et al. also revealed that METTL3-deficient macrophages produced subnormal levels of tumor necrosis factor α (TNFα) when stimulated with lipopolysaccharide (LPS) in vitro and increased susceptibility to bacterial infection and tumor growth [225]. In contrast to m6A writers’ positive roles in macrophages, knocking down the m6A reader YTHDF2 promotes macrophages to express LPS-induced inflammatory cytokines, implying that YTHDF2 plays a negative regulatory role in LPS-induced inflammatory responses of macrophages [226]. In HCC, ALKBH5 was shown to regulate MAP8K6 expression in an m6A-dependent manner, boosting the recruitment of PD-L1+ tumor-associated macrophages, implying that ALKBH5 overexpression also has a role in regulating the tumor immune microenvironment [227].
T cells
T cells offer important protection against viral infection and tumor cells [228]. They are generally classified into two groups based on whether their cell surface receptor is CD4 or CD8 [229]. It has been found that METTL3 depletion in mouse T cells may affect the homeostasis and differentiation of naive T cells [230]. However, METTL3 loss suppresses the function and stability of Treg cells by inhibiting IL-2/STAT5 signaling and promoting the cytokine secretion of T effector cells, resulting in enhancement of the anti-tumor immune responses in the tumor immune environment. METTL3 has also been demonstrated to upregulate PD-L1 expression and preserve its mRNA stability in an m6A-IGF2BP3-dependent manner, thereby inhibiting the activation of anti-tumor T cells and enabling immunological escape from tumors [231]. In cholangiocarcinoma, METTL14 directly regulates its downstream target seven in absentia homolog 2 (SIAH2) through promoting the mRNA degradation of SIAH2 mediated via YTHDF2, inhibiting T cell expansion, and mediated immunological escape [205]. In HCC, the lnc RNA 942 recruits the RNA-binding protein IGF2BP3 in an m6A-dependent manner and enhances the stability of SLC7A11 mRNA, which promotes the proliferation and immunosuppression of Treg cells [232].
In general, it has been shown that the association of m6A-related enzymes with the tumor immune system is crucial for the treatment of clinical tumors. However, there is currently a paucity of systematic and thorough reviews on the roles of m6A-associated enzymes in the tumor immune microenvironment and their impact on human tumor immunotherapy. More extensive experimental researches are required to investigate the potential processes of m6A-related enzymes in tumor immunotherapy, and to give a solid theoretical basis and unique insight for tumor immunotherapy.
Small-molecule compounds targeting RNA m6A regulators
Accumulating evidence indicates a strong association between m6A level and the occurrence and development of tumors [233]. Hence, the use of small compounds to target m6A key proteins and regulate their expression holds promise for the treatment of malignancies. Over the past decade, researchers have made constant attempts to screen and discover new small compounds that target m6A regulators, and they have shown good antitumor efficacy in vitro and in vivo. In the subsequent section, we discuss the potential significance of small molecules that target m6A modification in cancer therapy, as shown in Table 3.
Table 3
Molecules targeting RNA m6A regulators and their clinical application
| Compound | Target | IC50 | Cancer Type | Mechanism | Clinical Trial | Targeted Disease | ClinicalTrials gov ID | Reference |
|---|---|---|---|---|---|---|---|---|
| STM2457 | METTL3 | 16.9 μM | AML | STM2457 inhibits the activity of METTL3, thereby reducing the level of intracellular m6A modification, leading to the differentiation and apoptosis of AML cells | no | no | / | [240] |
| STC-15 | METTL3 | Unknown | AML | STC-15 has also been shown to inhibit tumor growth through mechanisms involving anti-cancer immune responses such as changes in interferon signaling and synergy with T-cell checkpoint blockade | Phase I | AML | NCT05584111 | / |
| UZH2 | METTL3 | 0.28 μM | AML | UZH2 can enter cells and bind to METTL3 in a targeted manner, thereby inhibiting its enzymatic activity and reducing m6A levels in MOLM-13 and PC-3 tumor cell lines, inhibiting tumor cell proliferation | no | no | / | [241] |
| CDIBA-43n | METTL3/14 | 16.9 μM | AML | CDIBA-43n inhibits AML cell proliferation by inhibiting METTL3-14 complex and reducing the level of m6A modification of mRNAs | no | no | / | [242] |
| Eltrom-Bopag | METLL3/14 | 3.65 μM | AML | Eltrombopag inhibits AML cell proliferation by inhibiting METTL3 activity and decreasing m6A levels | Phase II Phase III | LymphomaAML | NCT05961410 NCT03701217 | [243] |
| Metformin | METTL3 | Unknown | BC | Metformin inhibits breast cancer cell proliferation by targeting the miR-483-3p/METTL3/m6A/p21 pathway | Phase II Phase II Phase II | BC SCLC, LUAD | NCT01042379 NCT03994744 NCT03709147 | [244] |
| Baicalin | METTL3, METTL14 | Unknown | NPC | Baicalin elevated METTL3 and METTL14 levels to augment m6A methylation of Suv39H1 mRNA.Enhanced m6A methylation facilitated diverse Suv39H1 cleavage, influencing genomic stability in cancer cells and yielding anti-tumor effects | no | no | / | [235] |
| Fusaric acid (FA) | METTL3, METTL14 | Unknown | HCC | FA increased hypermethylation of the p53 gene promoter, impeding p53 transcription, and diminished the synthesis of the m6A methyltransferases METTL3 and METTL14, hence decreasing the m6A modification of p53 mRNA and P53 expression | no | no | / | [237] |
| 2-((1-hydroxy-2-oxo-2-phenylethylthio)-acetic acid | ALKBH5 | 0.84 μM | AML | Compounds 7 and 8 bind with the ALKBH5 protein. At low micromolar levels, there was a notable reduction in cell proliferation and survival | no | no | / | [248] |
| 4-((furan-2-ylmethyl)-amino) | ALKBH5 | 1.79 μM | AML | |||||
| DDO-2728 | ALKBH5 | 2.97 μM | AML | Compound 10 interacts with ALKBH5 by occupying the m6A-binding pocket. This interaction leads to the suppression of AML cell growth by influencing the cell cycle, E2F targets, G2M checkpoints, and MYC targets | no | no | / | [249] |
| ALK-04 | ALKBH5 | Unknown | Melanoma | The combination of compound 11 (ALK-04) with the PD-1 antibody and GVAX has demonstrated a substantial inhibitory effect on the growth of B16 tumors in melanoma by blocking the function of the ALKBH5 enzyme | no | no | / | [211] |
| MV1035 | ALKBH5 | Unknown | GBM | MV1035 competes with 2-oxoglutarate for the binding site of ALKBH5, essential for its catalytic function, hence diminishing CD73 expression and restricting GBM cell invasion and migration | no | no | / | [250] |
| MO-I-500 | FTO | 1.51 μM | TNBC | MO-I-500 possesses both anticonvulsant characteristics and the ability to efficiently inhibit the survival and growth of drug-resistant triple-negative breast cancer (TNBC) cells, specifically the SUM149-MA cell line, via blocking FTO | no | no | / | [252] |
| Diacerein | FTO | 8.7 μM | BC | Diacerein could hinder the IL-6/IL-6R signaling route, leading to the down-regulation of anti-apoptotic proteins Bcl-2 and Bcl-xL, and the up-regulation of the pro-apoptotic protein Bax, so causing death in tumor cells. Additionally, it inhibits the STAT3, MAPK, and Akt signaling pathways, which suppresses tumor cell growth | no | no | / | [253, 254] |
| 18077 | FTO | 1.43 μM | BC | The administration of 18077 and 18097 resulted in a substantial increase in the quantity of m6A-modified mRNA in cancer cells. This increase improved the stability of SOCS1 mRNA and stimulated the p53 signaling pathway, hence enhancing the cells' sensitivity to chemotherapy such as cisplatin and doxorubicin | no | no | / | [255] |
| 18097 | FTO | 0.64 μM | BC | |||||
| Meclofenamic acid | FTO | 17.4 μM | NSCLC | Administration of Meclofenamic acid demonstrated a significant synergistic effect in GE-resistant non-small cell lung cancer (NSCLC) cells with the combination of Meclofenamic acid and geftinib (GE) | no | no | / | [256, 257] |
| ZLD115 | FTO | 2.3 μM | AML | ZLD115 can upregulate RARA and downregulate MYC levels in MOLM13 cells, inhibiting the oncogenic FTO signaling pathway in AML cells, thereby demonstrating anti-leukemic activity | no | no | / | [259] |
| FB23 | FTO | 0.06 μM | AML | FB23-2 triggers apoptosis, and increases the expression of ASB2 and RARA, which are direct targets of FTO, thereby exerting anti-tumor effects | no | no | / | [258] |
| FB23-2 | FTO | 1.6-16 μM | AML | |||||
| Dac51 | FTO | 0.4 μM | Melanoma, CRC | Dac51 enhances CD8+ T cell infiltration and IFN-γ release in melanoma, hence restricting tumor development. The combination of Dac51 and anti-PD-L1 therapy significantly prolongs the longevity of MC38 colon cancer murine models. | no | no | / | [260] |
| FTO-02 | FTO | 2.18 μM | GBM | FTO-04 impedes the development of neutrospheres produced from GSCs, but does not impact the proliferation of neutrospheres produced from normal neural stem cells. | no | no | / | [261] |
| FTO-04 | FTO | 3.39 μM | ||||||
| FTO-43N | FTO | 1.0 μM | GC | Administration of FTO-43N is associated with the downregulation of the Wnt and PI3K-Akt signaling pathways, while exhibiting no growth toxicity to normal colon cells. | no | no | / | [262] |
| Zantrene | FTO | Unknown | AML, CRCC | Zantrene has been identified as a potent targeted inhibitor of FTO, and inhibition of FTO via Zantrene administration can overcome PD-1 immune checkpoint resistance in mouse melanoma models. | Phase I | AML, melanoma, and CRCC | NCT05456269 | / |
| Entacapone | FTO | Unknown | Osteos-arcoma (OS) | Elevated FTO levels in OS may signify a worse prognosis. FTO accelerated OS development and metastasis both in vitro and in vivo. Entacapone, a treatment for Parkinson's disease, inhibited oxidative stress via m6A-mediated regulation of FTO and DACT1. | Early Phase I | Gastrointestinal stromal tumors | NCT04006769 | / |
| Saiko-saponin | FTO | Unknown | Leu-kemia | Saikosaponin D stopped FTO and fixed m6A hypomethylation in MYC and RARA. After these effects, MTHFR and BCL2 became less stable, which made MV4-11- or Kas-1-resistant human myeloid mononuclear leukemia cells more sensitive to tyrosine kinase inhibitors. | no | no | / | [246] |
| CuB | IGF2BP1 | 1.7 μM | HCC | CuB has been demonstrated to obstruct the recognition of c-Myc mRNA by IGF2BP1 through allosteric mechanisms. As a result, this results in the activation of apoptosis, the reestablishment of the immunological response, and the manifestation of anti-hepatoma properties. | no | no | / | [264] |
| Tegaserod | YTHDF1 | 13.82 μM | AML | Tegaserod inhibits the YTHDF1-regulated translation of cyclin E2 and blocks the G1 phase of CD34+ cells. | no | no | / | [265] |
| DC-Y13-27 | YTHDF2 | 21.8±1.8 μM | CRC | DC-Y13-27 can augment anti-PD-L1 treatment in MC38 murine models. Meanwhile, DC-Y13-27 could suppress YTHDF2 expression to counteract IR-induced immunosuppression and enhance adaptive immunity, facilitating IR in combination treatment. | no | no | / | [266] |
| JX5 | IGF2BP2 | Unknown | T-ALL | JX5 can inhibit the NOTCH1 signaling pathway by suppressing the binding of IGF2BP2 to NOTCH1, thereby impeding the proliferation of T-cell acute lymphoblastic leukemia (T-ALL) cells. | no | no | / | [267] |
| Quercetin | METTL3 | Unknown | Cervical cancer | METTL3 promotes the proliferation and metastasis of cervical cancer cells. Quercetin enhances the sensitivity of cervical cancer cells to cisplatin by inhibiting METTL3 protein expression, hence diminishing tumor progression and improving chemotherapeutic efficacy. | Phase II Phase II Phase II | TNBC HNSC TSCC | NCT06355037 NCT05724329 NCT05456022 | [234] |
| Simvastatin | METTL3 | Unknown | Lung cancer | In lung cancer, simvastatin decreased the expression of METTL3 and consequently the m6A levels of EZH2 mRNA, thus impeding the movement and infiltration of A549 cells. | Phase II Phase II Phase II Phase II | PRAD, PAAD, BC, SCLC | NCT06437574 NCT05821556 NCT05550415 NCT04698941 | [239] |
Targeting RNA m6A writers
Natural products from traditional medicines
METTL3 is crucial in the malignant biological processes that cervical cancer cells exhibit, including proliferation and metastasis. The compound 1 queerctin increases the responsiveness of cervical cancer cells to cisplatin by suppressing the expression of METTL3, therefore impeding tumor proliferation and enhancing the effectiveness of treatment [234]. Through the upregulation of METTL3 and METTL14 synthesis, the traditional Chinese compound baicalin was shown to elevate the m6A methylation level of Suv39H1 mRNA. Increased m6A methylation promoted distinct cleavage of Suv39H1, therefore affecting the genomic stability of cancer cells and generating anti-tumor effects [235].
Fusaric acid (FA) is a mycotoxin produced by Fusarium species [236]. While FA caused hypermethylation of the p53 gene promoter, therefore preventing the transcription of p53, it also lowered the expression of the m6A methyltransferases METTL3 and METTL14. This reduction in m6A modification of p53 mRNA consequently decreased the production of P53 translation [237].
Simvastatin is a synthetic derivative of a compound produced via the fermentation of Aspergillus terreus [238]. In lung cancer, it decreased the expression of METTL3 and consequently the m6A levels of EZH2 mRNA, thus impeding the movement and infiltration of A549 cells [239].
Synthesized molecules targeting RNA m6A writers
Yankova et al. announced a METTL3 competitive inhibitor 2 (STM2457) with highly strong inhibitory action (METTL3 IC50 =
= 16.9 nM) and poor inhibitory activity against other kinases in 2021, based on high-throughput screening of 250,000 distinct drug-like compounds [240]. STORM has advanced the development of oral-available compound 3 (STC-15), expanding on compound 2. It is now undergoing Phase I clinical trials due to its strong effectiveness in leukemia models. Inhibition of METTL3 by Compound 3 leads to immunomodulatory effects, modulation of interferon signaling, and synergistic blocking of T-cell checkpoints.
16.9 nM) and poor inhibitory activity against other kinases in 2021, based on high-throughput screening of 250,000 distinct drug-like compounds [240]. STORM has advanced the development of oral-available compound 3 (STC-15), expanding on compound 2. It is now undergoing Phase I clinical trials due to its strong effectiveness in leukemia models. Inhibition of METTL3 by Compound 3 leads to immunomodulatory effects, modulation of interferon signaling, and synergistic blocking of T-cell checkpoints.
Moroz Omori et al. reported a selective and cell-permeable METTL3 competitive inhibitor 4 (UZH2, METTL3 IC50 =
= 0.28 μM) in 2021 after conducting drug design and structural optimization based on an adenine library-based screen. Compound 4 demonstrated its promise as an anticancer drug by inhibiting the proliferation of tumor cells by effectively reducing m6A levels in AML MOLM-13 cells (m6A IC50
0.28 μM) in 2021 after conducting drug design and structural optimization based on an adenine library-based screen. Compound 4 demonstrated its promise as an anticancer drug by inhibiting the proliferation of tumor cells by effectively reducing m6A levels in AML MOLM-13 cells (m6A IC50 =
= 7 μM) and osteosarcoma U2OS cells (m6A IC50
7 μM) and osteosarcoma U2OS cells (m6A IC50 =
= 9 μM) [241]. Meanwhile, as a reversible and noncompetitive allosteric inhibitor, compound 5 (CDIBA-43n, METTL3/14 IC50
9 μM) [241]. Meanwhile, as a reversible and noncompetitive allosteric inhibitor, compound 5 (CDIBA-43n, METTL3/14 IC50 =
= 2.81 μM) was able to inhibit proliferation of a variety of acute myeloid leukemia (AML) cells (MOLM-13 cell GI50
2.81 μM) was able to inhibit proliferation of a variety of acute myeloid leukemia (AML) cells (MOLM-13 cell GI50 =
= 14.6 μM, MOLM-14 cell GI50
14.6 μM, MOLM-14 cell GI50 =
= 13.1 μM, THP-1 cell GI50
13.1 μM, THP-1 cell GI50 =
= 21.6 μM, HL60 cell GI50
21.6 μM, HL60 cell GI50 =
= 15.5 μM). Furthermore, thrombopoietin receptor (TPO-R) agonist compound 6 (Eltrombopag, METTL3 IC50
15.5 μM). Furthermore, thrombopoietin receptor (TPO-R) agonist compound 6 (Eltrombopag, METTL3 IC50 =
= 3.65 μM) approved by FDA was also reported as a noncompetitive allosteric inhibitor of METTL3/14. Both of these compounds have been shown to selectively block the enzymatic activity of the METTL3/14 complex, leading to a decrease in the gene expression of m6A in mRNA. Ultimately, this results in an inhibitory influence on the growth of AML cells [242, 243].
3.65 μM) approved by FDA was also reported as a noncompetitive allosteric inhibitor of METTL3/14. Both of these compounds have been shown to selectively block the enzymatic activity of the METTL3/14 complex, leading to a decrease in the gene expression of m6A in mRNA. Ultimately, this results in an inhibitory influence on the growth of AML cells [242, 243].
Given its excellent pharmacological effectiveness and simple oral administration, compound 7 (metformin) has garnered significant interest as a primary therapy for type 2 diabetic mellitus (TDM2) [244]. Cheng et al. found a potential connection between m6A modification and the therapeutic efficacy of compound 7 in treating breast cancer. Compound 7 inhibits the proliferation of breast cancer cells by downregulating METTL3 by manipulating miR-483-3p, which in turn lowers m6A methylation levels and regulates the synthesis of p21 [245].
Targeting RNA m6A erasers
Natural products from traditional medicines
Saikosaponin is a traditional triterpenoid that is taken from Radix Bupleuri, which possesses anti-inflammatory and anticancer activities [246]. Saikosaponin D stopped FTO and fixed m6A hypomethylation in MYC and RARA. After these effects, MTHFR and BCL2 became less stable, which made MV4-11- or Kas-1-resistant human myeloid mononuclear leukemia cells more sensitive to tyrosine kinase inhibitors [247].
Synthesized molecules targeting RNA m6A erasers
ALKBH5 selective inhibitors include compound 8 (2-((1-hydroxy-2-oxo-2-phenylethyl)thio) acetic acid, ALKBH5 IC50 =
= 0.84 μM, LE
0.84 μM, LE =
= 0.44 kcal/mol), compound 9 (4-((furan-2-ylmethyl)amino) tetrahydropyridazine-3,6-dione, ALKBH5 IC50
0.44 kcal/mol), compound 9 (4-((furan-2-ylmethyl)amino) tetrahydropyridazine-3,6-dione, ALKBH5 IC50 =
= 1.79 μM, LE
1.79 μM, LE =
= 0.32 kcal/mol), and compound 10 (DDO-2728, ALKBH5 IC50
0.32 kcal/mol), and compound 10 (DDO-2728, ALKBH5 IC50 =
= 2.97 μM). Compounds 8 and 9 were found to interact with the ALKBH5 protein, resulting in the formation of a stable complex. At low micromolar levels, there was a notable reduction in cell survival, along with a potent inhibitory effect on the proliferation of cancer cells. Notably, compounds 8 and 9 demonstrated targeted inhibition of leukemia cells at lower levels, although they may cause particular harmful effects on healthy cells at higher concentrations. Further inquiries are required to assess the safety and therapeutic scope of these compounds, in order to determine their potential as anti-leukemia drugs [248]. In contrast to the first two compounds, compound 10 interacts with ALKBH5 by occupying the m6A-binding pocket. This interaction leads to the suppression of AML cell growth by influencing the cell cycle, E2F targets, G2M checkpoint, and MYC targets (MOLM-13 IC50
2.97 μM). Compounds 8 and 9 were found to interact with the ALKBH5 protein, resulting in the formation of a stable complex. At low micromolar levels, there was a notable reduction in cell survival, along with a potent inhibitory effect on the proliferation of cancer cells. Notably, compounds 8 and 9 demonstrated targeted inhibition of leukemia cells at lower levels, although they may cause particular harmful effects on healthy cells at higher concentrations. Further inquiries are required to assess the safety and therapeutic scope of these compounds, in order to determine their potential as anti-leukemia drugs [248]. In contrast to the first two compounds, compound 10 interacts with ALKBH5 by occupying the m6A-binding pocket. This interaction leads to the suppression of AML cell growth by influencing the cell cycle, E2F targets, G2M checkpoint, and MYC targets (MOLM-13 IC50 =
= 0.49 μM, MV4-11 IC50
0.49 μM, MV4-11 IC50 =
= 1.2 μM) [249].
1.2 μM) [249].
The combination of compound 11 (ALK-04) with the PD-1 antibody and GVAX has demonstrated a substantial inhibitory effect on the growth of B16 tumors in melanoma. The results are consistent with the notion that compound 11 improves the effectiveness of immunotherapy by blocking the function of the ALKBH5 enzyme, as seen by the observed enhancement of immunotherapy when ALKBH5 is suppressed [211].
Compound 12 (MV1035) was found to possess anticancer effects due to its ability to inhibit ALKBH5 function. From a molecular perspective, it may be postulated that compound 12 competes with 2-oxoglutarate for the binding site on ALKBH5, which is an essential component for ALKBH5 to perform its catalytic activity, leading to a reduction in CD73 expression and inhibiting the invasion and migration of GBM cells [250].
With the increasing acknowledgement of the significance of FTO in the development of cancer and its role in the scientific community, the progress in creating drugs that particularly target FTO has been consistently and thoroughly impressive. In 2014, Zheng et al. designed an FTO inhibitor compound 13 (MO-I-500, FTO IC50 =
= 8.7 μM) [251]. Furthermore, Singh proved that compound 13 possesses both anticonvulsant characteristics and the ability to efficiently inhibit the survival and growth of drug-resistant triple-negative breast cancer (TNBC) cells, specifically the SUM149-MA cell line, via blocking FTO [252]. Meanwhile, compound 14 (Diacerein, FTO IC50
8.7 μM) [251]. Furthermore, Singh proved that compound 13 possesses both anticonvulsant characteristics and the ability to efficiently inhibit the survival and growth of drug-resistant triple-negative breast cancer (TNBC) cells, specifically the SUM149-MA cell line, via blocking FTO [252]. Meanwhile, compound 14 (Diacerein, FTO IC50 =
= 1.51 μM) is also an apoptosis-inducible molecule by mediating the interleukin-6 signaling pathway and exerting anti-BC effects [253, 254]. Xie identified two selective FTO inhibitors, compound 15 (18,077, FTO IC50
1.51 μM) is also an apoptosis-inducible molecule by mediating the interleukin-6 signaling pathway and exerting anti-BC effects [253, 254]. Xie identified two selective FTO inhibitors, compound 15 (18,077, FTO IC50 =
= 1.43 μM) and compound 16 (18,097, FTO IC50
1.43 μM) and compound 16 (18,097, FTO IC50 =
= 0.64 μM), which have cellular action and can enhance chemosensitivity in BC. The administration of compounds 15 and 16 resulted in an elevation of SOCS1 mRNA and stimulated the p53 signaling pathway via enhanced m6A modification, hence improving the cells' sensitivity to chemotherapy such as cisplatin and doxorubicin [255]. In order to enhance the specificity of FTO inhibitors, Huang et al. identified a specific FTO inhibitor compound 17 (Meclofenamic acid, MA, FTO IC50
0.64 μM), which have cellular action and can enhance chemosensitivity in BC. The administration of compounds 15 and 16 resulted in an elevation of SOCS1 mRNA and stimulated the p53 signaling pathway via enhanced m6A modification, hence improving the cells' sensitivity to chemotherapy such as cisplatin and doxorubicin [255]. In order to enhance the specificity of FTO inhibitors, Huang et al. identified a specific FTO inhibitor compound 17 (Meclofenamic acid, MA, FTO IC50 =
= 17.4 μM) and demonstrated a significant synergistic effect in GE-resistant non-small cell lung cancer (NSCLC) cells with the combination of compound 17 and gefitinib (GE) [256, 257]. Huang’s team further developed FTO inhibitor compound 18 (FB23, FTO IC50
17.4 μM) and demonstrated a significant synergistic effect in GE-resistant non-small cell lung cancer (NSCLC) cells with the combination of compound 17 and gefitinib (GE) [256, 257]. Huang’s team further developed FTO inhibitor compound 18 (FB23, FTO IC50 =
= 0.06 μM) and compound 19 (FB23-2, FTO IC50
0.06 μM) and compound 19 (FB23-2, FTO IC50 =
= 2.6 μM). Regarding its anti-tumor properties, compound 19 (IC50
2.6 μM). Regarding its anti-tumor properties, compound 19 (IC50 =
= 1.6–16 μM) was shown to suppress the growth of AML cells by triggering apoptosis and increasing the expression of ASB2 and RARA [258]. Additionally, FTO inhibitor compound 20 (ZLD115, FTO IC50
1.6–16 μM) was shown to suppress the growth of AML cells by triggering apoptosis and increasing the expression of ASB2 and RARA [258]. Additionally, FTO inhibitor compound 20 (ZLD115, FTO IC50 =
= 2.3 μM) increases RARA and decreases MYC in MOLM13 cells, blocking the oncogenic FTO signaling pathway in AML cells. Meanwhile, compound 20 holds the promise for pharmacological application of its metabolic stability and lack of cytotoxicity [259]. The excellent properties of compounds 18 and 19 attracted interest from Liu, who further optimized them to develop a more potent FTO inhibitor compound 21 (Dac51, FTO IC50
2.3 μM) increases RARA and decreases MYC in MOLM13 cells, blocking the oncogenic FTO signaling pathway in AML cells. Meanwhile, compound 20 holds the promise for pharmacological application of its metabolic stability and lack of cytotoxicity [259]. The excellent properties of compounds 18 and 19 attracted interest from Liu, who further optimized them to develop a more potent FTO inhibitor compound 21 (Dac51, FTO IC50 =
= 0.4 μM). At the cellular level, Dac51 has been observed to exhibit limited toxicity to epithelial cells, fibroblasts, and T cells. By inhibiting FTO and increasing the methylation level of transcripts, compound 21 can inhibit the glycolytic capacity of tumor cells and exert antitumor proliferation activity [260].
0.4 μM). At the cellular level, Dac51 has been observed to exhibit limited toxicity to epithelial cells, fibroblasts, and T cells. By inhibiting FTO and increasing the methylation level of transcripts, compound 21 can inhibit the glycolytic capacity of tumor cells and exert antitumor proliferation activity [260].
By analyzing the binding site of compound 17 with FTO, Sarah Huff et al. searched for potential compounds to assist in the development of FTO inhibitors and finally screened a competitive inhibitor of FTO, compound 22 ((FTO-02, FTO IC50 =
= 2.18 μM) and compound 23 (FTO-04, FTO IC50
2.18 μM) and compound 23 (FTO-04, FTO IC50 =
= 3.39 μM). In cellular assays, compound 23 exerts antitumor effects by increasing m6A levels in cells and interfering with Glioblastoma Stem Cells (GSCs) renewal. Significantly, compound 23 demonstrated the ability to impede the development of neutrospheres produced from GSCs, but did not impact the proliferation of normal neutrospheres. These findings indicate that it has the potential to be a new and innovative lead molecule for treating gliomas [261]. After developing compound 23, Huff further designed the oxetanyl FTO inhibitor compound 24 (FTO-43 N, FTO IC50
3.39 μM). In cellular assays, compound 23 exerts antitumor effects by increasing m6A levels in cells and interfering with Glioblastoma Stem Cells (GSCs) renewal. Significantly, compound 23 demonstrated the ability to impede the development of neutrospheres produced from GSCs, but did not impact the proliferation of normal neutrospheres. These findings indicate that it has the potential to be a new and innovative lead molecule for treating gliomas [261]. After developing compound 23, Huff further designed the oxetanyl FTO inhibitor compound 24 (FTO-43 N, FTO IC50 =
= 1.0 μM). In subsequent studies of anti-tumor activity, compound 24 effectively inhibited the growth of gastric cancer AGS, KATOIII, and SNU-16 cell lines (AGS EC50
1.0 μM). In subsequent studies of anti-tumor activity, compound 24 effectively inhibited the growth of gastric cancer AGS, KATOIII, and SNU-16 cell lines (AGS EC50 =
= 20.3 μM, KATOIII EC50
20.3 μM, KATOIII EC50 =
= 35.9 μM, SNU-16 EC50
35.9 μM, SNU-16 EC50 =
= 17.7 μM) associated with the downregulation of the Wnt and PI3K-Akt signaling pathways, while exhibiting no growth toxicity to normal colon cells [262]. As of now, the FTO-selective inhibitor Zantrene, developed by Race Oncology, has undergone a Phase I clinical study aimed at assessing its effectiveness in FTO-driven AML, melanoma, and CRCC. Furthermore, the FTO-inhibitor entacapone approved by the FDA has been conducted into Early Phase I clinical research in conjunction with Imatinib to evaluate the treatment effectiveness for gastrointestinal stromal tumors [263].
17.7 μM) associated with the downregulation of the Wnt and PI3K-Akt signaling pathways, while exhibiting no growth toxicity to normal colon cells [262]. As of now, the FTO-selective inhibitor Zantrene, developed by Race Oncology, has undergone a Phase I clinical study aimed at assessing its effectiveness in FTO-driven AML, melanoma, and CRCC. Furthermore, the FTO-inhibitor entacapone approved by the FDA has been conducted into Early Phase I clinical research in conjunction with Imatinib to evaluate the treatment effectiveness for gastrointestinal stromal tumors [263].
Targeting RNA m6A readers
Natural products from traditional medicines
Fusaric acid reduces the expression of p53 in HepG2 cells by decreasing the m6A methylation of p53 mRNA. This is achieved by the lowering of METTL3 and METTL14, as well as the downregulation of YTHDF1, YTHDC2, and YTHDF3 [237]. Among the natural products, the tetracyclic triterpenoid cucurbitacin B (compound 25, CuB, IGF2BP1 IC50 =
= 1.7 μM) was found to target IGF2BP1 to exert inhibitory effects. Compound 25 has been demonstrated to obstruct the recognition of c-Myc mRNA by IGF2BP1 through allosteric mechanisms. This results in the activation of apoptosis, the reestablishment of the immunological response, and the manifestation of anti-hepatoma properties [264].
1.7 μM) was found to target IGF2BP1 to exert inhibitory effects. Compound 25 has been demonstrated to obstruct the recognition of c-Myc mRNA by IGF2BP1 through allosteric mechanisms. This results in the activation of apoptosis, the reestablishment of the immunological response, and the manifestation of anti-hepatoma properties [264].
Synthesized molecules targeting RNA m6A readers
A novel potential YTHDF1 inhibitor for the treatment of irritable bowel syndrome with constipation has been identified in a recent report as compound 26 (Tegaserod, YTHDF1 IC50 =
= 13.82 μM). This compound has the capacity to obstruct YTHDF1 from binding m6A-modified mRNA and YTHDF1-dependent protein translation.
13.82 μM). This compound has the capacity to obstruct YTHDF1 from binding m6A-modified mRNA and YTHDF1-dependent protein translation.
As compound 26 inhibits the YTHDF1-regulated translation of cyclin E2 and prevents the G1 phase of CD34+ cells, the viability of patient-derived AML cells is decreased and the survival of patient-derived transplanted tumor models is enhanced [265]. In 2023, Wang et al. performed fluorescence polarization based high-throughput screening on its internal compound library and discovered a YTHDF2 inhibitor compound 27 (DC-Y13-27, YTHDF2 IC50 =
= 21.8
21.8 ±
± 1.8 μM, YTHDF1 IC50
1.8 μM, YTHDF1 IC50 =
= 165.2
165.2 ±
± 7.7 μM, KD
7.7 μM, KD =
= 37.9
37.9 ±
± 4.3 μM). The role of compound 27 in combination therapy has been demonstrated to markedly enhance the tumor-suppressive effect of ionizing radiation (IR). By suppressing YTHDF2 expression, Compound 27 mechanistically counterbalances the rise in immunosuppressive cells generated by IR and promotes the adaptive immunity, thus supporting IR in combination therapy [266].
4.3 μM). The role of compound 27 in combination therapy has been demonstrated to markedly enhance the tumor-suppressive effect of ionizing radiation (IR). By suppressing YTHDF2 expression, Compound 27 mechanistically counterbalances the rise in immunosuppressive cells generated by IR and promotes the adaptive immunity, thus supporting IR in combination therapy [266].
T-cell acute lymphoblastic leukemia (T-ALL) has a solid relationship with the overexpression of NOTCH1. In order to tackle this problem, scientists have created the chemical 28 (JX5). It can inhibit the NOTCH1 signaling pathway by suppressing the binding of IGF2BP2 to NOTCH1, thereby impeding the proliferation of T-ALL cells. Despite the therapeutic potential of JX5 (25 μM), it only suppressed the growth of around 50% of T-ALL cells, and certain T-ALL malignancies may develop a phenotype that is resistant to JX5. Therefore, more research on the mechanism of action of JX5 is necessary to provide a more comprehensive knowledge of its possible adverse effects [267].
Moreover, the potential adverse effects of these m6A-targeted pharmacological agents may stem from their specificity in targeting tumor cells, which could lead to possible harm to normal organs. Additionally, the equilibrium between toxicity and efficacy of these pharmaceuticals is a critical factor in their side effects. For instance, although the obstruction of METTL3 via STC-15 impeded the tumor progression, its inhibition may interfere with hematopoiesis and other immunological or dermatological responses, contributing to the occurrence of thrombocytopenia, rashes, or pruritus. Overall, the adverse reactions of STC-15 are reported to be dose-dependent and generally manageable, but further investigation is needed to optimize its therapeutic window [268]. Zantrene has been explored in clinical trials for treating various FTO-driven tumors. Although designed as a less cardiotoxic substitute for anthracyclines, it continues to exhibit specific cardiovascular complications throughout clinical trials, such as alterations in cardiac rhythm [269]. While common side effects such as nausea, vomiting, and diarrhea, though manageable with supportive care, can significantly affect patient quality of life during treatment [270]. Clinical trials for gastrointestinal stromal tumors (GIST) have shown that entacapone can cause gastrointestinal side effects such as diarrhea and abdominal pain by disrupting Catechol-O-methyltransferase (COMT). Additionally, its modulation of dopamine pathways is associated with neurological effects including dyskinesia and hallucinations. Evidence of urine discoloration has also been observed [271]. In conclusion, alongside their therapeutic effects on tumor cells, the off-target effects also interfere with critical cellular pathways and lead to metabolic disruptions affecting normal cell function. Consequently, further investigation is needed to optimize the appropriate therapeutic window and adverse effect management of these three drugs.
Immunotherapy enhancement via m6A-mediated ferroptosis
In the preceding discussion, we determined that m6A regulators such as METTL3, METTL14, and YTHDF1 directly contribute to the control of anti-tumor immunity in several ways. Meanwhile, aberrant expression of m6A regulators in tumor cells creates an immunosuppressive tumor-associated milieu, hastening cancer progression. Furthermore, as previously stated, there is a tight correlation between m6A and ferroptosis. Therefore, targeting m6A-mediated ferroptosis might be a novel strategy for cancer immune escape and immunotherapy.
Previous studies have predicted that the immune checkpoint molecule PD-1 is positively correlated with the expression level of ACSL4 but negatively correlated with the expression levels of GPX4 and HSPB1 in clear cell renal cell carcinoma [17]. In line with this possibility, Liao et al. have demonstrated an increased overall survival or progression-free survival in patients with high ACSL4 expression following immune checkpoint blockade therapy [195]. More convincing evidence is that PD-L1 blockade treatment directly led to an increase of ROS in CD45-ID8 cells and effectively reduced tumor growth [16]. Recent findings suggest that tumor immunotherapy involves M2 repolarization of tumor-associated macrophages. Notably, it has recently been demonstrated that M2 macrophages change into M1 macrophages via the ferroptosis pathway, which can boost anti-PD1 immunotherapy in HCC [114]. These observations further support that tumor immune checkpoint inhibitor therapy based on ferroptosis is expected to provide a new strategy for tumor immunotherapy.
As we mentioned above, there is a tight correlation between m6A-mediated ferroptosis and tumor progression. On the one hand, it is important to note that the induction of ferroptosis can be controlled through m6A modification; On the other hand, there is a growing interest in the immune checkpoints that are modified by m6A. For example, It was found that deletion of METTL3 in bone marrow cells promotes tumor growth in vivo, even enhancing tumor invasion and metastasis, leading to an attenuation of PD-1 blockade therapy [224]. Meanwhile, PD-L1 is directly regulated by METTL3 in terms of the m6A modification of its mRNA and is regarded as a downstream target of METTL3 via the METTL3/IGF2BP3 axis [203]. In addition, METTL3 can regulate the expression of FSP1 in a m6A-mediated manner, hence suppressing ferroptosis [179]. Meanwhile, activated CD8+ T cells secrete IFNγ during immunotherapy, which downregulates the expression of SLC7A11 (and its regulatory partner SLC3A2) and inhibits cystine uptake in cancer cells, thereby augmenting lipid peroxidation and ferroptosis. While SLC7A11 is also regarded as a target for m6A modification, suggesting a combination of immune checkpoint inhibitors (ICIs) with cyst(e)inase potentiated tumor ferroptosis and T cell-mediated antitumor immune responses in vivo [16]. Recent mechanical research indicated YTHDF1 can both alleviate ferroptosis and decrease CD8+ T cytotoxicity via PD-L1 upregulation [272]. Collectively, understanding the mechanism of m6A-mediated ferroptosis and immunotherapy can provide us with a novel perspective on immunotherapy, as shown in Fig. 5.

Immunotherapy efficacy improvement via m6A-mediated ferroptosis. The immunosuppressive TME is the primary barrier to effective immunotherapy. The involvement of m6A modification in regulating immune checkpoints and immune cells has been highlighted. On the one hand, RNA m6A modification could influence immune responses by regulating the expression of immune checkpoints such as PD1 and PD-L1. On the other hand, RNA m6A modification could directly affect the functional activities of immune cells, thus modulating immune responses. Therefore, regulating m6A-mediated ferroptosis could enhance the efficacy of immunotherapy to some extent. This figure was created with BioRender.com
Conclusion and future prospects
Along with the application of novel technological advances, including single-cell RNA sequencing and multiplexed histological assays, our understanding of tumor microenvironment and tumor progression has become increasingly comprehensive and profound. Therefore, research into immunotherapy for malignant tumors has grown exponentially, leading to a significant shift in the treatment paradigm. Despite the growing importance of ICIs in cancer therapy, only about one-third of patients in most cancer types respond to ICIs, significantly limiting their use. To circumvent the limits of immunotherapy, we investigated the relationship between ferroptosis and antitumor immunity. Ferroptosis, a form of cell death characterized by iron-dependent phospholipid peroxidation, is gaining increasing attention. Targeted manipulation of ferroptosis represents a promising avenue for enhancing the efficacy of immunotherapy. Based on previous preclinical models, the administration of FINs represents a promising approach to ferroptosis-based anticancer strategies [273–275]. Nevertheless, the timing and frequency of administration are subject to variation in practice, the underlying mechanism remains unexplored, and the side effects of long-term administration, potential drug resistance, and the side effects associated with the destruction of anti-tumor immune cells are not well understood. Therefore, it is imperative to develop cell-specific precision targeting strategies in order to improve the efficacy of ferroptosis-induced treatment.
As RNA m6A modification is the most prevalent post-transcriptional modification, a more comprehensive investigation of the correlation between tumor progression and RNA m6A modification offers innovative opportunities for the development of combination therapeutic approaches [276]. In the meantime, RNA m6A methylation plays a vital role in ferroptotic sensitivity and also impacts the function and phenotype of immune cells in the tumor microenvironment. More precisely, the combination of small molecules targeting m6A regulators with first-line therapies and ICI not only enhances the therapeutic response by modulating the tumor microenvironment (TME), but also successfully tackles treatment resistance in different types of cancer. Consequently, these diverse m6A regulators could serve as therapeutic targets for immunotherapy through m6A-mediated ferroptosis.
Despite the extensive documentation of several inhibitors and activators that target RNA m6A modification, only a small number have been granted clinical approval for cancer therapy. The following factors may be accountable for this predicament: First and foremost, the influence of m6A regulators on tumor growth remains a topic of controversy due to the absence of research on the precise regulation of m6A modifications (both global and targeted) and the complex and combined functions of RNA m6A regulators in various tumors or even within the same tumor. Second, there is limited therapeutic practicality since tumor heterogeneity and infrequent predictors provide an obstacle between targeted drugs and specific tumors. Due to the extensive range of subtyping stages and distinct features at various cancer stages, it is necessary to further elucidate and stratify the particular suitability of small molecules. Third, most studies have primarily concentrated on the inhibitory activity of small compounds, neglecting the optimization of their absorption, distribution, metabolism and elimination (ADME) properties, as well as lipophilicity and solubility. Further improvement is also needed to boost the inhibitory efficacy, intracellular activity, and concentration of certain small molecules, mostly because of the selectivity of cell absorption [277]. Additionally, there is a deficiency in the incorporation of significant fields in antitumor research, such as ferroptosis-based therapy, ICI therapy, and other combined therapies. Finally, the current research methodologies focusing on RNA m6A small molecules include medication repurposing, computer-aided drug design (CADD), and natural product screening, while there is an insufficient use of innovative methodologies such as Artificial Intelligence (AI) and Machine Learning (ML) technologies to develop more targeted small molecules [278, 279]. Consequently, more evaluation of prospective agents is required. We hold the belief that the advances in drug screening technology and applications of clinical study are still in the infancy of m6A-mediated anti-tumor therapy.
The joint advancement of ferroptosis research is also set to usher in a period of substantial improvement. This review wraps up current breakthroughs in ferroptosis regulation and the challenges associated with immunotherapy, as well as suggesting a novel method for m6A-mediated ferroptosis in immunotherapy. It is advisable to vigorously promote clinical trials of this combination therapy to evaluate its effectiveness and safety, therefore establishing a basis for further comprehensive investigations that will be advantageous for clinical patients. Despite substantial research into the processes of RNA m6A modification and recent therapeutic advancements in tumor progression, the broad use of medications targeting m6A regulation remains limited. The diverse range and intricate nature of interactions associated with m6A-regulated tumors impede the practical use of drugs that target m6A regulators in clinical therapy. Nevertheless, the precise mechanism of m6A-regulated ferroptosis in different types of tumors has been carefully investigated and is expected to be further improved. To optimize immunotherapy by inducing ferroptosis and modulating the tumor immune microenvironment in various tumor cells, forthcoming pharmaceutical research should give priority to crucial targets of m6A-regulated ferroptosis. Hence, it is anticipated that this innovative immunotherapy based on m6A-mediated ferroptosis will be formulated and implemented in the foreseeable future.
Acknowledgements
We thank Biorender (https://www.biorender.com/) for the assistance for the illustration.
Abbreviations
| ACC | Acetyl-CoA carboxylase |
| ACSL3 | Acyl-CoA synthetase long-chain family member 3 |
| ACSL-4 | Acyl-CoA synthetase long-chain family member 4 |
| AIFM2 | Apoptosis-inducing factor mitochondrial-related 2 |
| ALKBH5 | AlkB homolog 5 |
| ALOX | ACSL4-LPCAT3-arachidonic acid lipoxygenase |
| AML | Acute myeloid leukemia |
| APC | Antigen-processing cells |
| APOC1 | Apolipoprotein C1 |
| BAP1 | BRCA1-associated protein 1 |
| BC | Breast cancer |
| BDNF | Brain-derived neurotrophic factor |
| BMSCs | Bone marrow stem cells |
| CAR-T cells | Chimeric antigen receptor T cells |
| CCA | Cholangiocarcinoma |
| CISD1 | CDGSH iron sulfur domain 1 |
| CoQ10 | Ubiquinone |
| CRC | Colorectal cancer |
| CRCC | Clear cell renal cell carcinoma |
| CSCs | Cancer stem cells |
| CTLA-4 | Cytotoxic T lymphocyte antigen 4 |
| CuB | Cucurbitacin B |
| CYPs | Cytochrome P450 enzymes |
| DAMP | Damage-associated molecular pattern |
| DCs | Dendritic cells |
| DLL3 | Delta-like ligand 3 |
| DNMT3A | DNA methyltransferase 3A |
| EHT | Endothelial-to-hematopoietic transition |
| eIF3 | Eukaryotic initiation factor 3 |
| ERK | Extracellular signal regulated kinase |
| ETC | Electron transfer chain |
| FA | Fusaric acid |
| FAO | Fatty acid oxidation |
| FASL | Fas-Fas ligand |
| FGFR4 | Fibroblast growth factor receptor 4 |
| FLT4 | Fms Related Tyrosine Kinase 4 |
| FSP1 | Ferroptosis inhibitor protein 1 |
| FTH1 | Ferritin heavy chain 1 |
| FTMT | Ferritin mitochondrial |
| FTO | Fat mass and obesity-associated protein |
| FZD7 | Frizzled 7 |
| GBM | Glioblastoma |
| GE | Geftinib |
| GSCs | Glioblastoma Stem Cells |
| H2Aub | Histone 2A ubiquitination |
| HCC | Hepatocellular carcinoma |
| HES1 | Hairy and enhancer of split 1 |
| HINT-2 | Histidine triad nucleotide-binding protein 2 |
| HMGB1 | High-mobility group box 1 |
| HNRNPA2B1 | Heterogeneous nuclear ribonucleoprotein A2B1 |
| HNRNPC | Heterogeneous nuclear ribonucleoprotein C |
| HNSCC | Head and neck squamous cell carcinoma |
| HOXA13 | Homeo box A13 |
| ICIs | Immune checkpoint inhibitors |
| IDO | Indoleamine 2,3-dioxygenase-1 |
| IEG | Immediate-Early Gene |
| IFNγ | Interferon gamma |
| IGF2BP1 | Insulin-like growth factor 2 mRNA binding protein 1 |
| IGF2BP2 | Insulin-like growth factor 2 mRNA binding protein 2 |
| IGF2BP3 | Insulin-like growth factor 2 mRNA binding protein 3 |
| IR | Ionizing radiation |
| IRFA | Inadequate radiofrequency ablation |
| KEAP1 | Kelch-like ECH-associated protein 1 |
| LIP | Labile iron pool |
| L-KYN | L-kynurenine |
| LPS | Lipopolysaccharide |
| LUDA | Lung adenocarcinoma |
| m6A | N6-methyladenine |
| MA | Meclofenamic acid |
| MDSCs | Myeloid-derived suppressor cells |
| MHC | Major histocompatibility complex |
| MHC-II | Major histocompatibility complex class II |
| NEAT1 | Nuclear paraspeckle assembly transcript 1 |
| NET | Neutrophil extracellular trap |
| NK cells | Natural killer cells |
| NKAP | NF-κB activating protein |
| NOTCH3 | Neurogenic locus notch homolog protein 3 |
| NOX | NADPH oxidase |
| NPM1 | Nucleophosmin 1 |
| NRF2 | Nuclear factor erythroid factor 2-related factor 2 |
| NSCLC | Non-small cell lung cancer |
| PAAD | Pancreatic Adenocarcinoma |
| PD-1 | Programmed cell death 1 |
| PDAC | Pancreatic ductal carcinoma |
| PD-L1 | Programmed cell death ligand 1 |
| POR | P450 oxidoreductase |
| PTGS2 | Post-transcriptional gene silencing |
| PUFAs | Polyunsaturated fatty acids |
| RCD | Regulated cell death |
| ROS | Reactive oxidation species |
| SIAH2 | Seven in absentia homolog 2 |
| SLC11A2 | Solute carrier family 11 member 2 |
| SLC25A28 | Solute carrier family 25 member 28 |
| SLC25A37 | Solute carrier family 25 member 37 |
| SLC7A11 | Solute carrier family 7 member 11 |
| STAT1 | Signal transducer and activator of transcription 1 |
| STEAP3 | Six-transmembrane epithelial antigen of the prostate 3 |
| T-ALL | T-cell acute lymphoblastic leukaemia |
| TAMs | Tumor-associated macrophages |
| Tc9 | T lymphocyte subset 9 |
| TCA | Tricarboxylic acid |
| TDM2 | Type 2 diabetic mellitus |
| TF | Transferrin |
| TFR1 | Transferrin receptor 1 |
| Th17 | T helper cell 17 |
| TME | Tumor microenvironment |
| TNBC | Triple-negative breast cancer |
| TP53INP2 | Tumor protein p53 inducible nuclear protein 2 |
| TPO-R | Thrombopoietin receptor |
| Tregs | Regulatory T cells |
| TSCC | Tongue Squamous Cell Carcinoma |
| VIRMA | Vir-like m6A methyltransferase associated |
| WTAP | Wilms tumor 1-associated protein |
| YAP | Yes-associated protein |
| YTHDC1 | YTH domain-containing proteins 1 |
| YTHDC2 | YTH domain-containing proteins 2 |
| YTHDF1 | YTH domain family members 1 |
| YTHDF2 | YTH domain family members 2 |
| YTHDF3 | YTH domain family members 3 |
| ZC3H13 | Zinc finger CCCH-type containing 13 |
| α-KG | α-Ketoglutarate |
| γ-Glu-AAs | γ-Glutamyl peptides |
Authors’ contributions
YW, HL, and FY designed the review; JS, ZZ, HY, XP, CL, QL, JZ, WZ and FL searched for literature and wrote the manuscript; JS, YW, ZZ, HY, and XP drew the figures; YW, FY, HY, XP, and HL helped edit and revise the manuscript. HL, and FY provided funding support. All authors have read and approved the article.
Funding
This work was supported by the National Science and Technology Major Project (Nos. 2023ZD0500102), the National Natural Science Foundation of China (Nos. 82270634), and Clinical Young Talent Project, Eagle breeding Team of Meng Chao Tengfei Project (Eastern Hepatobiliary Surgery Hospital).
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Not applicable.
All authors have approved to publish this manuscript.
The authors declare no competing interests.
Footnotes
The original online version of this article was revised: Following publication of the original article [1], the authors noticed that the Funding information was not indicated in the article. The details of Funding were included in the revised manuscript that was submitted by the author to production system. The Funding information is given in the article correction "10.1186/s12943-024-02144-2".
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jun-xiao Shi, Zhi-chao Zhang, Hao-zan Yin, and Xian-jie Piao contributed equally to this work.
Change history
10/7/2024
The original online version of this article was revised: Following publication of the original article [1], the authors noticed that the Funding information was not indicated in the article. The details of Funding were included in the revised manuscript that was submitted by the author to production system. The Funding information is given in the article correction "10.1186/s12943-024-02144-2".
Change history
10/8/2024
A Correction to this paper has been published: 10.1186/s12943-024-02144-2
Contributor Information
Fu Yang, Email: nc.ude.umms@7991qsufgnay.
Yue-fan Wang, Email: moc.361@5102nafeuygnaw.
Hui Liu, Email: moc.liamtoh@ggiuhuil.
References
Articles from Molecular Cancer are provided here courtesy of BMC
Citations & impact
Impact metrics
Article citations
Correction: RNA m6A modification in ferroptosis: implications for advancing tumor immunotherapy.
Mol Cancer, 23(1):225, 08 Oct 2024
Cited by: 0 articles | PMID: 39380024 | PMCID: PMC11460104
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
Clinical Trials (Showing 6 of 6)
- (1 citation) ClinicalTrials.gov - NCT05961410
- (1 citation) ClinicalTrials.gov - NCT03994744
- (1 citation) ClinicalTrials.gov - NCT03701217
- (1 citation) ClinicalTrials.gov - NCT01042379
- (1 citation) ClinicalTrials.gov - NCT05584111
- (1 citation) ClinicalTrials.gov - NCT03709147
Show less
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Ferroptosis: a critical mechanism of N6-methyladenosine modification involved in carcinogenesis and tumor progression.
Sci China Life Sci, 67(6):1119-1132, 28 Feb 2024
Cited by: 0 articles | PMID: 38811442
Review
The role of RNA methylation in tumor immunity and its potential in immunotherapy.
Mol Cancer, 23(1):130, 20 Jun 2024
Cited by: 4 articles | PMID: 38902779 | PMCID: PMC11188252
Review Free full text in Europe PMC
m6A modification of RNA in cervical cancer: role and clinical perspectives.
RNA Biol, 21(1):49-61, 01 Jan 2024
Cited by: 0 articles | PMID: 39344658 | PMCID: PMC11445900
Review Free full text in Europe PMC
The regulatory mechanisms of N6-methyladenosine modification in ferroptosis and its implications in disease pathogenesis.
Life Sci, 355:123011, 23 Aug 2024
Cited by: 0 articles | PMID: 39181316
Review

 2,3,4
2,3,4 

