Abstract
Background
Colorectal cancer (CRC) remains a significant healthcare burden worldwide, characterized by a complex interplay between obesity and chronic inflammation. While the relationship between CRC, obesity and altered lipid metabolism is not fully understood, there are evidences suggesting a link between them. In this study, we hypothesized that dysregulated lipid metabolism contributes to local accumulation of foam cells (FC) in CRC, which in turn disrupts antitumor immunosurveillance.Methods
Tumor infiltrating FC and CD8+ were quantified by digital pathology in patients affected by T2-T4 CRC with any N stage undergoing radical upfront surgery (n=65) and correlated with patients' clinical outcomes. Multiparametric high-resolution flow cytometry analysis and bulk RNAseq of CRC tissue were conducted to evaluate the phenotype and transcriptomic program of immune cell infiltrate in relation to FC accumulation. The immunosuppressive effects of FC and mechanistic studies on FC-associated transforming growth factor-beta (TGF-β) and anti-PD-L1 inhibition were explored using an in-vitro human model of lipid-engulfed macrophages.Results
FC (large CD68+ Bodipy+ macrophages) accumulated at the tumor margin in CRC samples. FChigh tumors exhibited reduced CD8+ T cells and increased regulatory T cells (Tregs). Functional transcriptional profiling depicted an immunosuppressed milieu characterized by reduced interferon gamma, memory CD8+ T cells, and activated macrophages mirrored by increased T-cell exhaustion and Treg enrichment. Furthermore, FChigh tumor phenotype was independent of standard clinical factors but correlated with high body mass index (BMI) and plasma saturated fatty acid levels. In CD8low tumors, the FChigh phenotype was associated with a 3-year disease-free survival rate of 8.6% compared with 28.7% of FClow (p=0.001). In-vitro studies demonstrated that FC significantly impact on CD8 proliferation in TFG-β dependent manner, while inhibition of TGF-β FC-related factors restored antitumor immunity.Conclusions
FC exert immunosuppressive activity through a TGF-β-related pathway, resulting in a CD8-excluded microenvironment and identifying immunosuppressed tumors with worse prognosis in patients with primary CRC. FC association with patient BMI and dyslipidemia might explain the link of CRC with obesity, and offers novel therapeutic and preventive perspectives in this specific clinical setting.Free full text

Cancer-associated foam cells hamper protective T cell immunity and favor tumor progression in human colon carcinogenesis
Abstract
Background
Colorectal cancer (CRC) remains a significant healthcare burden worldwide, characterized by a complex interplay between obesity and chronic inflammation. While the relationship between CRC, obesity and altered lipid metabolism is not fully understood, there are evidences suggesting a link between them. In this study, we hypothesized that dysregulated lipid metabolism contributes to local accumulation of foam cells (FC) in CRC, which in turn disrupts antitumor immunosurveillance.
Methods
Tumor infiltrating FC and CD8+ were quantified by digital pathology in patients affected by T2–T4 CRC with any N stage undergoing radical upfront surgery (n=65) and correlated with patients’ clinical outcomes. Multiparametric high-resolution flow cytometry analysis and bulk RNAseq of CRC tissue were conducted to evaluate the phenotype and transcriptomic program of immune cell infiltrate in relation to FC accumulation. The immunosuppressive effects of FC and mechanistic studies on FC-associated transforming growth factor-beta (TGF-β) and anti-PD-L1 inhibition were explored using an in-vitro human model of lipid-engulfed macrophages.
Results
FC (large CD68+ Bodipy+ macrophages) accumulated at the tumor margin in CRC samples. FChigh tumors exhibited reduced CD8+ T cells and increased regulatory T cells (Tregs). Functional transcriptional profiling depicted an immunosuppressed milieu characterized by reduced interferon gamma, memory CD8+ T cells, and activated macrophages mirrored by increased T-cell exhaustion and Treg enrichment. Furthermore, FChigh tumor phenotype was independent of standard clinical factors but correlated with high body mass index (BMI) and plasma saturated fatty acid levels. In CD8low tumors, the FChigh phenotype was associated with a 3-year disease-free survival rate of 8.6% compared with 28.7% of FClow (p=0.001). In-vitro studies demonstrated that FC significantly impact on CD8 proliferation in TFG-β dependent manner, while inhibition of TGF-β FC-related factors restored antitumor immunity.
Conclusions
FC exert immunosuppressive activity through a TGF-β-related pathway, resulting in a CD8-excluded microenvironment and identifying immunosuppressed tumors with worse prognosis in patients with primary CRC. FC association with patient BMI and dyslipidemia might explain the link of CRC with obesity, and offers novel therapeutic and preventive perspectives in this specific clinical setting.
Introduction
Despite significant advancements in the diagnosis and treatment of colorectal cancer (CRC), it remains the third leading cause of cancer-related death worldwide.1 Emerging evidences suggest that dyslipidemia, increased body mass index (BMI), and obesity are major risk factors for CRC2 indicating potential involvement of lipid-related disorders in gut carcinogenesis.3 Conversely, the protective role of infiltrating CD8+ T cells, assessed by their spatial distribution using the Immunoscore,4 has been validated as a crucial feature in CRC, essential for adaptive antitumor immunity and for controlling tumor growth in immunogenic CRC.5 Despite extensive research has explored the complex biological networks underlying human gut carcinogenesis,6 the potential interplay between the tumor-favoring effects of dysregulated lipid metabolism, the protective T cell-mediated adaptive immunity, and their implications for CRC development remain poorly investigated.
Various immune cells located at the intestinal epithelium play pivotal roles in maintaining gut homeostasis.7 In particular, colonic macrophages are increasingly acknowledged as key guardians, protecting the host from gastrointestinal pathogens while also promoting immune tolerance towards food and microbiota antigens.8 9 The multifunctional properties of colonic macrophages, which are finely tuned both at local and systemic levels, are involved in the pathogenesis of numerous chronic gastrointestinal diseases, including inflammatory conditions and cancers.10 11 Macrophages rely on lipid metabolism not only for cellular energy but also to adapt their phenotype and function.12 By expressing scavenger receptors, these cells accumulate lipids in cytosolic droplets,13 developing what is known as the “foam cells” (FC) morphology. While FC have been widely studied in the context of atherosclerosis14 and tuberculosis,15 they are increasingly recognized as a hallmark of chronic inflammation and is found in selected cancers, as well as in various metabolic, infectious, and autoimmune diseases.16 Although the presence of FC in the bowel has been reported in specific pathological conditions, such as pediatric microscopic colitis,17 and more recently, in scattered cases of CRC,18 their immunological properties, particularly their impact on adaptive antitumor T cell immunity, remain largely unexplored. In non-cancerous pathological conditions, FC are known to exert immune-suppressive activity. This activity is associated with the induction of regulatory T cells (Tregs), a reduction in antigen-presenting capacity, and direct inhibition of CD8+ T cell effectors through the release of transforming growth factor-β (TGF-β) and other immunomodulating cytokines and chemokines.19 20 Notably, many of these functional features are shared with tolerogenic colonic macrophages21 and closely resemble the immunosuppressive activity observed in macrophages within the tumor microenvironment (TME).22 This study aimed to investigate the role of FC and their altered lipid metabolism in impairing the protective effect of CD8 T cell immunity in human CRC, exploring also their prognostic impact. The hypothesis driving this study is that colonic macrophages, engulfed by lipid excess, modulate T-cell infiltration to establish an immune-tolerant milieu that could facilitate cancer development and progression.
Methods
Patients and sample collection
The study included prospective and retrospective cohorts of 65 patients (INT127/19 and INT149/19) with T2–T4, any N stage CRC treated with upfront surgery (online supplemental table S1). In-vitro mechanic studies were conducted using peripheral blood mononuclear cells (PBMCs) from healthy donors (HDs) (INT61/20).
Immunohistochemistry and digital pathology assessment
Tumor specimens were assessed for the presence of CD68+ and CD8+ T lymphocytes, by immunohistochemistry. Formalin-fixed, paraffin-embedded (FFPE) tissue sections were evaluated using antigen retrieval, primary antibody incubation, and 3,3′-diaminobenzidine staining (EnVision FLEX System). High-resolution images were digitally scanned (Aperio Scanscope XT) and the whole tissues area was segmented using MiaQuant,23 24 to quantify the cell marker-pixel densities in the tumor core (TC), adjacent mucosa (AM), invasive margin (IM) and distinct 400 µm-thick region of interest, internal (intra) and peripheral (peri) from the IM. FC were identified as CD68+ elements with an area greater than 100 μm2. Immunofluorescence (IF) staining was also used to detect CD68+ Bodipy493/503+ cells, CD8+ T infiltrate, PD-1, Foxp3, TGF-β and Ki67 markers. Images were captured by an Olympus BX63 microscope (online supplemental tables S2 and S3).
Cell cultures
In vitro-derived FC were generated by culturing sorted CD14+ cells from PBMCs of HDs and patients with CRC with macrophage colony-stimulating factor in absence or presence of oxLDL for 48 hours. T cell proliferation study was performed using CFSE staining prior TCR stimulation with anti-human CD3/CD28 mAb-conjugated beads and then cocultured at ratio 1:1 for 5 days with in vitro generated FC or monocyte-derived early macrophages as control, in presence or absence of anti-TGF-β1,2,3 and anti-PD-L1 mAbs (online supplemental table S3). Lymphocytes cultured alone were used as internal reference.
Flow cytometry analyses
Flow cytometry assays were used to analyze single-cell suspensions derived from surgical specimens (n=21 CRC) and in vitro cell cultures. Surface antigens and intracellular lipid uptake were stained with fluorochrome-conjugated antibodies and reagents (online supplemental tables S3 and S4). Cytokine quantification was performed by Cytometric Bead Array. Samples were analyzed using a CytoFLEX S flow cytometry (Beckman Coulter). Data analyses were conducted using the Kaluza Software (Beckman Coulter).
Transcriptomic analyses
RNA was isolated from FFPE intestinal tissue sections of patients with CRC who underwent surgery and from cultured CD14+ cells in the absence or presence of oxLDL for 48 hours. Library preparation was performed using Illumina Stranded Total RNA Prep according to the manufacturer’s instructions. Sequencing and bioinformatics analyses (online supplemental table S5) were performed and annotated in the Gene Expression Omnibus (GEO) Super Series: GSE227206 and GSE273106.
Lipidomic analyses
Total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), and triglycerides levels were analyzed using a Cobas Roche Automated Clinical Chemistry Analyzer (Roche Diagnostics), following standard clinical procedures. Plasma esterified fatty acids were analyzed as methyl esters. Quantification was performed using gas chromatograph equipped with a flame ionization detector, using the chromatographic peak area according to the internal standard method.
Statistical analyses
Statistical analyses were performed using R software V.4.2.0 and GraphPad Prism (V.8.4.3), using either the Wilcoxon signed-rank test, two-tailed Mann Whitney U-test, two-way analysis of variance or Fisher’s exact test. Correlation analyses were performed using Spearman’s test. Statistical significance was set at p<0.05. For survival analyses, Kaplan-Meier survival curves were generated, and a log-rank test was performed to assess statistical significance.
Detailed procedures are provided in online supplemental material section.
Results
Baseline patients’ characteristics
Mean age of patients with CRC (n=65) was 69.1 (±10.8) years, 37 of which (56.9%) were male and 28 (43.1%) female. Mean BMI was 26.5 kg/m2 (online supplemental table S1). Most patients had pT3 (70.8%), pN0 (55.4%), and G2 (75.4%) CRC. A right colectomy was performed in 18 cases (27.7%), a left colectomy in 20 (30.8%), and an anterior rectal resection in 27 patients (41.5%). None of them received neoadjuvant treatment. Adjuvant chemotherapy or chemo-radiotherapy was instead administered in 29 patients (44.6%) (online supplemental table S1).
Macrophages accumulate lipid-droplets in primary CRC tissue
First, we investigated the presence of macrophages with lipid droplets in patients with stage I–III CRC. In tumor lesions, a subset of CD68+ cells displayed a specific morphology of large polygonal cells with optically empty vacuoles-rich cytoplasm and centrally placed faint-colored nuclei. The cytoplasmic vacuoles stained strongly positive for neutral lipids, and weakly positive for acidic mucins (figure 1A).

The accumulation of Bodipy+ lipid droplets positively correlate with enhanced size of CD68+ cells (p<0.0001), resembling macrophages turned into FC. Contrarily, CD68+ macrophages displaying no lipid droplet content, which were reproducibly small in size, were defined as non-FC (figure 1B). Thus, a specific algorithm for digital pathology was developed to quantify FC and non-FC based on the morphology and size of CD68+ elements, in the whole FFPE tissue section from the retrospective cohort, discriminating tumor core (TC), invasive margin (IM), and adjacent mucosa (AM) localization (figure 1C). Results from this analysis showed that FC accumulated at IM, displaying a “barrier-like” distribution. In contrast, non-FC were more ubiquitous, involving also intratumoral localizations and AM, and more homogeneously detected in the majority of patients (figure 1D(i)). Specifically, FC clustering was 54.2-fold higher at the IM than TC, while non-FC marker density showed an increase of 6.7-fold at the IM compared with TC (p<0.0001) (figure 1D(ii)).
Although clearly detectable along the tumor IM, the FC infiltrate was highly heterogenous among different patients (IQR: 0.006–0.035), allowing us to define a median cut-off value (0.02), and dichotomize the patients into the FChigh (43%) versus FClow (57%) groups. While no association was observed between FC abundance and the main clinical and pathological variables, including CEA and CA19.9 levels, pT versus pN stages, and grading (online supplemental figure S1), a significant positive correlation was observed between FC accumulation in tissue and BMI (figure 1E). Conversely, BMI did not show any correlation with non-FC (online supplemental figure S1) and did not display a significant relationship with cancer-associated variables (online supplemental table S6). On this basis, we then investigated the association between FC infiltrate and plasma lipid profiles. FChigh CRC exhibited a clustering with higher triglycerides and lower HDL in plasma compared with FClow CRC, while cholesterol and LDL did not show statistically significant differences between the two groups (online supplemental figure S2). Moreover, a thorough characterization of lipid species revealed elevated levels of saturated fatty acid, including palmitic acid (C16:0; p=0.04) in FChigh CRC. This group also displayed an enrichment of monounsaturated fatty acid (p<0.0002), specifically of oleic acid (C18:1; p<0.0003). In contrast, polyunsaturated fatty acids (PUFAs) were significantly lower in FChigh tumors (p<0.0076), with no significant differences in omega-6 (n-6) PUFA arachidonic acid (C20:4) while there a remarkable decrease of eicosapentaenoic acid (C20:5; p<0.001), docosapentanoic acid (C22:5; p<0.0006), and docosahexaenoic acid (C22:6; p<0.02), both belonging to omega-3 (n-3) PUFA, in FChigh versus FClow CRC (figure 1F, online supplemental figure S2).
FC accumulation at the tumor edge prevents CD8+ intratumoral infiltration
Next, CD8+ T-cells were also quantified by digital pathology and tumors divided into CD8high (>0.025 median cut-off) and CD8low (<0.025) with respect to their spatial distribution at IM. FChigh CRC showed a significantly lower density of CD8+ T cells, compared with FClow cases (p=0.04) (figure 2A(i)), and reciprocally, FC accumulation at IM was significantly higher in CD8low CRC (p=0.01) (figure 2A(ii)).
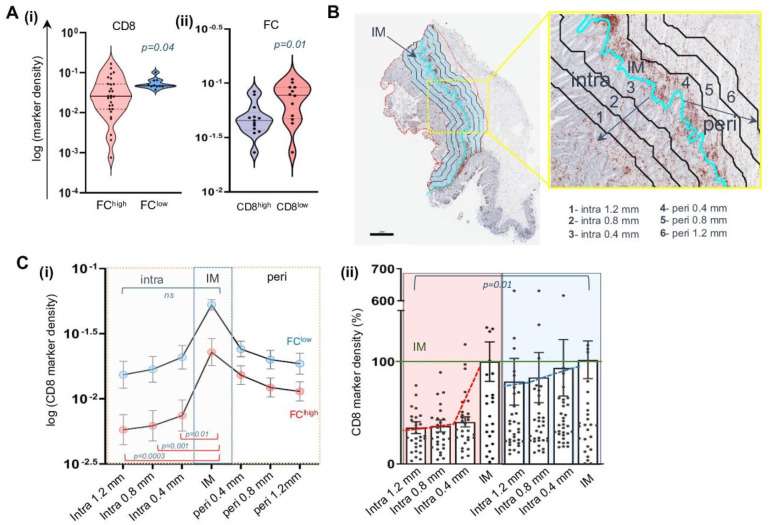
The quantification of CD8 gradient from peri-tumoral (peri) to intra-tumoral (intra) localization by applying a 400 µm width intervals (figure 2B) showed that in FChigh tumors, CD8+ T cells progressively declined from the lesion edge towards the internal localization, with the marker density decreasing to 0.017 (±0.004; p=0.01), 0.014 (±0.003; p=0.001), and 0.013 (±0.003: p=0.0003) at 0.4, 0.8, and 1.2 mm, respectively. Contrarily, the CD8 quantification was not significantly modulated with regards to the IM in FClow cancer (figure 2C(i)). Globally, only 35% of the CD8 infiltrate present in the IM could reach the TC (1.2 mm) in FChigh with respect to the 80% of the FClow setting (figure 2C(ii)). Summarizing, this detailed special analysis showed that the disparity in CD8 intratumor infiltrate detected in our CRC case set is strictly influenced by FC abundance at the IM, indicating a potential role of these cells in restraining the influx of T-cell effectors deep into the TME.
FC-wall assumes a clinical significance in stage I–III CRC
The impact of the FC versus CD8+ T cells interplay on disease-free survival (DFS) was evaluated using Kaplan-Meier analyses. First, to elucidate the reciprocal impact of FC and CD8+ T cells on DFS, patients with a high versus low FC/CD8 ratio (cut-off 2.6) were compared. Patients with high FC/CD8 ratio exhibited a significant poor DFS (log-rank p=0.002 (online supplemental figure S3A). Subsequently, Kaplan-Meier curves were applied separately to assess this impact in poorly and highly immunogenic tumors. CRC with high CD8+ cells in IM (cut-off 0.025) showed a 3-year DFS rate of 63.6% versus 20.8% in CD8low CRC (log-rank p=0.009) (figure 3A). However, within the CD8low patients, the presence of the FChigh phenotype (cut-off 0.02) was associated with a significantly lower 3-year DFS rate (8.6%) compared with FClow cases, which showed a 28.7% 3-year DFS (log-rank p=0.001) (figure 3A). In contrast, no prognostic difference was observed between low and high non-FC density at IM (cut-off 0.107) in CD8low CRC (log-rank p=0.19 (online supplemental figure S3B)), nor between FChigh and FClow density in CD8high patients (log-rank p=0.17 (online supplemental figure S3C)). This likely suggests that in the CD8high group, the density of FC was not sufficient to counteract the antitumor activity of effector CD8+ T-cells, which is associated with improved survival.
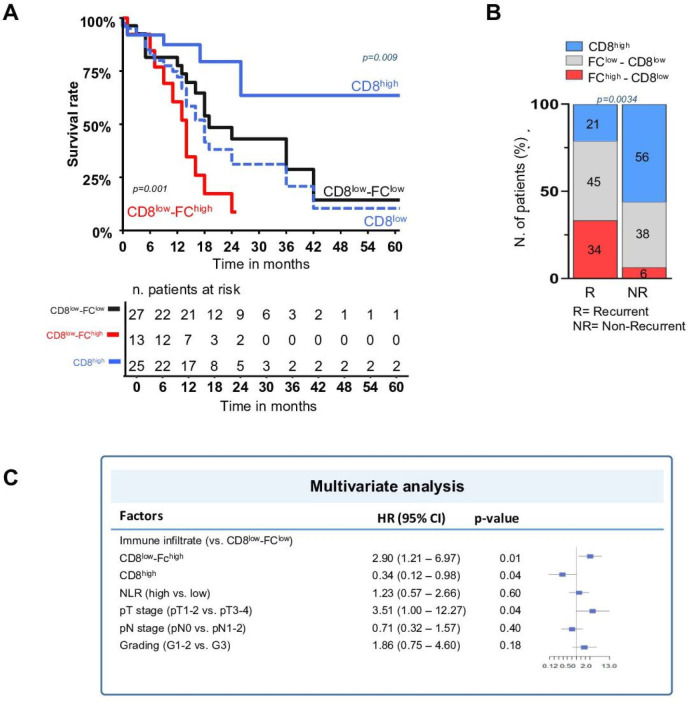
Clustering patients by the presence (n=33) or absence (n=32) of recurrence at 3-year follow-up showed a notable enrichment of FChigh CD8low and general reduction of CD8high cases within the recurrent population compared with disease-free patients (figure 3B).
Univariate Cox regression analysis indicated that FChigh CD8low phenotype was one of the strongest negative prognostic factors associated with DFS (HR 2.47; p=0.02), together with CEA (HR 1.14; p=0.002), Ca19.9 (HR 1.57; p=0.004), neutrophil-to-lymphocyte ratio (HR 1.13; p=0.025), extramural vascular invasion (HR 2.55; p=0.01), and perineural invasion (HR 2.12; p=0.038). In contrast, CD8high phenotype showed a potent protective effect (HR 0.29; p=0.01) (online supplemental table S7), in line with the validated role of the Immunoscore.4 On multivariate Cox analysis, the only independent factors associated with DFS were pT stage (HR 3.5; p=0.04), FChigh CD8low (HR 2.9; p=0.01), and the CD8high cells (HR 0.33; p=0.04). Harrell’s C-index was 0.66 (figure 3C).
These findings confirm the clinical relevance of the immunosuppressive features of FC, and these cells significantly impact on disease progression when protective CD8-mediated immunosurveillance is lacking.
Tissue FC associated with distinct intratumoral immune cell contexture
Multiparametric flow cytometry analysis performed on fresh single cell suspension from tumor lesions of our CRC case set (n=21) depicted an overall enrichment of CD14+ (4.9±4.3 vs 0.98±1; p=0.0001) and CD4+ T cells (39.2±15.1 vs 14.3±7.7; p<0.0001) paired with reduced CD8+ (17.5±7.3 vs 28.5±16.8; p=0.01), compared with patient-matched unaffected mucosa (figure 4A(i)).
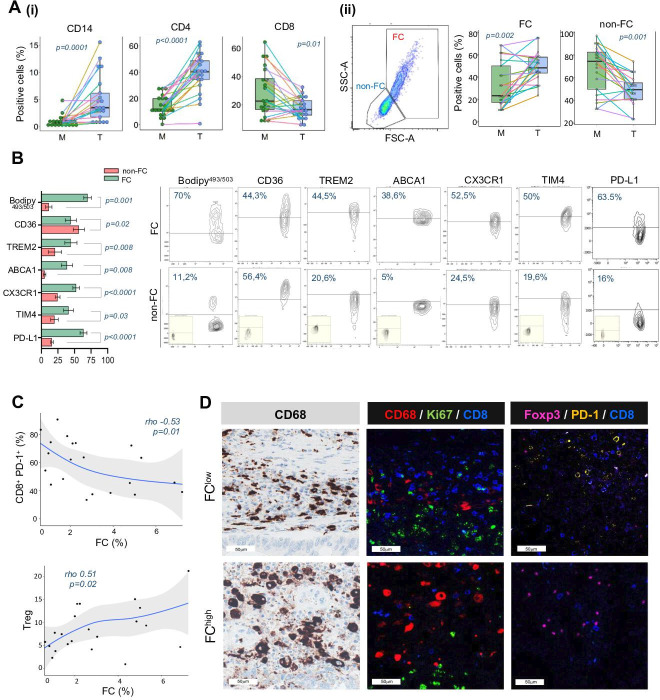
As expected, the whole immune context of the tumor lesion was remarkably different from that of the corresponding normal colon mucosa (online supplemental figure S4), with immunosuppressive and regulatory populations being enriched along with reduced antitumor immune effectors. Indeed, CRC displayed enhanced infiltrate of the monocytic MDSC (CD14+ HLA-DR−) (17.7±19.1 vs 9±10.9; p=0.004) and reduced frequency of CX3CR1+ CD14+ cells (39.5±25 vs 53.4±33; p=0.02), which are associated with resident macrophages in steady-state colon.25 Within T-subsets, activated PD-1+ (49.7±21 vs 29±22.4; p=0.003) and regulatory HLA-DR+ (40.3±21.7 vs 17.7±14.5; p=0.004) CD8+ T-cells were expanded in tumors, while the resident memory CX3CR1+ CD8+ T-cells26 significantly declined (4.9±4.1 vs 8.4±8; p=0.02). CRC specimens were also characterized by increased frequency of regulatory CD4+CD25hiCD127− T cells (Tregs) (20.7±8 vs 9±6.1; p=0.0004) and CCR4+ effector Treg (21.2±18.3 vs 15±14.8; p=0.004), along with a contraction of Th1 CD4+CXCR3+ (9.2±10.4 vs 25.4±15.2; p<0.0001) T cell subsets (online supplemental figures S4 and S5). We also detected FC within the tumor-infiltrating CD14+ myeloid population based on the larger morphology and the higher structural complexity with respect to the non-FC counterpart. Remarkably, FC were significantly more abundant in tumor specimens compared with matched distant mucosa (49.29±13.22 and 32.67±21.10, respectively; p=0.002), while non-FC were significantly accumulated in mucosa (68.09±20.67) compared with tumor (49.16±13.00; p=0.001) (figure 4A(ii)).
The results obtained from multiparametric flow cytometry revealed that FC were characterized by the expression of distinct metabolic and functional markers associated with the induction of tumor immune tolerance. The modulation of these markers differed significantly compared with their non-FC counterparts (figure 4B). Specifically, Bodipy493/503 staining, indicating cellular lipid content, was expressed in 70% of FC, compared with only in 11.1% of non-FC (p=0.001). Mirroring their active lipid metabolism, FC exhibited modulated expression of lipid transporter proteins, including upregulation of TREM2 (44.5%; p=0.008) and ABCA1 (38.6%; p=0.008), and downmodulation of CD36 (44.3%; p=0.02), in comparison with their non-FC counterpart (20.6 %, 4.9%, and 58.4%, respectively) (figure 4B). Additionally, FC showed significantly higher expression of the phosphatidylserine (PS) receptor Tim4 (40.9%; p=0.03), the chemokine receptor CX3CR1 (52.5%; p<0.0001) and the inhibitory immune checkpoint PD-L1 (63.5%; p<0,0001) compared with non-FC (19.6%, 24.5%, and 16%, respectively) (figure 4B), defining features of long-lived resident macrophages27 with a potential role in impairing antitumor T cell response and inducing regulatory subsets.28 Along with the increased expression of the myeloid lineage markers CD68 and CD163, FC notably exhibited higher expression of CD206 (80.7%; p=0.06) and PD-L1 (compared with the non-FC, as an additional immunosuppressive pathway to blunt antitumor immunity (online supplemental figure S6). Notably, the abundance of FC was found to negatively correlate with the presence of activated CD8+PD-1+ effector subsets (p=0.01), while it was positively associated with Tregs (p=0.02) (figure 4C). In contrast, no correlation between non-FC and effector or regulatory T cell subsets was observed (online supplemental figure S7). In summary, the immunological hallmarks of FC-enriched CRC indicate a tolerogenic and pro-tumor environment, characterized by the exclusion of activated CD8+ T cells and the predominance of Tregs. This observation was also supported by confocal analyses, revealing lack of the PD-1 activation marker in CD8+ T cells, decreased total CD8+ T cell infiltration, and increased presence of FoxP3+ Tregs in FChigh tumors. Furthermore, CD8+ T cells in FChigh CRC did not colocalize with the proliferative marker Ki67 (figure 4D), thus revealing poor proliferative potential.
Transcriptional profile of FC-enriched CRC is compatible with a multimodal dampening of tumor immunosurveillance
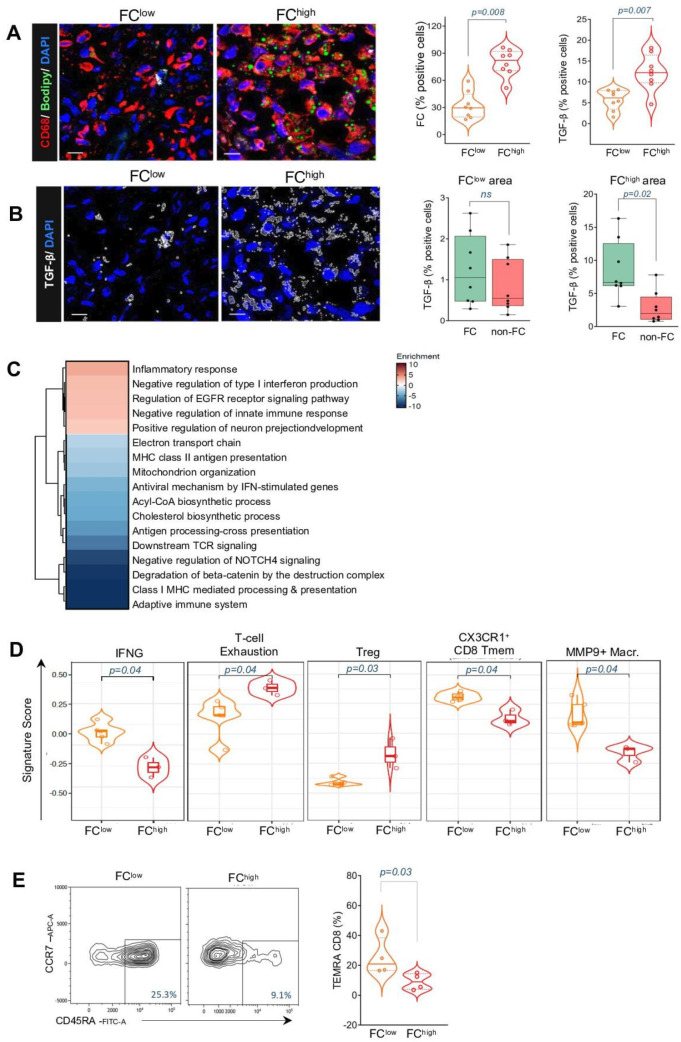
TGF- β is an acknowledged player of the immunosuppressive activity mediated by FC in chronic inflammatory setting such as atherosclerosis,29 and a key immune escape mediator in TME.30 Hence, our subsequent objective was to assess whether TGF- β could be linked to the restrained T-cell immunity associated with FC. IM CRC regions with an average area of 0.05 mm2 were analyzed by IF. The FChigh area, marked by a substantial accumulation of Bodipy+ and CD68+ FC, was compared with matched FClow area, where the average FC accumulation was 80% vs. 32% (figure 5A). The FChigh area exhibited a twofold higher TGF-β level compared with the FClow area (13% vs 5.7%; p=0.007) (figure 5A). Furthermore, TGF-β was found to be independently associated with FC compared with non-FC elements across different observed areas, reaching statistical significance particularly in FChigh area (p=0.02) (figure 5B).
Our next step was to functionally characterize FC immune suppressive features by assessing the transcriptional profile of FChigh and FClow CRC, selected from the digital pathology quantification (online supplemental table S5A).
Analyses from bulk RNAseq data revealed 2900 differentially expressed (DE) genes (FDR<0.05) from FChigh and FClow CRC tissue specimens (onlinesupplemental figure S8A table S5B). Specific FC-associated transcriptomic programs were identified by crossing the expression levels with cell-state specific genes listed in micro-dissected FC-enriched and deprived tumors.31 881 genes with coherent differential expression were identified (onlinesupplemental figure S8B table S5B). Specifically, 676 genes were upregulated, whereas 205 were downregulated in FChigh CRC in both studies (FDR<0.05). Unsupervised enrichment analyses based on upregulated and downregulated coherently modulated genes (online supplemental table S5C) revealed a scenario resembling a state of immune hyporeactivity in FChigh CRC. Selected terms from the Reactome Gene Set and GO Biological Processes (figure 5C, online supplemental table S5D), showed upregulation of negative type I interferon production and innate immune response as detected by the genes RNF125, HAVCR2, NLRC3, PTPN2 and CD96, as well as, enhanced cell proliferation as depicted by the upregulation of “EGFR signaling pathway” and downregulation of “degradation of β-catenin.”
Additionally, FChigh CRC showed significantly negative enrichment of a core set of genes implicated in class I-II MHC mediated antigen presentation and processing, such as AP2B1, ARF1, DYNC1I2, KIF5B, DYNLL1, SEC24C, BLMH, TPP2). Moreover, the term related to “downstream TCR signaling” was also significantly negative enriched (PSMA4, AP2B1, ARF1, KIF3B, SEC24C, URB4, TUBA1B), indicating that the functions and cross-talk between adaptive and innate immunity are inhibited in FC-enriched CRC.
Alongside the altered antitumor immunity, FChigh CRC exhibited changes in lipid metabolism, indicated by the significant negative enrichment of the term “cholesterol biosynthesis process,” including downregulation of ACLY, DHCR24, HMGCR, IDI1, EBP, NSDHL, and PLPP6 genes. Furthermore, this process was accompanied by mitochondrial dysfunction, as evidenced by the significant downmodulation of a core set of gene involved in mitochondrion organization and biosynthesis (TIMM8A, HSPA4, NDUFA8, VDAC2, COA1, ACACA, ACAT1, and HMGCR).
Next, we examined immune-related gene signatures in FChigh and FClow CRC. The interferon gamma (IFNγ) activating signature (IFNG_score_21050467),32 comprising genes associated with effective immunosurveillance and favorable prognosis in patients with cancer was found to be reduced in FChigh CRC (figure 5D, online supplemental table S5E). Conversely, there was an increase in signature associated with T-cell exhaustion (inhibitory)32 and Tregs26 in FChigh CRC. Additionally, compared with FClow tumors, transcriptional pathways indicative of memory CD8 (CX3CR1+ CD8 Tmem) and various subsets of activated colonic macrophages (MMP9+ inflammatory macrophage) were downregulated in FChigh samples26 (figure 5D, online supplemental table S5E). To validate the findings of the dysfunctional state of antitumor immunosurveillance in the presence of FC, tumor cell suspensions were assessed for abundance of differentiated effector cells. FChigh CRC were characterized by lower proportion of terminally differentiated effector memory CD8+ T cells (TEMRA) compared with FClow CRC (figure 5E). This suggests that weak T cell receptor engagement and limited immune response to tumor antigens may occur within the TME in the presence of lipid-engulfed macrophages.
Overall, these findings suggest that FC-enriched CRC are characterized by immunosuppressive milieu that, through the impaired CD8 T-cell proliferation and activity, accrual of regulatory CD4, and loss of homeostatic function in resident myeloid cells, leads to the complete subversion of local immunosurveillance.
TGF-β targeting could restore the activation of effector T cell
To investigate the FC-associated immunosuppression, we exploited in vitro-generated human FC. Early macrophages, derived from CD14+ isolated from PBMCs of HDs, were engulfed with oxLDL for 48 hours (figure 6A). To determine whether in vitro-generated FC accurately reflect the characteristic of in vivo lipid-engulfed macrophages, their transcriptional profiles were evaluated by bulk RNAseq (GSE273106 GEO identifier). Early macrophages treated with oxLDL showed 161 DE genes (FDR<0.05) compared with controls (online supplemental table S5F). Subsequently, DE genes of in vitro FC were then compared with those identified in vivo. A total of 429 DE genes in FChigh versus FClow CRC were coherently DE with genes identified in micro-dissected FC-enriched tumors31 and in vitro-derived FC. Enrichment analyses of these 429 DE genes revealed 338 significantly enriched terms outlining the overall functional state of FC (online supplemental table S5G). Among the 20 top enriched terms (online supplemental figure S9A), pathways related to lipid metabolism were notably modulated. Specifically, 40 coherently DE genes enriched the term “metabolism of steroids” (R-HSA- 8957322, FDR<0.001) were further investigated. Unsupervised hierarchic clustering of these genes across the in vitro and in vivo FC demonstrated similar gene modulation in lipid-engulfed macrophages, indicating that, despite potential differences in their biogenesis, in vitro FC closely resemble their in vivo counterparts (figure 6B). Consistent with the in vivo findings where FChigh CRC tissue exhibited significantly higher TGF-β staining compared with FClow tissue, in vitro-generated FC also produced significantly higher levels of TGF-β (261±97 vs 195±68; p=0.03) compared with non-engulfed CD14+ cells (figure 6C). This results underscores TGF-β, as a potential key modulator in the FC-mediated inhibition of antitumor immunosurveillance, supporting its role in the immune evasion mechanism observed in CRC.
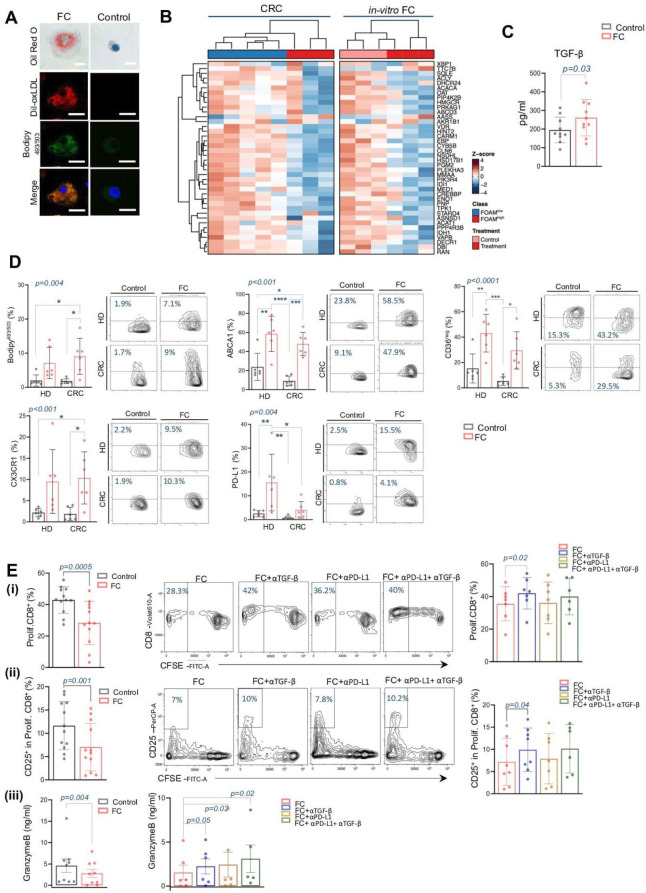
To further bear the role of lipid-engulfed macrophages in promoting an immunosuppressive milieu within TME, in vitro-generated FC derived from both HD and CRC were thoroughly characterized by their phenotype. Overall, lipid engulfment resulted in comparable marker modulation in CD14+ cells in both healthy and disease conditions, closely resembling the FC phenotype observed in CRC tissue (figure 6D). Specifically, the abundance of cells stained positive for Bodipy493/503, was significantly increased in oxLDL-treated early macrophages compared with controls (1.9%±1.6 vs 7.1±4.5 in HD and 1.7%±0.7 vs 9%±5.2 in CRC; p=0.004). Mirroring tissue FC, in vitro lipid-loaded cells exhibited higher expression of ABCA1 transporter (23.8%±14.1 vs 58.5%±18.5 in HD and 9.1±5 vs 47.9%±12.1 in CRC; p<0.001) and a reduction of the scavenger receptor CD36, as indicated by the enhancement of the CD36-negative fraction (15.3%±11.4 vs 43.2%±14.9 in HD and 5.3%±3.3 vs 29.5%±14.7 in CRC; p<0.0001). The chemokine receptor CX3CR1 was also increased in lipid engulfed macrophages compared with control (2.1%±0.9 vs 9.5%±7.5 in HD and 1.8%±1.5 vs 10.3%±6.1 in CRC; p<0.001). Similarly, the inhibitory immune check point PD-L1 was enhanced in FC counterparts (2.5%±1.3 vs 15.5%±11.8 in HD and 0.8%±0.7 vs 4.1%±3.5 in CRC, p=0.004).
Additional phenotypic features, such as the increase in CD163 marker expression and the decreased expression of the costimulatory molecule CD86 (online supplemental figure S9B), further support the lipid-associated polarization towards immunosuppressive myeloid cells.
To evaluate the mechanisms hindering effective CD8-T cell-mediated antitumor immunity associated with lipid engulfment in the CD14+ population, TCR-stimulated autologous T cells were cocultured with in vitro-induced FC or control cells. A significantly reduced CD8+ T-cell proliferation was detected by CFSE assay in FC-conditioned cultures compared with non-engulfed CD14+ cells (28.3±13.8 vs 43±8.4; p=0.0005) (figure 6E(i)). We also assessed the frequency of CD25+ subsets on proliferating CD8+, as an indicator of cell activation. We found that in FC coculture, the frequency of proliferating CD8 T-cells expressing CD25 marker was significantly lower compared with control condition (7±5.1 vs 11.6±5.1; p=0.001) (figure 6E (ii)). In parallel, the concentration of the cytotoxic factor, Granzyme B, was significantly lower in T-cell:FC cocultures compared with the control group (2.8±2.7 vs 4.6±4.7; p=0.004) (figure 6E (iii)).
Given the decrease in PD-1 positive T-cells in FChigh tumor regions and the increased expression of PD-L1 in tissue and in vitro foamy macrophages, as well as the increased production of FC-associated TGF-β immunosuppressive factor, we additionally conducted immunosuppression assays with the in vitro generated FC pretreated with single or simultaneously inhibition of TGF-β and anti-PD-L1 signaling.
TGF-β blocking in human-derived FC, significantly restored the proliferation of CD8+ T-cells (42±9.7 vs 28.3±13.7; p=0.02) (figure 6E(i)), and significantly enhanced the activation of proliferating CD8+ (13%±6.6 vs 7%±5.1; p=0.04) (figure 6E(ii)), compared with T-cell:FC condition.
The anti-PD-L1 blockade did not enhanced CD8+ T-cells proliferation (36.2%±12.8) and nor increase the frequency of CD25+ CD8+ cells (7.9%±5.7) in coculture with FC (figure 6E(i,ii)). However, the dual blockade of TGF- β and PD-L1 did not restore inhibition of CD8 T-cell proliferation, suggesting that the inhibition is likely due only to TGF-β, while PD-L1 is probably not involved (figure 6E(i,ii)). Notably, neither inhibitory treatment did not enhance the proliferation or activation of TCR-stimulated T-cells cultured alone (online supplemental figure S10A). This suggests that the activity of effector T-cells was dependent on inhibitory factors secreted by dyslipidemic macrophages.
The modulation of T-cell effector function in presence of FC, with singular or simultaneously TGF-β and PD-L1 inhibition, was confirmed by quantifying released cytotoxic factors. In this case, the ability to produce Granzyme by T-cells was significantly restored in both single TGF-β and PD-L1 blocking (figure 6E(iii)). However, their dual inhibition enhanced by twofold increase in Granzyme B production compared with T-cell:FC condition (3.1±3.5 vs 1.5±2; p=0.02) (figure 6(iii)), suggesting that FC-associated TGF-β signaling could be able to alter the anti-PD-L1 cell response, in line with recent findings.33 34 Single or simultaneously TGF-β and PD-L1 blocking did not show any modulation in T-cell:non-engulfed macrophages cocultured (online supplemental figure S10A,B).
These data collectively highlight the role of FC-secreted TGF-β as a critical factor in impeding protective CD8 antitumor immunity and underscore a potential targetable pathway for restoring effective T cell function.
Conclusions
Primary CRC is a human malignancy in which local immunity, estimated by the quantification and spatial distribution of CD8+ T cells, acts as a positive prognostic factor and the best predictor of DFS, more efficiently than the tumor-node-metastasis staging.35 This strong adaptive immune response is believed to reflect the rich T cell neoantigen repertoire expressed by CRC tumors as a consequence of specific genetic profiles (eg, MSI-H/dMMR).36 However, a high Immunoscore is detectable in merely 30% of CRCs,4 while tumor DNA alterations potentially leading to T cell epitopes, but showing limited evidence of T cell infiltration, are found in a vast majority of cases.37 These observations indicate that additional immune components of the CRC TME might be involved in hindering tumor T cell immunogenicity, and may deserve investigations for implementing immune-mediated control of this disease.
Several attempts have been made to identify myeloid subsets that correlate with CRC progression, including the proportion of CD163 or CD206 positive subpopulation on the CD68 pan-macrophages marker.38 However, the identification of detrimental subsets could be very challenging, considering their ontogenically heterogeneous nature and above all their high plasticity.27 39 Indeed, by rescuing catabolites imbalance within the TME, such as tumor secreted-lipids, macrophages rewired their metabolism and reshuffle their lipid composition40 and phenotype, thus representing an appealing immunosuppressive mediator.41
This study provides valuable insights into the complex interplay between altered lipid metabolism, colonic macrophages and T-cell immunity in the context of stage I–III CRC, shedding the light on their collective impact on disease progression.
Given the recently findings on the association of lipid-engulfed macrophages in controlling metabolic disorders,42 we have first demonstrated that FC, characterized by their lipid-laden phenotype, are associated with patients’ BMI and specific systemic lipid composition, suggesting for the first time their linking role between obesity and CRC. Specifically, FC associated with increased palmitic and oleic acids and decreased omega-3 PUFA, despite the lack of macroscopic clinical dyslipidemia. In this regard, dietary palmitic acid has recently been associated with metastatic potential in oral carcinomas,43 and it is well known that the unbalanced ratio between saturated and unsaturated lipids in membrane phospholipids could alter membrane fluidity and affect effector functions such as phagocytosis.
Then we have elucidated the significant role of FC in creating an immunosuppressive microenvironment within the colon, potentially fostering an immune milieu fueling tumor growth and progression. Specifically, FC identify CRC cases with a higher risk of recurrence after radical resection in patients with low immunogenic tumors. Indeed, the quantification of FC allowed for the identification of a subset of patients with CRC with a CD8low FChigh phenotype, who displayed the shortest DFS with respect to patients with CD8low FClow and obviously with CD8high tumors. Interestingly, considering the balance of FC and CD8, 2.6-fold higher abundance of FC on CD8 is predictive of worse DFS, demonstrating that the burden of FC is relevant even in patients with presence of CD8 infiltrate.
In this context, FC are paired with a specific immune context, consisting of reduced CD8+PD1+ T cells and enriched CD4+ Treg and infiltration, which indicates an active role of FC in promoting local immune tolerance. Interestingly, this scenario is highly reminiscent of the FC described in atherosclerotic plaques, where they have been proven to actively induce Treg accrual and concomitant inhibition of pathogenic CD8+ T cell effectors, resulting in plaque stabilization through the onset of an immunosuppressive milieu.44 The MiaQuant digital pathology algorithm23 24 allowed us to capture FC within the entire macrophage population based on their specific morphology and size characteristics. Analysis of the spatial distribution of FC with respect to CD8+ T cells in CRC lesions revealed a distinctive behavior of cancer-associated FC. The “barrier-like” distribution of FC controls the immune cells entering the neoplastic nests from the AM. High FC frequency at the CRC IM was thus confirmed to be associated with a significant decrease in the frequency of inner CD8 T cells, a correlation that was not detected when non-FC macrophages were evaluated. In line with this evidence, Peranzoni et al recently demonstrated the ability of macrophages to interfere with CD8 T cell motility and prevent them reaching tumor regions in lung squamous cell carcinoma.45 We further reinforced the potential immunosuppressive activity of CRC-associated FC by reporting that CRC features of FChigh infiltrate displayed enhanced dysregulation of TGF-β signaling, potentially contributing to immune evasion and disease progression. The transcriptional program showed reduced expression of genes associated with IFNγ-related pathways32 and CD8 T memory cells,26 while showing enrichment in terms related to Treg26 and T cell exhaustion.32 Furthermore, the presence of terminally differentiated effectors alongside FC delineates an impaired antitumor immunosurveillance.
Additionally, multiparametric flow cytometry analysis of CRC cell revealed a more complex dysfunctional immune environment coexisting with FC accumulation. To the already mentioned reduced frequency of CD8+PD1+ activated T cells and the increased effector Treg accrual,46 FChigh tumors were thus found characterized by a loss of predominance of immunoregulatory cellular elements, including an increase of regulatory CD8+HLA-DR+ subset47 together with a decrease in CD4+CXCR3+, which underlies the impairment of the generation of Th1 effector.48
Notably, a remarkable decrease in CX3CR1 expression in myeloid and lymphoid components was detected in FChigh tumors. CX3CR1 and its ligand, CX3CL1 (fractalkine), are pillar mediators of inflammation and autoimmune diseases.49 When expressed in macrophages of the colonic lamina propria, the CX3CR1/CX3CL1 axis mediates T cells activation leading to colitis,50 indicating a key role for this pathway in the accrual of intestinal T-cell effectors and suggesting its loss as a potential mechanism of immune escape in CRC.
Further, our investigation delves into the intricate interplay between FC and the potential interventions to restore effective antitumor immunosurveillance. TGF-β emerges as a promising therapeutic strategy to disrupt FC-mediated immunosuppression, potentially restoring effective antitumor immunity.
Additionally, TGF-β inhibition was found to enhance the ability of anti-PD-L1 in restoring T-cell activity. This aligns with recent preclinical studies showing that simultaneous targeting of both pathways enhances tumor infiltrating T-cell in tumors with a CD8 desert phenotype.33 34
The present study has some limitations. First, the sample size of accrued patients is relatively small, which may limit the ability to fully verify the strength of FC as a negative prognostic biomarker, particularly in CD8high CRC cases. Additionally, the FC model established here represents a simplified version compared with the in vivo FC, which arise from chronic exposure of TME macrophages to local and systemic modulating factors. These in vivo FC may secrete other inhibitory factors that could be involved in the suppression of CD8 effector activity. Furthermore, conducting immunosuppression assays by stimulating T cells with tumor antigens, which more closely mimic the physiological TME, could reflect a more realistic condition and potentially results in even greater observed immunosuppression.
In conclusion, our study showed that FChigh CD8low CRC, representing 20% of patients, have the highest risk of recurrence after radical surgery, suggesting the possibility of implementing Immunoscore with FC quantification to identify patients who may have worse clinical outcomes. Additionally, this study sheds light on the critical role of lipid-laden macrophages in mediating immune modulation in CRC pathogenesis, providing a novel avenue for therapeutic intervention in CD8 T-cell excluded patients. This offers a promising opportunity to improve therapeutic outcomes and patient survival in CRC. Finally, the link observed between FC tissue accrual, BMI and specific lipid profiles in patient plasma supports the use of interventions targeting lipid metabolism as a complementary approach to enhance effective tumor immunosurveillance by interfering with macrophage lipid metabolism in CRC treatment and prevention.
supplementary material
online supplemental file 1
online supplemental file 2
online supplemental file 3
Footnotes
Funding: This work was funded by AIRC Project IG 2017 Id.20752. The research leading to these results has received funding from AIRC under IG 2022 - ID. 27841 project to RL (PI). This work was supported in part by 5 X 10005 Funds—2014 MIUR—to MV. ED was supported as postdoctoral fellow by Umberto Veronesi Foundation for the year 2022–2024 and Pezcoller-SIC Foundation for the year 2021.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Consent obtained directly from patient(s).
Ethics approval: This study involves human participants and was approved by Ethics Committee of the Fondazione IRCCS, Istituto Nazionale dei Tumori, Milan (INT), Italy (INT127/19, INT149/19, INT61/20). Informed consent was obtained from all patients and HDs.
Data availability free text: Data are available upon reasonable request. All data associated with this study and its supplementary data files are available within the article. Sequencing and bioinformatics analyses were annotated in Gene Expression Omnibus Super Series: GSE227206 and GSE273106.
References
Articles from Journal for Immunotherapy of Cancer are provided here courtesy of BMJ Publishing Group
Citations & impact
This article has not been cited yet.
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/169342914
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
GEO - Gene Expression Omnibus (2)
- (2 citations) GEO - GSE273106
- (2 citations) GEO - GSE227206
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Targeting tumor-infiltrating CCR8+ regulatory T cells induces antitumor immunity through functional restoration of CD4+ Tconvs and CD8+ T cells in colorectal cancer.
J Transl Med, 22(1):709, 30 Jul 2024
Cited by: 1 article | PMID: 39080766 | PMCID: PMC11290082
A Novel TGF-β-Related Signature for Predicting Prognosis, Tumor Microenvironment, and Therapeutic Response in Colorectal Cancer.
Biochem Genet, 62(4):2999-3029, 07 Dec 2023
Cited by: 2 articles | PMID: 38062276
Association of CD8+TILs co-expressing granzyme A and interferon-γ with colon cancer cells in the tumor microenvironment.
BMC Cancer, 24(1):869, 19 Jul 2024
Cited by: 0 articles | PMID: 39030523 | PMCID: PMC11265531
The Macrophages-Microbiota Interplay in Colorectal Cancer (CRC)-Related Inflammation: Prognostic and Therapeutic Significance.
Int J Mol Sci, 21(18):E6866, 18 Sep 2020
Cited by: 23 articles | PMID: 32962159 | PMCID: PMC7558485
Review Free full text in Europe PMC
Funding
Funders who supported this work.
Associazione Italiana per la Ricerca sul Cancro (2)
Grant ID: ID. 20752 to LR
Grant ID: ID.27841 to LR
Fondazione Pezcoller (1)
Grant ID: post doctoral fellow (2021) to ED
Fondazione Veronesi (1)
Grant ID: post doctoral fellow (2022-2024) to ED
Ministero dell'Istruzione, dell'Università e della Ricerca (1)
Grant ID: 5x1000 funds to MV

 1
1 

