Abstract
Background
Mechanobiological mechanisms of osteoarthritis (OA) are unclear. Our objectives were to explore: 1) changes in knee joint physiology using a large panel of synovial fluid biomarkers from before to one year after high tibial osteotomy (HTO) surgery, and 2) the association of changes in the synovial fluid biomarkers with the changes in MRI measures of knee effusion-synovitis and articular cartilage composition.Methods
Twenty-six patients with symptomatic knee OA and varus alignment underwent synovial fluid aspirations and 3 T MRI before and one year after medial opening wedge HTO. Cytokine and growth factor levels in synovial fluid were measured with multiplex assays. Ontology and pathway enrichment was assessed using data protein sets with gene set enrichment analysis (GSEA), and analyzed using linear mixed effects models. MRIs were analyzed for effusion-synovitis and T2 cartilage relaxation time using manual segmentations. Changes in biomarker concentrations were correlated to changes in MRI effusion-synovitis volume and articular cartilage T2 relaxation times.Results
Decreased enrichment in Toll-like receptor and TNF-α signalling was detected one year after HTO. The leading contributors to this reduction included IL-6, TNF-α and IL-1β, whereas the highest contributors to positive enrichment were EGF, PDGF-BB and FGF-2. Effusion-synovitis volume decreased (mean [95%CI]) one year after HTO (-2811.58 [-5094.40, -528.76mm3]). Effusion-synovitis volume was moderately correlated (r [95% CI]) with decreased MMP-1 (0.44 [0.05; 0.71]), IL-7 (0.41 [0.00; 0.69]) and IL-1β (0.59 [0.25; 0.80]) and increased MIP-1β (0.47 [0.10; 0.73]). Medial tibiofemoral articular cartilage T2 relaxation time decreased (mean [95% CI]) one year after HTO (-0.33 [-2.69; 2.05]ms). Decreased T2 relaxation time was moderately correlated to decreased Flt-3L (0.61 [0.28; 0.81]), IL-10 (0.47 [0.09; 0.73]), IP-10 (0.42; 0.03-0.70) and increased MMP-9 (-0.41 [-0.7; -0.03]) and IL-18 (-0.48 [-0.73; -0.10]).Conclusions
Decreased aberrant knee mechanical loading in patients with OA is associated with decreased biological and imaging measures of inflammation (measured in synovial fluid and on MRI) and increased anabolic processes. These exploratory findings suggest that improvement in knee loading can produce long-term (one year) improvement in joint physiology.Free full text

Changes and associations between synovial fluid and magnetic resonance imaging markers of osteoarthritis after high tibial osteotomy
Associated Data
Abstract
Background
Mechanobiological mechanisms of osteoarthritis (OA) are unclear. Our objectives were to explore: 1) changes in knee joint physiology using a large panel of synovial fluid biomarkers from before to one year after high tibial osteotomy (HTO) surgery, and 2) the association of changes in the synovial fluid biomarkers with the changes in MRI measures of knee effusion-synovitis and articular cartilage composition.
Methods
Twenty-six patients with symptomatic knee OA and varus alignment underwent synovial fluid aspirations and 3 T MRI before and one year after medial opening wedge HTO. Cytokine and growth factor levels in synovial fluid were measured with multiplex assays. Ontology and pathway enrichment was assessed using data protein sets with gene set enrichment analysis (GSEA), and analyzed using linear mixed effects models. MRIs were analyzed for effusion-synovitis and T2 cartilage relaxation time using manual segmentations. Changes in biomarker concentrations were correlated to changes in MRI effusion-synovitis volume and articular cartilage T2 relaxation times.
Results
Decreased enrichment in Toll-like receptor and TNF-α signalling was detected one year after HTO. The leading contributors to this reduction included IL-6, TNF-α and IL-1β, whereas the highest contributors to positive enrichment were EGF, PDGF-BB and FGF-2. Effusion-synovitis volume decreased (mean [95%CI]) one year after HTO (-2811.58 [-5094.40, -528.76mm3]). Effusion-synovitis volume was moderately correlated (r [95% CI]) with decreased MMP-1 (0.44 [0.05; 0.71]), IL-7 (0.41 [0.00; 0.69]) and IL-1β (0.59 [0.25; 0.80]) and increased MIP-1β (0.47 [0.10; 0.73]). Medial tibiofemoral articular cartilage T2 relaxation time decreased (mean [95% CI]) one year after HTO (-0.33 [-2.69; 2.05]ms). Decreased T2 relaxation time was moderately correlated to decreased Flt-3L (0.61 [0.28; 0.81]), IL-10 (0.47 [0.09; 0.73]), IP-10 (0.42; 0.03–0.70) and increased MMP-9 (-0.41 [-0.7; -0.03]) and IL-18 (-0.48 [-0.73; -0.10]).
Conclusions
Decreased aberrant knee mechanical loading in patients with OA is associated with decreased biological and imaging measures of inflammation (measured in synovial fluid and on MRI) and increased anabolic processes. These exploratory findings suggest that improvement in knee loading can produce long-term (one year) improvement in joint physiology.
Introduction
It is well established that biological processes mediate the effects of biomechanical stresses on joint tissues in knee osteoarthritis (OA), but the development of a disease modifying OA intervention is hindered by the complexity of the interactions between biomechanics and joint biology in humans. In a healthy synovial joint, there is a balance of anabolic, catabolic, inflammatory, and anti-inflammatory processes that work together to maintain homeostasis [1, 2]. OA skews this balance by lowering anabolic homeostasis in favour of inflammatory and catabolic processes [3]. Products of cartilage breakdown that are released into the synovial fluid are likely to perpetuate synovial inflammation in OA. In response, synoviocytes produce pro-inflammatory mediators which attract immune cells, increase angiogenesis and impair joint physiology leading to increases in cell stress in all joint tissues. This creates a vicious cycle, as chondrocytes produce additional cytokines and proteolytic enzymes that increase cartilage degradation and induce further synovial inflammation. Subsequently, the synovium and cartilage may produce anti-inflammatory cytokines, although they are often not sufficient to stop the disease process [2, 4, 5].
Aberrant mechanical loading exacerbates OA pathogenesis and in some cases may even initiate it [6, 7]. Human gait biomechanics studies suggest that proxy measures representing dynamic knee joint loading are associated with articular cartilage degradation and inflammation assessed by imaging [8–11]. Joint imaging techniques primarily analyze morphological changes, although recent advances in MRI sequences targeting water content in cartilage permit insights into cartilage composition. Importantly, these methods have limited ability to assess biological activity in joint tissues and would be aided by contemporaneous analysis of biochemical markers in synovial fluid. Associations between changes in joint mechanics and anabolic and pro-inflammatory joint physiology are not well-understood. Anabolic and inflammatory changes in joint physiology may be studied by measuring synovial fluid cytokines, chemokines, growth factors and matrix metalloproteinases (MMPs), which are key mediators of OA pathophysiology. Although these factors may be altered in the long-term after load-altering interventions for knee OA, such changes and their associations with morphological and compositional MRI measures in joint tissues have not been investigated.
Knee load-altering surgical interventions can be used as models to investigate potential links between changes in joint biomechanics and biological processes [12, 13]. Watt et al. [12]observed increases in synovial fluid concentrations of interleukin (IL)-8, fibroblast growth factor (FGF)-2, and transforming growth factor-beta (TGFβ)-1 six weeks after knee joint distraction surgery. Kumagai et al. [13], observed decreases in synovial fluid concentrations of IL-6, IL-8, MMP-2, MMP-3, MMP-13, vascular endothelial growth factor (VEGF) and cartilage oligomeric matrix protein (COMP) 17 months after high tibial osteotomy (HTO). Although these studies have identified changes in individual factors following load-altering surgeries, larger panel analyses would facilitate a broader understanding of the physiological consequences of load-altering interventions, including pathways that play a key role in OA pathogenesis [14]. Further, paired analyses of associations between changes in joint imaging and biochemical panel data are enticing approaches to better understand the effects of load-alteration on OA pathophysiology.
We have previously observed improvements in magnetic resonance imaging (MRI) measures of knee effusion-synovitis [9] and articular cartilage composition (T2 relaxation) [10] after HTO. Load-altering interventions such as HTO produce changes in knee loads, [9, 15, 16], but it remains unclear if these MRI changes are associated with changes in joint physiology, represented by changes in the composition of synovial fluid. Using medial opening wedge HTO as a model of substantially altered knee joint mechanical loading, the objectives of this study were to explore: 1) changes in knee joint physiology using a large panel of synovial fluid biomarkers from before to one year after surgery, and 2) the association of changes in the synovial fluid biomarkers with the changes in MRI measures of knee effusion-synovitis and articular cartilage composition.
Methods
Participants
A subgroup of consecutive patients recruited for an ongoing prospective cohort study investigating medial opening wedge HTO agreed to undergo additional assessents including synovial fuid biochemical marker testing and MRI, both completed preoperatively and one year postoperatively [15]. We identified the first 36 participants with complete pre and postoperative MRI data, based on 80% power to detect a statistically significant (p <
< 0.05) within-patient change of moderate effect size (d
0.05) within-patient change of moderate effect size (d =
= 0.5) with α
0.5) with α =
= 0.05. [17] That sample size provided the ability to detect a Pearson r correlation coefficient of
0.05. [17] That sample size provided the ability to detect a Pearson r correlation coefficient of ≥
≥ 0.45 between change scores, with 80% power and α
0.45 between change scores, with 80% power and α =
= 0.05. [17] Participants with synovial fluid samples
0.05. [17] Participants with synovial fluid samples <
< 100 ul at either pre- or post-operative time points were excluded.
100 ul at either pre- or post-operative time points were excluded.
We recruited participants from the Fowler Kennedy Sport Medicine Clinic (London, Ontario, Canada) during consultation with an orthopedic surgeon. Patients were eligible if they met the American College of Rheumatology clinical classification criteria for knee OA [18], had varus alignment and radiographic signs of OA primarily affecting the medial tibiofemoral compartment. Patients with worse radiographic joint space narrowing in the lateral or patellofemoral compartment were excluded. Baseline demographics, including Kellgren-Laurence (KL) grades [19], OsteoArthrits Research Society International (OARSI) [20]grading and Knee Osteoarthritis Outcome Score (KOOS) [21] were all collected. All participants provided informed consent, and study was approved by the Western University Health Sciences Research Ethics Board #1187.
Intervention
Medial opening-wedge HTO was performed using a previously described technique [22]. A preoperative hip-to-ankle standing anteroposterior radiograph was used to template a correction angle required to create very slight valgus alignment postoperative (mechanical axis angle =
= 0-to-3 degrees). The osteotomy was fixed using either a polyethyletherketone insert and screws, which did not produce artifact on MRI [23], or a titanium locking plate with cortical and cancellous screws that were removed at approximately six months postoperative. The removal was done through a percutaneous incision at the original incision site. Postoperative management included the use of a hinged brace and feather weight-bearing with crutches for six weeks, and a previously described rehabilitation program [9].
0-to-3 degrees). The osteotomy was fixed using either a polyethyletherketone insert and screws, which did not produce artifact on MRI [23], or a titanium locking plate with cortical and cancellous screws that were removed at approximately six months postoperative. The removal was done through a percutaneous incision at the original incision site. Postoperative management included the use of a hinged brace and feather weight-bearing with crutches for six weeks, and a previously described rehabilitation program [9].
Synovial fluid biomarkers
Synovial fluid was drawn from the operative knee immediately before surgery in the operating room, and again at the one-year follow-up visit in the clinic. The synovial fluid samples were immediately centrifuged at 2800 g for 15 min to remove cells and debris and the supernatants were collected and stored at -80 °C. Samples were treated with hylaluronidase immediately before multiplex assay in duplicate. Commercial multiplex bead-based immunoassays [Human Cytokine/Chemokine Array 48-plex, TGF-β/tissue inhibitor of metalloproteinases (TIMP) panel and MMP panel] were used to measure biomarker concentrations (Eve Technologies, Calgary, AB, Canada). Biomarker concentrations [pg/mL or fluorescence intensity (FI) units] that were out of range of above or below the standard curve were imputed with the highest or lowest interpolated concentration for that analyte, respectively.
Magnetic Resonance Imaging (MRI)
Imaging was taken before surgery, and at the one-year follow up. MRI and synovial fluid extractions were attempted to be taken as close as possible, however; due to patient and surgeon availability, there was some variability. Each participant was scanned using a Siemens MAGNETOM Prisma Fit whole-body 3-Tesla MRI scanner and 15-channel knee RF coil. To analyze effusion-synovitis concentrations, we used the 3D Dual Echo Steady-State (DESS) pulse sequence, which consists of 160 slices, a slice thickness of 0.7 mm and 0.37  >
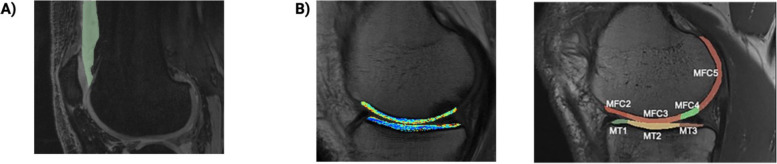
> 0.80] [9]. The borders of effusion-synovitis in the suprapatellar pouch were segmented in the sagittal view on a slice-by-slice basis, and affirmed in the transverse and frontal views. A signal intensity threshold for each image was then applied to eliminate hypointense tissue within the synovial volume (Fig. (Fig.11a).
0.80] [9]. The borders of effusion-synovitis in the suprapatellar pouch were segmented in the sagittal view on a slice-by-slice basis, and affirmed in the transverse and frontal views. A signal intensity threshold for each image was then applied to eliminate hypointense tissue within the synovial volume (Fig. (Fig.11a).
To analyze T2 relaxation, the same protocol was followed as previously described [10]. The T2 mapping sequence was a sagittal multi-slice multi-echo spin echo sequence with seven echoes (TE =
= 10, 20, 30, 40, 50, 60, 70 ms), and a repetition time of 2700 ms. T2 relaxation values were calculated using Siemens MapIt software (Siemens Medical Solutions, Erlangen, Germany). One reader, trained by a musculoskeletal radiologist and rheumatologist and blinded to timepoint, manually segmented three consecutive slices for each region. Each loadbearing region of the medial femur, medial tibia, lateral femur, lateral tibia and patella was identified using a standardized anatomical atlas [25] and created using 3D Slicer (3D slicer, http://www.slicer.org). [24] We previously demonstrated excellent intra- and inter-reliability for pre- and postoperative volume segmentation (lower ends of all ICC 95% CIs
10, 20, 30, 40, 50, 60, 70 ms), and a repetition time of 2700 ms. T2 relaxation values were calculated using Siemens MapIt software (Siemens Medical Solutions, Erlangen, Germany). One reader, trained by a musculoskeletal radiologist and rheumatologist and blinded to timepoint, manually segmented three consecutive slices for each region. Each loadbearing region of the medial femur, medial tibia, lateral femur, lateral tibia and patella was identified using a standardized anatomical atlas [25] and created using 3D Slicer (3D slicer, http://www.slicer.org). [24] We previously demonstrated excellent intra- and inter-reliability for pre- and postoperative volume segmentation (lower ends of all ICC 95% CIs >
> 0.81) [10]. The medial tibial and femoral (MT
0.81) [10]. The medial tibial and femoral (MT +
+ MF) and lateral tibial and femoral (LT
MF) and lateral tibial and femoral (LT +
+ LF) articular cartilage measures were combined together and analyzed as compartmental regions of interest (medial compartment and lateral compartment) to determine overall cartilage changes in those compartments (Fig. (Fig.11b).
LF) articular cartilage measures were combined together and analyzed as compartmental regions of interest (medial compartment and lateral compartment) to determine overall cartilage changes in those compartments (Fig. (Fig.11b).
Statistical analyses
Baseline demographic and clinical characteristics for the sample were described using means and standard deviations for continuous data and frequencies and percentages for categorical data. We calculated means and standard deviations for pre and one-year postoperative measures for each individual biochemical marker concentration and MRI marker value, then calculated the mean change with 95% CIs and standardized response means (SRM). The SRMs of 0.2, 0.5 and 0.8 were considered low, moderate and strong, respectively [26]. Furthermore, pre and one-year post HTO values were compared statistically using paried t-tests. The correlations between change in biomarker concentration and MRI measures pre and one year post HTO were analyzed using Pearson’s correlation coefficients (r), where r =
= 0.1, 0.3 and 0.5 were considered weak, moderate and strong, respectively [27].
0.1, 0.3 and 0.5 were considered weak, moderate and strong, respectively [27].
For biomarker data pre-processing, multiple normalizations were performed, but a Box-Cox transformation and +
+ 2 offset normalization were performed on the FI values using the R packages BiocManager and bestNormalize, respectively as they best suited this data [28]. QQ plots were examined to confirm normality. The full biomarker dataset was queried using gene set enrichment analysis (GSEA, UC San Diego and Broad Insitute [29, 30]) to perform an enrichment analysis of biological pathways and ontologies in our protein biomarker panels, using a two-group comparison (baseline and one year post HTO). GSEA was chosen because it can be used to analyze enrichment of expression data of any type of marker, including gene and protein data [31]. A leading edge analysis (LEA) was performed to identify top candidates enriched in numerous pathway sets at each timepoint. Biomarker FI values were queried against Kyto Encyclopedia of Genes and Genomes (KEGG) and Hallmark databases [29]. False discovery rate (FDR) cut off was set at 0.25, which is commonly used in bioinformatic studies [29]. Linear mixed effects models (LMM) were performed on the data using the R package lme4 [32], where timepoint (baseline and one year post HTO) was a fixed effect, and participant was a random effect to account for variation between patients.
2 offset normalization were performed on the FI values using the R packages BiocManager and bestNormalize, respectively as they best suited this data [28]. QQ plots were examined to confirm normality. The full biomarker dataset was queried using gene set enrichment analysis (GSEA, UC San Diego and Broad Insitute [29, 30]) to perform an enrichment analysis of biological pathways and ontologies in our protein biomarker panels, using a two-group comparison (baseline and one year post HTO). GSEA was chosen because it can be used to analyze enrichment of expression data of any type of marker, including gene and protein data [31]. A leading edge analysis (LEA) was performed to identify top candidates enriched in numerous pathway sets at each timepoint. Biomarker FI values were queried against Kyto Encyclopedia of Genes and Genomes (KEGG) and Hallmark databases [29]. False discovery rate (FDR) cut off was set at 0.25, which is commonly used in bioinformatic studies [29]. Linear mixed effects models (LMM) were performed on the data using the R package lme4 [32], where timepoint (baseline and one year post HTO) was a fixed effect, and participant was a random effect to account for variation between patients.
Results
Twenty six patients had sufficient volume of synovial fluid at both time points and were included in this analysis. Baseline demographic and clinical characteristics are summarized in in Table Table1.1. The MAA changed from -6.4 (SD =
= 2.3) to
2.3) to +
+ 0.88 (SD
0.88 (SD =
= 2.9) degrees, indicating a substantial change in alignment from varus to approximately neutral. MRI and synovial fluid markers were assessed on average within 5.8 (SD
2.9) degrees, indicating a substantial change in alignment from varus to approximately neutral. MRI and synovial fluid markers were assessed on average within 5.8 (SD =
= 5.9) weeks pre-operatively and within 3.3 (SD
5.9) weeks pre-operatively and within 3.3 (SD =
= 5.2) weeks post-operatively.
5.2) weeks post-operatively.
Table 1
Patient baseline demographics and clinical characteristics
| Characteristic | Participants (n = = 26) 26) |
|---|---|
| Sex, male | 21 (81%) |
Age, years | 53.9 ± ± 5.4 5.4 |
| BMI (kg/m2) | 30.2 ± ± 4.2 4.2 |
| MAA, (degrees) | -6.4 ± ± 2.3 2.3 |
| K/L grade | |
 1 1 | 3 (11%) |
 2 2 | 7 (27%) |
 3 3 | 16 (62%) |
| OARSI medial narrowing | |
 1 1 | 5 (20%) |
 2 2 | 10 (40%) |
 3 3 | 10 (40%) |
| OARSI lateral narrowing | |
 0 0 | 20 (80%) |
 1 1 | 5 (20%) |
| KOOS Pain | 51.7 ± ± 12.9 12.9 |
| KOOS ADL | 63.4 ± ± 16.0 16.0 |
| KOOS Symptoms | 49.3 ± ± 12.4 12.4 |
| KOOS Sport | 32.6 ± ± 23.2 23.2 |
| KOOS QoL | 25.0 ± ± 15.0 15.0 |
Represented as means ± standard deviations or frequency and percent. BMI Body mass index, MAA Mechanical axis angle, K/L Kellgren and Lawrence, OARSI Osteoarthritis Research Society International, KOOS Knee Injury and Osteoarthritis Outcome Score, ADL Activities of Daily Living, QoL Quality of Life
Changes in biomarker concentration and MRI measures of joint pathology
Changes in biomarker concentration are reported in Table Table2.2. From baseline to one year after HTO, there were small-to-moderate decreases (SRM 0.4–0.6) in concentrations of most MMPs, TIMP-1 and some pro-inflammatory cytokines, and small increases in growth factors (SRM <
< 0.2). From baseline to one year post HTO, there was a reduction in MRI measured effusion synovitis (SRM
0.2). From baseline to one year post HTO, there was a reduction in MRI measured effusion synovitis (SRM =
= -0.50). Additionally, there was a shortening in medial compartment T2 relaxation times (SRM
-0.50). Additionally, there was a shortening in medial compartment T2 relaxation times (SRM =
= -0.87) post HTO. There were no significant changes in lateral compartment T2 relaxation time, nor patellar relaxation time (Table (Table22).
-0.87) post HTO. There were no significant changes in lateral compartment T2 relaxation time, nor patellar relaxation time (Table (Table22).
Table 2
Baseline, one year, and pre-to-post changes (post minus pre) in synovial fluid biomarker concentration, effusion-synovitis and T2 relaxation times
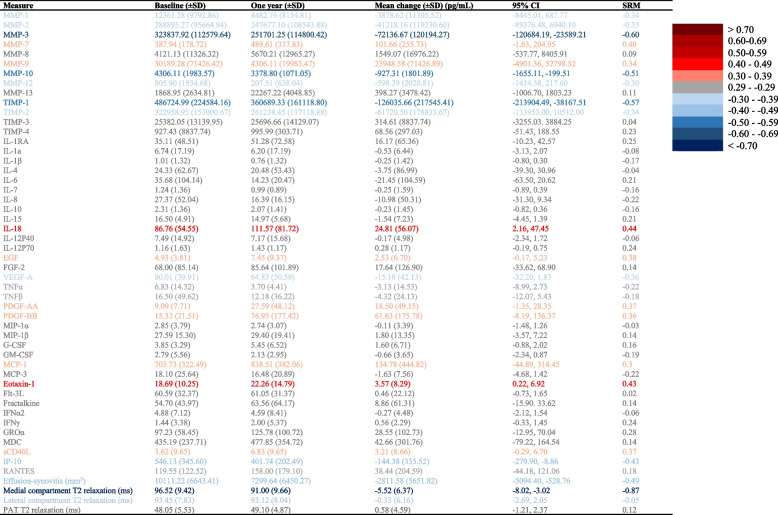
SD standard deviation, CI confidence interval, SRM standardized response mean, MT + MF medial tibial + medial femoral, LT + LF lateral tibial + lateral femoral, PAT patellar
Expression of synovial fluid biomarkers
LMM suggested (estimate for variance [95% CI], pg/mL), MMP-3 (-90,517.2 [-148125.3; 22,756.4]), TIMP-1 (-155,912.1 [-281124.4; -29,397.2]) and interferon gamma-induced protein 10 (IP-10, aka CXCL10) (-152.4 [-271.7; -32.6]) decreased after HTO, whereas MMP-7 (179.7 [43.2; 319.9]), PDGF-AA (25.2 [4.3; 46.1]) and soluble (s)CD40L (5.3 [0.9; 9.6]) increased after HTO.
Enrichment of pathways in synovial fluid one year after HTO
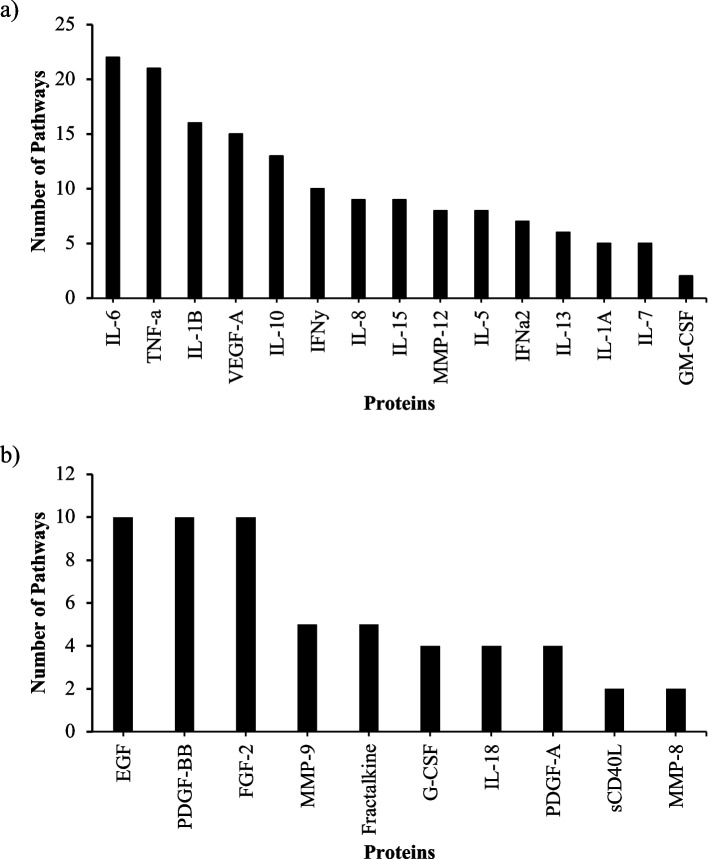
Several protein marker sets showed negative enrichment post HTO (i.e. decreased after HTO). These included markers related to inflammation such as immune and leukocyte responses and signaling pathways [toll like receptors (TLRs), tumor necrosis factor alpha (TNF-α)] (Table (Table3).3). LEA revealed that the highest contributors to negative enrichment (lower 1 year after HTO) included inflammatory markers IL-6, TNF-α and IL-1β (Fig. (Fig.2a).2a). Additionally, LEA at one year post HTO identified epidermal growth factor (EGF), PDGF-BB and fibroblast growth factor-2 (FGF-2) as top positively enriched markers post HTO, suggesting a switch from a pro-inflammatory to pro-anabolic/regenerative state (Figs. (Figs.22b).
Table 3
Enrichment of pathways from pre-to-post HTO
| Pathway | NES | NOM p-val | FDR q-val | |
|---|---|---|---|---|
| Negatively Enriched one year post HTO (higher at baseline) | ||||
| KEGG | Toll like receptor signaling pathway | -1.72 | 0.01 | 0.17 |
| JAK STAT signaling pathway | -1.67 | 0.02 | 0.13 | |
| Nod like receptor signaling pathway | -1.66 | 0.03 | 0.10 | |
| Graft vs host disease | -1.59 | 0.02 | 0.13 | |
| Leishmania infection | -1.57 | 0.04 | 0.11 | |
| Hematopoietic cell lineage | -1.50 | 0.06 | 0.16 | |
| Intestinal immune network for IGA production | -1.47 | 0.09 | 0.16 | |
| T-cell receptor signalling pathway | -1.44 | 0.11 | 0.17 | |
| Cytokine-cytokine receptor interaction | -1.43 | 0.05 | 0.17 | |
| Type I diabetes mellitus | -1.42 | 0.09 | 0.15 | |
| FC epsilon Ri signaling pathway | -1.41 | 0.10 | 0.14 | |
| Allograft rejection | -1.42 | 0.13 | 0.21 | |
| Cytosolic DNA sensing pathway | -1.30 | 0.16 | 0.21 | |
| Asthma | -1.26 | 0.19 | 0.23 | |
| Rig I-like receptor signaling pathway | -1.26 | 0.21 | 0.23 | |
| Hallmark | TNF-α signaling via NFκB | -1.62 | 0.03 | 0.20 |
| IL-6 JAK STAT3 signaling | -1.61 | 0.03 | 0.11 | |
| Allograft rejection | -1.50 | 0.03 | 0.15 | |
| IFNγ response | -1.49 | 0.09 | 0.12 | |
| Apoptosis | -1.42 | 0.07 | 0.14 | |
| IL-2 STAT5 signaling | -1.37 | 0.11 | 0.14 | |
| Positively Enriched one year post HTO (higher after surgery) | ||||
| KEGG | Pathways in cancer | 1.16 | 0.23 | 0.86 |
| MAPK signaling pathway | 1.07 | 0.35 | 0.56 | |
| Bladder cancer | 0.96 | 0.5 | 0.52 | |
| Hallmark | Coagulation | 1.44 | 0.09 | 0.31 |
| Complement | 0.97 | 0.50 | 1.0 | |
| KRAS signaling down | 0.88 | 0.62 | 0.84 | |
| KRAS signaling up | 0.60 | 0.94 | 0.94 | |
NES nominal enrichment score, FDR false discovery rate
Associations between changes in synovial fluid protein expression and MRI measures of joint pathology
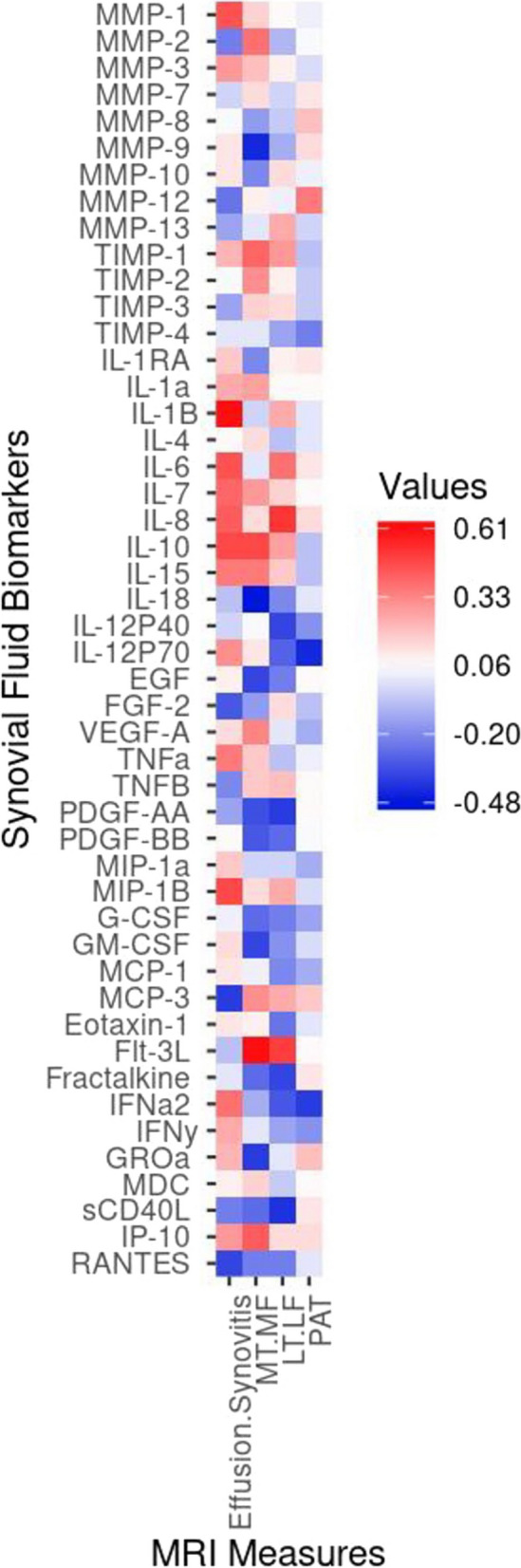
Pearson correlations describing the associations of changes in biomarker concentration to changes in MRI measures of joint pathology were generally very low (Fig. (Fig.3).3). The decrease in effusion-synovitis was moderately correlated (r, [95% CI]) with the decreases in MMP-1 (0.44 [0.05; 0.71]), IL-7 (0.41 [0.001; 0.69]) and IL-1β (0.59 [0.25; 0.80]) and increase in macrophage inflammatory protein (MIP)-1β (0.47 [0.10; 0.73]). The decrease in medial compartment T2 relaxation time was strongly correlated with a decrease in Flt-3L (0.61 [0.28; 0.81]) and moderately correlated to a decrease in IL-10 (0.47; 0.09, 0.73), IP-10 (0.42; 0.03–0.70) and an increase in MMP-9 (-0.4 [-0.7; -0.03]) and IL-18 (-0.48 [-0.73; -0.10]). The slight decrease in lateral compartment T2 relaxation time was moderately correlated with a decrease in FMS-like tyrosine kinase 3 ligand (Flt-3L) (0.48 [0.10; 0.73]) and IL-8 (0.51 [0.14; 0.75]) and an increase in sCD40L (-0.40 [-0.69; -0.01]). The slight increase in patellar T2 relaxation time was moderately correlated with a decrease in IL-12p70 (-0.43 [-0.71; -0.04]) .
Discussion
In this study, we observed small-to-moderate decreases in the concentrations of synovial fluid MMPs, TIMP-1 and pro-inflammatory cytokines, and small increases in growth factors one year after HTO. These physiologic changes were accompanied by large decreases in MRI measures of joint pathology, including effusion-synovitis and medial compartment T2 relaxation times. Taken together, these findings suggest that load adjustment with HTO favourably modifies joint physiology.
Additionally, key individual markers and inflammatory pathways were reduced one year after HTO, reflecting improvements in immune responses, leukocyte responses and signalling pathways. As HTO creates substantial changes in alignment and knee moments during walking that are associated with improvements on Knee Outcome Osteoarthritis Score (KOOS)-4, [15] the present results suggest that intereventions that alter mechanical loading may result in changes in mechanobiological processes that could be biologically- and clinically-meaningful. Specifically, the decrease in mechanical load was associated with a decrease in inflammation in the knee, demonstrated by both MRI (effusion-synovitis) and biochemical analyses (pro-inflammatory biomarkers), and signs of improved articular cartilage composition in the medial compartment. The results of this study further support the notion of mechanically-driven inflammation in patients with knee OA, and suggest that inflammation can be modified with corrective mechanical interventions. Interventions that reduce aberrant joint mechanical loads may result in improvements in inflammation and increased anti-inflammatory and anabolic processes, suggesting an overall improvement in joint health. This may also suggest that modifying a range of pathophysiological pathways is likely to be more effective then targeting a single cytokine.
From a biological standpoint, many of the observed changes in biomarkers are intuitive. For example MMP-1 is activated in inflammation, particularly vascular remodelling, which supports the association between a decrease in effusion-synovitis and a decrease in MMP-1 [33]. We observed a decrease in IL-6 post-HTO, which makes sense as IL-6 is a general marker of inflammation and increases with cartilage injury and aging. IL-6 expression is activated by hypoxia through hypoxia-inducible factor two alpha (HIF-2α) signalling and stimulates increases in MMP-3 and -13, which are associated with cartilage damage [34]. Blocking IL-6 in animal models reduces OA progression [35]; therefore, the reduction of IL-6 one year after HTO is a promising finding. We saw an increase in EGFR signaling post HTO, which can be supported by the notion that EGFR signalling is associated with OA as an endogenous repair mechanism that increases cartilage growth [36], although its role in OA is likely context-specific [37]. Finally, LEA revealed a decrease in IL-1β post HTO. While most clinical trials have not shown a benefit for targeting biomarkers for therapy, a secondary analysis of the CANTOS trial demonstrated that IL-1β inhibition was associated with a reduction in joint replacement surgeries [38]. Regardless of their efficacy as a therapeutic target, reduction in pro-inflammatory biomarkers can be used as an indicator of physiologic responses to a biomechanical intervention such as HTO. This supports a mechanoinflammation hypothesis of OA [39], and demonstrates that structural and physiological disease modification and overall improvement of joint health in response to redistributing aberrant knee loads. Although these results are encouraging, notably, not all OA-related catabolic factors decreased. For instance, we observed trends toward increases in MMP-13, despite observing improvements in MRI measures of synovitis and cartilage. While HTO has improved malalignment, a significant OA risk factor, these patients still have on-going OA pathogenic processes, which may be the reason not all our observed changes were intuitive. Therefore, although there are signs of improved joint physiology, the OA process has not been completely abrogated by load alteration. More work is required to investigate the impact of HTO on joint physiology and OA pathogenesis.
Three previous investigations have used knee load altering interventions as models to investigate changes in biomarkers, as well as potential links between changes in joint mechanics and biology. Kumagai et al. observed significant decreases in the concentrations of IL-6, IL-8, matrix metalloprotease (MMP)-2, -3, -13, vascular endothelial growth factor (VEGF) and cartilage oligomeric matrix protein (COMP) two years after HTO, as well as an improvement in cartilage health [13]. Similarly, Zhao et al. observed significant decreases in pain, and decreased concentration of IL-1β, IL-6 and Il-17 at one, three and six months in an HTO plus arthroscopy group versus HTO alone, although serum inflammatory biomarker concentrations decreased post-surgery in both groups [40]. Furthermore, Watt et al. observed increases in (TGFβ-1), monocyte chemoattractant protein-1 (MCP-1), IL-6 and FGF-2 six weeks after joint distraction surgery. Additionally, those that achieved the minimal clinically important difference of 10 points on the KOOS4 over six months showed greater increases in FGF-2 and TGFβ-1 than the non-responders [12]. We also observed decreased concentrations in similar biomarkers, such as ILs -1β, -6, and -8 and some MMPs as well as increases in FGF-2 after HTO. Furthermore, we observed positive changes in MRI measures joint pathology, as well as correlations between biochemical and MRI measures. Taken together, this demonstrates that multiple measures of should be considered when painting a picture of overall joint health.
It is unlikely that a single biomarker is able to capture the complexity of the pathophysiological processes involved in OA. Our panel-based approach therefore allowed us to assess changes in biological pathways in synovial fluid to gain a greater understanding of changes in OA pathophysiology after HTO. Additionally, some cytokines can be both pro-inflammatory and anti-inflammatory under certain conditions [41], also highlighting the importance of analyzing biomarker sets in addition to individual factors. Decreases in TNF-α signalling via nuclear factor kappa B (NFκB), TLR and nod-like receptor (NLR) signalling pathways occurred after HTO. When activated, TNF-α signalling via NFκB leads to increased production of a multitude of inflammatory markers, including MMPs -1, -3 and -13, IL-6 and -8, CCL5 and VEGF, increasing catabolism in the joint and leading to cartilage matrix remodelling, chondrocyte apoptosis and synovial inflammation [42, 43]. TLRs and NLRs participate in the induction of innate immunity and adaptive immune responses in OA [44]. Innate immune mechanisms play an important mediating role in OA pathophysiology by producing multiple proinflammatory mediators including cytokines, chemokines, MMPs and aggrecanases to induce pro-catabolic events and promote synovitis [45]. Additionally, TLRs also have implications in pain, cartilage degeneration, and apoptosis [46]. For instance, apoptotic synovial cells accumulating in OA are associated with increased cytokines in synovial fluid [47]. Therefore, the negative enrichment of these pathway suggests a decreased pro-inflammatory state in the local knee environment when aberrantly high mechanical loading is lessened. This suggests that there has been an improvement in OA pathophysiology.
Strengths of this study include the prospective design including analysis of synovial fluid biochemical markers paired with MRI measures of inflammation and articular cartilage composition, before and after a known load-altering intervention. Using bioinformatics, some studies have provided new insights into the molecular mechanism of OA, [48–50] but to our knowledge, this is the first study to use GSEA to analyze protein biomarker data to assess enrichment of pathways in OA synovial fluid as an indicator of joint physiologic state. Limitations include the lack of a control group, therefore causation cannot be determined from the present changes and associations. The relatively small sample size that included mostly males, is also acknowledged. Results are only generalizable to similar patients with varus alignment and medial dominant knee OA (Table (Table1)1) undergoing HTO. We could not conduct subgroup analyses due to the small sample size. Futhermore, we did not control for multiple comparisons due to the exploratory nature of this study. Also, we cannot rule out selection bias as about one third (10/36; 27.8%) of participants included at baseline were unable to provide sufficient synovial fluid to evaluate changes afer HTO; however, since these patients likely had the largest benefit from treatment by demonstrating resolution of their pre-operative effusion-synovitis, this selection bias may have led to an underestimation of the effect size seen in our results. Finally, the screw removal and variability in the acquisition of the imaging and synovial fluid markers may have impacted results. Future research is required to confirm the present findings.
Conclusion
In conclusion, this study suggests that decreasing aberrant mechanical load on the medial compartment of the osteoarthritic knee in patients with varus alignment is associated with an overall improvement in biological measures of joint health, including decreases in inflammation (measured on MRI and in synovial fluid) and increases in some anti-inflammatory and anabolic processes. Analysis of group/system-level changes in biomarkers can be used to assess physiologic changes pre-to-post interventions, and suggest changes in loading may take the joint from a “supra-pathophysiological” loading environment to a more normal or physiologic joint loading state.
Acknowledgements
We would like to thank the participants for their time. We would also like to thank Kristyn Leitch and the Wolf Orthopaedic Biomechanics Laboratory for their assistance with data collection. Additionally, we would like to thank Dawn Bryce and Holly Dupuis for their assistance with synovial fluid preparation for analysis.
Abbreviations
| OA | Osteoarthritis |
| HTO | High tibial osteotomy |
| MRI | Magnetic resonance imaging |
| GSEA | Gene set enrichment analysis |
| LEA | Leading edge analysis |
| IL | Interleukin |
| TNF-α | Tumor necrosis factor-alpha |
| EGF | Epidermal growth factor |
| PDGF | Platelet derived growth factor |
| FGF-2 | Fibroblast growth factor-2 |
| MIP | Macrophage inflammatory protein |
| KOOS | Knee Osteoarthritis Outcome Score |
| Flt-3L | FMS-like tyrosine kinase 3 ligand |
| IP-10 | Interferon-gamma-induced protein-10 [aka CXC motif chemokine ligand (CXCL10)] |
| MMP | Matrix metalloproteinase |
| TGF-β | Transforming growth factor-beta |
| VEGF | Vascular endothelial growth factor |
| COMP | Cartilage oligomeric matrix protein |
| MAA | Mechanical axis angle |
| TIMP | TGF-β/tissue inhibitor of metalloproteinases |
| FI | Fluorescence intensity |
| DESS | Dual echo steady-state |
| ICCs | Intraclass correlation |
| CIs | Confidence intervals |
| MT + MF | Medial tibial + medial femoral |
| LT + LT | Lateral tibial + lateral femoral |
| SRM | Standardized response mean |
| TLR | Toll-like receptor |
| NFκB | Nuclear factor kappa B |
| NLR | Nod-like receptor |
| RANTES | Regulation on activation, normal T cell expressed and secreted |
| GM-CSF | Granylocyte-macrophage colony-stimulating factor |
| IFN | Interferon |
Authors’ contributions
JMS had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Conception and design of the study: JMS, TBB, CTA, FB, JRG and FB. Analysis and interpretation of data: JMS, TBB, CTA, HTP, HFA and FB. Drafting of the article: JMS, TBB, HTP, HFA, FB and CTA. Final approval of all the manuscript: All authors.
Funding
This study was supported in part by the Canadian Institutes of Health Research (TBB, FB, JRG) and the Canada Research Chairs program (TBB, FB).
JMS was funded by the Ontario Graduate Scholarship and the Western University Bone and Joint Institute.
Availability of data and materials
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request.
Declarations
The study was approved by the Western Health Sciences Ethics Research Board (#1187). Written informed consent was obtained from all participants.
Not applicable.
CTA is a consultant for Abbvie, Amgen, Bristol Myers Squibb, Celgene, Fresenius Kabi, Gilead, Janssen, Merck, Novartis, Pfizer, Hoffmaan LaRoche, Sandoz, Sanofi-Genzyme, and UCB.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Articles from Arthritis Research & Therapy are provided here courtesy of BMC
Citations & impact
This article has not been cited yet.
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/169170837
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Changes of synovial fluid biomarker levels after opening wedge high tibial osteotomy in patients with knee osteoarthritis.
Osteoarthritis Cartilage, 29(7):1020-1028, 25 Mar 2021
Cited by: 7 articles | PMID: 33774186
Cartilage degeneration of patellofemoral joint occurs in open wedge high tibial osteotomy, rather than in hybrid closed wedge high tibial osteotomy, during the early postoperative period: A qualitative analysis using MRI T2 mapping.
J Orthop Surg (Hong Kong), 31(1):10225536221151132, 01 Jan 2023
Cited by: 0 articles | PMID: 36757867
High tibial osteotomy to neutral alignment improves medial knee articular cartilage composition.
Knee Surg Sports Traumatol Arthrosc, 30(3):1065-1074, 16 Mar 2021
Cited by: 9 articles | PMID: 33723653

 1,2,3
1,2,3