Abstract
Free full text

Occurrence of human infection with Salmonella Typhi in sub-Saharan Africa
Abstract
Typhoid fever, caused by Salmonella enterica serovar Typhi, results in over 1.2 million cases and 29 thousand deaths annually from sub-Saharan Africa. Combating this disease requires various intervention approaches, such as typhoid conjugate vaccines and improving water, sanitation, and hygiene. Enhancing the effectiveness of these strategies necessitates a deeper understanding of the variation of the typhoid fever across the target region. Although the magnitude and variation of typhoid fever at the country level have been studied globally, sub-national variation remains underexplored. To address this gap, we collected data from 229 published reports on typhoid fever occurrences in sub-Saharan Africa between January 2000 and December 2020. The dataset includes information on the year and geographical location of observation, diagnostic tests used, and the type of studies in which typhoid fever was reported. By analyzing this dataset, we can gain insights into the sub-national heterogeneity of typhoid fever’s burden in the region. This knowledge will be instrumental in designing more effective intervention strategies to combat the disease.
Background & Summary
Typhoid fever is a systemic bacterial infection caused by Salmonella enterica serovar Typhi (Salmonella Typhi or S. Typhi). Patients usually present sustained fever (39–40 °C) and other symptoms include weakness, stomach pain, headache, diarrhea or constipation, cough, and loss of appetite. Severe forms of illness include illeal perforation, which can lead to death. S. Typhi is transmitted via fecally-contaminated food and water and the majority of typhoid fever incidence is known to occur in low- and middle-income countries (LMIC)1–6.
°C) and other symptoms include weakness, stomach pain, headache, diarrhea or constipation, cough, and loss of appetite. Severe forms of illness include illeal perforation, which can lead to death. S. Typhi is transmitted via fecally-contaminated food and water and the majority of typhoid fever incidence is known to occur in low- and middle-income countries (LMIC)1–6.
Globally, typhoid fever causes estimated 12 million cases and 130 thousand deaths according a recent modelling study in which incidence rate data come from population-based longitudinal surveillance studies conducted at 22 sites in 14 different countries between 1978 and 20171. Earlier estimates of the global burden of typhoid fever were based on a relatively simplistic approach of extrapolating the incidence rates observed in one setting to the entire country or to neighbouring countries where data is unavailable2–4. However, the surveillance is often conducted at sites where the disease incidence has already been reported and therefore would not necessarily well represent the country5. Later studies used sophisticated modelling techniques to adjust observed values using the distribution of geospatial variables that potentially affect the transmission of typhoid fever rather than simple extrapolaton1,6.
While they clearly show variation at the country level, existing studies fail to emphasize that the burden of typhoid fever also shows significant sub-national variation for each country. Outbreaks often show district-level variation of typhoid incidence7–9 and country-level surveys show sub-national heterogeneity of incidence10–13. Understanding idiosyncratic behaviour of typhoid transmission between communities will be critical for a country to implement intervention programs such as campaign vaccination against endemic or epidemic typhoid fever more efficiently and effectively. Efficiency and effectiveness of an intervention program can improve, for instance, if the interventions are targeted on high-burden areas. Identifying those high-burden areas will depend on how well we understand the spatial variation of the burden of typhoid fever across the target country.
Although population-based prospective studies serve as the basis for existing estimates of country-level and global burden of typhoid fever, report of sporadic cases and outbreaks provide wider spatial coverage and hold information on sub-national variation of occurrence of typhoid fever14. In this study, we provide the data set on the occurrence of typhoid fever extracted from peer-reviewed literature. We focus on sub-Saharan Africa, where systematic surveillance has shown that the burden is substantial1,2,5,6. The dataset we report provides information on the occurrence of typhoid fever at the hospital, district, or higher sub-national administrative levels. While these datasets alone may not fully capture sub-national variations in typhoid fever, they can be enhanced by grey literature. Nonetheless, they hold potential to improve our understanding of sub-national heterogeneity in typhoid fever, especially when combined with other methods15.
Methods
Search and data sources
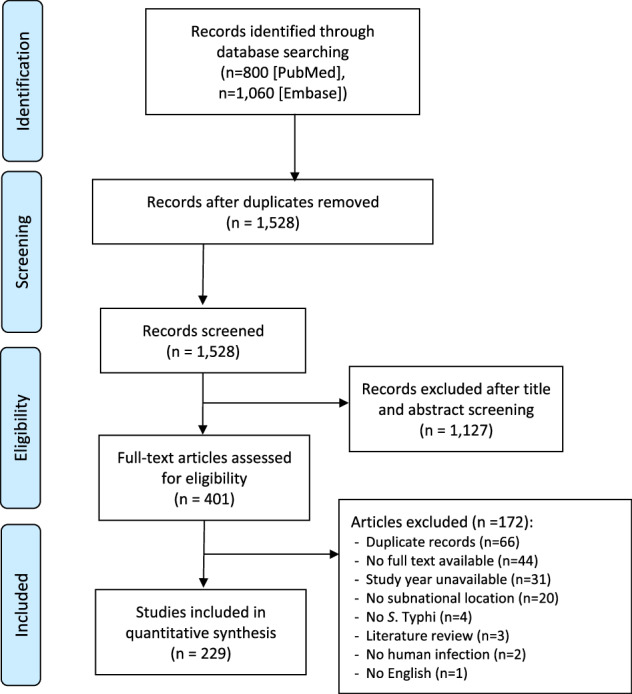
Data on the occurrence of typhoid fever were extracted from peer-reviewed research articles indexed in PubMed or Embase published between January 1, 2000 and October 31, 2020. Systematic review of the literature followed a standard procedure and the overview of the procedure in a Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram (Fig. 1). The search term used for querying PubMed and Embase was designed to capture different uses of typhoid fever in the literature such as typhoid fever, Salmonella typhi, S. Typhi, or enteric fever and limit to the countries from sub-Saharan Africa. The exact search terms appear in the Supplement.
Eligibility criteria and study selection
A study, regardless of study types, was eligible for inclusion in this analysis if it clearly reports date and location of occurrence as well as diagnostic method used for confirmation of the occurrence of human typhoid cases. We included reports of polymerase chain reaction (PCR) or culture-confirmed typhoid fever where S. Typhi was isolated from normally sterile sites such as blood, urine, bone marrow, or cerebrospinal fluid. We also included studies that confirm typhoid fever through serologic tests (e.g., Widal test or enzyme-linked immunosorbent assay [ELISA]) or suspected clinically (e.g., ileal perforation) while acknowledging that those case definitions are less reliable compared with culture or PCR confirmation. We excluded the studies that are based on the analyses of existing data and do not report novel occurrence of typhoid fever in humans. For instance, some articles report results of experiments using the existing isolates (e.g., susceptibility to antimicrobials or other medicinal plants) and were therefore excluded. Other studies fail to provide serovars while reporting infection Salmonella.
Data extraction, study variables, and analytic approach
Two authors (J-HK and PP) reviewed the literature and extracted the data. Where there was discordance among the two reviewers, the first author decided after discussions. Extracted data included year of observation, location (smallest sub-national area possible), diagnostic method, and the number of typhoid cases reported. In reports of typhoid cases from observations that span multiple years without further details broken down by year, we assumed that at least 1 episode of typhoid fever case occurred each year. We defined a typhoid fever occurrence as a report of at least 1 episode of typhoid fever excluding any duplicate reports from the same cohort. For imported cases, we assumed that the typhoid episode occurred in the country in which the infection occurred according to the report. If the imported cases led to local transmission, we assumed that typhoid occurred in both the country of infection origin and the country of report. An outbreak was defined based on the definition used in the report.
Data Records
Occurrence data were stored in an Excel spreadsheet where each row indicates an occurrence event of typhoid fever for specific year and location. Some occurrence events reported from a larger region may overlap those from smaller sub-regions within a region. Columns indicate data source (e.g., title of the article), years of observation and report, diagnostic method, number of cases reported, sub-national region (up to the smallest units available), study type, longitude and latitude which were acquired through Google Map. Locations may refer to the arbitrary central point of the region (e.g., neighbourhoods, village) or the location of a healthcare facility. Study types were categorized into review of hospital records, case report, outbreak reports, and longitudinal studies. The dataset is available at the Open Science Framework16.
Technical Validation
To ensure the overall quality of the data, a thorough review process was implemented. Two independent reviewers meticulously examined the data. The reported locations of typhoid occurrences were verified by cross-referencing information from the organization’s website and other reliable sources, including Wikipedia. Additionally, the dates of occurrence were confirmed by carefully reviewing the texts and tables provided in the original articles. This multi-step validation process was crucial to ensure the accuracy and reliability of the data used in this study. We provide an overview of the included studies by country and year of publication. Also, we provide more details on the diagnostic methods used to confirm the infection, types of studies in which typhoid fever were reported, year, and sub-national location of occurrence of typhoid fever.
Frequency of studies by year and country
Occurrence of confirmed typhoid fever were reported in 31 countries from Jan 2000 to Dec 2020 with overall reporting frequency increasing over time (Fig. 2A). The number of reports varies by study and year of publication (Fig. 2B) and by year in which the study began (Fig. 2C) considerably. Nigeria consistently reported the highest number of reports on the occurrence of typhoid fever over the period. For countries like Angola, Benin, Burundi, Guinea-Bissau, Liberia, Madagascar, Niger, Uganda, Zambia and Zimbabwe, typhoid fever has been reported after 2010 considering the period from 2000 to 2020.
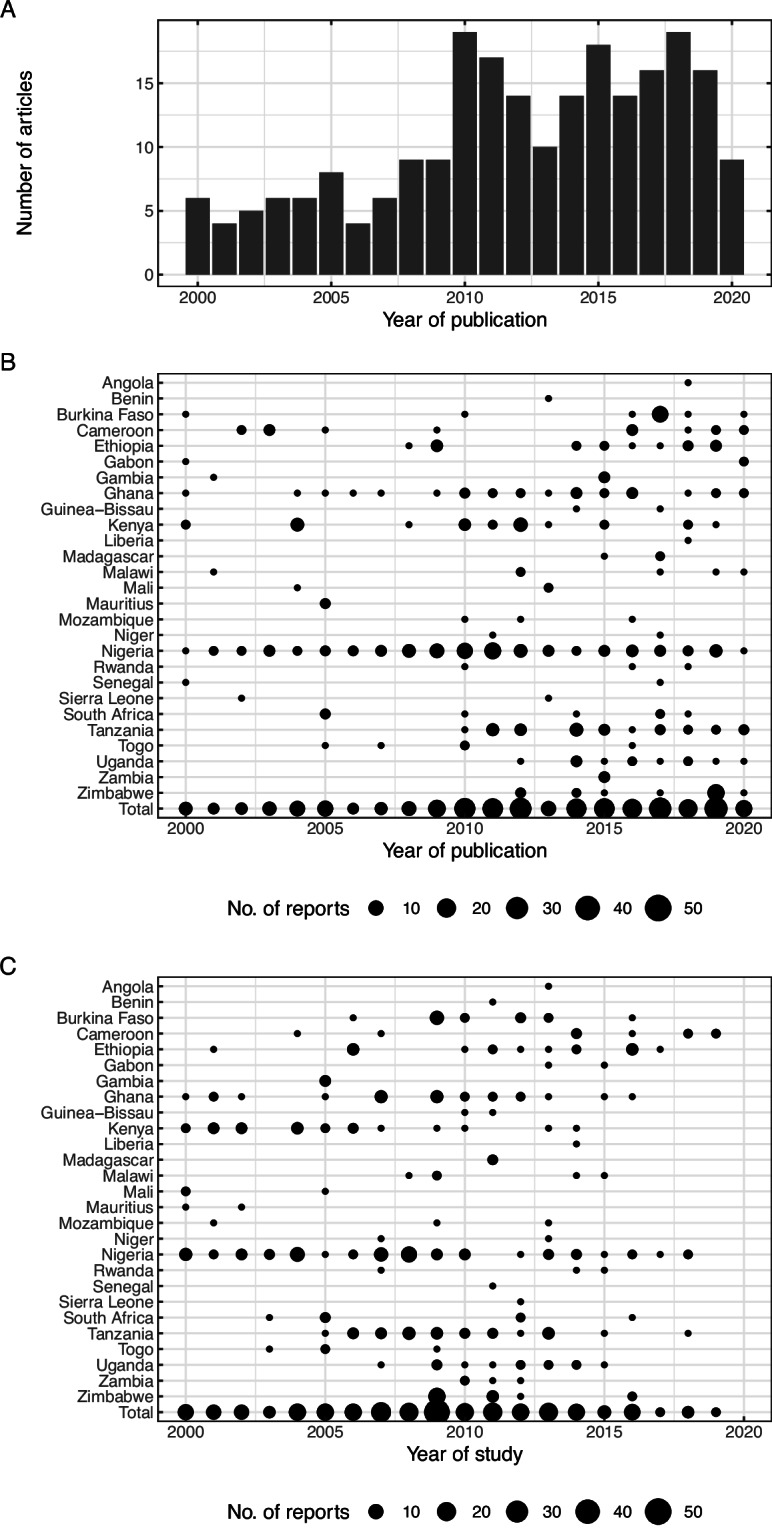
Number of included reports by year and country. The overall number of reports of typhoid occurrence in sub-Saharan Africa increases over time with considerable variation by year of publication (A) and country (B). The number of studies showed substantial variation also by country and the year in which the study began (C).
Diagnostic methods
We categorized the studies based on the diagnostic method employed to identify typhoid fever (Table 1). In instances where a single study utilized multiple diagnostic methods (e.g., clinical criteria, blood culture, and bone marrow sample culture), we prioritized the method with the higher performance. Specifically, if the study included the method such as the culture of bone marrow or cerebrospinal fluid samples, which is recognized as the highest sensitivity, we selected this method as the representative method of the study. Similarly, a study incorporated blood culture but did not included methods like the bone marrow culture, we chose the blood culture as the representative diagnostic method. If the methods included other culture methods, we chose the methods in the order of culture of urine samples, culture of stool samples, and culture of other body fluids. Other studies did not specifically mention the samples used to culture but implied the culturing method (e.g., “bacterial isolation” or “microbiologically confirmed”). In this case, we created a category called “Culture”. For studies whose diagnostic methods do not include culturing method, we chose the method in an order of Rapid tests, Widal, biochemical tests, and clinical symptoms. While we sought to categories by the precision of the tests, this category maybe somewhat arbitrary. The reader can access the original terms that indicated multiple methods used for each study.
Table 1
Frequency of studies by diagnostic methods. CSF =
= Cerebrospinal fluid.
Cerebrospinal fluid.
| Diagnostic method | Frequency [n (%)] | Samples of actual terms used in the literature |
|---|---|---|
| Culture of blood samples | 91 (39.7) | “urine, blood, or stool culture”, “blood or stool culture, or Widal-Felix”, “blood/urine/stool culture”, etc. |
| Clinical symptoms | 66 (28.8) | “typhoid perforation”, “intestinal perforation”, “clinical diagnosis”, “digestive perforation”, “typhoid gall bladder perforation”, “cholecystitis”, “clinical”, “hospital record”, “ileal perforation”, “multiple jejunal perforations “, “perforated enteritis”, “peritonitis”, “small bowel perforation”, “enterocutaneous fistula”, “septicaemia”, “case files”, “cases reported”, “death records”, “typhoid fever”, etc. |
| Widal test | 24 (10.5) | “Widal test”, “agglutination and titration (>4-fold)”, “Widal-Felix”, “Widal and Weilfelix direct card agglutination tests (DCAT)”, “Tube agglutination test”, etc. |
| Culture of stool samples | 14 (6.1) | “stool culture”, etc. |
| Culture | 12 (5.2) | “Isolation of Salmonella typhi”, “culture”, “isolated”, “bacterial isolation”,“microbiologically confirmed”, “laboratory confirmed”, “confirmed”, etc. |
| Unclear | 9 (3.9) | “Survey questionnaire”, “surveillance data”, “National Health Laboratory Services data”, “standard methods with blood samples”, “travel or tropical medicine clinics record”, “history based on self report”, “questionaire”, “report at medical record”, “self reported” |
| Culture of CSF samples | 3 (1.3) | “cerebrospinal fluid culture”, “spinal fluid culture”, “isolation from CSF, blood, or stool”, etc. |
| Autopsy | 3 (1.3) | “post morten report”, “autopsy”, “coroner’s autopsies” |
| Culture of bone marrow samples | 2 (0.9) | “blood, stool or bone marrow culture”, “blood and bone marrow cultures”, etc. |
| Rapid test | 2 (0.9) | “rapid typhoid test”, “Blood-RDT”, “rapid diagnostic test”, “RDT”, etc. |
| Culture of other body fluids | 1 (0.4) | “aspirates culture”, “culture of pus”, “wound culture”, “wound swab culture”, “Ear and throat culture”, “Sputum culture”, “rectal swab specimens examined bacteriologically”, etc. |
| Biochemical test | 1 (0.4) | “biochemical”, “biochemical testing of urine sample”, “biochemical tests of environmental samples”, etc. |
| Culture of urine samples | 1 (0.4) | “urine culture” |
According to this categorization, studies based on culturing blood samples were most frequent (n =
= 91, 39.7%). (Table 1) while still numerous studies relied on less reliable methods, which include clinical signs (e.g., illeal perforation) (n
91, 39.7%). (Table 1) while still numerous studies relied on less reliable methods, which include clinical signs (e.g., illeal perforation) (n =
= 66, 26.8%) or Widal test (or agglutination) (n
66, 26.8%) or Widal test (or agglutination) (n =
= 24, 10.5%).
24, 10.5%).
Study types
Study design was diverse and included case reports, outbreak investigation, cross-sectional studies, retrospective studies (i.e., review of hospital records), and prospective studies including multi-year longitudinal surveillance studies. While population-based longitudinal surveillance studies can provide incidence rates of typhoid fever, they are few (n =
= 5) and were conducted around 15 sub-national areas of 10 countries (Table 2). On the other hand, there are other studies that report occurrence of typhoid fever and wider spatial coverage in the dataset. Prospective studies including clinical trials were most common (n
5) and were conducted around 15 sub-national areas of 10 countries (Table 2). On the other hand, there are other studies that report occurrence of typhoid fever and wider spatial coverage in the dataset. Prospective studies including clinical trials were most common (n =
= 93), followed by retrospective (n
93), followed by retrospective (n =
= 74), cross-sectional (n
74), cross-sectional (n =
= 33), outbreak investigation (n
33), outbreak investigation (n =
= 13), case-control (n
13), case-control (n =
= 8), and case report (n
8), and case report (n =
= 8) studies.
8) studies.
Table 2
Population-based longitudinal surveillance of typhoid fever in sub-Saharan Africa since 2000.
| Country | Sub-national region | Surveillance period | Ref |
|---|---|---|---|
| Burkina Faso | Nanoro, Boulkiemde | 2013–2014 | 18 |
| Burkina Faso | Nioko II, Polesgo | 2012–2013 | 5 |
| Ethiopia | Butajira | 2012–2014 | 5 |
| Ghana | Asante Akim North | 2007–2009 | 19 |
| Ghana | Asante Akim North | 2010–2012 | 5 |
| Guinea-Bissau | Bandim | 2011–2013 | 5 |
| Kenya | Lwak, Kibera | 2006–2009 | 13 |
| Kenya | Kibera | 2012–2013 | 5 |
| Madagascar | Imerintsiatosika, Isotry | 2011–2013 | 5 |
| Senegal | Pikine | 2011–2013 | 5 |
| South Africa | Pietermaritzburg | 2012–2014 | 5 |
| Sudan | East Wad Medani | 2012–2013 | 5 |
| Tanzania | Pemba Island | 2010–2012 | 20 |
| Tanzania | Moshi urban, Moshi rural | 2011–2013 | 5 |
Geographical locations
Occurrence data cover entire continent although occurrences are more frequent in East and West Africa. Top three countries in terms of the number of unique 20 km by 20
km by 20 km grids where the occurrence was reported were Nigeria (n
km grids where the occurrence was reported were Nigeria (n =
= 65), Kenya (n
65), Kenya (n =
= 24), and United Republic of Tanzania (n
24), and United Republic of Tanzania (n =
= 23), and Ghana (n
23), and Ghana (n =
= 19) (Fig. 3). Sub-national locations in the dataset includes household, primary clinic, tertiary hospitals, district, or provinces. Occurrence of typhoid fever has been reported in small number of large hospitals and cities with substantial variation across countries. In Angola, National Institute of Public Health of Angola located in the Capital city, Luanda is the only place in which blood culture-confirmed typhoid fever was reported. On the other hand, more than 55 health centers and hospitals spread out through the country.
19) (Fig. 3). Sub-national locations in the dataset includes household, primary clinic, tertiary hospitals, district, or provinces. Occurrence of typhoid fever has been reported in small number of large hospitals and cities with substantial variation across countries. In Angola, National Institute of Public Health of Angola located in the Capital city, Luanda is the only place in which blood culture-confirmed typhoid fever was reported. On the other hand, more than 55 health centers and hospitals spread out through the country.
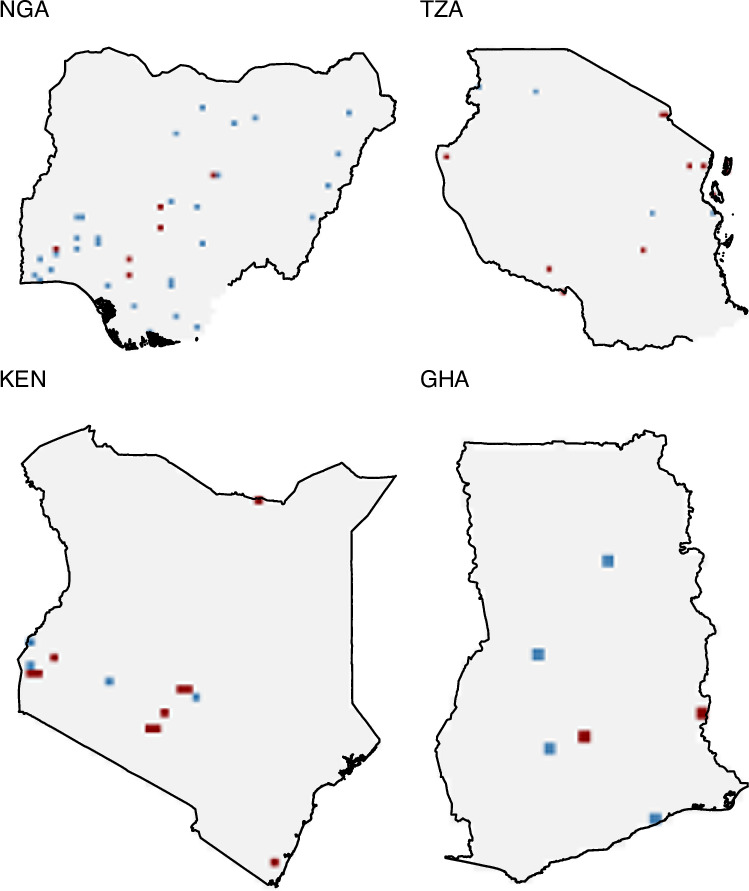
Geographical locations of occurrence of typhoid fever for four countries where the number of reports of typhoid fever is highest. The map has 20 km by 20
km by 20 km resolution. Red and blue grids represent locations of typhoid fever confirmed through culture and other methods, respectively. NGA
km resolution. Red and blue grids represent locations of typhoid fever confirmed through culture and other methods, respectively. NGA =
= Nigeria; TZA
Nigeria; TZA =
= United Republic of Tanzania; KEN
United Republic of Tanzania; KEN =
= Kenya; GHA
Kenya; GHA =
= Ghana.
Ghana.
Acknowledgements
This work was supported, in whole or in part, by Gavi, the Vaccine Alliance, and the Bill & Melinda Gates Foundation, via the Vaccine Impact Modelling Consortium (Grant Number OPP1157270/INV-009125) and the Severe Typhoid Fever in Africa Program (Grant Number OPP1127988). The funders were not involved in the study design, data analysis, data interpretation, and writing of the manuscript. The authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy, or views of their affiliated organizations.
Author contributions
J.-H.K. designed the study, performed the literature search, reviewed the articles, extracted the data, and wrote the manuscript. P.P. reviewed the articles, extracted data, and reviewed the manuscript. All authors reviewed the final version of the manuscript and approved its submission.
Code availability
All the codes used to generate the figures and tables are available in the GitHub repository17
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41597-024-03912-x.
References
Articles from Scientific Data are provided here courtesy of Nature Publishing Group
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
What Have We Learned From the Typhoid Fever Surveillance in Africa Program?
Clin Infect Dis, 62 Suppl 1:S1-3, 01 Mar 2016
Cited by: 13 articles | PMID: 26933014 | PMCID: PMC4772830
Global diversity and antimicrobial resistance of typhoid fever pathogens: Insights from a meta-analysis of 13,000 Salmonella Typhi genomes.
Elife, 12:e85867, 12 Sep 2023
Cited by: 21 articles | PMID: 37697804 | PMCID: PMC10506625
Incidence of invasive salmonella disease in sub-Saharan Africa: a multicentre population-based surveillance study.
Lancet Glob Health, 5(3):e310-e323, 01 Mar 2017
Cited by: 164 articles | PMID: 28193398 | PMCID: PMC5316558
Typhoid fever in sub-Saharan Africa: challenges of diagnosis and management of infections.
J Infect Dev Ctries, 2(6):443-447, 01 Dec 2008
Cited by: 10 articles | PMID: 19745521
Review
Funding
Funders who supported this work.
Bill and Melinda Gates Foundation (1)
Grant ID: OPP1157270
Bill and Melinda Gates Foundation (Bill & Melinda Gates Foundation) (1)
Grant ID: OPP1157270

 1
1