Abstract
Purpose
The number of elderly patients with trauma is increasing; therefore, precise models are necessary to estimate the mortality risk of elderly patients with trauma for informed clinical decision-making. This study aimed to develop machine learning based predictive models that predict 30-day mortality in severely injured elderly patients with trauma and to compare the predictive performance of various machine learning models.Methods
This study targeted patients aged ≥65 years with an Injury Severity Score of ≥15 who visited the regional trauma center at Chungbuk National University Hospital between 2016 and 2022. Four machine learning models-logistic regression, decision tree, random forest, and eXtreme Gradient Boosting (XGBoost)-were developed to predict 30-day mortality. The models' performance was compared using metrics such as area under the receiver operating characteristic curve (AUC), accuracy, precision, recall, specificity, F1 score, as well as Shapley Additive Explanations (SHAP) values and learning curves.Results
The performance evaluation of the machine learning models for predicting mortality in severely injured elderly patients with trauma showed AUC values for logistic regression, decision tree, random forest, and XGBoost of 0.938, 0.863, 0.919, and 0.934, respectively. Among the four models, XGBoost demonstrated superior accuracy, precision, recall, specificity, and F1 score of 0.91, 0.72, 0.86, 0.92, and 0.78, respectively. Analysis of important features of XGBoost using SHAP revealed associations such as a high Glasgow Coma Scale negatively impacting mortality probability, while higher counts of transfused red blood cells were positively correlated with mortality probability. The learning curves indicated increased generalization and robustness as training examples increased.Conclusions
We showed that machine learning models, especially XGBoost, can be used to predict 30-day mortality in severely injured elderly patients with trauma. Prognostic tools utilizing these models are helpful for physicians to evaluate the risk of mortality in elderly patients with severe trauma.Free full text

Predicting 30-day mortality in severely injured elderly patients with trauma in Korea using machine learning algorithms: a retrospective study
Abstract
Purpose
The number of elderly patients with trauma is increasing; therefore, precise models are necessary to estimate the mortality risk of elderly patients with trauma for informed clinical decision-making. This study aimed to develop machine learning based predictive models that predict 30-day mortality in severely injured elderly patients with trauma and to compare the predictive performance of various machine learning models.
Methods
This study targeted patients aged ≥65 years with an Injury Severity Score of ≥15 who visited the regional trauma center at Chungbuk National University Hospital between 2016 and 2022. Four machine learning models—logistic regression, decision tree, random forest, and eXtreme Gradient Boosting (XGBoost)—were developed to predict 30-day mortality. The models’ performance was compared using metrics such as area under the receiver operating characteristic curve (AUC), accuracy, precision, recall, specificity, F1 score, as well as Shapley Additive Explanations (SHAP) values and learning curves.
Results
The performance evaluation of the machine learning models for predicting mortality in severely injured elderly patients with trauma showed AUC values for logistic regression, decision tree, random forest, and XGBoost of 0.938, 0.863, 0.919, and 0.934, respectively. Among the four models, XGBoost demonstrated superior accuracy, precision, recall, specificity, and F1 score of 0.91, 0.72, 0.86, 0.92, and 0.78, respectively. Analysis of important features of XGBoost using SHAP revealed associations such as a high Glasgow Coma Scale negatively impacting mortality probability, while higher counts of transfused red blood cells were positively correlated with mortality probability. The learning curves indicated increased generalization and robustness as training examples increased.
Conclusions
We showed that machine learning models, especially XGBoost, can be used to predict 30-day mortality in severely injured elderly patients with trauma. Prognostic tools utilizing these models are helpful for physicians to evaluate the risk of mortality in elderly patients with severe trauma.
INTRODUCTION
Background
Trauma has been viewed as a common cause of disability and death in patients [1,2]. Approximately 500,000 elderly patients are admitted to trauma centers after injury annually in the United States [3]. In Korea, there are as many as 200,000 severe trauma patients annually, about 40% of whom aged ≥60 years. Recently, the proportion of elderly patients with trauma has been steadily increasing [4]. The care and management of critically ill elderly patients with severe trauma poses unique challenges for healthcare professionals, necessitating innovative approaches to improve outcomes and enhance patient care [5].
Many trauma scoring systems and predictors have been developed, such as the Injury Severity Score (ISS), Revised Trauma Score, Trauma and Injury Severity Score, and Geriatric Trauma Outcome Score (GTOS) [3,6,7]. The above scores provide clinical importance, but require the assumption of linear relationships between factors. However, in many cases, relationships exist beyond simple linear relationships, and multicollinearity problems often exist.
Machine learning (ML) learns models from past data to predict future data [8]. ML models show better predictive power when multiple variables show complicated and nonlinear relationships [9–13]. ML methods can extract various important factors that influence the prognosis of patients [14]. Using such technology can help improve the speed and accuracy of medical decisions, particularly in critically ill patients where swift decision-making is vital [15]. Many ML models, such as support vector machines, decision trees, logistic regression, random forests, eXtreme Gradient Boosting (XGBoost), and others, have been developed in various medical fields. In several studies, ML models have demonstrated high predictive accuracy in critically ill trauma patients [16–18].
Objectives
In this study, we developed a model to predict 30-day mortality in severely injured elderly patients with trauma, evaluated its performance, and analyzed risk factors.
METHODS
Ethics statement
This study was approved by the Institutional Review Board of Chungbuk National University Hospital (No. 2024-01-034-001). The requirement for informed consent was waived due to the retrospective nature of the study.
Study population
This retrospective study was conducted at a single level I trauma center at Chungbuk National University Hospital (Cheongju, Korea) between January 2016 and December 2022. Data from the medical records of patients admitted to this hospital and registered in the Korean Trauma Database were collected and analyzed.
The inclusion criteria were patients aged ≥65 years, admitted to traumatic intensive care unit with ISS ≥15. Those who were hopelessly discharged, died at the time of arrival, did not survive after cardiopulmonary resuscitation, died after 30 days after injury, or died unrelated to trauma were excluded from the study.
Study design
The patients were divided into two groups: survivor and nonsurvivor groups. We compared baseline characteristics, vital signs, and mental status upon arrival at the emergency room, Abbreviated Injury Score (AIS), ISS, Glasgow Coma Scale (GCS), need for emergency surgery, transfusion of red blood cells (RBCs) within 24 hours, number of transfused RBCs, and type of accident.
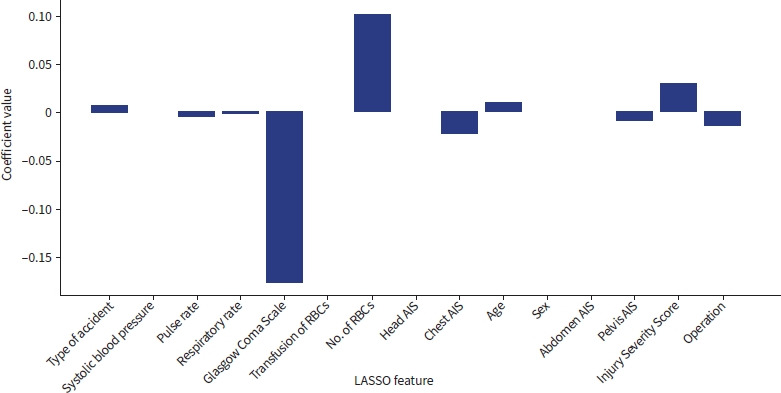
Finally, we selected 10 potential risk factors after the univariate analysis through least absolute shrinkage and selection operator (LASSO) regression [19]. We selected risk factors with nonzero LASSO coefficient as a feature (Fig. 1). The 10 risk factors were age, pulse rate, GCS score, respiratory rate, need for surgery, chest AIS, pelvis AIS, ISS, number of transfused RBCs, and type of accident. Potential risk factors were used as the same features to compare the models equally.
ML modeling establishment
The original dataset was divided into training (80%) and testing (20%). Four different ML models (logistic regression, decision tree, random forest, and XGBoost) were used to create and test the models for predicting mortality in elderly patients with severe trauma. Each model was trained on the training set and assessed using the test set. To obtain the best prediction model, the optimal hyperparameters of the models were selected and tuned using a grid search method with a 10-fold cross-validation procedure. The 10-fold cross-validation was used to evaluate the models by dividing the dataset into 10 subsamples, training and testing the models 10 times, and providing a more reliable estimate of their generalization performance [11,16]. The best estimator model was evaluated using the test set.
Model performance and comparison
The model was evaluated using a confusion matrix, the area under the receiver operating characteristic curve (AUC), accuracy, precision, recall (sensitivity), specificity, and F1 score. Shapley Additive Explanations (SHAP) is a popular technique in the field of ML for explaining the output of a model. This provided a way to understand the contribution of each feature to the prediction of the model [20]. To demonstrate the importance of features, we use the SHAP method. We used a learning curve to represent of the model’s performance on a task as a function of the amount of training data or training iterations [21,22].
Statistical analysis
Statistical analyses were performed using Python ver. 3.10.5 (Python Software Foundation) and R ver. 4.2.3 (R Foundation for Statistical Computing). For categorical variables, we used the chi-square test or Fisher exact test to determine significance. For continuous variables, we used the t-test or Mann-Whitney U-test. A P-value of <0.05 was considered statistically significant. ML models were constructed using Python and several Python modules (pandas, numpy, sklearn, xgboost, matplotlib, and shap).
RESULTS
Clinical characteristics of the study population
A total of 555 patients were included in the study. Of the 555 patients, 430 (77.5%) survived, and 125 (22.5%) died. The differences in baseline characteristics between survivors and nonsurvivors are shown in Table 1. The nonsurvivor group was significantly older than the survivor group (79 years vs. 75 years, P=0.021). The GCS score was significantly lower in the nonsurvivor group than in the survivor group (6 vs. 14, P<0.001). The nonsurvivor group had a greater proportion of patients with hypotension (20.8% vs. 11.4%, P=0.011), need for RBC transfusion (66.4% vs. 44.7%, P<0.001), and number of transfused RBCs (5 units vs. 3 units, P<0.001) than the survivor group. ISS (26 vs. 22, P<0.001) and GTOS (162 vs. 141, P<0.001) were also higher in the nonsurvivor group than in the survivor group. The number of patients exhibiting higher head and chest AIS scores was significantly higher in the nonsurvivor group (Table 2).
Table 1.
Basic characteristics of the study population
| Characteristic | Total (n=555) | Survivor (n=430) | Nonsurvivor (n=125) | P-value |
|---|---|---|---|---|
| Age (yr) | 75 (70–81) | 75 (70–80) | 79 (71–82) | 0.021 |
| Male sex | 357 (64.3) | 273 (63.5) | 84 (67.2) | 0.499 |
| Injury mechanism | 0.095 | |||
 Pedestrian TA Pedestrian TA | 124 (22.3) | 89 (20.7) | 35 (28.0) | |
 Bicycle TA Bicycle TA | 35 (6.3) | 24 (5.6) | 11 (8.8) | |
 Motorcycle TA Motorcycle TA | 85 (15.3) | 63 (16.8) | 22 (17.6) | |
 Car TA Car TA | 75 (13.5) | 67 (15.6) | 8 (6.4) | |
 Slip down Slip down | 84 (15.1) | 63 (14.7) | 21 (14.7) | |
 Fall from height Fall from height | 88 (15.9) | 74 (17.2) | 14 (11.2) | |
 Stuck by object Stuck by object | 25 (4.5) | 20 (4.7) | 5 (4.0) | |
 Other Other | 39 (7.0) | 30 (6.9) | 9 (7.2) | |
| Pulse rate (beats/min) | 85 (72–101) | 84 (72–99) | 92 (78–112) | 0.004 |
| Respiratory rate (breaths/min) | 20 (20–24) | 20 (20–24) | 22 (18–24) | 0.913 |
| Systolic blood pressure (mmHg) | 130 (102–158) | 130 (104–154) | 134 (100–170) | 0.463 |
| Glasgow Coma Scale | 14 (8–15) | 14 (11–15) | 6 (3–10) | <0.001 |
| Hypotension | 75 (13.5) | 49 (11.4) | 26 (20.8) | 0.011 |
| RBC transfusion | 275 (49.5) | 192 (44.7) | 83 (66.4) | <0.001 |
| No. of transfused RBCs (U) | 4 (2–6) | 3 (2–5) | 5 (4–11) | <0.001 |
| Injury Severity Score | 25 (18–27) | 22 (17–26) | 26 (25–35) | <0.001 |
| Trauma and Injury Severity Score | 0.881 (0.500–0.881) | 0.881 (0.731–0.953) | 0.500 (0.269–0.731) | <0.001 |
| Geriatric Trauma Outcome Score | 144 (129–164) | 141 (125–158) | 162 (144–186) | <0.001 |
| Emergency operation | 187 (33.7) | 139 (32.3) | 48 (38.4) | 0.247 |
Values are presented as median (interquartile range) or number (%). Percentages may not total 100 due to rounding.
TA, traffic accident; RBC, red blood cell.
Table 2.
Comparison of AIS of patients who survived or not
| AIS | No. of patients (%) | P-value | ||
|---|---|---|---|---|
| Total (n=555) | Survivor (n=430) | Nonsurvivor (n=125) | ||
| Head | <0.001 | |||
 0 0 | 142 (25.6) | 130 (30.2) | 12 (9.6) | |
 1 1 | 0 | 0 | 0 | |
 2 2 | 36 (6.5) | 31 (7.2) | 5 (4.0) | |
 3 3 | 89 (16.0) | 76 (17.7) | 13 (10.4) | |
 4 4 | 118 (21.3) | 98 (22.8) | 20 (16.0) | |
 5 5 | 170 (30.6) | 95 (22.1) | 75 (60.0) | |
 6 6 | 0 | 0 | 0 | |
| Chest | 0.006 | |||
 0 0 | 258 (46.5) | 186 (43.3) | 72 (57.6) | |
 1 1 | 7 (1.3) | 4 (0.9) | 3 (2.4) | |
 2 2 | 32 (5.8) | 25 (5.8) | 7 (5.6) | |
 3 3 | 209 (37.7) | 176 (40.9) | 33 (26.4) | |
 4 4 | 41 (7.4) | 35 (8.1) | 6 (4.8) | |
 5 5 | 7 (1.3) | 4 (0.9) | 3 (2.4) | |
 6 6 | 1 (0.2) | 0 | 1 (0.8) | |
| Abdomen | 0.297 | |||
 0 0 | 374 (67.4) | 285 (66.3) | 89 (71.2) | |
 1 1 | 0 | 0 | 0 | |
 2 2 | 91 (16.4) | 74 (17.2) | 17 (13.6) | |
 3 3 | 62 (11.2) | 52 (12.1) | 10 (8.0) | |
 4 4 | 24 (4.3) | 17 (4.0) | 7 (5.6) | |
 5 5 | 4 (0.7) | 2 (0.5) | 2 (1.6) | |
 6 6 | 0 | 0 | 0 | |
| Pelvis | 0.081 | |||
 0 0 | 308 (55.5) | 231 (53.7) | 77 (61.6) | |
 1 1 | 4 (0.7) | 2 (0.5) | 2 (1.6) | |
 2 2 | 121 (21.8) | 97 (22.6) | 24 (19.2) | |
 3 3 | 65 (11.7) | 57 (13.3) | 8 (6.4) | |
 4 4 | 38 (6.8) | 31 (7.2) | 7 (5.6) | |
 5 5 | 19 (3.4) | 12 (2.8) | 7 (5.6) | |
 6 6 | 0 | 0 | 0 | |
Percentages may not total 100 due to rounding.
AIS, Abbreviated Injury Scale.
Performance evaluation of the models
The accuracy, precision, recall, specificity, F1 score, and AUC of the ML models for predicting mortality in severely injured elderly patients with trauma were validated using the test dataset. In the test-set analysis, the AUCs of the logistic regression, decision tree, random forest, and XGBoost models were 0.938, 0.863, 0.919, and 0.934, respectively (Fig. 2). The AUC was slightly higher in the logistic regression than in XGBoost; however, XGBoost exhibited better results in terms of accuracy, precision, recall, specificity, and F1 score (0.91, 0.72, 0.86, 0.92, and 0.78, respectively) (Table 3).
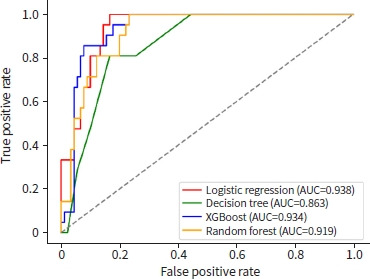
Receiver operating characteristic curves of various machine learning models. AUC, area under the receiver operating curve; XGBoost, eXtreme Gradient Boosting.
Table 3.
Model performances of the test dataset
| Prediction model | Accuracy | Precision | Recall | Specificity | F1 score | AUC |
|---|---|---|---|---|---|---|
| Logistic regression | 0.86 | 0.58 | 0.86 | 0.86 | 0.69 | 0.938 |
| Decision tree | 0.83 | 0.53 | 0.81 | 0.83 | 0.64 | 0.863 |
| Random forest | 0.86 | 0.61 | 0.81 | 0.88 | 0.69 | 0.919 |
| XGBoost | 0.91 | 0.72 | 0.86 | 0.92 | 0.78 | 0.934 |
AUC, area under the receiver operating curve; XGBoost, eXtreme Gradient Boosting.
To analyze the impact of important features on the model, a SHAP summary figure was used to show how features affected the probability of mortality. The colors and locations of the points indicate whether a certain feature has a higher or lower value than the other features. Using the XGBoost model as an example, a high GCS score is negatively associated with the probability of mortality, whereas a high number of transfused RBCs positively correlates with the probability of mortality. Lower GCS score, higher number of transfused RBCs, higher ISS, and old age positively impacted on 30-day mortality prediction (Fig. 3).

Shapley Additive Explanations (SHAP) value of the eXtreme Gradient Boosting (XGBoost) model output. RBC, red blood cell; AIS, Abbreviated Injury Severity.
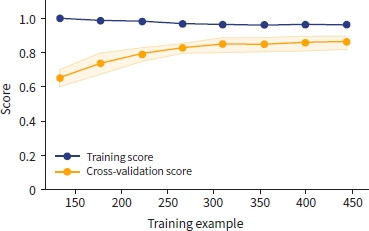
The learning curve of XGBoost showed that the training score decreased, and the validation score increased as the number of training examples increased (Fig. 4). With repeated training and validation sets, the difference between the training and validation sets decreased, indicating that the model became more generalized and robust.
DISCUSSION
Predicting a patient's prognosis with insufficient patient information in an emergency plays a vital role in patient evaluation and the utilization of limited medical resources in trauma. However, owing to information and time limitations in emergencies, emergency physicians and trauma surgeons are often unable to obtain all features for predicting mortality. Traditional trauma scoring systems, although clinically important, often assume linear relationships between explanatory variables. ML models, which use robust mathematical methodologies, exhibit better predictive capabilities, especially for handling complicated, nonlinear, and covariate factors. This provides an opportunity in the field of trauma to improve predictive accuracy.
This study focused on the application of ML models in predicting 30-day mortality in severely injured elderly patients with trauma, presenting an innovative approach for handling complex multiple variables. Mortality prediction models for elderly trauma patients with ISS ≥15 were established based on four ML algorithms.
AUC is a metric used to evaluate the performance of a ML model in binary classification tasks, such as predicting mortality. An AUC value over 0.9 is generally considered indicative of excellent discriminatory ability in a predictive model [23]. Three of the four models (logistic regression, random forest, and XGBoost) showed outstanding predictive performance in our test dataset. AUC is a valuable metric for assessing the overall performance of a predictive model; however, it has some limitations. These limitations include a lack of interpretability of specific contributors, inability to identify feature importance, and limited insight into the prediction impact. Various metrics can be used to evaluate binary classification models. Using only a subset can provide a false impression of a model's actual performance, leading to unexpected results when applied in clinical settings. Therefore, it is crucial to interpret the performance holistically by combining multiple metrics [24]. Therefore, we used the accuracy, precision, recall, specificity, and F1 score with the AUC to evaluate the models. The F1 score is a metric commonly used in binary classification problems, such as evaluating the performance of a ML model. The F1 score is the harmonic mean of precision and recall, and provide a balanced measure of a model's accuracy, particularly when dealing with imbalanced datasets [25,26].
In our study, XGBoost exhibited the best performance in terms of accuracy, precision, recall, specificity, and F1 score. The F1 score of XGBoost was 0.78, the highest among the four models. An F1 score of 0.78 generally indicate a model is performing well, striking a balance between precision and recall. In the field of medicine, datasets often exhibit an imbalance, where certain classes or outcomes are underrepresented compared to others [27]. Gradient boosting, including algorithms such as XGBoost, can be strong for imbalanced datasets, even without explicitly oversampling the minority class. Gradient boosting inherently has mechanisms that make it suitable for handling imbalanced datasets [28].
We used the SHAP method to analyze the feature importance in the ML models. The SHAP method is crucial in ML for providing insightful explanations of model outputs by quantifying the contribution of each feature to predictions [29,30]. In this study, GCS was the most important feature, followed by the number of transfused RBCs within 24 hours, ISS, pulse rate, and age in the XGBoost model.
Learning curves provide valuable insights into the performance of ML models during training by visualizing how a model's performance changes with the amount of training data or iterations [22]. Our results demonstrated a tendency toward generalization as the number of iterations increased across all models.
Limitations
Our study has some limitations. First, it was a retrospective study. Our cohort was specific to elderly patients. Therefore, there may have been selection bias. Second, the sample size was relatively small, and there may be problems such as overfitting, requiring additional data and external validation. Lastly, the unbalanced nature of the dataset may affect model generalization and reliability.
Conclusions
This study demonstrated that ML models, particularly XGBoost, perform well in predicting 30-day mortality among severely injured elderly trauma patients. ML models for mortality prediction can aid in the early identification of mortality risk factors and facilitate early intervention, potentially reducing mortality rates. Further research with larger datasets and external validation is recommended to enhance the robustness and generalizability of these models.
Footnotes
Author contributions
Conceptualization: JH, SYY; Data curation: JH, JS, JYL, JSL, JBY, YS, SHK, HRK; Formal analysis: JH; Methodology: JH; Project administration: YS; Supervision: SYY; Writing–original draft: JH; Writing–review & editing: all authors. All authors read and approved the final manuscript.
Conflicts of interest
The authors have no conflicts of interest to declare.
Funding
The authors received no financial support for this study.
Data availability
Data analyzed in this study are available from the corresponding author and the first author upon reasonable request.
REFERENCES
Articles from Journal of Trauma and Injury are provided here courtesy of Korean Society of Traumatology
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Machine learning models predict triage levels, massive transfusion protocol activation, and mortality in trauma utilizing patients hemodynamics on admission.
Comput Biol Med, 179:108880, 16 Jul 2024
Cited by: 1 article | PMID: 39018880
Predictive model and risk analysis for peripheral vascular disease in type 2 diabetes mellitus patients using machine learning and shapley additive explanation.
Front Endocrinol (Lausanne), 15:1320335, 28 Feb 2024
Cited by: 1 article | PMID: 38481447 | PMCID: PMC10933094
Development of interpretable machine learning models to predict in-hospital prognosis of acute heart failure patients.
ESC Heart Fail, 11(5):2798-2812, 15 May 2024
Cited by: 1 article | PMID: 38751135 | PMCID: PMC11424291
Interpretable machine learning model for early prediction of 28-day mortality in ICU patients with sepsis-induced coagulopathy: development and validation.
Eur J Med Res, 29(1):14, 03 Jan 2024
Cited by: 2 articles | PMID: 38172962 | PMCID: PMC10763177