Abstract
Introduction
Growing evidence suggests a role for neuroinflammation in Alzheimer's disease (AD). We investigated complement pathway activity in AD patient cerebrospinal fluid (CSF) and evaluated its modulation by the anti-tau antibody semorinemab.Methods
Immunoassays were applied to measure CSF complement proteins C4, factor B (FB), C3 and their cleavage fragments C4a, C3a, and factor Bb (Bb) in AD patients and a separate cognitively unimpaired (CU) cohort.Results
All measured CSF complement proteins were increased in AD versus CU subjects, with C4a displaying the most robust increase. Finally, semorinemab did not have a significant pharmacodynamic effect on CSF complement proteins.Discussion
Elevated levels of CSF C4a, C4, C3a, C3, Bb, and FB are consistent with complement activation in AD brains. Despite showing a reduction in CSF soluble tau species, semorinemab did not impact complement protein levels or activity. Further studies are needed to determine the value of complement proteins as neuroinflammation biomarkers in AD.Highlights
Cerebrospinal fluid (CSF) complement proteins C4a, C3a, Bb, C4, C3, and factor B levels were increased in Alzheimer's disease (AD) patients compared to a separate cognitively unimpaired (CU) cohort. Baseline CSF complement protein levels were correlated with neuro-axonal degeneration and glial activation biomarkers in AD patients. The investigational anti-tau antibody semorinemab did not impact CSF complement protein levels or activity relative to the placebo arm.Free full text

CSF complement proteins are elevated in prodromal to moderate Alzheimer's disease patients and are not altered by the anti‐tau antibody semorinemab
Abstract
INTRODUCTION
Growing evidence suggests a role for neuroinflammation in Alzheimer's disease (AD). We investigated complement pathway activity in AD patient cerebrospinal fluid (CSF) and evaluated its modulation by the anti‐tau antibody semorinemab.
METHODS
Immunoassays were applied to measure CSF complement proteins C4, factor B (FB), C3 and their cleavage fragments C4a, C3a, and factor Bb (Bb) in AD patients and a separate cognitively unimpaired (CU) cohort.
RESULTS
All measured CSF complement proteins were increased in AD versus CU subjects, with C4a displaying the most robust increase. Finally, semorinemab did not have a significant pharmacodynamic effect on CSF complement proteins.
DISCUSSION
Elevated levels of CSF C4a, C4, C3a, C3, Bb, and FB are consistent with complement activation in AD brains. Despite showing a reduction in CSF soluble tau species, semorinemab did not impact complement protein levels or activity. Further studies are needed to determine the value of complement proteins as neuroinflammation biomarkers in AD.
Highlights
Cerebrospinal fluid (CSF) complement proteins C4a, C3a, Bb, C4, C3, and factor B levels were increased in Alzheimer's disease (AD) patients compared to a separate cognitively unimpaired (CU) cohort.
Baseline CSF complement protein levels were correlated with neuro‐axonal degeneration and glial activation biomarkers in AD patients.
The investigational anti‐tau antibody semorinemab did not impact CSF complement protein levels or activity relative to the placebo arm.
1. BACKGROUND
Alzheimer's disease (AD), the predominant form of dementia, is neuropathologically characterized by extracellular amyloid beta (Aβ) plaques and intracellular neurofibrillary tau tangles (NFTs). 1 In addition, activated glial cells have been observed around affected neurons and Aβ plaques and AD risk genes that are enriched in glia. 2 These findings suggest that neuroinflammation may represent a third hallmark of AD pathogenesis. 3 , 4
The complement system is a component of the innate immune system and comprises three pathways. While the classical pathway (CP) is activated by immune complexes and other structures, lectin pathway (LP) activation is promoted by carbohydrates on pathogen surfaces. Both separate pathways induce the cleavage of C4 into C4a and C4b, followed by the formation of a C3 convertase, cleaving C3 into C3a and C3b. The alternative pathway (AP) involves interactions between spontaneously hydrolyzed C3 with factor B (FB) and factor D, leading to the formation of a C3 convertase. All pathways converge onto the formation of a C5 convertase, which initiates the osmolysis of cells or pathogens through the terminal pathway (TP). C3a and C5a act as pro‐inflammatory anaphylatoxins, while the opsonins C4b and C3b promote the phagocytosis of invading organisms and altered self. 5
Transcriptomics and proteomics analyses reveal increased brain expression of CP components in humans with AD and mouse models of amyloidosis and tauopathy. 6 , 7 The inhibition/deletion of complement C1Q and C3 rescues synapse loss in AD mouse models, suggesting that the CP contributes to glia‐mediated synapse phagocytosis. 7 , 8 , 9 There is thus a growing interest to further characterize complement activation and evaluate complement‐targeting therapeutics in AD. Previous publications investigating complement proteins in AD cerebrospinal fluid (CSF) showed mixed results, with some (but not all) studies reporting increased complement levels in AD (vs. healthy controls). 10 , 11 Potential drivers of these inconsistencies include small study sizes and differences in diagnostic criteria, preanalytical procedures, and analytical methods. Notably, only a small number of studies investigated CSF levels of the complement activation (cleaved) products. 7 , 8 , 12 , 13 , 14 , 15 , 16
Semorinemab is a humanized immunoglobulin G (IgG)4 monoclonal antibody that binds to the N‐terminal region of tau. The safety and efficacy of semorinemab have been evaluated in two phase 2 studies, Tauriel (NCT03289143) and Lauriet (NCT03828747), in prodromal to mild (P2M) and mild to moderate (M2M) AD patients, respectively. 17 , 18 In Tauriel, semorinemab did not slow clinical progression relative to placebo, as measured by the primary endpoint, the Clinical Dementia Rating Sum of Boxes (CDR‐SB) score. 17 In Lauriet, the semorinemab arm had a significant reduction in cognitive decline measured by the Alzheimer's Disease Assessment Scale‐Cognitive Subscale 11 (ADAS‐Cog11, co‐primary endpoint) relative to placebo. However, no treatment effect was observed on the functional outcome of the Alzheimer's Disease Cooperative Study‐Activities of Daily Living scale (ADCS‐ADL; co‐primary endpoint) or on any of the secondary endpoints. Although CSF tau indices were significantly reduced in Tauriel and Lauriet, semorinemab did not prevent the accumulation of NFTs as measured by Genentech Tau Probe 1 ([18F]GTP1). The cognitive benefit observed in Lauriet, in the absence of a tau positron emission tomography (PET) effect, raises the hypothesis that semorinemab may alleviate cognitive dysfunction by reducing soluble toxic tau species. 18 Recent studies propose the presence of aberrant oligomeric tau species within synapses and their involvement in glia‐mediated synapse engulfment in the AD brain. 19 , 20 We hypothesized that semorinemab may impact synapse loss through a modulation of complement pathway activity.
Here, we examined complement proteins reflecting CP/LP activity (C4, C4a); AP activity (FB, Bb) as well as C3 activation downstream CP/LP/AP activity (C3, C3a) in CSF from cognitively unimpaired (CU) as well as P2M and M2M AD patients (Figure 1A). We investigated the relationship between CSF complement levels and neurodegeneration/neuroinflammation biomarkers, tau PET, magnetic resonance imaging (MRI), and clinical outcomes in AD patients at baseline. Moreover, we assessed the pharmacodynamic effect of semorinemab on the change from baseline of CSF complement proteins in AD patients.
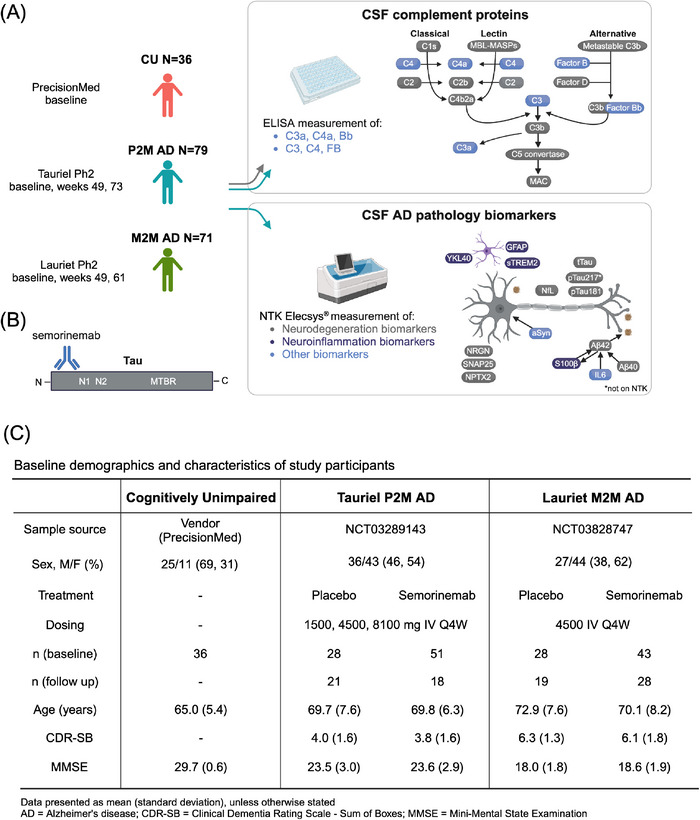
A, CSF complement protein levels from CU (n = 36), P2M (Tauriel, n = 79), and M2M (Lauriet, n = 71) participants were measured by immunoassay. CSF neurodegeneration and neuroinflammation biomarkers were measured for the two AD groups using the Roche NTK Elecsys in vitro diagnostic immunoassays (Aβ42, t‐tau, p‐tau181), and ELISA (p‐tau217). B, Semorinemab is an IgG4 antibody binding to the N‐terminal region (residues 6‐23) of all six isoforms of human tau, including phosphorylated species. C, Baseline demographics and characteristics of the CU, P2M AD, and M2M AD subjects included in this study. This figure was created with BioRender.com. Aβ, amyloid beta; AD, Alzheimer's disease; aSyn, a‐synuclein; CDR‐SB, Clinical Dementia Rating Sum of Boxes; CSF, cerebrospinal fluid; CU, cognitively unimpaired; ELISA, enzyme‐linked immunosorbent assay; GFAP, glial fibrillary acidic protein; IL‐6, interleukin 6; M2M, mild to moderate Alzheimer's disease; MMSE, Mini‐Mental State Examination; NfL, neurofilament light; NPTX2, neuronal pentraxin 2; NRGN, neurogranin; NTK, NeuroToolKit; P2M, prodromal to mild Alzheimer's disease; p‐tau, phosphorylated tau; S100b, S100 calcium‐binding protein B; SNAP25, synaptosomal associated protein 25; sTREM2, soluble triggering receptor expressed on myeloid cells 2; t‐tau, total tau; YKL‐40, chitinase‐3‐like protein.
2. METHODS
2.1. Participants and sample collection
2.1.1. CU subjects
CU CSF samples (from subjects with Mini‐Mental State Examination [MMSE] scores of ≥ 28) were procured from PrecisionMed. We confirmed the amyloid‐negative status by analysis of the CSF Aβ42/40 ratio. Only subjects with CSF Aβ42/40 ratios > 0.06 were included in this study (Figure S1 in supporting information 21 ). CSF was collected via lumbar puncture and any visibly blood‐contaminated samples were discarded prior to centrifugation for 10 minutes at 1200 g at room temperature (RT). The supernatant was stored in 1 mL aliquots at −80°C. A subset of samples were frozen prior to aliquoting.
2.1.2. P2M and M2M AD subjects from the semorinemab phase 2 Tauriel and Lauriet trials
AD CSF samples were collected from the semorinemab phase 2 studies Tauriel in P2M AD; and Lauriet in M2M AD. Tauriel, conducted from October 2017 to July 2020, included 457 participants in a randomized double‐blind 18‐month course of intravenous infusions of 1500, 4500, or 8100 mg of semorinemab (or placebo) every 2 weeks for the first three infusions, followed by infusions every 4 weeks.
17
The longitudinal analysis performed in this study only included the placebo and 4500 mg semorinemab arms. CSF was collected at baseline/screening, and follow‐up weeks 49 and 73. Lauriet, conducted from December 2018 to February 2020, included 272 participants in a randomized, placebo‐controlled double‐blind study of intravenous infusions of 4500 mg of semorinemab (or placebo) every 2 weeks for the first three infusions, followed by every 4 weeks for 48 or 60 weeks.
18
CSF was collected at baseline/screening, and follow‐up weeks 49 and 61. AD diagnosis was established based on confirmation of cognitive impairment by Clinical Dementia Rating Global Score (CDR‐GS) of 0.5 or 1 for Tauriel and 1 or 2 for Lauriet, MMSE clinical scores of 20 to 30 for Tauriel and 16 to 21 for Lauriet (inclusive), and amyloid pathology by PET scan or CSF Aβ(1‐42) ≤ 1000 pg/mL by Elecsys β‐Amyloid (1‐42) CSF immunoassay (Roche Diagnostics International Ltd).
17
,
18
CSF was collected at the study sites via lumbar puncture directly into sterile, low‐binding tubes. The first 1 to 2 mL, or more if necessary, of CSF, was discarded until blood cleared from the CSF. After collection, CSF was mixed by gently inverting the tubes, and then centrifuged for 10 minutes at 2000 × g at 4°C. Supernatant was collected and aliquoted in 0.5 mL amounts, then stored at −70°C.
For the cross‐sectional analyses, baseline CSF samples from 79 Tauriel and 71 Lauriet participants were used. The longitudinal analyses included 39 (21 placebo, 18 semorinemab 4500 mg) and 47 (19 placebo, 28 semorinemab 4500 mg) participants from Tauriel and Lauriet, respectively, where baseline and one of the follow‐up visits from week 49 or 73 for Tauriel and week 49 or 61 for Lauriet were used. Note that only a subset of samples with both baseline and follow‐up CSF were available at the time of this study. Thus, the sample number included in the longitudinal analysis differs from Teng et al. 17 and Monteiro et al. 18 Moreover, the number of available protein measurements per participant varies due to failed quality control (see acceptance criteria in the assay sections below).
2.2. Aβ42/40 ratio measurements in CU CSF samples
CSF concentrations of Aβ(1‐40) were measured using INNOTEST β‐AMYLOID(1‐40; Cat. No. 81585; Fujirebio) at Genentech. Dilution linearity testing with CU CSF demonstrated percent coefficient of variation (%CV) < 15% for dilution factors of 1:100 to 1:200 measured in duplicate. Samples were diluted 1:100 and measured following the manufacturer's protocol. Concentrations were interpolated from a 8‐point calibration curve ranging from 7.8 to 1000 pg/mL. Acceptance criteria required < 20%CV for duplicate sample measurements.
CSF concentrations of Aβ(1‐42) were measured using INNOTEST β‐AMYLOID(1‐42; Cat. No. 81583; Fujirebio) at Genentech. Samples were measured neat following the manufacturer's protocol. Concentrations were interpolated from a 6‐point calibration curve ranging from 62.5 to 4599.2 pg/mL. Acceptance criteria required < 20%CV for duplicate sample measurements.
2.3. p‐Tau217 measurements in P2M and M2M CSF samples
CSF concentrations of phosphorylated tau (p‐tau217) were measured at Genentech using a custom single molecule array assay (Simoa, Quanterix Corp). The reagents consisted of paramagnetic carboxylated beads coated with a rabbit monoclonal antibody specific for the phosphorylated T217 epitope of tau (Roche Diagnostics GmbH) and a biotinylated mouse monoclonal detection antibody specific for the mid‐domain of tau (125B11H3, Genentech, Inc.). Bead and antibody conjugations used the standard concentration and challenge ratios recommended by Quanterix, and the assays were run using a standard two‐step protocol on a Simoa HD‐1 instrument (Quanterix). In the protocol, antibody‐coated capture beads were incubated with diluted CSF (1:4) and the biotinylated detection antibody. After a wash step, the immunocomplexes were incubated with streptavidin‐conjugated β‐galactosidase (Quanterix), washed, re‐suspended in resorufin β‐D‐galactopyranoside (Quanterix), and then applied to Simoa discs. The HD‐1 analyzer was then used to read the resulting fluorescent signal and calculate the average number of enzymes per bead (AEB) for tested samples, which were analyzed against an 8‐point calibration curve constructed from GSK‐3β‐phosphorylated recombinant tau441 (SRP0689, Sigma‐Aldrich) ranging from 0.041 to 90 pg/mL. Samples were analyzed in duplicate using a single batch of reagents, with all available samples from a single participant measured in the same run. Qualification analyses demonstrated good detectability in human CSF (1.7–16.6 pg/mL, n = 10 samples), inter‐run precision (0.7%–12%, n = 10 samples, 2 runs), and parallelism (dilution linearity from 1:2 to 20, n = 10 samples). The assay was not impacted by the presence of semorinemab (tested up to 6 mg/mL, which was ≈ 1000 times the mean semorinemab trough concentration in CSF at weeks 49 and 73 after an 8100 mg dose). Acceptance criteria required < 20%CV for duplicate sample measurements.
2.4. Elecsys and NeuroToolKit measurements in P2M and M2M CSF samples
Concentrations of CSF Aβ42, total tau (t‐tau), and p‐tau181 were measured blinded with the CE‐marked Elecsys β‐Amyloid (1‐42) CSF, Total‐Tau CSF, and Phospho‐Tau(181P) CSF in vitro diagnostic immunoassays on fully automated cobas e 601 analyzers (Roche Diagnostics International Ltd). The assays were validated to the Clinical and Laboratory Standards Institute (CLSI) guidelines and exhibited a high level of within‐run (< 1.8%) and between‐run (< 2.3%) precision, which enabled singlicate measurements. Concentrations were interpolated from 2‐point calibration curves, with measuring ranges of 200 to 1700 pg/mL (β‐Amyloid [1‐42]), 80.0 to 1300 pg/mL (total‐tau), and 8.00 to 120 pg/mL (phospho‐tau[181P]). Acceptance criteria required two recombinant protein controls to quantify within 21% of their established concentrations. All measurements were performed in a blinded fashion at Covance CLS sites in Geneva (samples originating in Europe) and Indianapolis, Indiana (samples originating in the United States).
Concentrations of CSF Aβ40, α‐synuclein, glial fibrillary acidic protein (GFAP), interleukin (IL)‐6, neurogranin (NRGN), neurofilament light (NfL), neuronal pentraxin 2 (NPTX2), synaptosomal associated protein 25 (SNAP25), S100 calcium‐binding protein B (S100b), soluble triggering receptor expressed on myeloid cells 2 (sTREM2), and chitinase‐3‐like protein (YKL‐40) were measured using the NeuroToolKit (NTK) robust prototype assays on fully automated cobas e 411 and e 601 instruments (Roche Diagnostics International Ltd). NTK is a panel of exploratory prototype biomarker assays designed to specifically and robustly measure levels of soluble proteins associated with AD pathology, neuroinflammation, and neurodegeneration. The NTK robust prototype assays are not validated to CLSI standards and are not CE‐marked, but demonstrate high within‐run (< 5%) and between‐run (< 5%) precision, which also enabled singlicate measurements. Concentrations were interpolated from 5‐point calibration curves, and acceptance criteria required two recombinant protein controls to quantify within 21% of their established concentrations. The NTK measurements were performed in a blinded fashion at Microcoat Biotechnology (Bernried am Starnberger See, Germany) and Labcorp TBS (Greenfield, Indiana, USA).
2.4.1. Complement measurements in CU, P2M, and M2M AD CSF samples
Because freeze–thaw cycles affect complement protein concentrations, 22 the number of freeze–thaw cycles was matched for the CU and AD CSF. Previously developed and qualified enzyme‐linked immunosorbent assays (ELISAs) that measure C3, C3a, C4, and FB in CSF 22 were used for this study. The C3 assay measures intact C3 using an anti‐human C3a polyclonal antibody (R&D Systems, catalog AF3677) for capture and a polyclonal anti‐human C3 antibody (MP Bio, catalog 0855033) for detection. The C3a assay targets cleaved C3a and C3a desArg fragments, using a monoclonal anti‐human C3a antibody (R&D Systems, clone 354113) for capture and a monoclonal detection antibody specific to C3a/C3a desArg (BioLegend, clone K13/16). The C4 assay is designed to measure intact C4 using a polyclonal anti‐human C4c antibody (Abbexa, catalog abx102219) for capture and a monoclonal anti‐human C4c antibody (Quidel, catalog A211) for detection. During qualifications, it was demonstrated that the C4 assay exhibited cross‐reactivity with intact C4 and C4b fragments, yet it remains sensitive to ex vivo complement activation with heat aggregated gamma globulin (HAGG), indicating preferential detection of endogenous C4. 22 The Factor B ELISA measures intact FB using a monoclonal anti‐human FB antibody (AbCam, clone 6G11) for capture and a Genentech‐derived monoclonal anti‐human FB antibody for detection. For each ELISA, coating antibodies were applied to 96‐well half‐area high bind microplates (Corning), which were then incubated at temperatures between 2°C and 8°C for durations ranging from 18 to 48 hours. The plates were subsequently washed and blocked for a duration of 1 hour. Next, diluted standards, controls, and samples were introduced into the microplate wells and were allowed to incubate at RT for a period of 1.5 hours. Post‐wash, horseradish peroxidase (HRP)‐conjugated detection antibodies (Peroxidase Labeling Kit—NH2, Dojindo, cat. no. LK11) were incorporated into the C3, C3a, and C4 assays, and a biotinylated detection antibody (Biotin Labeling Kit—NH2, Dojindo, cat. no. LK03) was incorporated into the FB assay. After a 1 hour incubation at RT, the C3, C3a, and C4 assays underwent a final wash step. Meanwhile, a streptavidin poly‐HRP solution was incorporated into the FB assay post‐wash and allowed to incubate for an additional 30 minutes at RT. After the final series of washes, tetramethylbenzidine substrate solution was added into the microplate wells to develop color, a process arrested by adding 1 M phosphoric acid. The optical density of each well was calculated at 450 nm, using a reference wavelength subtraction of 650 nm, using a SpectraMax ELISA plate reader (Molecular Devices). A logistic (4‐PL) curve fit was applied to establish a standard regression curve. The concentrations of C4a were measured using the BD OptEIA Human C4a ELISA Kit (BD Biosciences, Cat. No. 550947), which uses a sandwich ELISA technique, as per the product guidelines. The concentrations of Bb were assessed using a custom single molecule array Quanterix Simoa assay, as has been previously reported. 23 Fit‐for‐purpose qualifications were previously performed for all six assays including ex vivo complement pathway activation and inhibition, plasma and CSF dilution linearity, specificity, and spike recovery. 22 Samples with insufficient volumes and measurements with a high %CV (> 30%) or below the limit of quantitation were removed from analyses.
2.4.2. Tauriel/Lauriet clinical outcome measures used for correlation analyses
Cognitive/functional impairments were assessed at baseline and follow‐up by ADCS‐ADL (co‐primary efficacy endpoint in Lauriet), ADAS‐Cog13 (Tauriel only), ADAS‐Cog11 (co‐primary efficacy endpoint in Lauriet), MMSE and CDR‐SB (primary efficacy endpoint in Tauriel) (see Teng et al. 17 and Monteiro et al. 18 for further details). The ADCS‐ADL scale quantifies the performance of activities of daily living (ADL) in patients with AD and is administered to the care partners (score range 0–78; higher scores indicate better function). 24 The ADAS‐Cog13 and ADAS‐Cog11 are 13‐ and 11‐item cognitive scales, respectively, administered to assess cognitive domains most often affected in AD (score range 0–85 for ADAS‐Cog13 and 0–70 for ADAS‐Cog11; lower scores indicate better cognitive performance). 25 The MMSE total score comprises subscores representing each cognitive domain: memory, orientation, attention, language, and construction (score range 0–30, lower scores indicate lower cognitive performance). 26 The CDR‐SB assesses six cognitive/functional domains (memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care). Scores range from 0 to 18, with higher scores indicating greater impairment. 27
MRI scans were performed at baseline and follow‐up (weeks 49, 61, or 73) volumetric MRI (vMRI) analysis was performed by a central vendor (NeuroRx) using 3D Sagittal T1‐weighted sequences to measure cortical gray matter whole volume (masking out the deep gray matter and cerebellum) at each visit (see Teng et al. 17 and Monteiro et al. 18 for further details).
A subset of participants underwent [18F]GTP1 tau PET imaging 28 at baseline and follow‐up at week 49 or 73 (for Tauriel), and at baseline and week 49 or 61 (for Lauriet). 17 , 18 A central PET vendor (Invicro) determined [18F]GTP1 standardized uptake value ratio (SUVR) across a whole cortical gray region of interest (excluding the deep gray matter and cerebellum) using cerebellar gray matter as reference. Images were not corrected for partial volume effect.
2.5. Statistical analyses
Density distribution plots were generated to determine the distribution of CSF complement levels for each disease group. The missing age of one subject from Tauriel (P2M AD) was imputed with the trial average age. Complement activities were evaluated by computing the ratios C4a:C4, C3a:C3, Bb:FB. Because skewed distributions were observed for complement protein concentrations (but not ratios) log2 transformations were applied to obtain normal analyte distributions and satisfy t test requirements. For the cross‐sectional analyses, age‐ and sex adjustment on reported CSF complement protein measurements were conducted by fitting linear regression models to the log2 transformed concentrations or ratios. Residuals of the models were computed and used to calculate a constant equal to the mean of the log2 transformed concentrations or ratios for each protein minus the mean of the residuals. The residuals were adjusted by adding the calculated constant values, and these adjusted residuals were used in the P2M and M2M AD versus CU comparisons. Two‐tailed Welch t tests were used to compare mean differences between groups with α = 0.05. Paired t tests were used to compare differences between log2 transformed complement concentrations or ratios at baseline and at follow‐up with α = 0.05. Boxes represent the median and interquartile range (IQR); the lower and upper hinges correspond to the first and third quartiles (the 25th and 75th percentiles). The upper whisker extends from the hinge to the largest value no further than 1.5 x IQR from the hinge. The lower whisker extends from the hinge to the smallest value at most 1.5 x IQR of the hinge. Individual data points are plotted over the box plots. Spearman rank correlation coefficient ρ was calculated for all correlation analyses with α = 0.05. When applicable, the Benjamini–Hochberg procedure was implemented to control the false discovery rate (FDR) in multiple comparisons.
Complement protein measurements from predose CSF from both the placebo and semorinemab arms were included in the correlation analysis of baseline complement proteins with baseline NTK proteins, vMRI, [18F]GTP1 SUVR, or cognitive/functional clinical scores (including CDR‐SB, ADAS‐Cog, MMSE, and ADCS‐ADL). The baseline correlation analyses between complement protein levels and ADAS‐Cog13 or ADAS‐Cog11 were represented by trial as Tauriel and Lauriet each only measured one of these scores (ADAS‐Cog13 for Tauriel, ADAS‐Cog11 for Lauriet). In the correlation analysis of the changes from baseline at follow‐up in complement proteins with the changes from baseline at follow‐up in NTK proteins, semorinemab‐treated patients were not included as treatment effects were observed longitudinally on the NTK proteins for both studies. 29 Due to the low N of placebo‐treated participants with both baseline and follow‐up measurements, semorinemab‐treated participants from Tauriel were included in the correlation analysis of changes from baseline at follow‐up of the complement proteins with the changes from baseline at follow‐up in vMRI, [18F]GTP1 SUVR, or cognitive/functional clinical scores (including CDR‐SB, ADAS‐Cog, MMSE, and ADCS‐ADL). Because no treatment effect was observed, we considered this approach reasonable. Semorinemab‐treated participants from Lauriet were excluded because of the treatment effect observed in ADAS‐Cog11. Unlike the baseline analysis, the correlation analysis with the change from baseline in ADAS‐Cog could not be represented by trial due to the low longitudinal sample count, so the percent change from baseline was used instead to account for the difference in the ADAS‐Cog13 and ADAS‐Cog11 score ranges. For correlation analyses of change from baseline at follow‐up in complement proteins with changes from baseline at follow‐up in NTK proteins and changes in cognitive/functional scores (except ADAS‐Cog), absolute changes were used.
All analyses were completed using publicly available packages in Python version 3.11.4 and R version 4.3.1.
3. RESULTS
3.1. Study design, participant characteristics, and CSF analytes
The present study included CSF samples from CU, P2M AD (Tauriel), and M2M AD cohorts (Lauriet; Figure 1A). For the CU cohort, CSF was collected at a single time point. Tauriel/Lauriet participant CSF was collected at baseline and after treatment with placebo or semorinemab (Figure 1A,B). Immunoassays were used to measure the levels of complement proteins C4, C3, and FB as well as their cleavage fragments C4a, C3a, and Bb (Figure 1A). Neurodegeneration and neuroinflammation biomarkers were evaluated using the NTK panel or by immunoassay
30
(Figure 1A). ,
, 
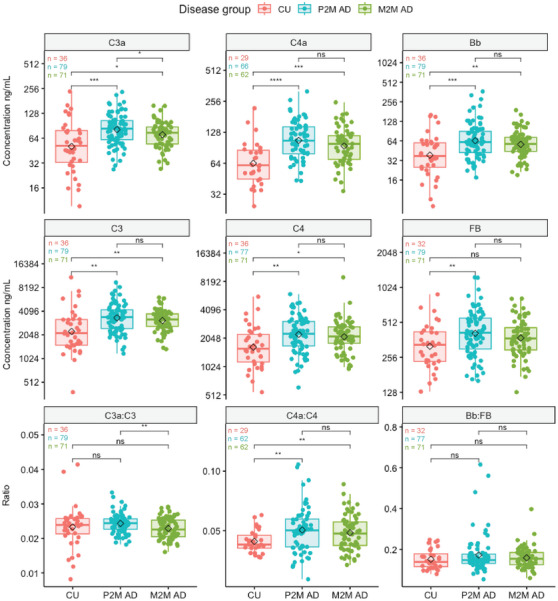
Box plots of age‐ and sex‐adjusted baseline CSF complement protein concentrations (ng/mL; on a log2 scale) or ratios from P2M AD, M2M AD, and CU participants. Black diamonds represent mean values of each disease group. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, ns (not significant) P ≥ 0.05, t test. See Table S1 in supporting information for absolute concentrations. CSF, cerebrospinal fluid; CU, cognitively unimpaired; M2M, mild to moderate Alzheimer's disease; P2M, prodromal to mild Alzheimer's disease.
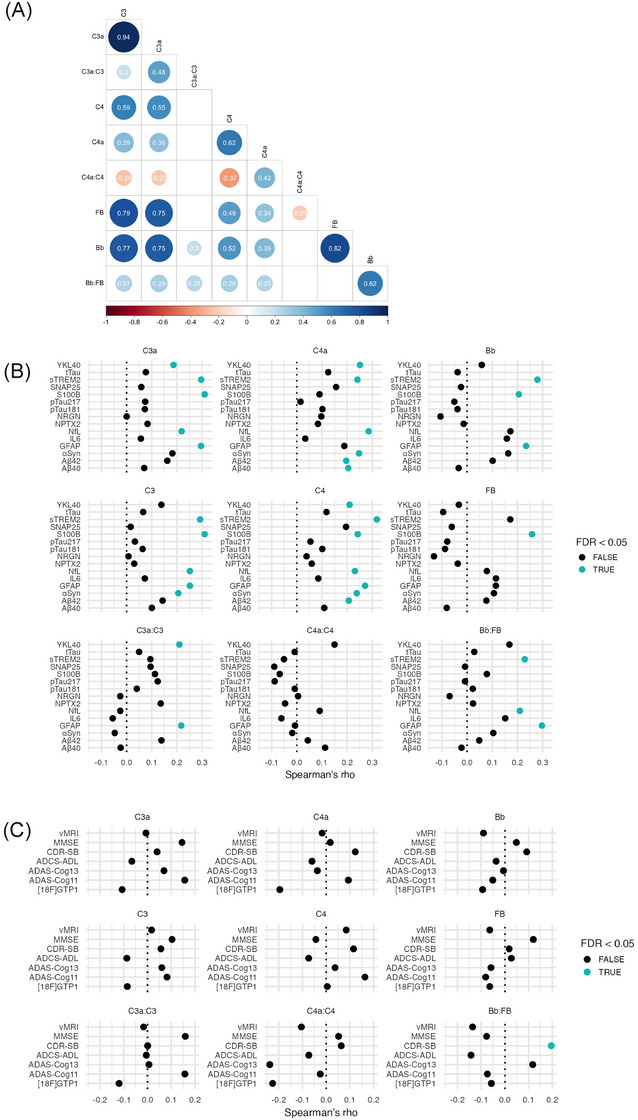
A, Heatmap of the correlation between CSF complement protein levels or ratios in baseline P2M AD and M2M AD participants. Spearman rank correlation coefficient values are displayed and circles represent significant correlations with FDR‐adjusted P values < 0.05. B, Spearman rank correlation between CSF complement and NTK proteins in P2M and M2M AD participants at baseline. Blue and black dots indicate the FDR adjusted P value less than or greater than 0.05, respectively. C, Spearman rank correlation between CSF complement and cognitive/functional scores, [18F]GTP1 SUVR, and whole brain vMRI scan measurements in P2M and M2M AD participants at baseline. ADAS‐Cog13 and ADAS‐Cog11 were used in Tauriel (P2M AD) and Lauriet (M2M AD), respectively. 17 , 18 Blue and black dots indicate the FDR‐adjusted P value less than or greater than 0.05, respectively. Aβ, amyloid beta; AD, Alzheimer's disease; ADAS‐Cog, Alzheimer's Disease Assessment Scale Cognitive subscale; aSyn, a‐synuclein; CSF, cerebrospinal fluid; CU, cognitively unimpaired; FDR, false discovery rate; GFAP, glial fibrillary acidic protein; IL‐6, interleukin 6; M2M, mild to moderate Alzheimer's disease; MMSE, Mini‐Mental State Examination; NfL, neurofilament light; NPTX2, neuronal pentraxin 2; NRGN, neurogranin; NTK, NeuroToolKit; P2M, prodromal to mild Alzheimer's disease; Ph2, phase 2; p‐tau, phosphorylated tau; S100b, S100 calcium‐binding protein B; SNAP25, synaptosomal associated protein 25; sTREM2, soluble triggering receptor expressed on myeloid cells 2; t‐tau, total tau; vMRI, volumetric magnetic resonance imaging; YKL‐40, chitinase‐3‐like protein.
The CU cohort included 69% males, while the P2M and M2M AD cohort included 46% and 38% males, respectively (Figure 1C). Despite attempts to age match the CU and AD cohorts, the CU participants were on average 4.7 to 7.9 years younger than the AD patients (Figure 1C). Because previous studies showed that peripheral complement protein levels were influenced by age and sex 31 we first sought to investigate the impact of these demographic characteristics on complement levels. CSF complement protein levels C3a, C3, Bb, and FB were mildly elevated in male subjects relative to females (Figure S2 in supporting information) with median fold differences of 1.17, 1.20, 1.42, and 1.22, respectively (Table S1 in supporting information). CSF C4a, C4, C3a, C3, Bb, C4a:C4 ratio, and Bb:FB ratio showed a weak (Spearman rho values ranging between 0.18 and 0.35) but significant correlation with age (Figure S3 in supporting information).
3.2. Baseline CSF complement measurements in CU and AD subjects
Next, we assessed the differences in age‐/sex‐adjusted CSF complement protein levels (see Methods section) between CU and baseline P2M or M2M AD subjects. Baseline CSF complement proteins C4a, C4, C3a, C3, and Bb levels were increased in P2M and M2M AD patients relative to CU subjects (Figure 2 and Figure S4 and Table S1 in supporting information). Among these analytes, C4a levels displayed the largest fold increase between CU and AD CSF, with a 2.2‐fold increase in P2M AD CSF, and a 1.9‐fold increase in M2M AD CSF (Figure 2, Table S1). CSF FB showed a mild but significant elevation in P2M but not M2M AD patient samples (Figure 2, Table S1). The ratio C4a:C4 was increased in P2M and M2M compared to CU (Figure 2). C3a:C3 and Bb:FB ratios were found unchanged between AD and CU subjects (Figure 2). C4a, C4, C3, Bb, and FB complement proteins did not significantly differ between P2M and M2M AD patients. C3a levels and C3a:C3 ratios were slightly lower in M2M versus P2M AD CSF (Figure 2, Table S1). Together, these data reveal elevated CSF C4a, C3a, Bb, C4, C3, and FB levels in AD patients (vs. CU), with the greatest difference observed for CP/LP activation marker C4a.
3.3. Baseline CSF complement proteins and neurodegeneration/neuroinflammation biomarkers in AD patients
Next, we investigated the intercorrelation patterns for different complement proteins as well as their association with neurodegeneration and neuroinflammation biomarkers in the CSF from P2M and M2M AD patients. As expected, the highest correlations were found between complement proteins C4, C3, FB, and their respective cleaved forms (Spearman rho > 0.6; Figure 3A). Moderate to strong associations were also found between different complement proteins: CSF C3a and C3 displayed the strongest correlation with Bb and FB (Figure 3A), likely due to the involvement of cleaved C3 in the AP activation (Figure 1A). CSF C4a and C4 were found to be more moderately associated with C3a, C3, and FB (Figure 3A).
When examining the correlations between complement proteins and neurodegeneration/neuroinflammation biomarkers, C4, C3a, and C3 were found to be modestly associated with NfL, GFAP, sTREM2, and S100b (Figure 3B, Spearman rho = 0.21–0.31). C4a was moderately correlated with Aβ40, Aβ42, NfL, sTREM2, and YKL‐40 (Figure 3B, Spearman rho = 0.2–0.29). C4a, C4, and C3 correlated with α‐synuclein (Figure 3B). CSF Bb levels were associated with GFAP, sTREM2, and S100b levels (Spearman rho = 0.2–0.27), while FB only correlated with S100b (Spearman rho = 0.26; Figure 3B). The C3a:C3 ratio correlated with YKL‐40 and GFAP (Spearman rho = 0.21–0.22), and the Bb:FB ratio correlated with sTREM2, NfL, and GFAP (Spearman rho = 0.21–0.3; Figure 3B). No significant correlations were observed between the tested complement proteins and tau indices (t‐tau, p‐tau181, and p‐tau217), NRGN, NPTX2, or SNAP25.
3.4. Baseline CSF complement proteins and MRI, tau PET, and clinical outcome measures in AD patients
None of the CSF complement proteins showed a meaningful, consistent correlation with whole brain vMRI measurements, [18F]GTP1 SUVR, or cognitive/functional clinical scores (including CDR‐SB, ADAS‐Cog [ADAS‐Cog13 for Tauriel and ADAS‐Cog11 for Lauriet], MMSE, and ADCS‐ADL) at baseline (Figure 3C). Together, our findings indicate that baseline CSF complement proteins correlate with baseline CSF neurodegeneration/neuroinflammation biomarker levels but not with brain atrophy or cognitive/functional scores.
3.5. Longitudinal changes in CSF complement levels in placebo and semorinemab‐ treated AD patients
In the placebo arms from Tauriel (P2M) and Lauriet (M2M), CSF C4a, C3a, Bb, C4, C3, FB, C4a:C4, C3a:C3, and Bb:FB did not significantly change from baseline to follow‐up (weeks 49, 61, or 73; Figure S5A,B in supporting information). No consistent, meaningful correlation patterns were found between the changes from baseline in complement proteins and changes from baseline in neurodegeneration or neuroinflammation biomarkers (Figure S6A in supporting information), [18F]GTP1 SUVR, whole brain vMRI, functional or clinical scores (Figure S6B). The change from baseline of CSF complement proteins C4a, C3a, Bb, C4, C3, FB, C4a:C4, C3a:C3, and Bb:FB did not significantly differ between semorinemab at 4500 mg and placebo in Tauriel P2M (Figure 4) or Lauriet M2M (Figure 5) AD patients. These data suggest that complement protein levels or activity do not significantly change from baseline at weeks 49, 61, or 73. Furthermore, we show that, despite the cognitive benefit observed in Lauriet, semorinemab does not modulate central nervous system (CNS) complement activity in P2M (Tauriel) or M2M (Lauriet) AD patients.
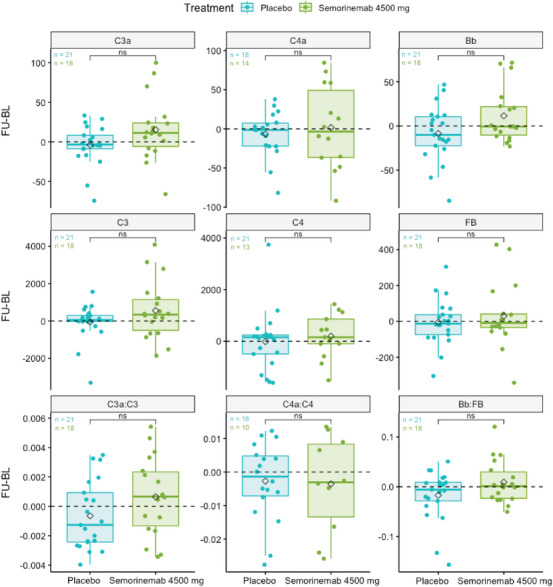
Box plots of the difference in complement protein concentration (ng/mL) or ratios at follow‐up (FU) visits from weeks 49 or 73 and baseline (BL) of Tauriel P2M AD patients treated with placebo or semorinemab at 4500 mg. Black diamonds represent mean values for each treatment per analyte. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, ns (not significant) P ≥ 0.05, t test. CU, cognitively unimpaired; M2M, mild to moderate Alzheimer's disease; P2M, prodromal to mild Alzheimer's disease.
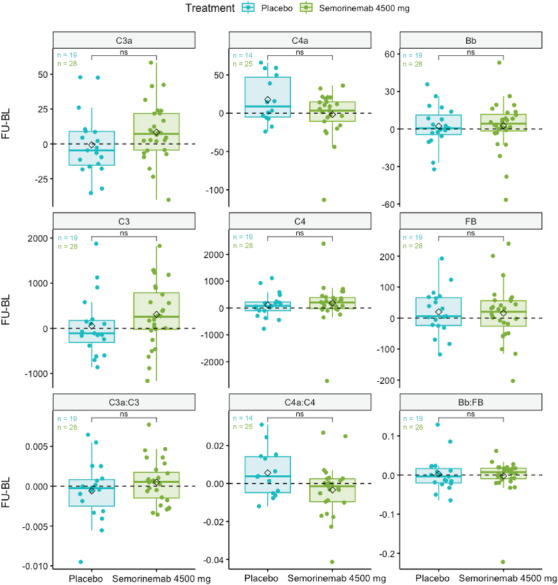
Box plots of the difference in complement protein concentration (ng/mL) or ratios at follow‐up (FU) visits from weeks 49 or 61 and baseline (BL) of Lauriet M2M AD patients treated with placebo or semorinemab at 4500 mg. Black diamonds represent mean values for each treatment per analyte. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, ns (not significant) P ≥ 0.05, t test. CU, cognitively unimpaired; M2M, mild to moderate Alzheimer's disease; P2M, prodromal to mild Alzheimer's disease.
4. DISCUSSION
The present study demonstrated elevated CSF complement protein C4a, C3a, Bb, C4, C3, and FB levels in P2M/M2M AD compared to CU, with the greatest difference observed for C4a. In AD patients, several CSF complement proteins were correlated with glial activation and neurodegeneration markers, including sTREM2, GFAP, YKL‐40, and NfL. Despite showing a reduction in soluble tau in CSF, semorinemab did not modulate complement proteins relative to placebo. Our results provide evidence for increased complement pathway activity in AD patients and could aid the development of therapeutic interventions targeting the CNS immune system.
Among all tested complement proteins, CSF C4a showed the most robust difference, with higher levels observed in AD patients compared to CU subjects. We also observed significant increases of CSF C4 and C4a:C4 ratios, suggesting increased CNS C4 levels and activity in AD patients. Elevated CSF C4 levels are consistent with previous reports of increased expression of complement pathway components in glia from AD patients and mouse models. 32 , 33 C4 processing occurs downstream of the CP and LP. 34 While the LP and AP are thought to contribute little to AD pathogenesis, numerous studies have reported increased levels of CP activator proteins in the CNS from AD patients as well as amyloid/tauopathy mouse models. 6 , 7 , 8 , 35 Mouse model experiments suggest that the CP initiator molecule C1Q targets synapses, induces the cleavage of C4 and C3, followed by C3b opsonization and glial engulfment of the synapses. 7 , 8 , 9 The deletion or inhibition of C1Q rescues microglia‐ and astrocyte‐mediated synapse phagocytosis in mouse models of amyloidosis and tauopathy. 7 , 8 , 9 Together, these data suggest that the increases in CSF C4a (and C3a) could reflect enhanced expression and activity of CP components in AD, although the contribution of the LP to C4/C3 cleavage cannot be excluded. It remains to be determined whether the levels and activity of TP components are altered in AD versus CU CSF.
No major differences were found in the complement levels/activity between P2M and M2M AD patient CSF, except for C3a and C3a:C3 ratio which were found to have a slight decrease in M2M versus P2M subjects. These findings contrast with previous studies reporting greater C1Q and C3 levels in brain homogenates from AD compared to early AD patients. 7 , 36 Nonetheless, the comparability between the studies is limited by potential differences in the study participant inclusion criteria (clinical diagnosis, amyloid and tau status).
Contrary to previous reports showing an association between CSF or brain C3 levels and tau, 7 , 37 we did not find a correlation between baseline CSF complement proteins and tau (both soluble CSF tau and aggregated tau measured by tau PET) in AD patients. The inconsistency between our results and previous reports could be explained by differences in the number of study participants, the participant disease stage, or the analytical methods. Notably, baseline CSF C4a, C3a, C4, and C3 correlated positively with the neurodegeneration biomarker NfL, consistent with recent findings in familial frontotemporal dementia. 38 Baseline AD CSF C4a, C3a, C4, and C3 also displayed a positive association with multiple glial activation biomarkers, including sTREM2, YKL‐40, and GFAP. Because brain complement pathway components are expressed and secreted by microglia and astrocytes, 39 these data support the hypothesis that changes to CSF complement protein levels and activity reflect CNS neuroinflammation in AD.
CSF complement levels did not change over 49, 61, or 73 weeks in AD patients and semorinemab demonstrated no pharmacodynamic effect on CSF complement protein changes from baseline at week 49 compared to placebo. Thus, despite evidence of target modulation in the CNS, there is no semorinemab‐mediated impact on CNS complement activity. This finding suggests that the significant reduction in cognitive decline measured by ADAS‐Cog11 in Lauriet may not be attributable to modulation of CNS complement activity.
Although our findings provide evidence for increased complement protein levels and/or activity in AD CSF, the present study has limitations. First, even though precautions were taken to align the preanalytical conditions for all groups as much as possible, the CSF collection and handling of the CU and Tauriel/Lauriet samples differed slightly. Second, the correlation analyses between CSF complement proteins and clinical outcomes were performed on a relatively small subset of AD subjects (Table S2 in supporting information). Third, there is a sex imbalance between the CU and AD groups, with greater numbers of male subjects in the CU and females in the AD cohorts. Fourth, due to the unavailability of appropriate assays, this study did not include CP‐, LP‐, or TP‐specific markers and thus cannot disentangle changes in the levels/activity of these pathway components in AD CSF. Together, these limitations warrant further studies to assess CSF C4a, C3a, Bb, C3, C4, FB, and CP‐/LP‐/TP‐specific markers in larger cohorts with equal biofluid preanalytical handling and accessible clinical/biomarkers from age‐ and sex‐matched CU and AD subjects.
Overall, our results provide in vivo evidence for increased complement pathway activity in AD patient CSF. Future longitudinal studies, including a presymptomatic patient population, will be essential to determine at which disease stage the CNS complement pathway activity starts to increase in AD patients. Moreover, it will be important to elucidate how the CSF complement protein levels relate to synapse loss.
AUTHOR CONTRIBUTIONS
Cosme Sandoval; Julie Lee; Johnny Gutierrez; Felix L. Yeh and Anne Biever: Study design. Cosme Sandoval; Edmond Teng; Cecilia Monteiro; Julie Lee; Johnny Gutierrez; Rajini Nagaraj; Stephen P. Schauer; Jennifer Hoffman; Emilia Calderon; Sandra M. Sanabria Bohórquez and Gwendlyn Kollmorgen: Sample and data acquisition. Julie Lee; Balazs Toth and Anne Biever: Data analysis. Cosme Sandoval; Julie Lee; Balazs Toth; Johnny Gutierrez; Jesse E. Hanson; Cecilia Monteiro; Edmond Teng; Felix L. Yeh and Anne Biever: Data interpretation. Cosme Sandoval; Julie Lee and Anne Biever: Manuscript writing—original draft. All authors: Manuscript writing—review and editing.
CONFLICT OF INTEREST STATEMENT
The NeuroToolKit is a panel of exploratory prototype assays designed to robustly evaluate biomarkers associated with key pathologic events characteristic of AD and other neurological disorders, used for research purposes only and not approved for clinical use (Roche Diagnostics International Ltd, Rotkreuz, Switzerland). Elecsys β‐Amyloid (1‐42), Total‐Tau, and Phospho‐Tau (181P) CSF assays are approved for clinical use. COBAS and ELECSYS are trademarks of Roche. All other product names and trademarks are the property of their respective owners. GK is a full‐time employee of Roche Diagnostics GmbH, Penzberg, Germany. All other authors are full‐time employees of Genentech Inc. (member of the Roche group). Edmond Teng is listed as a co‐inventor on the patent for semorinemab. Author disclosures are available in the supporting information.
CONSENT STATEMENT
All participants in the present study and/or their legally authorized representatives provided signed informed consent.
Supporting information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
We thank Dan Abramzon, Rod Mathews, Jeannette Lo, and Bill O'Gorman for comments on the manuscript. This work was funded by Genentech Inc.
Notes
Sandoval C, Lee J, Toth B, et al. CSF complement proteins are elevated in prodromal to moderate Alzheimer's disease patients and are not altered by the anti‐tau antibody semorinemab. Alzheimer's Dement. 2024;20:7940–7953. 10.1002/alz.14271 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
Cosme Sandoval and Julie Lee contributed equally to this work.
REFERENCES
Articles from Alzheimer's & Dementia are provided here courtesy of Wiley
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Impact of Preanalytical Procedures on Complement Biomarkers in Cerebrospinal Fluid and Plasma from Controls and Alzheimer's Disease Patients.
J Alzheimers Dis, 101(2):563-576, 01 Jan 2024
Cited by: 0 articles | PMID: 39213066 | PMCID: PMC11492022
Randomized Phase II Study of the Safety and Efficacy of Semorinemab in Participants With Mild-to-Moderate Alzheimer Disease: Lauriet.
Neurology, 101(14):e1391-e1401, 29 Aug 2023
Cited by: 16 articles | PMID: 37643887 | PMCID: PMC10573141
Semorinemab Pharmacokinetics and The Effect on Plasma Total Tau Pharmacodynamics in Clinical Studies.
J Prev Alzheimers Dis, 11(5):1241-1250, 01 Jan 2024
Cited by: 0 articles | PMID: 39350369
The relationship between complement factor C3, APOE ε4, amyloid and tau in Alzheimer's disease.
Acta Neuropathol Commun, 4(1):65, 29 Jun 2016
Cited by: 33 articles | PMID: 27357286 | PMCID: PMC4928261
Funding
Funders who supported this work.

 1
1


