Abstract
Background
Arterial stiffness is a degenerative modification in the arterial wall that significantly affects normal aging. Arterial hypertension is a major risk factor for cerebrovascular impairment. Pulse wave velocity (PWV) is an established gold standard for measuring arterial stiffness. Studies demonstrated that individuals with elevated blood pressure (BP) and PWV are more likely to experience worse cognitive decline compared to those with either condition alone. The aim of this review is to explore the clinical importance of arterial stiffness for cognitive function in older adults with hypertension.Methods
The systematic review was reported following the PRISMA 2020 guidelines and Cochrane protocol and was registered in NIHR PROSPERO. PubMed, Embase, Web of Science, CINAHL, and Cochrane databases were searched for relevant publications up to December 2022. Articles were filtered by age and type of study and only those including a sample size of at least 500 individuals were selected. Screening of abstracts and full-text review of selected articles were carried out through Covidence.Results
The full-text review included a total of 434 articles. Twenty-eight prospective studies have met the inclusion criteria. Selected studies used PWV as the main measurement of stiffness: 24 used carotid-femoral, 2 used brachial-ankle, 1 used aortic PWV, and 11 compared different measures. Studies demonstrated a strong association between increased BP and PWV with brain damage and cognitive deterioration among older adults. One study did not find an interaction with hypertension, while another study found that PWV but not BP was associated with cognitive decline. Few studies showed that the association between stiffness and cognitive outcomes was not significant after adjustment for BP. Several authors suggested that cognitive decline induced by stiff vasculature and hypertension benefited from antihypertensive therapy.Conclusion
The results of this review demonstrated that arterial hypertension is an important factor linking arterial stiffness to cognitive health in older individuals. BP plays a crucial role in brain integrity, whereas PWV was shown to be a strong measure associated with cognitive decline. Together, they can lead to disabling cognitive outcomes. Early screening of stiffness, BP control, and compliance with treatment are essential for cerebrovascular disease prevention.Trial registration
NIHR PROSPERO registry ID: CRD42022379887 .Free full text

Arterial stiffness measured by pulse wave velocity correlated with cognitive decline in hypertensive individuals: a systematic review
Abstract
Background
Arterial stiffness is a degenerative modification in the arterial wall that significantly affects normal aging. Arterial hypertension is a major risk factor for cerebrovascular impairment. Pulse wave velocity (PWV) is an established gold standard for measuring arterial stiffness. Studies demonstrated that individuals with elevated blood pressure (BP) and PWV are more likely to experience worse cognitive decline compared to those with either condition alone. The aim of this review is to explore the clinical importance of arterial stiffness for cognitive function in older adults with hypertension.
Methods
The systematic review was reported following the PRISMA 2020 guidelines and Cochrane protocol and was registered in NIHR PROSPERO. PubMed, Embase, Web of Science, CINAHL, and Cochrane databases were searched for relevant publications up to December 2022. Articles were filtered by age and type of study and only those including a sample size of at least 500 individuals were selected. Screening of abstracts and full-text review of selected articles were carried out through Covidence.
Results
The full-text review included a total of 434 articles. Twenty-eight prospective studies have met the inclusion criteria. Selected studies used PWV as the main measurement of stiffness: 24 used carotid-femoral, 2 used brachial-ankle, 1 used aortic PWV, and 11 compared different measures. Studies demonstrated a strong association between increased BP and PWV with brain damage and cognitive deterioration among older adults. One study did not find an interaction with hypertension, while another study found that PWV but not BP was associated with cognitive decline. Few studies showed that the association between stiffness and cognitive outcomes was not significant after adjustment for BP. Several authors suggested that cognitive decline induced by stiff vasculature and hypertension benefited from antihypertensive therapy.
Conclusion
The results of this review demonstrated that arterial hypertension is an important factor linking arterial stiffness to cognitive health in older individuals. BP plays a crucial role in brain integrity, whereas PWV was shown to be a strong measure associated with cognitive decline. Together, they can lead to disabling cognitive outcomes. Early screening of stiffness, BP control, and compliance with treatment are essential for cerebrovascular disease prevention.
Trial registration
NIHR PROSPERO registry ID: CRD42022379887.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12883-024-03905-8.
Introduction
The lifespan of humans has increased considerably during the last centuries. In the US, the number of adults older than 65 years was 54.1 million (16%) in 2019. By 2060 this number will increase to 25% (cdc.gov, 2022). Hypertension is a well-known risk factor for cerebral and cardiovascular diseases [1–5] and has continued to be a major public health problem for the last decades. Nearly half (47%, or 116 million) of the adults in the United States (US) have elevated BP. Aging is a heterogenic and dynamic process that progressively limits normal functioning and makes people susceptible to disease and death [6]. With advancing age and chronically increased BP, the elasticity of the arterial wall decreases, subsequently causing an increase in PWV. Elevated PWV is associated with the propagation of pulsatile flow to the brain, endothelial injury [7], decreased blood flow [7, 8], and a decreased ability of the brain to adapt to changes in blood flow [8]. This can lead to a decline in cognitive performance [9] and an increased risk of vascular dementia, although it varies between individuals. The incidence of cerebrovascular diseases (CeVD), particularly cerebral small vessel disease (CSVD), increases with extended life expectancy [10, 11], causing disability, mild cognitive impairment (MCI), and dementia [12, 13]. In the US, vascular cognitive impairment and hypertension are the top 5 causes of disability in the population older than 65 years (cdc.gov, 2022). 2013 European Society of Hypertension/European Society of Cardiology (ESH/ESC) [5] and 2015 American Heart Association (AHA) [14] recommended carotid-femoral PWV (cfPWV) as a gold standard for arterial stiffness research and an independent predictor for fatal and non-fatal cardiovascular events in hypertensive patients [15]. The process of vascular aging is exaggerated by concurred hypertension which has the strongest association with vascular events [16]. High BP, dyslipidemia, diabetes mellitus (DM), obesity, and other traditional cardiovascular risk factors are often not diagnosed timely and thus, remain undertreated or if treated, are poorly controlled [17].
Objectives of this review: (1) systematically review recent literature on arterial stiffness, hypertension, and cognitive function in aging and establish gaps where future research could be of benefit, (2) propose mechanistic links between arterial stiffness, hypertension, and cognitive function in aging, (3) assess the clinical ability of pulse wave velocity, as the measure of arterial stiffness, to predict cognitive decline in aging, (4) assess the potential of arterial stiffness to serve as a biomarker of cognitive decline.
Methods
Literature search
Search strategy
We searched PubMed, Embase, Cochrane CENTRAL, CINAHL, Web of Science, and Scopus platforms without data or language limits. We included abstracts from the database. The search strategy included a combination of subject headings and text words for the concepts of arterial stiffness, pulse wave velocity, cognitive decline, arterial hypertension, and their synonyms. The supplementary material presents an example of a search strategy in the PubMed database (Supplementary material, Search strategy). The age older than 45 years was used as a filter. There was no limit to the publication date.
Eligibility criteria
We screened all eligible studies, including clinical randomized trials, case-control, cohort, cross-sectional, longitudinal, experimental pilot, and community-based, as well as database analyses.
Inclusion criteria
We included studies published worldwide, studies using PWV and cognitive disorders measurements (neuropsychological tests and neuroimaging), studies on stroke-free and psychiatric disorders-free populations, articles reporting an odds ratio or hazard ratio for the relationship between exposure and outcome, and studies that included at least 500 participants older than 45 years.
Exclusion criteria
The exclusion criteria included studies that used a case report or case-series study design, articles without PWV measurement of arterial stiffness, articles that did not report a statistic for the association between arterial stiffness and cognitive changes, a sample size of less than 500 participants, and participants younger than 45 years.
Study selection
First, we completed the title and abstract screening to create a primary list. Then, the full texts were screened for additional information to decide if the studies were eligible. The duplicates and irrelevant articles were removed. The first reviewer (BA) assessed the eligibility criteria, and the second reviewer (TA) screened the articles and worked on the PRISMA flowchart and tables. A third reviewer (TR) was brought in to resolve any discrepancies in the selection. All relevant articles were collected in EndNote and screening was completed through the Covidence voting system.
Data extraction and analysis
We extracted data from the selected articles using pre-piloted data extraction forms prepared in Excel. The extracted data included: (1) subject characteristics (sample size, mean age, gender distribution, race and ethnicity distribution, BP), (2) exposure (stiffness measurements [cfPWV, brachial-ankle PWV (baPWV), aortic PWV (aoPWV), carotid-radial PWV (crPWV), or estimated PWV (ePWV)]), (3) outcome information based on neuropsychological tests of cognitive function [Mini-Mental State Examination (MMSE), modified MMSE (3MSE), and Montreal Cognitive Assessment (MoCA)] and imaging-based studies of the brain [computer tomography (CT) and magnetic resonance imaging (MRI)]. We considered appropriate inclusion/exclusion criteria when selecting the published articles.
Arterial hypertension, stiffness, and cognitive function were evaluated using the following measurements, techniques, guidelines, and devices:
Blood pressure: systolic (SBP) and diastolic (DBP), were measured by a sphygmomanometer, electronically calibrated manometer “Omron,” “Dinamap,” “Meditech,” 24 h ambulatory BP monitors. Mean arterial pressure (MAP) and pulse pressure (PP) were the calculated BP measurements. The units of BP measurement were mmHg. The percentage of hypertensive individuals and/or individuals on antihypertensive therapy throughout the studies is reflected in the summary table (Table 1).
Table 1
Summary
# Author Study name, country Study design Follow-up, years Population Sample size, n Gender, race, % Age, years Hypertension
[HTN, % HTNM, %, SBP, mmHg]
Arterial stiffness Cognition:
Neuroimaging, Neuropsychological tests
Conclusion 1 Hajjar I. et al., 2016 Emory University, USA Cohort longitudinal 4y Employees of Emory University 591 68%F
70%W
24%B
48.8 83% cfPWV (7.2) MDR
Memory recognition
VSL
SSTM
Pattern recall
DPR
Recognition of pattern
EFT
MF
DSST
FBDS
Symbol spotting
FSA
1. HTN + increased PWV were associated with a decline in executive score
2. In healthy adults increased cfPWV was superior to BP in predicting cognitive decline in all domains
3. The treatment of arterial stiffness in hypertensives is crucial for cognitive health prevention
2 Pase M.P. et al., 2016 FHS
(Framingham Heart Study), US
Population-based observational cohort since 1948 FHS G3 and offspring cohort 1101 54%F 69±6 58%
43% HTNM
cfPWV (10.6) DSMMD – Dementia (7%)
NINCDSAD – AD (5%) Petersen criteria – MCI (10%)
1. cfPWV predicts MCI in total sample, dementia and AD in non-diabetics.
2. Brachial PP is associated with increased risk of dementia
3 Nilsson E.D. et al., 2014 MDCS
(Malmo Diet and Cancer Study), Sweden
Population-based prospective cohort,
cross-sectional analysis
1991-start
5y
Sweden population 2637 60.8%F 72.1±5.6 135.6/75.6mmHg
47.7% HTNM
cfPWV (10.5±2.5) AQT MMSE 1.Increased cfPWV was inversely associated with cognition
2. There is a linear association between cfPWV and cognitive speed after adjusting for cardiovascular risk factors
4 Nilsson E.D. et al., 2017 MDCS
(Malmo Diet and Cancer Study), Sweden
Population-based prospective cohort 1991-start
5y
Sweden population 3056 43.1%M 61-85 137.8/74.6mmHg
60.8% HTNM
cfPWV (10.5±2.4) MMSE, AQT-color form DSSMD 1. Higher cfPWV is associated with dementia, before adjustment
2. No association between cfPWV and all-cause dementia and AD after adjustment to vascular factors
5 Watson N.L. et al., 2011 Health ABC Study, Cognitive Validity Substudy
(Aging and Body Composition Study), US
Prospective cohort 1997-enroll
9y
Pittsburg, PA; Memphis, TNMedicare 552 48%M
42%B
73.1±2.7 50% cfPWV (8.9±3.9) 3MSE Central AS contributes to cognitive decline independent of HTN and vascular risk factors 6 Menezes S.T. et al., 2019 ELSA-Brazil, (Brazilian Longitudinal Study of Adult Health) Longitudinal multicenter cohort 3.8y 6 Brazilian cities 6927 55%F 58.8±5.9
(baseline)
62.7±5.9 (follow-up)
SBP 130.2±18.9
39.4% HTNM
cfPWV (9.9±1.9) Memory test scoreVFTTrial-B 1. Increased cfPWV was associated with a sharper decline in cognitive performances, regardless of SBP among younger group.
2. Decline in cognition was faster among older cohorts
7 Araghi M. et al., 2020 Whitehall II, UK Longitudinal cohort Start in 1985
7y
London-based British civil servant 4300 25.3%F
92.3%W
65.3±5.7 124.5/70.7mmHg
32.7% HTNM
cfPWV
(3 groups:
<7.41; 7.41-8.91; >8.91)
AH4-I
global, memory, phonemic and semantic fluency
MMSE
1. Higher cfPWV was associated with faster cognitive decline
2. The highest third of PWV had the highest rate of HTN (41.6%)
8 Cooper L et al., 2016 AGES-Reykjavik (Age, Gene/Environmental Susceptibility Study), Iceland Cohort prospective, cross-sectional analysis 5y Iceland population 1820 60%F 80±5 144/64mmHg
71% HTNM
cfPWV (13.6±4.6) MRI
CVLT
DSST
Stroop test
EFT
WMH 15%
Microbleeds 29%
In older adults, cardiovascular resistance and WMH are associated with higher cfPWV and lower memory scores. MAP and segmental brain volumes were associated with cfPWV and memory scores, but not both measures 9 Mitchell G.F., et al., 2011 AGES-Reykjavik Study
(Age, Gene/Environmental Susceptibility Study), Iceland
Community-based prospective 1967 -start
4y
Iceland population 668 57%F 75±4F
76±4M
63%F
54%M
Carotid PP*
AI
Pix*
cfPWV*
(F 12.2±3.7
M 13.4±4.4)
icfPWV
MRI
MMSE
GDS score
1. Increased cfPWV and Pix were associated with higher volume of WMH, subcortical infarcts and reduced cognitive function
2. Stiff aorta was associated with lower wave reflection and lower cognitive scores
3. The higher level of BP and cfPWV are associated with diffuse brain damage and lower cognitive scores
10 RibaLlena I., et al., 2018 ISSYS (Investigating Silent Strokes in Hypertensives), Spain Community-based prospective ongoing 3y Hypertensive Spanish population 782 49.6%F 62.7±5.4 142.9/77.3mmHg
95.3% HTNM
cfPWV
(9.2-11.9)
MRI
7.2% -Lacunes
6.4% - microbleeds
6.7% WMH
24.5% EPVS in basal ganglia
40.1% EPVS in the centrum semiovale
In hypertensive population cfPWV is associated with total load of CSVD, especially EPVS 11 Maillard P., et al., 2017 FHS
(Framingham Heart Study), US
Population-based observational cohort Since 1948 FHS G3 and offspring cohort 2422 45.91%M 51.3±11.6 SBP 122.73±17.37mmHg
36.86% HTNM
cfPWV (7.9) MRI DTI 1. cfPWV is associated with higher FW, FA, WMH
2. the effect of SBP on FW is mediated by cfPWV
12 Benetos A. et al., 2012 PARTAGE
(Predictive Values of Blood Pressure and Arterial Stiffness in Institutionalized and Very Aged Population), France
Longitudinal multicenter prospective 3 year Nursing home 873 79%F 87±5 73%
95% HTNM
cfPWV (14.4 ±5) MMSE
Katz ADL
1. The higher cfPWV, the more profound the cognitive decline
2. PWV but not BP is associated with cognitive decline
13 Collin C. et al., 2010 3 City Dijon study, France Ongoing population-based longitudinal study, cross-sectional analysis Start in 1991
6 follow-ups over 10y
Bordeaux, Dijon, Montpellier 931 37.5%M 75±3.7 69.3% cfPWV
(m 15.2±3.3
f 14.3+3.1)
WMLV 1. In females the higher SBP was significantly associated with larger total WMLV
2. In males AS was significantly associated with higher periventricular WMLV after adjustment to MAP
14 Kim E.D. et al., 2017 PACE
(Predictors of Arrhythmic and Cardiovascular Risk in ESRD), US
Prospective population-based 1y Outpatient dialysis units in Baltimore 568 58%M72%B 55±13 100% cfPWV (10.0)
AI
cPP
TMT A, B
3MSE
Cognitive impairment 10%
1. Higher cfPWV and cPP were associated with TMT A, B, but association was attenuated after multivariable adjustment. 2. Higher AI and cPP were associated with cognitive decline in end-stage renal disease patients, but not cfPWV 15 Amier R.P., et al., 2021 HBCS
(Heart-Brain Connection Study), Netherlands
Multicenter prospective observational, cross-sectional analysis 09/2014-12/2017
3y3m
4 University medical center 559 35.8%F 67.8±8.8 56.8% aoPWV (8.4) (MRI) Dutch Parelsnoer Initiative (memory, language, speed, executive function MRI 1. Higher aoPWV, LVMi, and LVMVR were associated with the extent CSVD and cognitive impairment only in patients with cardiovascular diseases
2. The worse brain damage is correlated to SBP or HTN
3. Severity and duration of HTN are related to higher CSVD and cognitive impairment
16 Taniguchi Y. et al., 2015 Health examination, Japan Longitudinal prospective population-based Start 2008
3.4y
Community-dwelling older Japanese 526 57.8%F 71.7±5.6 36.3%
33.5% HTNM
baPWV (17.82) MMSE >26 points (94.1%)
Cognitive decline 16.2%
baPWV is independently associated with cognitive decline after adjustment for BP and HTN med 17 Han F. et al., 2021 Ongoing population based Shunyi cohort study, China Cross-sectional analysis 3y Suburb district of Beijing 933 63.7%F 55.5±9.1 49.8% baPWV (15.7±3.2) MMSE (26.4)
MRI DTI
Dementia 26%
1. baPWV is independently associated with white matter deterioration, decreased FA, increased mean, axial, radial diffusivity
2. General cognitive function worsened with increased baPWV after adjusting for hypertension
18 Palta P., et al., 2019 ARIC-NCS
(Atherosclerosis Risk in Communities-Neurocognitive Study), US
Community-based prospective 1987- start
2y
Washington County, MD; Forsyth County, NC; Minneapolis, MN; Jakson, MS 3703 60%F
20.8%B
75.2
(67-90)
63.5% cfPWV
cPP
MRI
DWR
DSST
TMT A, B
BNT
1. The higher cfPWV, the greater WMH, the lower cognitive scores
2. cPP was associated with brain damage and poorer cognitive performance
3. cfPWV and PP has the strongest association with executive function and speed
4. No significant interaction by hypertension is observed.
19 Meyer M. L. et al., 2017 ARIC-NCS
(Atherosclerosis Risk in Communities-Neurocognitive Study), US
Cross-sectional analysis Over 25y Washington County, MD; Forsyth County, NC; Minneapolis, MN; Jakson, MS 6538
4461 (for visit 5)
58.8%F 70-89y
Mean 75.4±5y
20.4%B
71.8%
72.4%
cfPWV (11.54-13.81)
PP
DSST
DWR
WF
MMSE
CDR
FAQ
MRI WMH
MCI: 19.8%W, 19.5%B
Dementia: 2.8%W, 4.3%B
Black and White adults with cerebrovascular disease have elevated cfPWV, central SBP, cPP. White individuals had higher central stiffness and pulsatility.
After adjustment to MAP the association of cfPWV with MCI or dementia is not significant
20 Waldstein S.R. et al., 2008 Baltimore longitudinal study of aging, US Prospective study of community-dwelling volunteers 1958-start
11y and 14y – follow up
Gerontology research center 1749
Subset of 582 had PWV
53.3%M79.4W 57.1 128.8/80.1mmHg
22.1% HTNM
cfPWV (7.1±2.7)
PP
MAP
MMSE
WAIS
CVLT
BVRT
TMT-A, B
BNT
I-M-C
1. PP, cfPWV longitudinally predicted cognitive decline before dementia.
2. AS was higher in patients with cardiovascular and metabolic risk factors (MAP), and lower cognitive function.
3. Increased PP was associated with prospective decline of cognitive tests
21 Tsao C.W. et al., 2013 FHS
(Framingham Heart Study), US
Population-based observational cohort, cross-sectional analysis Since 1948 FHS G3 and offspring cohort, Examination 7 1587 45%M 61±9 126/74mmHg
30% HTNM
cfPWV (9.0)
MAP
cPP
LMDR
Trials B-A
MRI
(TCBV=79±3, WMHV=0.05, Silent cerebral infarct= 10%)
1.Higher cfPWV was associated with lower TCBV, larger WMHV, prevalent silent cerebral infarct
2. MAP, cPP were associated with greater WMHV, lower TCBV, worse verbal memory
3. Increased stiffness and pressure pulsatility were associated with brain aging, AD
22 Tsao C.W. et al., 2016 FHS
(Framingham Heart Study), US
Population-based observational cohort Since 1948 FHS G3 and offspring cohort,
Examination7 and 8
1223 56%F 61±9 SBP 125±18
28% HTNM
cfPWV (9.0) AP
cPP
Trials B-A
MRI
1. Higher cfPWV, cPP were associated with greater progression of neurocognitive decline
2. Higher MAP, not cfPWV and cPP was associated with increased WMH
3. Increased cfPWV and PP were associated with longitudinal progression of subclinical brain injury and greater cognitive decline4. Treatment of arterial stiffness reduced cognitive decline
23 Poels M.M.F. et al., 2007 Rotterdam Study, Netherlands Population-based prospective cohort 1990-start
1997-1999
2002-2004
Population of Rotterdam 2767 42.3%M 72.0 MAP 106.7mmHg cfPWV (13.2±2.9)
Carotid distensibility
PP
MAP
MMSE – dementia
NINCDS-ADRDA
NINCDS-AIREN – VD
LDST
Stroop test
WF
1. No association between AS and cognitive decline or risk of dementia after adjustment for cardiovascular risk factors
2. Higher AS was associated with higher SBP, PP, atherosclerosis
24 Poels M.M.F. et al., 2012 Rotterdam Study, Netherlands Population-based prospective cohort,
cross-sectional analysis
1990-2004 Population of Rotterdam 1460 55.4%F 58.2 130/79.8mmHg
MAP 96.5mmHg
cfPWV (9.0±1.6) MRI
WM lesion=2.2ml
Lacunar infarct=4.3%
Cerebral microbleeds 10.1%
1.Increased cfPWV was associated with larger WMH volume2. Uncontrolled HTN, high aoPWV was associated with larger WMH and cerebral microbleeds 25 Zhong W.J. et al., 2014 EHLS-BDES
(Epidemiology of Hearing Loss Study – Beaver Dam Eye Study), US
Longitudinal cohort,
cross-sectional analysis
1989-start
1. 5y
2. 15y
Beaver Dam residents 1433 43%M 75 61.4-75.8%
65-71% HTNM
cfPWV (11.0±3.6)
crPWV (10.0±2.3)
MMSE
TMT-A, B
DSST
AVLT
VFT
1. cfPWV was associated with lower MMSE score, AVLT, TMT-B.2. crPWV was not associated with cognitive tests3. Large arteries stiffness was associated with worse cognitive function
4. Increased AS operates through HTN to decrease cognitive function
26 Lin CH, et al., 2022 LAST
(Longitudinal Aging Study of Taipei), Taiwan
Community-based prospective ongoing, cross-sectional analysis 6y Community in Taiwan 992 69.5%F 67.3 30.4%
25.7%HTNM
cfPWV (11.0±2.8)
Zc
carotid AI
carotid AP
Pf
Pb
XSPI, %XSPI
MoCA
MMSE
AS markers (XSPI/%XSPI), but not cfPWV were significantly higher in participants with low MoCA score due to vascular aging 27 Rensma S.R. et al., 2020 Maastrich Study, Netherlands Population-based observational cohort, cross-sectional analysis 3y S. Netherlands 2544 51%M 59.7 MAP 96.6mmHg
37.7% HTNM
cfPWV
carotid distensibility coefficient
MRI
VLT
SCWT part I-II-III
CST part A-B-C
LDST
Microbleeds=12.0
Lacunar infarct=5.3
1. Aortic stiffness, but not carotid stiffness was independently associated with worse cognition2. Increased aoPWV may lead to microvascular dysfunction via increased pulsatility load 28 Heffernan K.S. et al., 2022 NHANES
(National Health and Nutrition Examination Survey), US
Examination survey with serial cross-sectional design 1999-2002 enrollment.
3y
Noninstitutionalized population of all 50 states and Washington DC 3616 54.9%F
26.5%B
69.71 54% ePWV (11.02) DSST 1. PWV in older Black and White was inversely associated with DSST score (cognitive measure)2. BP has a mediating effect on AS and cognitive aging AD Alzheimer’s disease, ADL Activities of daily living, AH4-I Alice Heim 4-I, AI Augmentation index, aoPWV aortic pulse wave velocity, AS Arterial stiffness, AQT A quick test of cognitive speed, AVLT Auditory verbal learning test, B Black race, baPWV brachial-ankle pulse wave velocity, BNT Boston naming test, BP Blood pressure, BVRT Benton visual retention test, CDR Clinical dementia rating score, cfPWV– carotid-femoral pulse wave velocity, cPP central pulse pressure, CSVD Cerebral small vessel disease, CST part A-B-C Concept shifting test, CVLT – California verbal learning test, DPR Delayed pattern recall, DSSMD Diagnostic and statistical manual of mental disorders, DSST Digit symbol substitution test, DWR Delayed word recall, EFT Executive function test, EPVS Enlarged perivascular spaces, ePWV estimated pulse wave velocity, F Female, FA Fractional anisotropy, FAQ Functional activities questionnaire, FBDS Forward and backward digit span, FSA Focused and sustained attention, FW Free water, GDS Geriatric depression scale, HTN Hypertension, HTNM Hypertension medication, icfPWV inverse carotid–femoral pulse wave velocity, I-M-C blessed information-memory-concentration test, LDST Letter-digit substitution test, LMDR Logical memory-delayed recall, LVMi Left ventricle mass index, LVMVR Left ventricle mass-to-volume ratio, M Male, MAP Mean arterial pressure, MCI Mild cognitive impairment, MDR Memory delayed recall, MF Mental flexibility, MMSE Mini-mental state examination, MoCA Montreal cognitive assessment, MRI Magnetic resonance imaging, MRI DTI Magnetic resonance imaging diffusion tensor imaging, NINCDSAD National Institute of Neurological and Communicative Disorders and Stroke and the AD and Related Disorders Association, NINCDS-ADRDA - VD National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease Related Disorders Association – vascular dementia, NINDS-AIREN National Institute of Neurological Disorders and Stroke and the Association Internationale pour la recherche et l’Enseignement en Neurosciences, Pb Backward pressure amplitude, Pf Forward pressure amplitude, Pix Pulsatility index, PP Pulse pressure, PWV Pulse wave velocity, SBP Systolic blood pressure, SCWT Stroop color-word test, SSTM Spatial short-term memory, TCBV Total cerebral brain volume, TMT A, B Trail making test part A, B, VFT Verbal fluency test, VLT Verbal learning test, VSL Visual spatial learning, W White race, WAIS Wechsler adult intelligence scale, WF Word fluency, WMH White matter hyperintensity, WMHV White matter hyperintensity volume, WMLV White matter lesion volume, XSPI Excess pressure integral, Zc characteristic impedance, 3MSE modified mini-mental state test
Arterial stiffness was assessed by different techniques and devices: Complior, Sphygmocor, AtCor, DiaTecne srl, SMT Medical, pOpmetre, Vicorder, PulseTracePCA2 for PWV, VaSera for cardio-ankle vascular index (CAVI), and Doppler Sonography. The measurements of arterial stiffness included PWV, PP, PP amplification, ankle-brachial index, CAVI, augmentation index (AI), carotid AI, characteristic impedance, carotid augmented pressure, forward/backward pressure amplitude, excess pressure integral (XSPI), Young’s elastic modulus, and pulsatility index. We chose to use the following measurements of arterial stiffness: cfPWV, aoPWV, baPWV, crPWV, and ePWV. The means are listed in Table 2.
Table 2
The association of pulse wave velocities with cognitive function, cognitive decline, and brain damage markers. (Results section)
# Author, year Study Analysis PWV, m/s Results 1 Hajjar I. et al., 2015 Emory University, US Longitudinal cfPWV
7.2 (total sample)
7.68 (HTN group)
Individuals with hypertension and elevated PWV had worse decline in executive function score (p=0.004), working memory (p=0.039), memory score (p=0.018), independently of hypertension, SBP, and antihypertensive therapy. PWV was superior to BP in predicting cognitive decline and explained hypertension-executive function association. 2 Pase M.P. et al., 2016 FHS
(Framingham Heart Study), US
Longitudinal cfPWV
MCI group 10.6
(9.0-13.1)
Dementia group 10.7 (9.0-13.2)
After adjustment for MAP and hypertensive therapy, higher cfPWV was significantly associated with MCI (HR=1.41, p=0.01), but not dementia and Alzheimer’s disease. 3 Nilsson E.D. et al., 2014 MDCS
(Malmo Diet and Cancer Study), Sweden
Cross-sectional cfPWV
10.5±2.5
After adjustment for MAP, antihypertensive therapy:
1. the linear association between cfPWV and AQT, (ß=-0.37, p=0.039) was attenuated, but significant.
2. the association of cfPWV>13.8 was highly significant with AQT scores (ß=4.81, p=0.004) and MMSE (ß=-0.37, p=0.016).
4 Nilsson E.D. et al., 2017 MDCS
(Malmo Diet and Cancer Study), Sweden
Longitudinal cfPWV
Nondemented 10.5±2.4
Prevalent dementia
11.2±2.6
Incident dementia 11.2±2.7
Adjusted for MBP and medications, cfPWV was not associated with prevalent dementia (OR=0.95, p=0.40) or with incident dementia (OR=1.0, p=0.96) 5 Watson N.L. et al., 2011 Health ABC Study, Cognitive Validity Substudy
(Aging and Body Composition Study), US
Longitudinal cfPWV
8.86±3.89
1st tertile 3.29-6.73
2nd tertile 6.73-9.25
3rd tertile 9.25-28.23
After adjustment for MAP and hypertension, the higher cfPWV was significantly associated with:
1. worse global cognitive function, ß=-0.11; verbal memory, ß=-0.07; speed, ß=-0.09; perception, ß=-0.12
2. the greater risk of psychomotor speed decline, OR=1.42.
6 Menezes S.T. et al., 2019 ELSA-Brazil, (Brazilian Longitudinal Study of Adult Health) Longitudinal cfPWV
9.9±1.9
cfPWV was significantly associated with memory after adjustment for SBP at the baseline, ß=-0.03 and over time, ß=-0.02, p<0.01 7 Araghi M. et al., 2020 Whitehall II, UK Longitudinal cfPWV
Lowes third <7.41
Middle third 7.41-8.91
Highest third >8.91
1. The higher third of cfPWV was significantly associated with cognitive scores at the baseline (b=-0.12, p<0.05) and longitudinally (b=-0.06, p=0.01)
2. Individuals with cardiovascular factors had 11% higher cfPWV cross-sectionally and 4% longitudinally.
8 Cooper L. et al., 2015 AGES-Reykjavik (Age, Gene/Environmental Susceptibility Study), Iceland Cross-sectional cfPWV
13.6±4.6
After adjusting for antihypertensive medications:
1. cfPWV had stronger association with memory change, ß=-0.071±0.023, p=0.002, r2=0.19
2. there was a significant relationship between cfPWV and microbleeds, OR=1.12; cerebellar infarct, OR=1.30; subcortical infarct, OR=1.30
3. BP and stiffness have bidirectional relationship.
9 Mitchell G.F., et al., 2011 AGES-Reykjavik Study
(Age, Gene/Environmental Susceptibility Study), Iceland
Longitudinal cfPWV
F 12.2±3.7
M 13.4±4.4
After adjusting for MAP and hypertension medications, the association was significant between:
1. cfPWV and subcortical infarction (HR=1.62-1.71, p<0.001)
2. cfPWV with WMHV (ß=0.108±0.045, p=0.018)
4. cfPWV with lower memory score (ß=-0.095±0.043, p=0.028).
10 RibaLlena I., et al., 2018 ISSYS
(Investigating Silent Strokes in Hypertensives), Spain
Longitudinal cfPWV
9.2-11.9
After adjusting for MAP in hypertensive individuals, cfPWV was associated with CSVD load (OR=1.42, p<0.001), lacunes (OR =1.51, p=0.005), and PVS (OR=1.39, p=0.001) 11 Maillard P., et al., 2017 FHS
(Framingham Heart Study), US
Cross-sectional cfPWV
7.9
Significant association between SBP and free water was mediated by cfPWV, direct effect a=0.040, after adjustment for antihypertensive therapy and SBP. 12 Benetos A. et al., 2012 PARTAGE
(Predictive Values of Blood Pressure and Arterial Stiffness in Institutionalized and Very Aged Population), France
Longitudinal cfPWV
14.4 ±5.0
1st tertile 9.6±1.3
2nd tertile 13.5±1.2
3rd tertile 20.1±4.0
In individuals older than 80 years:
1. The higher PWV tertile, adjusted for MAP and medications, was associated with worse MMSE at the baseline and in 1 year (-2.20±3.98, p<0.03 and 21.3±6.0, p<0.05 respectively)
2. BP was not associated with cognitive decline (r=-0.005, p=0.88)
13 Collin C. et al., 2010 3 City Dijon study, France Longitudinal cfPWV
15.2±3.3 M
14.3+3.1 F
Adjusted for MAP:
1. cfPWV was significantly associated with WMLV in males, OR=1.48, p<0.05.
2. SBP was significantly associated with deep WMLV in females, OR=1.27, p<0.05.
14 Kim E.D. et al., 2017 PACE
(Predictors of Arrhythmic and Cardiovascular Risk in ESRD), US
Longitudinal cfPWV 10
(7.9-12.5)
In individuals on hemodialysis the association between PWV with cognitive scores was attenuated after adjustment for diastolic BP: 3MS score at the baseline (OR=4.68, p=0.20) and in 1 year (OR=0.12, p=0.43). 15 Amier R.P., et al., 2021 HBCS
(Heart-Brain Connection Study), Netherlands
Cross-sectional aoPWV
8.4
(6.8-10.8)
After corrections for SBP and hypertension:
1. aoPWV was associated with CSVD (OR=1.17, p=0.003)
2. The association between aoPWV and cognitive impairment was mediated by CSVD (p=0.004).
16 Taniguchi Y. et al., 2015 Health examination, Japan Longitudinal baPWV
17.82±3.62
1st tertile <15.91
2nd tertile 15.91-18.89
3rd tertile >18.89
After adjustment for SBP and HTNM, the higher tertile of baPWV was significantly associated with cognitive decline, OR=2.95. 17 Han F. et al., 2021 Ongoing population based Shunyi cohort study, China Cross-sectional baPWV
15.7±3.2
1. Hypertension has a direct effect on stiffness and white matter integrity.
2. The association of baPWV with white matter integrity, after adjustment for hypertension, was significant, p<0.05
3. The association between baPWV increase per SD and worse MMSE scores was significant after adjustment (ß=-0.093, p=0.011).
18 Palta P., et al., 2019 ARIC-NCS
(Atherosclerosis Risk in Communities-Neurocognitive Study), US
Cross-sectional cfPWV
Lowest quartiles
3.25-11.22
Highest quartiles
11.23-22.58
After adjusting for MAP:
1. The highest cfPWV levels was associated with faster cognitive decline, z-score=-0.17
2. The highest cfPWV was associated with WMH, total brain volume, AD.
3. No significant interaction by hypertension between stiffness and brain damage.
19 Meyer M. L. et al., 2017 ARIC-NCS
(Atherosclerosis Risk in Communities-Neurocognitive Study), US
Cross-sectional cfPWV
11.54-13.81
1. Increased cfPWV and SBP was associated with higher MCI and dementia among White individuals (OR=1.27 and OR=1.76 respectively) before adjustment.
2. There was no effect modification by hypertension and MAP of the association between cfPWV with MCI or dementia.
20 Waldstein S.R. et al., 2007 Baltimore longitudinal study of aging, US Longitudinal cfPWV
7.1±2.7
(3.03-19.42)
1. Increased PWV, adjusted for MAP and antihypertensive therapy, was associated with cognitive decline before dementia.
2. Hypertension management preserves cognitive health.
21 Tsao C.W. et al., 2013 FHS
(Framingham Heart Study), US
Cross-sectional cfPWV
9.0
(7.6-11.0)
In Model2, adjusted for MAP and hypertensive medication:
1.Increased cfPWV was associated with WMH volume (ß=-0.07±0.04, p<0.05), silent infarct (OR=1.45, p<0.01), and TCBV (ß=-0.07±0.03, p<0.05)
2. increased MAP was associated with WMH volume (ß=-0.07±0.04, p<0.01) and memory delay (ß=-0.05±0.03, p<0.05).
22 Tsao C.W. et al., 2016 (long) FHS
(Framingham Heart Study), US
Longitudinal cfPWV
9.0
(7.6-10.9)
In adjusted for antihypertensive medication model:
1. Higher cfPWV was associated with executive function decline (ß=-0.10±0.04, p<0.05)
2. Higher MAP was associated with WMH volume (ß=-0.07±0.03, p=0.017.
23 Poels M.M.F. et al., 2007 Rotterdam Study, Netherlands Longitudinal cfPWV
13.2±2.9
1. Adjusted for MAP, PWV was considerably associated with poorer Stroop’s scores (ß=1.13 per SD increase in PWV)
2. No association after adjustment between stiffness and cognitive decline (OR 0.93), dementia (OR=0.91), and AD (OR=0.90).
24 Poels M.M.F. et al., 2012 Rotterdam Study, Netherlands Cross-sectional cfPWV
9.0±1.6
After adjustment for MAP:
1. In uncontrolled hypertensive individuals cfPWV was associated with CSVD: WMLV difference=0.09 per SD increase; lacunar infarcts, OR=1.63; deep microbleeds, OR=2.13
2. There was no association between cfPWV and CSVD in controlled HTN and without HTN groups
25 Zhong W.J. et al., 2014 EHLS-BDES
(Epidemiology of Hearing Loss Study – Beaver Dam Eye Study), US
Cross-sectional cfPWV
11.0±3.6
crPWV
(10.0±2.3)
Adjusted for hypertension, cfPWV>12m/s was associated with lower cognitive scores: MMSE (ß=-0.31, p=0.005), AVLT (ß=-1.10, p<0.01).
This association was attenuated by hypertensive treatment.
26 Lin C.H., et al., 2022 LAST
(Longitudinal Aging Study of Taipei), Taiwan
Cross-sectional cfPWV
11.0±2.8
After adjusting for MAP and hypertensive treatment:
1. cfPWV was not associated with cognitive impairment (OR=1.04).
2. Excess pressure integrals remained significantly associated with lower MoCA score (OR=1.30).
27 Rensma S.R. et al., 2020 Maastrich Study, Netherlands Cross-sectional cfPWV
9.0±2.1
After adjustment for MAP and hypertension therapy, cfPWV was associated with lower cognitive scores (ß=-0.018) and CSVD (ß=-0.018). 28 Heffernan K.S. et al., 2022 NHANES
(National Health and Nutrition Examination Survey), US
Cross-sectional ePWV
11.02
(10.9-11.1)
Adjusted for hypertension ePWV was significantly associated with DSST scores in Black (ß=-3.47, p<0.001) and White (ß=-3.51, p<0.001) adults. AD Alzheimer’s disease, aoPWV aortic pulse wave velocity, AS Arterial stiffness, AQT A quick test of cognitive speed, AVLT Auditory verbal learning test, baPWV brachial-ankle pulse wave velocity, BP Blood pressure, cfPWV carotid-femoral pulse wave velocity, CSVD Cerebral small vessel disease, ePWV estimated pulse wave velocity, F Female, HTN Hypertension, HTNM Hypertension medication, HR Hazard ratio, M Male, MAP Mean arterial pressure, MCI Mild cognitive impairment, MMSE Mini-mental state examination, MoCA Montreal cognitive assessment, OR Odds ratio, PWV Pulse wave velocity, SBP Systolic blood pressure, WMH White matter hyperintensity, WMLV White matter lesion volume, 3MSE Modified mini-mental state test
The cognitive function was evaluated via a battery of multiple neurocognitive tests that are listed in the summary table (Table 1). The results of the studies are summarized in Table 2. The most widely used clinical screening tests for cognitive function assessment were MMSE, MoCA, and 3MSE. The assessment of brain damage was provided by neuroimaging studies, such as CT and MRI. The MRI classification of CSVD was: (1) recent small subcortical infarct classified as acute lacunar infarct, (2) white matter hyperintensity (WMH), (3) silent lacunar infarct, (4) cerebral microbleed, and (5) perivascular spaces (PVS) [18].
Definitions
Arterial stiffness is an aging process in the arterial wall characterized by degeneration of elastic fibers, an increase in collagenous material, and calcium deposition [19].
2017 ACC/AHA defined stage 1 hypertension as BP at or above 130/80 mmHg, and stage 2 hypertension at or above 140/90 mmHg [20].
MCI is an early stage of memory loss or other cognitive ability loss with the preserved ability to independently perform most activities of daily living; 5–53% of MCI cases progress into dementia, and 15% into Alzheimer’s disease (AD) [11].
Dementia is an impaired ability to remember, think, or make decisions that interfere with daily activities. It is caused by the degeneration and loss of neurons and neuronal connections in the brain. The affected area causes the symptoms of dementia. Dementia is not a part of normal aging. AD is the most common type of dementia [21].
Reporting bias assessment
We used the QualSyst tool to evaluate the studies [22]. Scores for the quality assessment of the studies were calculated based on a 14-item checklist provided in the tool (Supplementary material, Table s1). Scoring above 55% was recommended as the quality inclusion threshold the QualSyst.
Synthesis of results
Table 1 summarizes the chosen studies’ exposure and outcome data. Table 2 differentiates the PWV measurement by type and includes the results. The cognitive outcomes are compared with arterial stiffness to identify any correlations with increased blood pressure. The combined effect measures were not calculated due to the multiple types of neuropsychological tests used to score the outcome.
Results
Study selection
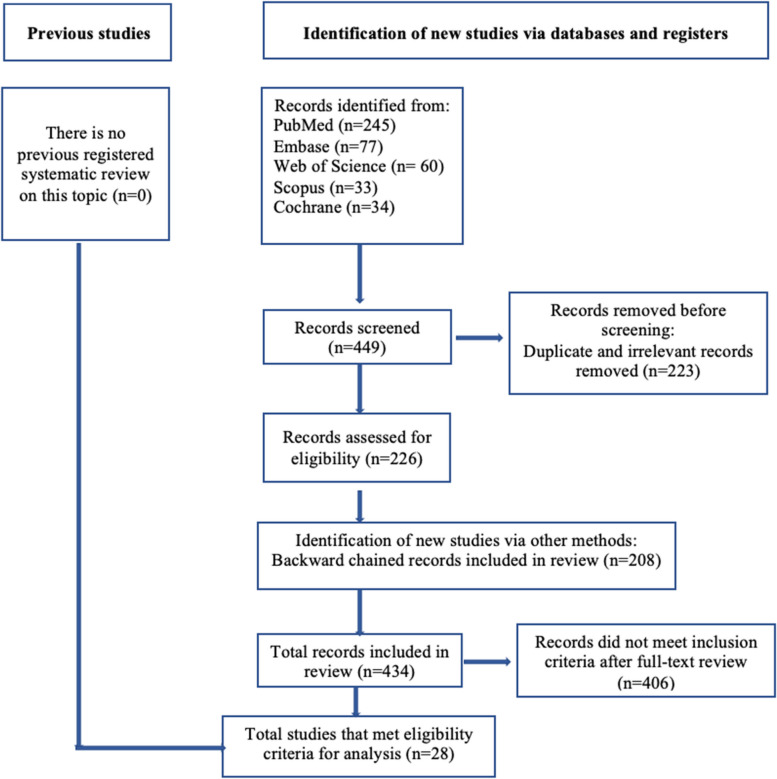
The PRISMA flow diagram (Fig. 1) was created using the PRISMA 2020 flow diagram template for systematic review [23].
Study characteristics and results of individual studies
Each of the included study characteristics, such as study design, follow-up, age, sex, and other variables, as well as outcome, are described in Table 1. Chosen studies were classified by:
Sample size: n
 >
> 1000 (17 studies), n
1000 (17 studies), n =
= 500–1000 (11 studies) participants. The total sample size in this review is n
500–1000 (11 studies) participants. The total sample size in this review is n =
= 56,858.
56,858.Years of publication: 24 papers were published from 2012 to 2022 and 4 papers were published before 2012. The first relevant paper matching the eligibility criteria was published in 2007.
Age: the youngest participant was 45 years old, the oldest was 92 years old, and the average age of participants was 66.9 years old.
Gender: 55.6% of participants were women.
The most known and oldest ongoing study was the Framingham Heart Study (FHS), which began 75 years ago. This review includes four studies of third-generation FHS offspring.
All the studies were prospective: 13 of them used cross-sectional analysis and 15 studies were assessed longitudinally. The follow-up period ranged from 1 to 25 years.
Risk of bias in studies
Our team used the QualSyst tool to evaluate the quality of quantitative studies [22] (Supplementary material, Table s1). All selected articles met the recommended threshold.
Data synthesis
The review of reports demonstrated an association between hypertension-related arterial stiffness and cognitive dysfunction. Chronically elevated BP causes the arterial wall to be more fibrotic, hypertrophic, and stiff. Subsequently, these structural changes exacerbate vascular remodeling and promote vascular aging [24–28]. Vascular stiffness and elevated BP cause microvascular brain damage [29–31] and contribute to stroke [32, 33], cognitive deterioration [34–36], and vascular dementia [37, 38]. The correlation between arterial stiffness and cognitive decline was reported in multiple studies. Hajjar et al. reported that hypertensive individuals with stiffness had a worse decline in executive function (p =
= 0.004), working memory (p
0.004), working memory (p =
= 0.039), and memory scores (p
0.039), and memory scores (p =
= 0.018). Stiffness was a better predictor of cognitive impairment than BP. Also, stiffness explained the association between hypertension and executive function [39]. After adjustment for MAP and hypertensive therapy, a significant association between higher cfPWV with MCI (HR
0.018). Stiffness was a better predictor of cognitive impairment than BP. Also, stiffness explained the association between hypertension and executive function [39]. After adjustment for MAP and hypertensive therapy, a significant association between higher cfPWV with MCI (HR =
= 1.41, p
1.41, p =
= 0.01), but not dementia or AD was shown by Pase et al. in the FHS [40]. Nilsson et al. analyzed the association of stiffness with dementia in the Malmo Diet and Cancer Study (MDCS). The results were similar: (1) cross-sectional analysis demonstrated the highly significant association between cfPWV
0.01), but not dementia or AD was shown by Pase et al. in the FHS [40]. Nilsson et al. analyzed the association of stiffness with dementia in the Malmo Diet and Cancer Study (MDCS). The results were similar: (1) cross-sectional analysis demonstrated the highly significant association between cfPWV >
> 13.8 m/s with MMSE (b=-0.37, p
13.8 m/s with MMSE (b=-0.37, p =
= 0.016) and a quick test of cognitive speed (AQT, b
0.016) and a quick test of cognitive speed (AQT, b =
= 4.81, p
4.81, p =
= 0.004) scores [35]; (2) longitudinally, after adjustment the association between cfPWV with prevalent dementia (OR
0.004) scores [35]; (2) longitudinally, after adjustment the association between cfPWV with prevalent dementia (OR =
= 0.95, p
0.95, p =
= 0.40) and incident dementia (OR
0.40) and incident dementia (OR =
= 1.0, p
1.0, p =
= 0.96) was not significant [41]. Watson et al. demonstrated the association between central stiffness and psychomotor speed decline (OR
0.96) was not significant [41]. Watson et al. demonstrated the association between central stiffness and psychomotor speed decline (OR =
= 1.42) independent of hypertension [42]. Menezes et al. indicated a faster decline in cognitive performance among older adults (verbal fluency test, b=-0.02, p
1.42) independent of hypertension [42]. Menezes et al. indicated a faster decline in cognitive performance among older adults (verbal fluency test, b=-0.02, p <
< 0.01) [43]. Araghi et al. showed that higher tertile of cfPWV (>
0.01) [43]. Araghi et al. showed that higher tertile of cfPWV (> 8.91 m/s) had the highest rate of hypertension (41.6%) and faster cognitive decline (b=-0.06, p
8.91 m/s) had the highest rate of hypertension (41.6%) and faster cognitive decline (b=-0.06, p =
= 0.01) [44].
0.01) [44].
White matter hyperintensities, enlarged PVS, and total cerebral brain volume were brain damage markers in MRI-based studies and considered causes of cognitive decline. The Age, Gene/Environmental Susceptibility Study (AGES-Reykjavik) demonstrated a bidirectional relationship between BP and stiffness associated with brain damage and cognitive impairment. The results showed a significant relationship between elevated cfPWV and microbleeds (OR =
= 1.12), cerebellar infarct (OR
1.12), cerebellar infarct (OR =
= 1.30), subcortical infarct (OR
1.30), subcortical infarct (OR =
= 1.30), and memory change (b=-0.071
1.30), and memory change (b=-0.071 ±
± 0.023, p
0.023, p =
= 0.002, r2
0.002, r2 =
= 0.19) [45]. Earlier, in the same study, Mitchel et al. found a significant association between elevated cfPWV with subcortical infarction (HR
0.19) [45]. Earlier, in the same study, Mitchel et al. found a significant association between elevated cfPWV with subcortical infarction (HR =
= 1.62–1.71, p
1.62–1.71, p <
< 0.001), WMHV (b
0.001), WMHV (b =
= 0.108
0.108 ±
± 0.045, p
0.045, p =
= 0.018), and lower memory score (b=-0.095
0.018), and lower memory score (b=-0.095 ±
± 0.043, p
0.043, p =
= 0.028) [46]. The Silent Stroke Study in hypertensive individuals reported the association between increased cfPWV and CSVD load (OR
0.028) [46]. The Silent Stroke Study in hypertensive individuals reported the association between increased cfPWV and CSVD load (OR =
= 1.42, p
1.42, p <
< 0.001), lacunes (OR
0.001), lacunes (OR =
= 1.51, p
1.51, p =
= 0.005), and PVS (OR
0.005), and PVS (OR =
= 1.39, p
1.39, p =
= 0.001) [47]. Maillard et al. demonstrated in the third-generation offspring of FHS that cfPWV has a direct mediating effect (a
0.001) [47]. Maillard et al. demonstrated in the third-generation offspring of FHS that cfPWV has a direct mediating effect (a =
= 0.040, p
0.040, p <
< 0.001) on the association between SBP and free water (a biomarker of cerebral injury contributing to white matter degeneration) [48].
0.001) on the association between SBP and free water (a biomarker of cerebral injury contributing to white matter degeneration) [48].
Routine assessment of older hypertensive individuals for cognitive decline was highly recommended to prevent and postpone cognitive burden by 2020 ESH/EGMS (European Geriatric Medicine Society) [49]. At very advanced age, people are prone to episodes of systolic hypotension [50], which, in conjunction with stiff vasculature, may cause severe cognitive impairment [51]. The Predictive Values of Blood Pressure and Arterial Stiffness in Institutionalized and Very Aged Population (PARTAGE) study [52] showed that PWV, but not BP, was associated with cognitive decline in institutionalized individuals older than 80 years (r=-0.005, p =
= 0.88). This is probably due to comorbidities, lower BP, and very low vascular compliance. The worse MMSE at the baseline and in 1 year (-2.20
0.88). This is probably due to comorbidities, lower BP, and very low vascular compliance. The worse MMSE at the baseline and in 1 year (-2.20 ±
± 3.98, p
3.98, p <
< 0.03 and 21.3
0.03 and 21.3 ±
± 6.0, p
6.0, p <
< 0.05 respectively) was associated with higher tertile of cfPWV (20.1
0.05 respectively) was associated with higher tertile of cfPWV (20.1 ±
± 4.0) [52]. Antihypertensive therapy consistently showed a significant improvement in BP and PWV levels in individuals with stiff vasculature [53, 54] and cognitive impairment [39, 55].
4.0) [52]. Antihypertensive therapy consistently showed a significant improvement in BP and PWV levels in individuals with stiff vasculature [53, 54] and cognitive impairment [39, 55].
Endocrinal causes may mediate the association between aging vasculature and cognitive performance. Collin et al. reported the differences between males and females in the MRI-based study: the larger white matter hyperintensity volume (WMLV) was significantly associated with higher central SBP among females (OR =
= 1.27, p
1.27, p <
< 0.05), whereas in males, the higher periventricular WMLV was significantly associated with higher aortic stiffness (OR
0.05), whereas in males, the higher periventricular WMLV was significantly associated with higher aortic stiffness (OR =
= 1.48, p
1.48, p <
< 0.05) [31].
0.05) [31].
Oxidative stress, vascular inflammation, autoimmunity activation, and atherosclerotic modulation promote arterial aging, cardiovascular events, and stroke [56]. A strong correlation between metabolic factors, neuroinflammatory markers, cerebral microvascular changes, and white matter lesions with cognitive decline was shown through several studies [57]. Global MARE Consortium (Metabolic Syndrome and Artery Research) considered metabolic syndrome as a mechanism explaining vascular aging, which in some individuals predisposes to earlier and in others to healthier vascular aging. The lower pulse wave velocities correspond to healthier vascular aging [58]. Supernormal vascular aging is a protective phenotype of low PWV values. It can be diagnosed in individuals with extremely low arterial stiffness for their age and sex [59]. Some populations, such as Yanomamo Indians, Papua New Guinea, and rural Kenyans, do not have an increased incidence of hypertension with advancing age [60]; they have a good aerobic load, low cholesterol, low sodium, and high fiber carbs diet [61]. Oppositely, early vascular aging syndrome, first described in 2008, explains the effect of premature vascular aging with abnormal arterial function [62, 63].
Increased vascular stiffness elevates cardiovascular risk [64–68] and demonstrates an association of cognitive decline with cardiovascular risk factors [69–72] and multiple end-organ damage [73–76]. Recently, Scuteri A. et al. defined SHATS (systemic hemodynamic atherosclerotic syndrome) as a combination of left ventricular hypertrophy, common carotid artery damage, and chronic kidney disease (CKD) [75–78]. Left ventricle remodeling and fibrosis can cause cerebrovascular hemodynamic changes with cognitive impairment [79] independently of blood pressure [80]. In recently diagnosed hypertensive individuals, stiffness was associated with microalbuminuria related to cerebral microcirculatory changes and, as a result, caused cognitive damage [81]. The Predictors of Arrhythmic and Cardiovascular Risk in End-stage Renal Disease (PACE) study found an association between cfPWV and lower cognitive test scores in end-stage renal disease patients. This association was attenuated after adjustment for DBP: 3MSE score at the baseline (OR =
= 4.68, p
4.68, p =
= 0.20) and in 1 year (OR
0.20) and in 1 year (OR =
= 0.12, p
0.12, p =
= 0.43) [78]. In individuals on hemodialysis, stiffness may deteriorate due to progressive calcification in the arterial wall [82]. Stiffness is a risk factor for cardiovascular disease, myocardial infarction, and stroke because of the strong association with atherosclerotic plaques and thickened intima-media [83]. A positive correlation between stiffness and aortic atherosclerosis was also confirmed in the autopsy study [84].
0.43) [78]. In individuals on hemodialysis, stiffness may deteriorate due to progressive calcification in the arterial wall [82]. Stiffness is a risk factor for cardiovascular disease, myocardial infarction, and stroke because of the strong association with atherosclerotic plaques and thickened intima-media [83]. A positive correlation between stiffness and aortic atherosclerosis was also confirmed in the autopsy study [84].
Studies used multiple biomarkers of arterial stiffness other than cfPWV, such as aoPWV, baPWV, crPWV, ePWV, PP, and pressure integrals. Amier et al. used cardiovascular MRI to measure aoPWV. The results showed that the severity and burden of hypertension were directly related to worse CSVD (OR =
= 1.17, p
1.17, p =
= 0.003) and cognitive impairment [85]. The baPWV was used to measure stiffness in Japanese [38] and Chinese [30] studies. Taniguchi et al. reported an independent association between the highest and middle tertiles of baPWV with cognitive decline (OR
0.003) and cognitive impairment [85]. The baPWV was used to measure stiffness in Japanese [38] and Chinese [30] studies. Taniguchi et al. reported an independent association between the highest and middle tertiles of baPWV with cognitive decline (OR =
= 2.95 and OR
2.95 and OR =
= 2.39 respectively) [38]. Han et al. used MRI-DTI to assess the association of baPWV with white matter integrity: the association was significant (p
2.39 respectively) [38]. Han et al. used MRI-DTI to assess the association of baPWV with white matter integrity: the association was significant (p <
< 0.05), and MMSE scores were worse in those with elevated PWV (b=-0.093, p
0.05), and MMSE scores were worse in those with elevated PWV (b=-0.093, p =
= 0.011) [30]. Atherosclerosis Risk in Communities (ARIC) study compared central PP with cfPWV, and the results were similar: participants with elevated cfPWV had larger WMH (p
0.011) [30]. Atherosclerosis Risk in Communities (ARIC) study compared central PP with cfPWV, and the results were similar: participants with elevated cfPWV had larger WMH (p <
< 0.007), smaller total brain volume, lower scores for executive function/processing speed (b=-0.04, p
0.007), smaller total brain volume, lower scores for executive function/processing speed (b=-0.04, p <
< 0.05) and global performance (b=-0.09, p
0.05) and global performance (b=-0.09, p <
< 0.05) [86]. Previously, in the same study, among White individuals, those with higher PP showed a higher prevalence of MCI (OR
0.05) [86]. Previously, in the same study, among White individuals, those with higher PP showed a higher prevalence of MCI (OR =
= 1.27) and dementia (OR
1.27) and dementia (OR =
= 1.76), as well as those with elevated cfPWV and SBP, had a higher prevalence of MCI (OR
1.76), as well as those with elevated cfPWV and SBP, had a higher prevalence of MCI (OR =
= 1.27). The estimates variance among Black participants with CSVD was large, and the association between cfPWV, cPP, and cSBP with MCI and dementia was not statistically significant. There was no effect modification by hypertension or diabetes after adjustment [36]. Similarly, in the Baltimore Aging Study, PP and cfPWV were significantly associated with lower cognitive scores (p
1.27). The estimates variance among Black participants with CSVD was large, and the association between cfPWV, cPP, and cSBP with MCI and dementia was not statistically significant. There was no effect modification by hypertension or diabetes after adjustment [36]. Similarly, in the Baltimore Aging Study, PP and cfPWV were significantly associated with lower cognitive scores (p <
< 0.05) before clinical symptoms of dementia [87].
0.05) before clinical symptoms of dementia [87].
Tsao et al. assessed the association between stiffness, measured with cfPWV, MAP, central PP, and neurocognitive outcomes cross-sectionally [88] and longitudinally [55]: increased cfPWV was associated with executive function decline (b=-0.10 ±
± 0.04, p
0.04, p <
< 0.05), elevated MAP was associated with larger WMHV (b=-0.07
0.05), elevated MAP was associated with larger WMHV (b=-0.07 ±
± 0.03, p
0.03, p =
= 0.017). The longitudinal results from the Rotterdam Study reported no association between stiffness and cognitive decline (OR
0.017). The longitudinal results from the Rotterdam Study reported no association between stiffness and cognitive decline (OR =
= 0.93), dementia (OR
0.93), dementia (OR =
= 0.91), or AD (OR
0.91), or AD (OR =
= 0.90) after adjustment for cardiovascular risk factors [89]. Later, the cross-sectional analysis showed that in uncontrolled hypertensive individuals, cfPWV was associated with CSVD: WMLV (difference in volume
0.90) after adjustment for cardiovascular risk factors [89]. Later, the cross-sectional analysis showed that in uncontrolled hypertensive individuals, cfPWV was associated with CSVD: WMLV (difference in volume =
= 0.09 per SD increase); lacunar infarcts (OR
0.09 per SD increase); lacunar infarcts (OR =
= 1.63), and deep microbleeds (OR
1.63), and deep microbleeds (OR =
= 2.13). However, in the group with controlled BP and without hypertension, there was no association between cfPWV and CSVD [90].
2.13). However, in the group with controlled BP and without hypertension, there was no association between cfPWV and CSVD [90].
Carotid-radial was compared with carotid-femoral PWV among residents of Madison, Wisconsin, by Zhong et al. The results failed to find an association between crPWV and cognitive scores. However, cfPWV >
> 12 m/s was significantly associated with lower MMSE (p
12 m/s was significantly associated with lower MMSE (p =
= 0.005), auditory verbal learning test (p
0.005), auditory verbal learning test (p =
= 0.01), and composite cognition scores (p
0.01), and composite cognition scores (p =
= 0.04) [91]. The association of cognitive function with XSPI and cfPWV was compared in the Taipei Study. The results reported no significance with cfPWV but a significance with XSPI (OR
0.04) [91]. The association of cognitive function with XSPI and cfPWV was compared in the Taipei Study. The results reported no significance with cfPWV but a significance with XSPI (OR =
= 1.30), likely, due to aortic pulsatility load [92]. Similarly, in the Maastrich Study, the aortic but not carotid stiffness was independently associated with worse cognitive scores (b=-0.018) and larger microvascular damage (b=-0.018, p
1.30), likely, due to aortic pulsatility load [92]. Similarly, in the Maastrich Study, the aortic but not carotid stiffness was independently associated with worse cognitive scores (b=-0.018) and larger microvascular damage (b=-0.018, p <
< 0.05) due to increased pulsatility load [93].
0.05) due to increased pulsatility load [93].
The estimation of PWV (ePWV) was calculated using age and BP and showed an association with increased incidence of CeVD in the Kailuan Study (China, n =
= 98,348) [94], the Systolic Blood Pressure Interventional Trial (SPRINT) (Greece, n
98,348) [94], the Systolic Blood Pressure Interventional Trial (SPRINT) (Greece, n =
= 8,450) [95], Danish Monitoring Trends and Determinants in Cardiovascular Disease (MONICA, n
8,450) [95], Danish Monitoring Trends and Determinants in Cardiovascular Disease (MONICA, n =
= 2,366) [96], and General Chinese Population Study (n
2,366) [96], and General Chinese Population Study (n =
= 7,012) [97]. Heffernan et al. indicated an inverse association between elevated ePWV and lower cognitive digit symbol substitution test scores among Black (b=-3.47, p
7,012) [97]. Heffernan et al. indicated an inverse association between elevated ePWV and lower cognitive digit symbol substitution test scores among Black (b=-3.47, p <
< 0.001) and White (b=-3.51, p
0.001) and White (b=-3.51, p <
< 0.001) adults [34].
0.001) adults [34].
It is important to mention that 24-hour blood pressure fluctuation associated with atherosclerotic arterial stiffness mostly impacts the carotid pool (113) and was suggested as a contributor to vascular dementia and AD [98]. In individuals with masked and white coat hypertension, BP alteration is associated with arterial structure and function, with greater concentric arterial remodeling among women [99].
Cerebral hypoperfusion was established as a cause of cognitive decline in multiple studies [100–102]. Whereas, in the aging population, pathological vascular mechanisms may be masked by parallel biological processes, routine PWV screening was recommended in the middle-aged population [35, 97, 103, 104]. Carotid-femoral PWV was shown as an independent predictor of mortality in individuals with essential hypertension [105], type 2 diabetes [106], and end-stage CKD [106, 107]. A 1 m/s elevation in PWV was significantly associated with 11% elevation of cardiovascular and 12% all-cause mortality [108]. However, PARTAGE [109], Pronostic Cardiovasculaire Optimization Therapeutique en Geriatric Study (PROTEGER) [110], and metanalysis of 17,635 individuals by Ben-Shlomo et al. [111]. showed that after adjustment for cardiovascular risk factors in older adults, PWV was not predictive of future fatal and nonfatal cardiovascular events.
Discussion
Age a priori is associated with a change in arterial geometry that leads to increased stiffness and blood pressure over time. The carotid-femoral PWV was found to be more predictive of cognitive decline, whereas hypertension plays a crucial role in cerebrovascular function and brain integrity.
Although some studies were included several times, they were conducted at different time points with different population sizes and addressed different research questions. We treated population studies and interventional studies in the same manner and acknowledged that this may be a potential limitation. However, the results seem to converge and lead to similar conclusions.
Arterial stiffness measured with PWV is significantly associated with cognitive decline in aging individuals with chronically elevated BP. The results show that arterial hypertension is one of the most important risk factors in this association. Multiple other factors contribute to the link between hypertension, stiffness, and cognitive dysfunction as well. These factors include hemodynamic, immunologic, metabolic, neuro, and vascular inflammatory processes, as well as cardiac and renal comorbidities. The mechanism behind cerebral damage and cognitive dysfunction is complex and manifests at micro- and macrovascular levels, such as white matter lesions, microinfarcts, microbleeds, enlarged PVS, and cortical atrophy and neurodegeneration.
Aging individuals are among the most vulnerable populations. They are at the highest risk for developing disabling cognitive impairment and sooner death due to multiple vascular risk factors and chronic comorbidities. Our review showed that accelerated arterial stiffness and higher blood pressure significantly lower cognitive abilities and mental functionality and predispose to worse cardiovascular outcomes and CSVD. Reducing the burden of cardio- and cerebrovascular events by lowering risk factors is complex and suboptimal [17]. Therefore, early screening of high-risk individuals, intensive treatment, and effective prevention of vascular risk factors and cognitive decline in the aging population should be implemented to provide a better quality of life, promote personal independence, and reduce social burden and healthcare costs [112, 113].
The goal of this review was achieved. We captured current relevant studies (Objective 1). There is a negative relationship between arterial stiffness and microvascular cerebral impairment with cognitive dysfunction. Further analysis of published longitudinal studies confirmed this negative association. The selected studies demonstrated a strong association between arterial stiffness, measured with pulse wave velocity, and cognitive decline. After controlling for covariates, such as age, sex, and blood pressure, the negative association between arterial stiffness and cognitive function was maintained in 25 studies. The consistency of this association was strengthened by the findings from studies, regardless of the duration of the follow-up periods.
The MDCS [41], Rotterdam [89], and ARIC-NC [36] studies reported no association between cfPWV with MCI, dementia, and AD after adjustment for cardiovascular risk factors. Later, Palta et al. analyzed data from the ARIC-NC study longitudinally and found that higher cfPWV was associated with AD; however, a significant interaction by hypertension was not observed [86]. Factors such as CKD, metabolic syndrome, and genetic predisposition influenced the relationship between cognitive function and arterial stiffness [78].
The Rotterdam Study (2007) with n =
= 2,767 (4.9% of the total sample size of all analyzed studies) reported no association between stiffness and cognitive decline after adjustment [89]. Later, the cross-sectional analysis indicated a correlation between higher levels of cfPWV and larger volumes of WMH after adjusting for MAP, heart rate, and cardiovascular risk factors [90].
2,767 (4.9% of the total sample size of all analyzed studies) reported no association between stiffness and cognitive decline after adjustment [89]. Later, the cross-sectional analysis indicated a correlation between higher levels of cfPWV and larger volumes of WMH after adjusting for MAP, heart rate, and cardiovascular risk factors [90].
Objective 2 was supported by the following results: (1) a stiff aorta promotes increased blood flow to the fragile cerebral small vessels contributing to microcirculatory impairment, (2) cerebral hypoperfusion may induce brain damage, such as WMH, lacunar infarcts, etc., and (3) endothelial dysfunction contributes to hypertension and, as a result, to stroke [44].
Clinical use of biomarkers depends on predictive value, technical availability, and cost of the procedure. Importantly, in analyzed studies, the gold standard for arterial stiffness cfPWV, as well as aoPWV, baPWV, and crPWV were measured noninvasively via specialized devices; ePWV was quantified from age and mean arterial pressure. In the prediction of cardiovascular events ePWV cannot substitute but rather be additive to cfPWV: the estimated measure is not predictive in individuals with high risk. Also, the formula of ePWV includes MAP and, therefore, could be influenced by treated hypertension [114]. The MONICA study reported that a 1 m/s increase in ePWV was associated with a 20% increase in mortality risk [115].
Results of the review support Objective 3: earlier screening of cfPWV improves prognoses of cerebrovascular events, cognitive decline, disability, morbidity, and mortality. Adequate treatment, adherence, and compliance can change the prognosis of the patient and reverse the stiffening process. Many decades of attempting to produce drugs that reduce dementia-related neurodegenerative pathways have failed to show significant clinical benefits [116]. Hajjar et al. [39] and Tsao et al. [55] showed that therapeutic management of hypertension-related arterial stiffness was beneficial for cognitive health. A healthy diet and lifestyle modification minimize cognitive decline in the aging population. Factors like high levels of education, body mass index, physical activity, intensive treatment of hypertension, as well as education programs are protective among non-compliant patients. Regular aerobic exercise and reduced sodium intake were clinically effective in the prevention and treatment of arterial stiffness [117].
To support Objective 4, it was found that a group of experts proposed to implement a classification and staging of aging-related diseases, as well as a scoring system of tissue and organ senescence to evaluate patients’ status and guide policy at the World Health Organization and government level [118]. The control of hypertension demands planned collective action and the adoption of actions at the National level [119]. The paradigm of effective prevention should be shifted from traditional risk factors to arterial aging [17]. The proclamation that calls for joint prevention of stroke and dementia, data harmonization, and translation into action was issued by the World Stroke Organization and endorsed by 23 international, regional, and national organizations [120, 121]. The guidelines for standardized clinical evaluation of cognitive function in hypertensive patients were elaborated by a group of experts from ESH and EGMS in 2020 [49].
The results from this review show that arterial stiffness, measured by PWV is a strong predictor of cognitive decline in hypertensive individuals older than 45 years, independent of any specific demographics. Pulse wave velocity is a non-invasive, reliable method to determine arterial stiffness and is a marker of brain health. The earlier onset of cognitive decline is associated with higher progression rates of worse cerebrovascular outcomes. Thus, a PWV assessment could be included as a routine examination for high-risk adults for the prevention of cardiovascular and cognitive events.
The strength of this review is its inclusion of comprehensive prospective studies with substantial sample sizes that were methodologically analyzed prospectively and cross-sectionally. In geographically, racially, and ethnically diverse elderly populations with comorbidities, the influence of arterial stiffness on cognitive health was confirmed. Out of the 28 studies, the majority (23) utilized gold standard carotid-femoral pulse wave velocity (cfPWV) for assessing arterial stiffness. Additionally, studies compared various measures of arterial stiffness, and the longitudinal analyses covered a period of up to 25 years. Furthermore, several studies suggested the beneficial effects of antihypertensive therapy on arterial stiffness and, consequently, on cognitive outcomes.
The limitations of this systematic review are: (1) the methods and tools used to measure cognitive function varied across the included studies, (2) heterogeneity in outcome measurements was found among the included studies, (3) cause-effect could not be inferred from the cross‐sectional analyses, (4) population sizes from different continents varied across studies, 5) multiple studies were included several times, however, they were conducted at different time points with different population sizes and addressed some different research questions.
Conclusion
Based on this systematic review, it was established that there is a negative association between arterial stiffness and cognitive function among older adults with hypertension. The future direction suggests that early screening of PWV could play a crucial role as a significant clinical biomarker for middle-aged individuals with hypertension and older asymptomatic individuals at high vascular risk for cognitive decline and stroke. It is imperative to implement interventions aimed at reducing and preventing cerebrovascular events in the aging population. This proactive strategy could significantly contribute to improving the overall brain health of at-risk individuals.
Registration and protocol
The protocol for the systematic review was registered on the NIHR PROSPERO.
Registry ID: CRD42022379887.
A systematic literature review protocol was provided based on the Cochrane Handbook for Systematic Reviews of Interventions, 2022 [122].
The systematic review was based on the PRISMA 2020 statement: An updated guideline for reporting systematic reviews [23].
Acknowledgements
We would like to thank John Reynolds, MLIS, Jorge E. Perez, MLIS, and Thilani Samarakoon, PhD, MSIS of the Louis Calder Memorial Library at the University of Miami Miller School of Medicine for consulting on the search strategy and review methodology, and Roni Klass, PhD, at the University of Miami Writing Center.
Abbreviations
| CINAHL | Cumulated index to nursing and allied health literature |
| NIHR PROSPERO | National Institute for Health Research International Prospective Register of Systematic Review |
| PRISMA | Preferred Reported Items for Systematic Reviews and Meta–Analyses |
Authors’ contributions
BA collected, synthesized, and analyzed studies. TA worked on the flowchart and tables. SM assisted in the review and writing. TR guided and assessed the process of reviewing and writing. All authors read and approved the final manuscript.
Funding
Financial support received from the Evelyn F. McKnight Brain Institute, University of Miami.
Authors’ contributions: BA collected, synthesized, and analyzed studies. TA worked on the flowchart and tables. SM assisted in the review and writing. TR guided and assessed the process of reviewing and writing. All authors read and approved the final manuscript.
Data availability
All data generated during this review are included in this published manuscript. The NIHR PROSPERO protocol is available at https://www.crd.york.ac.uk/PROSPERO/.
Declarations
Not applicable.
Not applicable.
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
 –
– 85. [Abstract]
85. [Abstract]Articles from BMC Neurology are provided here courtesy of BMC
Citations & impact
This article has not been cited yet.
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/169763962
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Aortic-Brachial Pulse Wave Velocity Ratio: A Blood Pressure-Independent Index of Vascular Aging.
Hypertension, 69(1):96-101, 07 Nov 2016
Cited by: 27 articles | PMID: 27821616
Roles of Arterial Stiffness and Blood Pressure in Hypertension-Associated Cognitive Decline in Healthy Adults.
Hypertension, 67(1):171-175, 02 Nov 2015
Cited by: 64 articles | PMID: 26527049 | PMCID: PMC4715367
Aortic Stiffness, Ambulatory Blood Pressure, and Predictors of Response to Antihypertensive Therapy in Hemodialysis.
Am J Kidney Dis, 66(2):305-312, 25 Mar 2015
Cited by: 14 articles | PMID: 25818679
The Association between Inflammation and Pulse Wave Velocity in Dyslipidemia: An Evidence-Based Review.
Mediators Inflamm, 2020:4732987, 18 Aug 2020
Cited by: 13 articles | PMID: 32908450 | PMCID: PMC7450307
Review Free full text in Europe PMC
Funding
Funders who supported this work.