Abstract
Background
Lung transplantation (LTx) remains the only efficient treatment for selected patients with end-stage pulmonary disease. The age limit for the acceptance of donor organs in LTx is still a matter of debate. We here analyze the impact of donor organ age and the underlying pulmonary disease on short- and long-term outcome and survival after LTx.Methods
Donor and recipient characteristics of LTx recipients at our institution between 03/2003 and 12/2021 were analyzed. Statistical analysis was performed using SPSS and GraphPad software.Results
In 230 patients analyzed, donor age ≥ 55 years was associated with a higher incidence of severe primary graft dysfunction (PGD2/3) (46% vs. 31%, p = 0.03) and reduced long-term survival after LTx (1-, 5- and 10-year survival: 75%, 54%, 37% vs. 84%, 76%, 69%, p = 0.006). Notably, this was only significant in recipients with idiopathic pulmonary fibrosis (IPF) (PGD: 65%, vs. 37%, p = 0.016; 1-, 5-, and 10-year survival: 62%, 38%, 16% vs. 80%, 76%, 70%, p = 0.0002 respectively). In patients with chronic obstructive pulmonary disease (COPD), donor age had no impact on the incidence of PGD2/3 or survival (21% vs. 27%, p = 0.60 and 68% vs. 72%; p = 0.90 respectively). Moreover, we found higher Torque-teno virus (TTV)-DNA levels after LTx in patients with IPF compared to COPD (X2 = 4.57, p = 0.033). Donor age ≥ 55 is an independent risk factor for reduced survival in the whole cohort and patients with IPF specifically.Conclusions
In recipients with IPF, donor organ age ≥ 55 years was associated with a higher incidence of PGD2/3 and reduced survival after LTx. The underlying pulmonary disease may thus be a relevant factor for postoperative graft function and survival.Trial registration number dkrs
DRKS00033312.Free full text

Donor age over 55 is associated with worse outcome in lung transplant recipients with idiopathic pulmonary fibrosis
Abstract
Background
Lung transplantation (LTx) remains the only efficient treatment for selected patients with end-stage pulmonary disease. The age limit for the acceptance of donor organs in LTx is still a matter of debate. We here analyze the impact of donor organ age and the underlying pulmonary disease on short- and long-term outcome and survival after LTx.
Methods
Donor and recipient characteristics of LTx recipients at our institution between 03/2003 and 12/2021 were analyzed. Statistical analysis was performed using SPSS and GraphPad software.
Results
In 230 patients analyzed, donor age ≥
≥ 55 years was associated with a higher incidence of severe primary graft dysfunction (PGD2/3) (46% vs. 31%, p
55 years was associated with a higher incidence of severe primary graft dysfunction (PGD2/3) (46% vs. 31%, p =
= 0.03) and reduced long-term survival after LTx (1-, 5- and 10-year survival: 75%, 54%, 37% vs. 84%, 76%, 69%, p
0.03) and reduced long-term survival after LTx (1-, 5- and 10-year survival: 75%, 54%, 37% vs. 84%, 76%, 69%, p =
= 0.006). Notably, this was only significant in recipients with idiopathic pulmonary fibrosis (IPF) (PGD: 65%, vs. 37%, p
0.006). Notably, this was only significant in recipients with idiopathic pulmonary fibrosis (IPF) (PGD: 65%, vs. 37%, p =
= 0.016; 1-, 5-, and 10-year survival: 62%, 38%, 16% vs. 80%, 76%, 70%, p
0.016; 1-, 5-, and 10-year survival: 62%, 38%, 16% vs. 80%, 76%, 70%, p =
= 0.0002 respectively). In patients with chronic obstructive pulmonary disease (COPD), donor age had no impact on the incidence of PGD2/3 or survival (21% vs. 27%, p
0.0002 respectively). In patients with chronic obstructive pulmonary disease (COPD), donor age had no impact on the incidence of PGD2/3 or survival (21% vs. 27%, p =
= 0.60 and 68% vs. 72%; p
0.60 and 68% vs. 72%; p =
= 0.90 respectively). Moreover, we found higher Torque-teno virus (TTV)-DNA levels after LTx in patients with IPF compared to COPD (X2
0.90 respectively). Moreover, we found higher Torque-teno virus (TTV)-DNA levels after LTx in patients with IPF compared to COPD (X2 =
= 4.57, p
4.57, p =
= 0.033). Donor age
0.033). Donor age ≥
≥ 55 is an independent risk factor for reduced survival in the whole cohort and patients with IPF specifically.
55 is an independent risk factor for reduced survival in the whole cohort and patients with IPF specifically.
Conclusions
In recipients with IPF, donor organ age ≥
≥ 55 years was associated with a higher incidence of PGD2/3 and reduced survival after LTx. The underlying pulmonary disease may thus be a relevant factor for postoperative graft function and survival.
55 years was associated with a higher incidence of PGD2/3 and reduced survival after LTx. The underlying pulmonary disease may thus be a relevant factor for postoperative graft function and survival.
Trial registration number DKRS
DRKS00033312.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12890-024-03317-x.
Introduction
For selected patients with end-stage chronic lung disease, lung transplantation is an established and effective treatment option [1]. Although survival rates have improved over the last decade, they are still lower compared to other solid organ transplantations due to a high number of complications, e.g., recurrent infection [1] or the development of chronic lung allograft dysfunction (CLAD) [2–4].
The 2013 report of the Registry of the International Society for Heart and Lung Transplantation shows an increase in the median age of lung transplant recipients from 45 to 55 years over the preceding decade [5]. This may increase morbidity and mortality after LTx since older patients are more likely to receive an organ [6]. The shortage of donor organs is a persistent problem resulting in a waiting list mortality of approximately 10–13% [3]. Therefore, not only the characteristics of the recipient are subject to discussion [7, 8], but also how to expand the donor pool and find the best possible match for the respective patient on the waiting list [9].
The debate on organ selection remains controversial regarding the use of older organs for transplantation [10, 11]. In previous studies, the ‘ideal donor’ is described as < 55 years of age [9, 10, 12], However, this statement does no longer reflect current practice and over the last decade the use of donors
55 years of age [9, 10, 12], However, this statement does no longer reflect current practice and over the last decade the use of donors ≥
≥ 55 years of age has been divergently handled by many transplant centres [10, 13–15].
55 years of age has been divergently handled by many transplant centres [10, 13–15].
Particularly the impact of the recipient’s underlying pulmonary disease on short and long-term outcome is incompletely understood. Fibrotic and obstructive lung diseases differ not only morphologically, but also regarding the underlying pathophysiology including different immunological alterations. Aside from idiopathic pulmonary fibrosis (IPF), there are many other causes of pulmonary fibrosis such as autoimmune/connective tissue diseases, e.g., rheumatoid arthritis, environmental exposures to coal or silica, or allergens, e.g., bird fancier’s lung [16, 17]. The pathogenesis of IPF is multifactorial, involving various processes that lead to dysregulation in wound healing with widespread scarring of the lungs [16]. It is considered an age-related disease, which usually occurs in patients older than 50 years, and is increasing in both, incidence, and prevalence [18, 19]. Moreover, altered innate and adaptive immune cell responses have both been linked to myofibroblast biology and fibrogenesis [18] and thus to fibrotic lung disease. In the context of lung transplantation, a dysregulated immune system might pose an additional challenge for the postoperative course and long-term survival. Monitoring immunosuppression after lung transplantation is challenging, and drug blood levels alone are insufficient to determine the individual status of immunosuppression [20, 21].
This study aims to analyse the impact of donor age, as well as the impact of the underlying pulmonary diseaseon graft function and short- and long-term outcome in our lung transplant cohort.
Methods
Design and study population
We identified all patients who underwent lung transplantation at the Department of Thoracic Surgery, Medical Centre – University of Freiburg between March 2003 and December 2021. Complete follow-up was available for 91% of the patients. Characteristics of recipients and donors were retrospectively analysed. Patients who underwent en-bloc heart-lung transplantation were excluded (Supplementary Figure S1). Most patients suffer from IPF or COPD as an underlying pulmonary disease. A detailed distribution is shown in Supplementary Figure S2. All patients were regularly followed up at our transplant outpatient centre. Basiliximab was used for induction therapy and standard immunosuppression consisted of prednisone, mycophenolate mofetil and tacrolimus, with adaptation depending on multiple factors, e.g., kidney function.
Moreover, all patients received the standard posttransplant antibiotic prophylaxis, CMV prophylaxis and antifungal prophylaxis. In case the donor and recipient were CMV negative, CMV prophylaxis was provided with Aciclovir for 3 months. Ganciclovir was given for 6 months in case both, or only the recipient, were CMV positive, and for 9 months in case of a high-risk constellation with the donor being positive and the recipient negative for CMV.
Recipient data, e.g., lung function analysis, bronchoscopic biopsy results as well as blood tests and clinical examination results were collected by checking electronic medical records, discharge reports and autopsy reports. Donor data were taken from the donor reports provided by Eurotransplant.
Definitions
The predicted total lung capacity (pTLC) of the donor was calculated according to the formula provided by the Eurotransplant 2013 annual report. For male donors: 7.99x(height) – 7.08 and for female donors: 6.60x (height) – 5.7 [22].
Primary graft dysfunction (PGD) was defined according to the ISHLT criteria based on the PaO2/FiO2 ratio and the presence of diffuse parenchymal infiltrates in the allograft on the chest radiograph within 72 h [23]. All patients were evaluated according to these criteria regardless of the time of transplantation. PGD grade 2 and higher (PGD2/3) was considered relevant for the clinical outcome.
Torque-Teno virus (TTV) copy numbers
TTV copy number kinetics after lung transplantation from a previous study were re-analyzed [24]. Torque-teno virus (TTV) copy numbers in plasma samples of 33 patients (22 patients with underlying idiopathic pulmonary fibrosis (IPF) and 11 patients with COPD) were compared. Samples were collected pre-transplant and every following month for up to one year after lung transplantation.
CMV reactivation
The CMV reactivation cut-off was defined as > 3000 copies/mL after the prophylaxis had ended, similar to other studies [25, 26].
3000 copies/mL after the prophylaxis had ended, similar to other studies [25, 26].
Statistical analysis
To estimate overall survival, we applied the Kaplan-Meier method. The log-rank test was used for comparison of the survival curves of the lung transplant recipients. Fischer’s exact test was used to analyse connections between different parameters in patients with and without underlying fibrotic lung disease. To analyse predictors of survival univariate and multivariate Cox proportional hazard regression analysis was used. To estimate the the difference in TTV load between patients with COPD and IPF a non-parametric Friedman Test was applied. All tests were two-tailed and a p-value <
< 0.05 was considered statistically significant. SPSS (Version 27, IBM Corporation, New York, NY, USA) and GraphPad Prism (Version 9, GraphPad Software, San Diego, CA 92108, USA) were used for all statistical analyses.
0.05 was considered statistically significant. SPSS (Version 27, IBM Corporation, New York, NY, USA) and GraphPad Prism (Version 9, GraphPad Software, San Diego, CA 92108, USA) were used for all statistical analyses.
Results
230 patients (120 male, 110 female) underwent lung transplantation at our institution between March 2003 and December 2021. 203 (88%) patients had a double lung transplantation, and 27 (12%) patients had a single lung transplantation. The overall 1-, 5- and 10-year-survival rate was 80%, 67% and 58% respectively (Fig. 1A). Basic donor and recipient characteristics are shown in Table 1.
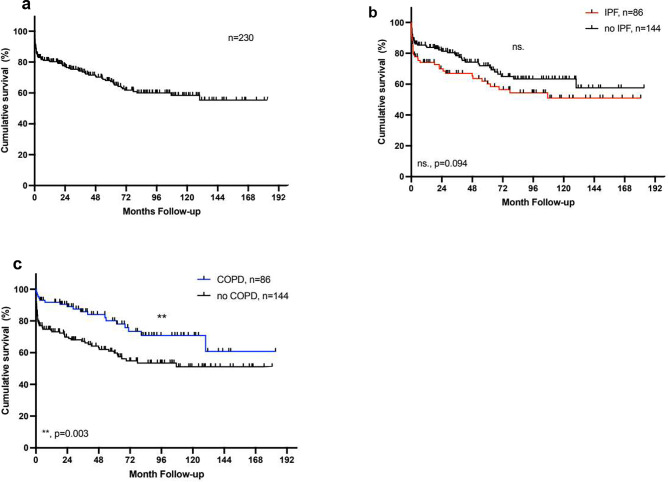
Kaplan-Meier Analysis of survival after lung transplantation. a) Overall post-transplant survival: 1-, 5- and 10-year survival: 80%, 67% and 58%. b) Survival with IPF: 1-, 5-, and 10-year survival 74%, 60%, and 51% vs. 91%, 75%, 66%. c) Survival with COPD: 1-,5- and 10-year survival 91%,80% and 70% vs. 74%, 60% and 51%. The log-rank test was used for the comparison of the survival curves
Table 1
Basic patient characteristics of donors and recipients
| Variable | Recipients | Corresponding Donors | ||||||
|---|---|---|---|---|---|---|---|---|
| Overall (n  = = 230) 230) | IPF (n  = = 86) 86) | COPD (n  = = 86) 86) | Other (n  = = 58) 58) | |||||
| Sex | ||||||||
 Male Male | 118 (51%) | 53 (38%) | 41 (48%) | 24 (43%) | 112 (49%) | 33 (62%) | 52 (60%) | 27 (47%) |
 Female Female | 112 (49%) | 33 (62%) | 45 (52%) | 34 (57%) | 118 (51%) | 53 (38%) | 34 (40%) | 31 (53%) |
| Age at LTX (years) | ||||||||
| 17 69 58 | 36 69 59 | 46 67 60 | 17 65 58 | 13 74 51 | 14 74 51 | 15 73 52 | 13 70 51 |
| Age groups (years) | ||||||||
| 1 (0.5%) 9 (4%) 7 (3%) 25 (11%) 91 (40%) 98 (43%) 0 (0%) | 0 (0%) 0 (0%) 2 (2%) 11 (13%) 34 (40%) 39 (45%) 0 (0%) | 0 (0%) 0 (0%) 0 (0%) 4 (5%) 37 (43%) 45 (52%) 0 (0%) | 1 (2%) 9 (16%) 5 (9%) 10 (17%) 20 (34%) 14 (29%) 0 (0%) | 10 (04%) 18 (08%) 26 (11%) 54 (23%) 61 (27%) 49 (21%) 12 (05%) | 2 (2%) 8 (9%) 12 (14%) 19 (22%) 18 (21%) 22 (26%) 5 (6%) | 3 (3%) 8 (9%) 11(13%) 19 (22%) 20 (23%) 20 (23%) 5 (6%) | 5 (9%) 2 (3%) 3 (5%) 16 (28%) 23 (40%) 7 (12%) 2 (3%) |
| BMI at LTX (kg/m2) | ||||||||
 Min Min | 14.8 | 14.8 | 15.9 | 15.2 | 14.2 | 14.1 | 18.5 | 16.3 |
 Max Max | 33.7 | 33.1 | 31.1 | 33.7 | 46.9 | 46.9 | 39.1 | 34.7 |
 Median Median | 23.1 | 24.2 | 22.1 | 23 | 24.8 | 24.2 | 25.4 | 24.8 |
| LAS at LTX | ||||||||
 Min Min | 31 | 32 | 31 | 32 | ||||
 Max Max | 96 | 95 | 83 | 96 | ||||
 Median Median | 39 | 45 | 34 | 39 | ||||
| Pre LAS* | 69 (30%) | 32(37%) | 21(24%) | 16 (28%) | ||||
 HU HU | 35 | 19 | 4 | 12 | ||||
 U U | 24 | 9 | 12 | 3 | ||||
 T T | 7 | 2 | 5 | 0 | ||||
Donor > > 55 (%) 55 (%) | ||||||||
 LAS era LAS era | 80 (50%) | 27 (50%) | 31 (48%) | 22 (40%) | ||||
 Pre-LAS era Pre-LAS era | 13 (19%) | 7 (20%) | 2 (10%) | 4 (7%) | ||||
| Comorbidity | ||||||||
 CHD CHD | 38 (17%) | 12 (14%) | 18 (21%) | 8 (14%) | ||||
 aHT aHT | 66 (29%) | 27 (31%) | 33 (38%) | 6 (10%) | ||||
 DM DM | 44(19%) | 17 (20%) | 15 (17%) | 12 (21%) | ||||
| Comorbidity Donor | ||||||||
| 34 (15%) 75 (33%) 3 447 | 8 (9%) 25 (29%) 3 460 | 8 (9%) 32 (37%) 3 440 | 18 (31%) 18 (31%) 3 450 | ||||
| Operation | ||||||||
| 203 (88%) 27 (12%) 82 (36%) 70 (85%) 9 (11%) 3 (4%) | 74 (86%) 12 (14%) 43 (50%) 36 (84%) 6 (14%) 1(2%) | 73 (85%) 13 (15%) 13 (15%) 12 (92%) 1(8%) 0(0%) | 56 (97%) 2 (3%) 26 (49%) 22 (38%) 2 (3%) 2 (3%) | ||||
Basic characteristics of donors and recipients. BMI =
= body Mass Index, COPD
body Mass Index, COPD =
= chronic obstructive pulmonary disease, IPF
chronic obstructive pulmonary disease, IPF =
= idiopathic pulmonary fibrosis, EAA
idiopathic pulmonary fibrosis, EAA =
= exogenous allergic alveolitis, AAT
exogenous allergic alveolitis, AAT =
= alpha-1 antitrypsin deficiency, CHD
alpha-1 antitrypsin deficiency, CHD =
= coronary heart disease. * in the pre-LAS era, there was no information on the HU/U/T Status for 2 patients in the IPF group
coronary heart disease. * in the pre-LAS era, there was no information on the HU/U/T Status for 2 patients in the IPF group
In the whole study cohort, a donor age of ≥ 55 years at the time of LTx was associated with reduced overall survival (1-, 5- and 10-year survival: 75%, 54%, 37% vs. 84%, 76%, 69%; p
55 years at the time of LTx was associated with reduced overall survival (1-, 5- and 10-year survival: 75%, 54%, 37% vs. 84%, 76%, 69%; p =
= 0.0006) and an increased incidence of PGD 2/3 (46% vs. 31%, p
0.0006) and an increased incidence of PGD 2/3 (46% vs. 31%, p =
= 0.03) (Fig. 2). The significantly reduced survival was consistent for donor lungs
0.03) (Fig. 2). The significantly reduced survival was consistent for donor lungs ≥
≥ 60 and ≥
60 and ≥ 65 years and the also incidence of PGD2/3 was increased for donors
65 years and the also incidence of PGD2/3 was increased for donors ≥
≥ 60 compared to younger donors (Supplementary Figure S3). PGD2/3 and donor age
60 compared to younger donors (Supplementary Figure S3). PGD2/3 and donor age ≥
≥ 55 years were found to be independent predictors of reduced survival in the whole cohort in multivariate analysis (p
55 years were found to be independent predictors of reduced survival in the whole cohort in multivariate analysis (p =
= 0.0005 and p
0.0005 and p =
= 0.017 respectively) (Table 2). There was no statistically significant impact of donor age on the development of CLAD (p
0.017 respectively) (Table 2). There was no statistically significant impact of donor age on the development of CLAD (p =
= 0.28), however, CLAD was identified as a predictor of reduced survival in univariate analysis (p
0.28), however, CLAD was identified as a predictor of reduced survival in univariate analysis (p =
= 0.019). Interestingly, the recipient’s age at the time of LTx did not correlate with the incidence of PGD or long-term survival in either group.
0.019). Interestingly, the recipient’s age at the time of LTx did not correlate with the incidence of PGD or long-term survival in either group.
Table 2
Univariate and multivariate Cox proportional hazard regression analysis to identify predictors of survival
| Variables (n  = = 230) 230) | Univariate, HR (95% CI) | p | Multivariate, HR (95% CI) | p |
|---|---|---|---|---|
| Sex | ||||
 Gender (female) Gender (female) | 1.653 (1.052–2.597) | 0.046 | 1.247(0.732–2.126) | 0.42 |
| Underlying disease | ||||
| 1.463 (0.919–2.327) 0.572 (0.365–0.896) | 0.094 0.003 | 1.489 (0.847–2.719) | 0.12 |
| CLAD* | ||||
| 2.399 (1.154–4.990) 1.824 (0.581–7.924) 1.741 (0.512–5.917) | 0.019 0.24 0.31 | 1.076 (0.585–1.934) | 0.84 |
| PGD2/3 | ||||
| 3.225 (1.985–5.239) 2.202 (1.146–4.234) 3.757 (1.511–9.345) | < 0.0124 0.017 | 2.324 (1.447–3.752) 1.808 (0.897–3.744) 0.696 (0.331–1.320) | 0.0005 0.10 0.30 |
Recipient ≥ ≥ 55 years 55 years | ||||
| 0.865 (0.539–1.386) 1.114 (0.546–2.273) 0.721 (0.255–2.037) | 0.55 0.77 0.50 | ||
Donor ≥ ≥ 55 years 55 years | ||||
| 2.277 (1.420–3.660) 3.311 (1.646–6.663) 1.065 (0.400 | 0.0006 0.0002 0.90 | 1.817 (1.115–2.975) 2.969 (1.431–6.380) | 0.017 0.004 |
| Recipient comorbidity | ||||
| 1.295 (0.735–2.282) 0.903 (0.591–1.378) | 0.37 0.64 | ||
| Donor comorbidity | ||||
| 0.997 (0.624–1.593) 1.576 (0.751–3.308) | 0.99 0.23 | ||
| Sex | ||||
Surgery
| ||||
| 1.016 (0.520–1.983) 1.033 (0.297–3.597) 1.246 (0.514–3.021) | 0.96 0.96 0.60 | ||
| 2.480 (1.559–3.943) 3.063 (0.898–10.44) 1.405 (0.731–2.699) |
< 0.013 0.31 | 2.036 (1.245–3.378) 1.145 (0.542–2.190) | 0.005 0.70 |
Predictors of survival. IPF =
= idiopathic pulmonary fibrosis, COPD
idiopathic pulmonary fibrosis, COPD =
= chronic obstructive pulmonary disease, ECMO
chronic obstructive pulmonary disease, ECMO =
= extracorporeal membrane oxygenation, CHD
extracorporeal membrane oxygenation, CHD =
= coronary heart disease, CLAD
coronary heart disease, CLAD =
= chronic lung allograft dysfunction, *includes all patients that survived at least 6 months. If not mentioned otherwise, the values are calculated for all patients. The information on PGD is missing for one patient
chronic lung allograft dysfunction, *includes all patients that survived at least 6 months. If not mentioned otherwise, the values are calculated for all patients. The information on PGD is missing for one patient
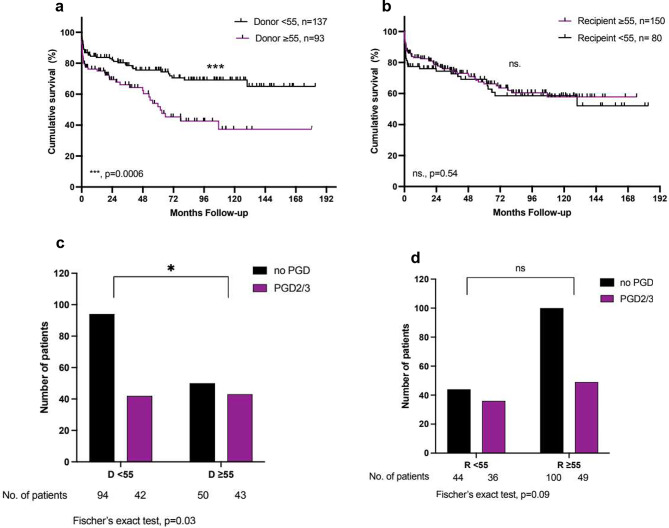
a +
+ b: Kaplan-Meier Analysis of survival after lung transplantation: for donors and recipients
b: Kaplan-Meier Analysis of survival after lung transplantation: for donors and recipients <
< 55 and ≥
55 and ≥ 55 years of age. c
55 years of age. c +
+ d: Incidence of primary graft dysfunction grade 2 or 3 in donors and recipients
d: Incidence of primary graft dysfunction grade 2 or 3 in donors and recipients ≥
≥ 55 years of age. For one patient there is no information available on PGD
55 years of age. For one patient there is no information available on PGD
In the next step, we analysed if there was a differential impact of donor age depending on the recipients’ underlying pulmonary disease. We identified 86 (37%) patients with idiopathic pulmonary fibrosis (IPF) and 86 (37%) patients with chronic obstructive pulmonary disease (COPD). Basic donor and recipient characteristics were comparable in both groups (Table 1). In general, overall survival after LTx was similar in patients with IPF (p =
= 0.094) and increased in patients with COPD (p
0.094) and increased in patients with COPD (p =
= 0.003) (Fig. 1B
0.003) (Fig. 1B +
+ C). Notably, only in patients with IPF, a donor age
C). Notably, only in patients with IPF, a donor age ≥
≥ 55 years was associated with a higher incidence of severe primary graft dysfunction (PGD2/3) (65% vs. 37%; p
55 years was associated with a higher incidence of severe primary graft dysfunction (PGD2/3) (65% vs. 37%; p =
= 0.016) and with a considerably reduced long-term survival (1-, 5-, and 10-year survival: 62%, 38%, 16% vs. 80%, 76%, 70%; p
0.016) and with a considerably reduced long-term survival (1-, 5-, and 10-year survival: 62%, 38%, 16% vs. 80%, 76%, 70%; p =
= 0.0002) (Fig. 3). On the other hand, a donor age
0.0002) (Fig. 3). On the other hand, a donor age ≥
≥ 55 had no impact on survival nor the incidence of PGD2/3 in patients with COPD (68% vs. 72%, p
55 had no impact on survival nor the incidence of PGD2/3 in patients with COPD (68% vs. 72%, p =
= 0.90 and 21% vs. 27%, p
0.90 and 21% vs. 27%, p =
= 0.60 respectively) (Fig. 4). Again, the older age of the recipients was not associated with reduced overall survival or the incidence of PGD2/3 in either group. For patients with IPF, a donor age
0.60 respectively) (Fig. 4). Again, the older age of the recipients was not associated with reduced overall survival or the incidence of PGD2/3 in either group. For patients with IPF, a donor age ≥
≥ 55 years was identified as an independent predictor of reduced survival in multivariate Cox regression analysis (p
55 years was identified as an independent predictor of reduced survival in multivariate Cox regression analysis (p =
= 0.004) (Table 2). The development of CLAD was not affected by donor or recipient age in either subgroup. As expected, the median LAS score was higher in the IPF group, however, there was no significant difference in the percentage of donor organs
0.004) (Table 2). The development of CLAD was not affected by donor or recipient age in either subgroup. As expected, the median LAS score was higher in the IPF group, however, there was no significant difference in the percentage of donor organs >
> 55 years between the groups in the LAS era (Table 1).
55 years between the groups in the LAS era (Table 1).
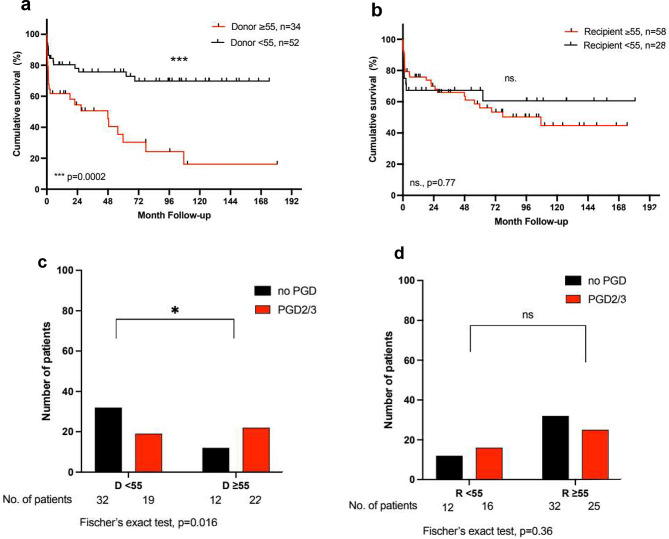
a +
+ b: Kaplan-Meier Analysis of survival after lung transplantation in patients with IPF for donors and recipients
b: Kaplan-Meier Analysis of survival after lung transplantation in patients with IPF for donors and recipients <
< 55 and ≥
55 and ≥ 55 years of age. c
55 years of age. c +
+ d: Incidence of primary graft dysfunction grade 2 or 3 PGD2/3 in patients with IPF for donors and recipients
d: Incidence of primary graft dysfunction grade 2 or 3 PGD2/3 in patients with IPF for donors and recipients <
< 55 and ≥
55 and ≥ 55 years of age. The log-rank test was used for the comparison of the survival curves. For one patient there is no information available on PGD. D
55 years of age. The log-rank test was used for the comparison of the survival curves. For one patient there is no information available on PGD. D =
= donor, R
donor, R =
= recipient
recipient
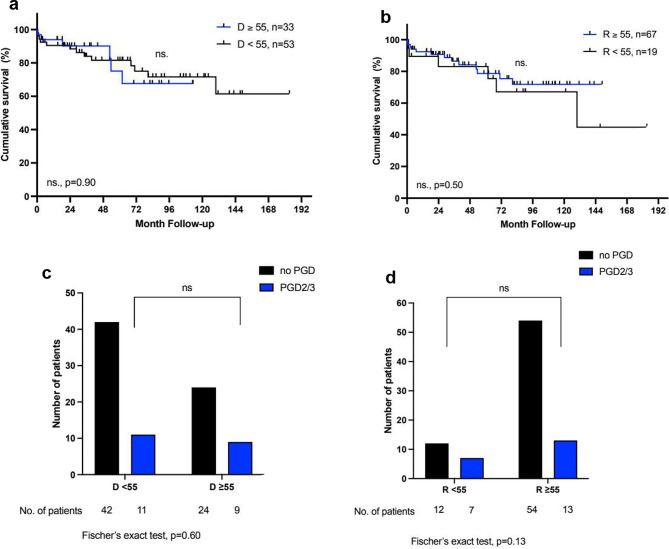
a +
+ b: Kaplan-Meier Analysis of survival after lung transplantation in patients with COPD for donors and recipients
b: Kaplan-Meier Analysis of survival after lung transplantation in patients with COPD for donors and recipients <
< 55 and ≥
55 and ≥ 55 years of age; c
55 years of age; c +
+ d: Incidence of primary graft dysfunction grade 2 or 3 (PGD2/3 ) in patients with COPD for donors and recipients
d: Incidence of primary graft dysfunction grade 2 or 3 (PGD2/3 ) in patients with COPD for donors and recipients <
< 55 and ≥
55 and ≥ 55 years of age. The log-rank test was used for the comparison of the survival curves. For one patient there is no information available on PGD. D
55 years of age. The log-rank test was used for the comparison of the survival curves. For one patient there is no information available on PGD. D =
= donor, R
donor, R =
= recipient
recipient
Single lung transplantation was performed in 12 (14%) patients with IPF and 13 (15%) patients with COPD. There was no difference in survival in either group compared to patients who underwent double lung transplantation. While more patients in the IPF subgroup were transplanted on ECMO support (43/86), there was no statistically significant association between the use of perioperative ECMO and survival (p =
= 0.31), unlike in the COPD subgroup (p
0.31), unlike in the COPD subgroup (p =
= 0.013) and the whole cohort (p
0.013) and the whole cohort (p =
= <
< 0.0001) (Table 2).
0.0001) (Table 2).
The cause of death in LTx recipients with IPF was sepsis and consecutive multi-organ failure in 34/49 (69%) of the patients in that subgroup). To further corroborate this hypothesis, we re-analyzed the Torque-teno virus (TTV) load as a possible endogenous marker to reflect immune function in selected patients from our cohort [24]. Interestingly, we found higher TTV-DNA levels after lung transplantation in patients with IPF compared to COPD patients (X2 =
= 4.57, p
4.57, p =
= 0.033; Fig. 5). The incidence of CMV reactivation was similar in both groups, COPD and IPF patients (42% vs. 36%). While there was a higher percentage of CMV reactivations in IPF patients with donor organs
0.033; Fig. 5). The incidence of CMV reactivation was similar in both groups, COPD and IPF patients (42% vs. 36%). While there was a higher percentage of CMV reactivations in IPF patients with donor organs ≥
≥ 55 years, this did not reach statistical significance (55% vs. 33%, p
55 years, this did not reach statistical significance (55% vs. 33%, p =
= 0.16).
0.16).
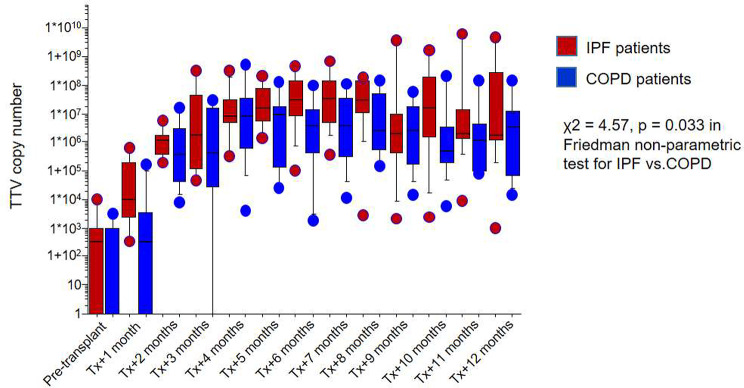
Torque-Teno Virus copy number in patients with IPF and COPD. . Increased copies of Torque-Teno Virus after lung transplantation in patients with IPF compared to patients with COPD as an underlying disease
Discussion
Selecting the best donor for a patient remains a challenge in lung transplantation [9]. In our lung transplant recipient cohort, a donor age of ≥ 55 years was associated with impaired organ function and reduced survival in patients with IPF.
55 years was associated with impaired organ function and reduced survival in patients with IPF.
Donor and recipient age
Due to the general shortage of organs, the possibility of an expansion of the donor pool using older organs has been analysed in several studies [14, 15, 27–29]. The results are controversial, and the study populations are mostly heterogeneous. While some studies report comparable short-term and long-term outcome in older donor lungs, Sommer et al. found that spirometry findings after transplant were better in emphysema recipients than in IPF recipients with donor lungs >
> 70 years of age [28]. Data regarding the differential outcome of patients depending on the underlying pulmonary disease, specifically in patients with IPF is scarce. In this context, the most striking finding in our cohort was that donor age has an impact on severe PGD as a short-term complication, and reduced survival as a long-term consequence in patients with IPF. In contrast to recipients with IPF, the transplantation of a donor organ
70 years of age [28]. Data regarding the differential outcome of patients depending on the underlying pulmonary disease, specifically in patients with IPF is scarce. In this context, the most striking finding in our cohort was that donor age has an impact on severe PGD as a short-term complication, and reduced survival as a long-term consequence in patients with IPF. In contrast to recipients with IPF, the transplantation of a donor organ ≥
≥ 55 years of age for patients with underlying COPD did not affect the incidence of PGD or long-term survival. This is in accordance with another study from Shigemura and colleagues, where COPD patients, which comprised the majority of the cohort, had a similar outcome with organs
55 years of age for patients with underlying COPD did not affect the incidence of PGD or long-term survival. This is in accordance with another study from Shigemura and colleagues, where COPD patients, which comprised the majority of the cohort, had a similar outcome with organs >
> 55 years of age [15]. One might speculate that underlying immunological alterations [17] and/or an accelerated ageing process in patients suffering from IPF may increase the susceptibility to infections and, that these patients become even more vulnerable to infections if also the transplanted organ (and the immune cells within) are older. The incidence of CLAD was not affected by donor or recipient age, similar to the results from other studies [27, 30].
55 years of age [15]. One might speculate that underlying immunological alterations [17] and/or an accelerated ageing process in patients suffering from IPF may increase the susceptibility to infections and, that these patients become even more vulnerable to infections if also the transplanted organ (and the immune cells within) are older. The incidence of CLAD was not affected by donor or recipient age, similar to the results from other studies [27, 30].
While evaluating the importance of donor age depending on the underlying lung disease, we also analysed surgical factors that might impact the study’s results. Single lung transplantation may be a risk factor for increased incidence of post-transplant infection due to bacteria in the remaining native lung. However, there was no statistically significant difference in survival in either group.
ECMO is a significant risk factor for PGD and may also have contributed to the high incidence of PGD in patients with IPF compared to patients with COPD. On the other hand, 58% (25/43) of the donors of the ECMO patients in the IPF group were < 55 years old and there was no statistically significant difference in overall survival with and without ECMO in IPF patients.
55 years old and there was no statistically significant difference in overall survival with and without ECMO in IPF patients.
Lung fibrosis and immunological response
Telomere-related gene mutations have a high prevalence in patients with IPF [31] leading to accelerated ageing of the lung. Over the last decade, shorter telomere length has been associated with impaired immune function [32] and a worse outcome after LTx [33]. Transplant recipients are more susceptible to neutropenia [34] under immunosuppressive medication and more prone to lower airway infections. Older donor organs do not have the same self-repair mechanisms compared to their younger counterparts mainly due to endothelial senescence [35]. They additionally have reduced autophagy [18] and impaired pulmonary endothelial protective mechanisms [35, 36]. All of this results in an increased vulnerability to infection, mitigated DNA repair mechanisms, deficits in cell repair and regeneration and thus attenuated parenchymal healing [37].
Most likely, both conditions, the older age of donor organs and the presence of fibrosis as a surrogate of accelerated aging in the recipient [17] are responsible for the observed unfavourable outcome. The hypothesis of aging and immunological processes underlying fibrosis might explain the reduced survival after the transplantation of an older organ with potentially reduced regeneration abilities. This is further supported by the fact that sepsis was the leading cause of death in our cohort in patients with fibrotic lung diseases and older donor age.
Over the last decade, Torque-Teno Virus or Transfusion-transmitted Virus (TTV) has gained increasing attention as a possible endogenous marker to reflect immune function in solid organ transplantation [24, 38], with higher TTV-DNA copy numbers indicating a more suppressed immune system [20]. There is evidence that the TTV-DNA load may correlate with the level of medical immunosuppression individually after transplantation [20, 39]. In accordance with our hypothesis, there is a significant difference towards higher TTV-DNA levels after lung transplantation in patients with IPF compared to COPD patients, as shown in re-analyzed data from our cohort [24]. However, we were only able to analyze a small sub-cohort of our patients, and the role of TTV load as a marker for immunosuppression is disputable and needs further verification [38], therefore, we cannot draw a definite conclusion here. IPF lung transplant recipients were shown to have an increased incidence of CMV viremia episodes and other CMV complications [40]. However, the association of post-transplant CMV reactivation with TTV copy numbers is controversial [41, 42]. While there was no statistically significant increase in CMV reactivations in patients with IPF compared to patients with COPD in our cohort, this might be due to small numbers.
Yet, one may carefully hypothesize that dysregulation of the immune response in patients with IPF, and potentially in patients with fibrotic lung diseases in general, might impact short- and long-term outcomes after LTx. With these data, we cannot analyse the impact of older donor organs on TTV copy numbers in IPF and COPD patients due to small numbers. Ultimately, the combination of a donor organ with age-related impaired regeneration abilities in donor lungs aged ≥
≥ 55 years, may contribute to reduced survival in these patients.
55 years, may contribute to reduced survival in these patients.
Limitations of the study
This a retrospective single-centre study covering nearly 20 years. Due to the retrospective nature of our study, older donors who have not been used for transplantation, were not considered. The observations made in this study may therefore be affected by a selection bias. Despite the long analysis period, the immunosuppressive regime for all patients was determined by our outpatient centre and did not significantly change over time. Changes in surgical technique and ECMO management certainly improved ever since which may have influenced patient outcome.
Unfortunately, data on telomere length was not available for the IPF patients in our cohort, therefore we could not further analyse the hypothesis of accelerated aging and reduced immune function after LTx due to shorter telomeres.
Only two subgroups, IPF and COPD patients, were analysed in depth and the overall cohort is heterogeneous. While the effect of donor age might be relevant for patients with underlying fibrotic lung disease in general, not just with IPF (data not shown), more studies are needed that analyse subgroups of pulmonary diseases leading to transplantation differentially.
Lastly, TTV-DNA levels were taken from a small sub-cohort of IPF and COPD patients. More detailed studies with higher patient numbers are warranted to further elucidate the impact on immune function and the association with immunosuppression drug levels. Although some hypotheses support an altered immune function in IPF patients, potentially contributing to reduced survival in IPF recipients with older donor organs, our data is not sufficient to draw a definite conclusion here. Moreover, due to small numbers, TTV DNA levels in IPF/COPD recipients with donors ≥
≥ 55 years of age cannot be sufficiently evaluated in this study.
55 years of age cannot be sufficiently evaluated in this study.
Conclusions
In lung transplant recipients with IPF, a donor ≥
≥ 55 years of age was associated with a higher incidence of primary graft dysfunction. Moreover, it was revealed as an independent risk factor for reduced survival in the whole cohort and in patients with IPF specifically. Thus, precise recipient selection is crucial. Although we may only hypothesise an altered immune function in selected recipients with IPF, from our viewpoint, older donor organs should be carefully evaluated. Since most studies are based on a heterogeneous recipient cohort, further studies are needed to analyse the impact of the underlying pulmonary disease, as this may be a relevant factor for postoperative graft function and survival.
55 years of age was associated with a higher incidence of primary graft dysfunction. Moreover, it was revealed as an independent risk factor for reduced survival in the whole cohort and in patients with IPF specifically. Thus, precise recipient selection is crucial. Although we may only hypothesise an altered immune function in selected recipients with IPF, from our viewpoint, older donor organs should be carefully evaluated. Since most studies are based on a heterogeneous recipient cohort, further studies are needed to analyse the impact of the underlying pulmonary disease, as this may be a relevant factor for postoperative graft function and survival.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Abbreviations
| AAT | Alpha-1 Antitrypsin |
| AHT | Arterial Hypertension |
| BMI | Body Mass Index |
| CHD | Coronary Heart Disease |
| COPD | Chronic Obstructive Pulmonary Diseases |
| CLAD | Chronic Lung Allograft Dysfunction |
| DLTx | Double Lung Transplantation |
| DM | Diabetes Mellitus |
| EAA | Exogenous Allergic Alveolitis |
| IPF | Idiopathic Pulmonary Fibrosis |
| LTx | Lung Transplantation |
| PGD | Primary Graft Dysfunction |
| PGD2/3 | Primary Graft Dysfunction Grade 2 or 3 |
| SLTx | Single Lung Transplantation |
| TTV | Torque-Teno Virus |
Author contributions
IM and WJ concepted the study. EDO, AK and IM collected the data. SB and BCF contributed the detection and analysis of the TTV-DNA. EDO and IM performed the statistical analysis and IM wrote the manuscript with input from DS, SF, WJ and BCF. Visualisation: IM and WJ. BP, MC, DS, BCF and WJ reviewed and revised the manuscript.
Funding
No external funding was used for this study.
Open Access funding enabled and organized by Projekt DEAL.
Data availability
The datasets analysed during the current study are available from the corresponding author on reasonable request.
Declarations
The study was conducted following the guidelines proposed in the Declaration of Helsinki and approved by the Medical Centre – University of Freiburg’s local ethics committee. It is registered at the German Registry for Clinical Trials (DRKS) under the trial registration number 00021501. Due to the study’s retrospective nature and the associated minimal risk, a waiver of consent was granted.
Not applicable.
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wolfgang Jungraithmayr and Bjoern Christian Frye contributed equally to this work.
References
 2019; focus theme: Donor and recipient size match. J Heart Lung Transpl. 2019;38(10):1056–66. [Europe PMC free article] [Abstract] [Google Scholar]
2019; focus theme: Donor and recipient size match. J Heart Lung Transpl. 2019;38(10):1056–66. [Europe PMC free article] [Abstract] [Google Scholar] 2020; focus on deceased donor characteristics. J Heart Lung Transpl. 2020;39(10):1016–27. [Europe PMC free article] [Abstract] [Google Scholar]
2020; focus on deceased donor characteristics. J Heart Lung Transpl. 2020;39(10):1016–27. [Europe PMC free article] [Abstract] [Google Scholar]Articles from BMC Pulmonary Medicine are provided here courtesy of BMC
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Increased Donor Organ Size and Age is Associated with Reduced Survival in Female Lung Transplant Recipients.
Transplant Proc, 56(6):1429-1435, 23 Jul 2024
Cited by: 0 articles | PMID: 39048476
Lung Transplant Type and Donor Age in Idiopathic Pulmonary Fibrosis: A Single Center Study.
J Surg Res, 271:125-136, 11 Dec 2021
Cited by: 0 articles | PMID: 34902736
Impact of Acute Exacerbation of Idiopathic Pulmonary Fibrosis on Lung Transplant Outcomes.
Transplantation, 108(6):1460-1465, 23 May 2024
Cited by: 1 article | PMID: 38291576
Lung transplant in idiopathic pulmonary fibrosis.
Arch Surg, 146(10):1204-1209, 01 Oct 2011
Cited by: 34 articles | PMID: 22006881
Review
Funding
Funders who supported this work.

 1
1 

