Abstract
Free full text

Stepwise release of Activin-A from its inhibitory prodomain is modulated by cysteines and requires furin coexpression to promote melanoma growth
Abstract
The Activin-A precursor dimer can be cleaved by furin, but how this proteolytic maturation is regulated in vivo and how it facilitates access to signaling receptors is unclear. Here, analysis in a syngeneic melanoma grafting model shows that without furin coexpression, Activin-A failed to accelerate tumor growth, correlating with failure of one or both subunits to undergo cleavage in signal-sending cells, even though compensatory processing by host cells nonetheless sustained elevated circulating Activin-A levels. In reporter assays, furin-independent cleavage of one subunit enabled juxtacrine Activin-A signaling, whereas completion of proteolytic maturation by coexpressed furin or by recipient cells stimulated contact-independent activity, crosstalk with BMP receptors, and signal inhibition by follistatin. Mechanistically, Activin-A processing was modulated by allosteric disulfide bonds flanking the furin site. Disruption of these disulfide linkages with the prodomain enabled Activin-A binding to cognate type II receptors independently of proteolytic maturation. Stepwise proteolytic maturation is a novel mechanism to control Activin-A protein interactions and signaling.
Introduction
The transforming growth factor-β (TGF-β) family member activin A (here referred to as Activin-A) is synthesized as a precursor dimer (preproactivin-A) of two inhibin βA chains encoded by the INHBA gene. Alternatively, a βA subunit can heterodimerize with βB or βC to form activin AB or activin AC1,2. Activin-A signaling is mediated by complexes of activin receptors (ActR)-IIA or -IIB with ActR-IB (also known as ALK4) which stimulate SMAD2/3 transcription factors and SMAD-independent signal transduction3. In addition, Activin-A can compete against bone morphogenetic protein-2 (BMP-2) and BMP-4 for the BMP type II receptor (BMPR-II), and against BMP-7 for ActR-I, to thereby dampen SMAD1/5/8 signaling4,5. Binding to cognate activin type II receptors can be blocked by inhibins that consist of heterodimers of β subunits with inhibin α encoded by INHA3. Alternatively, Activin-A signaling can be inhibited by the secreted antagonist follistatin (FST) which binds and masks type I and II receptor-binding epitopes6.
Elevated levels of circulating Activin-A in plasma can provoke a cancer-associated systemic muscle wasting syndrome known as cachexia, and they correlate with poor prognosis in multiple tumor types7–9. Alterations in Activin-A signaling also associate with fatal outcomes of SARS-2 coronavirus infection and contribute to reproductive decline, aging-related heart failure, autoimmune disorders, and allergy10,11. In many cancer types, Activin-A expression or perturbations in activin signaling can inhibit or promote several cancer hallmarks, depending on the context12. For example, treatment of melanocytes with Activin-A inhibits cell proliferation and induces apoptosis13,14, and a transgene expressing a ligand-independent mutant ALK4 in B16-F1 mouse melanoma grafts inhibited their tumor growth15. ALK4 signaling is also anti-proliferative in many epithelial cell types12. By contrast, in cancers evading growth inhibition, Activin-A is implicated in facilitating tumor progression by regulating cell de-differentiation, migration, and invasion, epithelial-to-mesenchymal transitions (EMT), or drug resistance16–21. Furthermore, analysis in mouse melanoma models revealed that paracrine Activin-A signaling in the tumor microenvironment (TME) promotes immune evasion and resistance to immunotherapies both in gain and loss of function models15,22,23. These findings are likely clinically relevant also in melanoma patients since elevated Activin-A transcription correlates with resistance to anti-PD1 immunotherapy 22, and increased Activin-A protein staining in cancer cells and in macrophages, and low levels of FST mRNA associate with poor prognosis15,24. In addition, blockade of endogenous Activin-A in human melanoma xenografts protects mice against muscle wasting15. Therefore, insights into how Activin-A matures from its precursor, and how this process is regulated to control pleiotropic signaling outputs are urgently needed.
22, and increased Activin-A protein staining in cancer cells and in macrophages, and low levels of FST mRNA associate with poor prognosis15,24. In addition, blockade of endogenous Activin-A in human melanoma xenografts protects mice against muscle wasting15. Therefore, insights into how Activin-A matures from its precursor, and how this process is regulated to control pleiotropic signaling outputs are urgently needed.
Following signal sequence cleavage in the endoplasmic reticulum (ER), proteolytic processing of proActivin-A during or after secretion releases a disulfide-linked homodimer of the C-terminal mature region from a non-covalent dimer of the N-terminal prodomain25–27. When expressed without the prodomain, Activin-A aggregates in the ER due to aberrant disulfide bond formation28. Specifically, three conserved intrachain disulfides in the mature region are essential for correct Activin-A folding and secretion, consistent with their role in forming the cystine knot structure characteristic of TGF-β family members29,30. In addition, Activin-A signaling requires an interchain disulfide bond of cysteine C390 in the mature region29. Endoproteolytic processing of proActivin-A occurs after arginine 310 at the distal end of a conserved penta-arginine motif. This motif matches the consensus recognition sequence [R/K-(X)n-R/K-R↓] of furin and of related basic residue-specific proprotein convertases (PC) of the subtilisin/kexin (PCSK) family in the secretory apparatus, with the arrow indicating the scissile bond, and n being equal to 1, 2, 4, or 631. Targeted inactivation of Furin in murine B16-F1 melanoma cells largely inhibited the maturation of transiently transfected Activin-A32. However, the same study showed that an Activin-A processing intermediate corresponding in size to a dimer of one cleaved and one uncleaved βA subunit formed even independently of furin. An analogous half-processed dimer is secreted alongside mature Activin-A by multiple cell lines where precursor processing has been characterized26,27. How this “hemicleavage” is regulated and whether it influences Activin-A binding to signaling receptors is unknown. A related open question is whether Activin-A relies on furin to promote tumor growth, or whether furin-independent processing or precursor cleavage in the TME might be sufficient for Activin-A to signal locally or in the circulation.
Furin is active in multiple subcellular compartments. After autocleavage in the ER, it cycles between the trans-Golgi network (TGN) and the cell surface via endosomes, where the acidic pH facilitates the dissociation from the inhibitory propeptide and the release of soluble furin from its trans-membrane and cytosolic domains31,33,34. Imaging in B16-F1 cells expressing a biosensor confirmed that endogenous furin activity was below detection in the ER, but present in the TGN, at the cell surface, and in endosomes32. PC7, the only furin-related activity in B16-F1 cells is dispensable for the furin-independent Activin-A hemicleavage32. Therefore, and since no other basic residue-specific PC activities appeared to be expressed in B16-F1 cells, furin-independent Activin-A hemicleavage likely relies on an unknown PC-like protease (PCLP).
Here, we asked if furin-independent cleavage of one βA subunit of Activin-A also occurs in vivo and whether it influences receptor binding and signaling. We show that without furin coexpression, Activin-A fails to accelerate tumor growth in syngeneic melanoma grafts, correlating with impaired maturation within melanoma cells, even though processing in the tumor microenvironment sustained high levels of mature Activin-A in the circulation. Furthermore, analysis in cultured cells shows that whereas hemicleaved Activin-A can initiate juxtacrine signaling, the completion of proteolytic processing by coexpressed furin in cis, or by co-cultured cells in trans is necessary to potentiate cell contact-independent signaling via cognate activin receptors and to bind to BMPR-II. Mechanistically, we show that the effect of furin on the bioavailability of Activin-A and on the reservoir of its precursor is modulated by allosteric disulfide bonds between the mature region and the prodomain that we found to be regulated by cysteines C314 and C322 near the furin recognition motif. Thus, besides validating furin expression as a potential therapeutic target in melanoma cells, our findings indicate that Activin-A maturation involves at least one additional protease and stepwise proteolytic processing that is modulated by the connectivity of disulfide bonds between the mature region and the prodomain.
Results
A PC-like protease (PCLP) activity partially cleaves proActivin-A independently of furin and PC7 at the multibasic S1 site
Endoproteolytic maturation of Activin-A and of a half-processed intermediate migrating with an apparent molecular weight of approximately 70 kDa can be inhibited by deleting a cluster of five conserved arginines (R306-R310) at the distal end of the N-terminal prodomain27 (Fig. S1A). To validate the importance of this processing site 1 (S1) for the maturation of Activin-A in melanoma cells, we substituted it by five alanines. The resulting mS1 mutant or wild-type INHBA (βA) were transfected into B16-F1 melanoma cells, or into CRISPR-edited Furin knockout (FurKO), or Furin and Pcsk7 double knockout (DKO) B16-F1 subclones. We chose B16-F1 cells because they do not secrete endogenous activin or TGF-β that could interfere with our assays22. As described previously, βA-transfected control B16-F1 cells secrete mature Activin-A (≥24
kDa can be inhibited by deleting a cluster of five conserved arginines (R306-R310) at the distal end of the N-terminal prodomain27 (Fig. S1A). To validate the importance of this processing site 1 (S1) for the maturation of Activin-A in melanoma cells, we substituted it by five alanines. The resulting mS1 mutant or wild-type INHBA (βA) were transfected into B16-F1 melanoma cells, or into CRISPR-edited Furin knockout (FurKO), or Furin and Pcsk7 double knockout (DKO) B16-F1 subclones. We chose B16-F1 cells because they do not secrete endogenous activin or TGF-β that could interfere with our assays22. As described previously, βA-transfected control B16-F1 cells secrete mature Activin-A (≥24 kDa) together with a 58
kDa) together with a 58 kDa form that were designated A30 and A60, respectively, to readily distinguish them from uncleaved 104
kDa form that were designated A30 and A60, respectively, to readily distinguish them from uncleaved 104 kDa proActivin-A (A110) and from a 66
kDa proActivin-A (A110) and from a 66 kDa processing intermediate (A70) in culture supernatants (SN) of βA-transfected FurKO and DKO cells32 (Fig. 1A). Deleting a myc epitope present in our βA construct between residues E313 and C314 did not suppress the furin-independent A70 formation (Fig. S1B), confirming published data that also the wild-type sequence is subject to hemicleavage27. By contrast, mutating the S1 site (mS1) stabilized A110, coinciding with the loss of A70 and of detectable furin- and PC7-independent A30 formation. A110 was also enriched at the expense of A70 in SNs of FurKO or DKO cells that were treated with a high dose (50
kDa processing intermediate (A70) in culture supernatants (SN) of βA-transfected FurKO and DKO cells32 (Fig. 1A). Deleting a myc epitope present in our βA construct between residues E313 and C314 did not suppress the furin-independent A70 formation (Fig. S1B), confirming published data that also the wild-type sequence is subject to hemicleavage27. By contrast, mutating the S1 site (mS1) stabilized A110, coinciding with the loss of A70 and of detectable furin- and PC7-independent A30 formation. A110 was also enriched at the expense of A70 in SNs of FurKO or DKO cells that were treated with a high dose (50 µM) of the membrane-permeable furin inhibitor decanoyl-RVKR-chloromethylketone (CMK). Since the RVKR peptide mimics multibasic recognition motifs35, this result confirms that PCLP cleaves proActivin-A at the S1 site. To verify that A70 formation is no transfection artifact, we stably transduced B16-F1, FurKO, and DKO cells with lentiviral INHBA. Three independent FurKO clones expressed the βA transgene at levels comparable to those in control B16F1-βA cells (Fig. S1C). DKO-βA cells incidentally expressed 2-fold higher βA mRNA levels. However, neither FurKO nor DKO cells transcribed any basic residue-specific PCs other than furin/Pcsk3 or PC7/Pcsk7, regardless of the βA transgene (Fig. S1D). βA expression or loss of furin and PC7 also did not significantly alter cell proliferation (Fig. S1E). However, in FurKO-βA and DKO-βA cell lines, A30 and A60 formation was largely inhibited, leading to the build-up of A70 alongside A110 (Fig. 1B). Importantly, A110 and A70 were also stabilized after FURIN knockdown in human melanoma cell lines expressing endogenous INHBA (Fig. 1C). Besides corroborating our previous observation that A30 and A60 formation in melanoma cells relies primarily on furin32, these results suggest that the S1 site can be partially cleaved independently of furin or related basic residue-specific PCs to generate the half-processed A70.
µM) of the membrane-permeable furin inhibitor decanoyl-RVKR-chloromethylketone (CMK). Since the RVKR peptide mimics multibasic recognition motifs35, this result confirms that PCLP cleaves proActivin-A at the S1 site. To verify that A70 formation is no transfection artifact, we stably transduced B16-F1, FurKO, and DKO cells with lentiviral INHBA. Three independent FurKO clones expressed the βA transgene at levels comparable to those in control B16F1-βA cells (Fig. S1C). DKO-βA cells incidentally expressed 2-fold higher βA mRNA levels. However, neither FurKO nor DKO cells transcribed any basic residue-specific PCs other than furin/Pcsk3 or PC7/Pcsk7, regardless of the βA transgene (Fig. S1D). βA expression or loss of furin and PC7 also did not significantly alter cell proliferation (Fig. S1E). However, in FurKO-βA and DKO-βA cell lines, A30 and A60 formation was largely inhibited, leading to the build-up of A70 alongside A110 (Fig. 1B). Importantly, A110 and A70 were also stabilized after FURIN knockdown in human melanoma cell lines expressing endogenous INHBA (Fig. 1C). Besides corroborating our previous observation that A30 and A60 formation in melanoma cells relies primarily on furin32, these results suggest that the S1 site can be partially cleaved independently of furin or related basic residue-specific PCs to generate the half-processed A70.
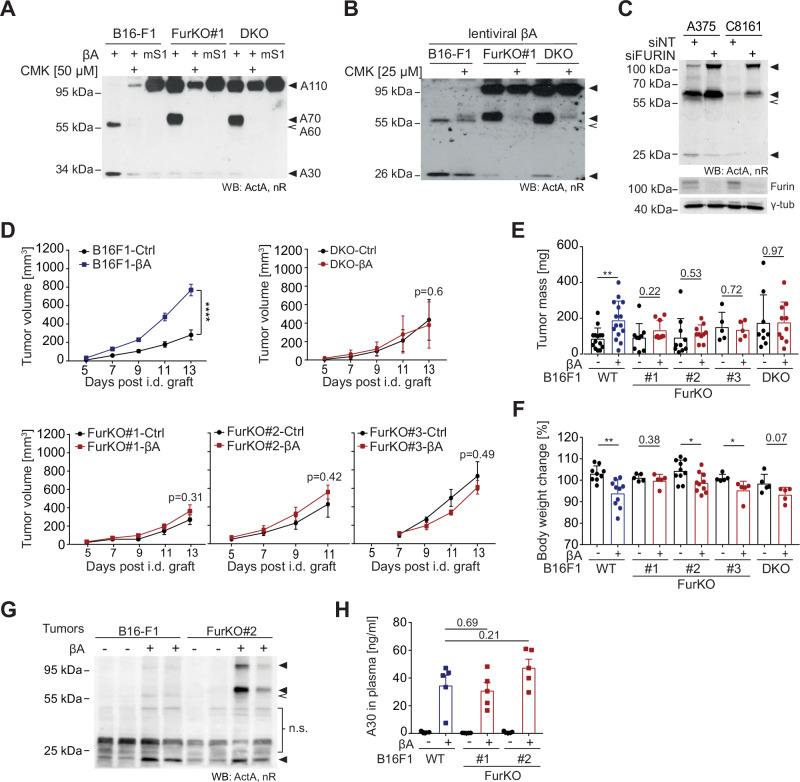
A Parental B16-F1 mouse melanoma cells and previously validated single or double knockout subclones lacking Furin (FurKO#1) or Furin and PC-7 (DKO)32 were transfected with INHBA (βΑ). Where indicated, the furin site 1 (S1) of βA was mutated from RRRRR to AAAAA to block its cleavage by PCs (mS1). After incubating the transfected cells for 72 h with or without the pan-PC inhibitor decanoyl-RVKR-chloromethylketone (CMK, 50
h with or without the pan-PC inhibitor decanoyl-RVKR-chloromethylketone (CMK, 50 µM), culture supernatants (SN) were analyzed by anti-Activin-A (ActA) Western blot (WB) under non-reducing (nR) conditions. B Western blot of B16F1-, FurKO#1- and DKO-βΑ cell SNs from cultures treated with CMK (25
µM), culture supernatants (SN) were analyzed by anti-Activin-A (ActA) Western blot (WB) under non-reducing (nR) conditions. B Western blot of B16F1-, FurKO#1- and DKO-βΑ cell SNs from cultures treated with CMK (25 μM) or empty vehicle for 48
μM) or empty vehicle for 48 h. C Western blot of human A375 and C8161 melanoma cell SNs 48
h. C Western blot of human A375 and C8161 melanoma cell SNs 48 h after transfection with non-targeted (nt) control or FURIN nt siRNAs. Reprobing with furin and γ-tubulin (tub) antibodies confirmed furin knockdown and equal loading. D Tumor growth curves of control- or βA-transduced B16F1, DKO, and FurKO melanoma grafts in C57BL/6J syngeneic mice. Comparison of (E) tumor masses and (F) changes in body weight of each tumor recipient analyzed in (D) at the endpoint (day 13 or, in the case of the fastest growing CRISPR clone FurKO#2, day 11) relative to its body weight at the time of grafting. Error bars, SEM (n
h after transfection with non-targeted (nt) control or FURIN nt siRNAs. Reprobing with furin and γ-tubulin (tub) antibodies confirmed furin knockdown and equal loading. D Tumor growth curves of control- or βA-transduced B16F1, DKO, and FurKO melanoma grafts in C57BL/6J syngeneic mice. Comparison of (E) tumor masses and (F) changes in body weight of each tumor recipient analyzed in (D) at the endpoint (day 13 or, in the case of the fastest growing CRISPR clone FurKO#2, day 11) relative to its body weight at the time of grafting. Error bars, SEM (n =
= 5–15 per group from 3 independent experiments, each dot represents one mouse); *p
5–15 per group from 3 independent experiments, each dot represents one mouse); *p <
< 0.05, **p
0.05, **p <
< 0.01, ***p
0.01, ***p <
< 0.001, ****p
0.001, ****p <
< 0.0001, Student’s t-test. G Anti-Activin-A Western blots of lysates of control or Furin KO (FurKO#2) B16F1 tumor grafts expressing βA (+) or empty control (−) lentivirus at the endpoint. H Quantification of mature Activin-A in tumor extracts by ELISA. Error bars, SEM (n
0.0001, Student’s t-test. G Anti-Activin-A Western blots of lysates of control or Furin KO (FurKO#2) B16F1 tumor grafts expressing βA (+) or empty control (−) lentivirus at the endpoint. H Quantification of mature Activin-A in tumor extracts by ELISA. Error bars, SEM (n =
= 5 per group); *p
5 per group); *p <
< 0.05, **p
0.05, **p <
< 0.01, ***p
0.01, ***p <
< 0.001, ****p
0.001, ****p <
< 0.0001, Student’s t-test.
0.0001, Student’s t-test.
Loss of furin in melanoma grafts impairs A110 and A70 cleavage and the associated tumor growth, yet without depleting circulating mature Activin-A
To assess the role of Activin-A processing and its regulation by furin in vivo, we analyzed syngeneic grafts of B16-F1, FurKO, or DKO melanoma cells expressing βA versus empty control vectors in C57BL/6J mice. Analysis of tumor growth curves and tumor weights at the endpoint revealed that compared to Ctrl, B16F1-βA grafts had a marked growth advantage and significantly diminished the body weights of the host (Fig. 1D–F), as described previously15,22. By contrast, in FurKO-βA and DKO-βA grafts lacking Furin, Activin-A failed to accelerate tumor growth compared to Ctrl groups. Importantly, a comparison of body weight of the hosts before tumor grafting and at the endpoint revealed that the systemic effect of βA expression was maintained nonetheless, as clearly seen in FurKO#2 and FurKO#3 tumors (Fig. 1F). In hosts of βA-expressing FurKO#1 and DKO tumors, a similar trend did not reach statistical significance. These two CRISPR clones form tumors more slowly than parental βA-expressing cells (Fig. 1D), despite comparable cell proliferation in vitro (Fig. S1E), suggesting they did not quickly enough form tumors of sufficient size for cachexia to manifest before reaching the endpoint.
To test if half-processed Activin-A forms in vivo, tumor lysates and plasma samples of tumor-bearing mice were analyzed by Activin-A Western blots. We found that B16F1-βA tumors contained mainly A30 together with A60. By contrast, FurKO-βA tumors lacked detectable A60 and instead accumulated A70 together with A110, confirming that no other PC substituted for furin within the tumor cells. Nevertheless, A30 formation persisted in the FurKO-βA tumors (Fig. 1G). Immunoblots of plasma, and an ELISA assay detecting specifically A30 revealed that the FurKO-βA tumors also still released circulating mature Activin-A but no half-processed or uncleaved precursor (Figs. 1H and S1F, G). Collectively, these results indicate that hosts of FurKO-βA tumors rescued both A30 formation and the associated loss of body weight. By contrast, to stimulate tumor growth, Activin-A depends on the coexpression of endogenous furin in cis that cleaves A110 and A70 within the tumor cells.
In the absence of cell contacts, half-processed Activin-A depends on further extracellular cleavage by the recipient cells to robustly activate SMAD3
Whereas furin is active both in intra- and extracellular compartments, the localization of PCLP activity is unknown. Therefore, to further characterize the furin-independent Activin-A cleavage and its role in melanoma, we first asked where it takes place. To address this, βA-expressing cells were treated with CMK, or with membrane-impermeable hexa-D-arginine (D6R) (Fig. 2A). While D6R had no effect even at the maximal concentration (200 µM), treatment with 13–25
µM), treatment with 13–25 µM CMK abolished the PCLP-mediated A70 formation in FurKO#1-βA cells, as well as A60 and A30 formation in B16F1-βA cells. CMK treatment also stabilized A110 in Furin wild-type cells, albeit only at elevated concentrations of 50–100
µM CMK abolished the PCLP-mediated A70 formation in FurKO#1-βA cells, as well as A60 and A30 formation in B16F1-βA cells. CMK treatment also stabilized A110 in Furin wild-type cells, albeit only at elevated concentrations of 50–100 µM, and incompletely compared to Furin-deleted cells. These data suggest that furin and PCLP activities in B16-F1 cells cleave Activin-A intracellularly.
µM, and incompletely compared to Furin-deleted cells. These data suggest that furin and PCLP activities in B16-F1 cells cleave Activin-A intracellularly.
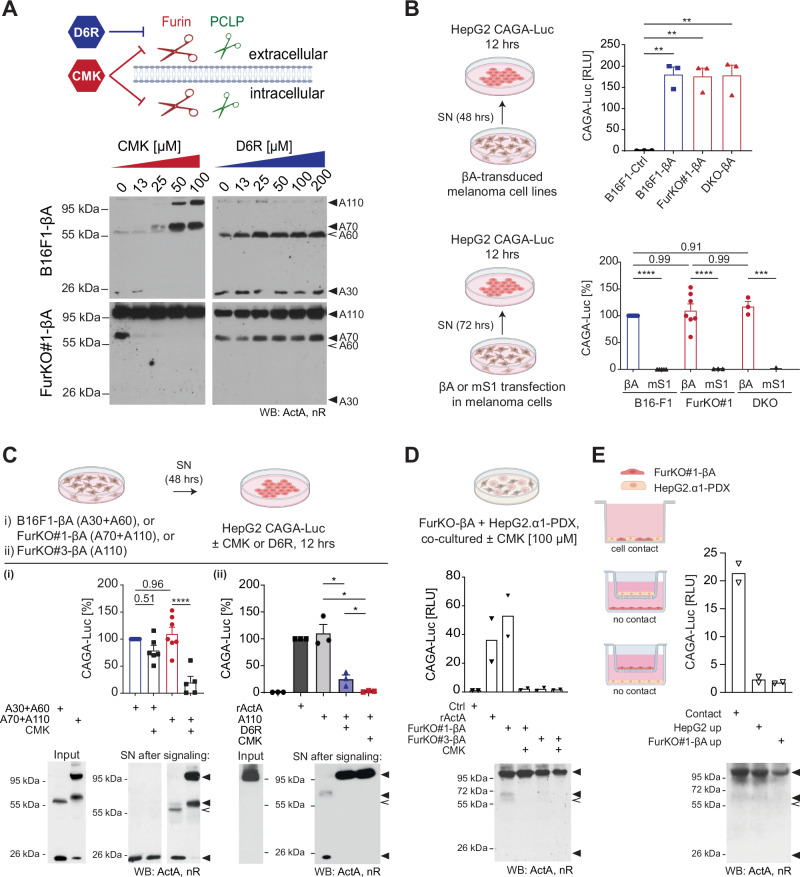
A Western blot of Activin-A in SNs of B16F1-βΑ (top) and FurKO#1-βΑ cells (bottom) that were treated with increasing doses of CMK or with the membrane-impermeable PC inhibitor D6R for 36 h. B Luciferase reporter assay to test if loss of furin-mediated cleavage within melanoma cells blocks Activin-A signaling. HepG2 reporter cells were incubated for 12
h. B Luciferase reporter assay to test if loss of furin-mediated cleavage within melanoma cells blocks Activin-A signaling. HepG2 reporter cells were incubated for 12 h with 10-fold diluted SNs of the B16-F1, FurKO#1, or DKO melanoma cell lines shown in Fig. 1A, B which express βA or control (ctrl) lentivirus (top), or which were transfected with wild-type or S1 site mutant (mS1) βA plasmids (bottom). Induction of the SMAD3 reporter CAGA-Luc by SNs of transiently transfected melanoma cells is shown as a percentage relative to the fold induction by SN of βA-transfected Furin wild-type control cells. Error bars, SEM (n
h with 10-fold diluted SNs of the B16-F1, FurKO#1, or DKO melanoma cell lines shown in Fig. 1A, B which express βA or control (ctrl) lentivirus (top), or which were transfected with wild-type or S1 site mutant (mS1) βA plasmids (bottom). Induction of the SMAD3 reporter CAGA-Luc by SNs of transiently transfected melanoma cells is shown as a percentage relative to the fold induction by SN of βA-transfected Furin wild-type control cells. Error bars, SEM (n =
= 3–7 experiments); **p
3–7 experiments); **p <
< 0.01, ordinary one-way ANOVA with Šídák’s multiple comparisons test. C Analysis of Activin-A precursor processing after 12
0.01, ordinary one-way ANOVA with Šídák’s multiple comparisons test. C Analysis of Activin-A precursor processing after 12 h incubation with HepG2 CAGA-Luc reporter cells with or without the furin inhibitors CMK (100
h incubation with HepG2 CAGA-Luc reporter cells with or without the furin inhibitors CMK (100 µM) or D6R (50
µM) or D6R (50 µM). SNs of the melanoma cell lines producing the indicated forms of Activin-A were each diluted 10-fold to not exceed the dynamic range of the assay. Induction of CAGA-Luc was normalized relative to (i) the fold induction by control SN (A30
µM). SNs of the melanoma cell lines producing the indicated forms of Activin-A were each diluted 10-fold to not exceed the dynamic range of the assay. Induction of CAGA-Luc was normalized relative to (i) the fold induction by control SN (A30 +
+ A60) of Furin wild-type B16F1-βA cells (left panel) or (ii) to the activity of 10
A60) of Furin wild-type B16F1-βA cells (left panel) or (ii) to the activity of 10 ng/ml recombinant Activin-A (right panel). Western blot analysis of SNs after their incubation on HepG2 is shown below. To treat HepG2 cells with only uncleaved A110, we used the SN of FurKO#3-βA cells as input, because A70 formation can be inhibited in this clone by culturing it at subconfluent cell density (see supplementary Fig. S2). Error bars, SEM (n
ng/ml recombinant Activin-A (right panel). Western blot analysis of SNs after their incubation on HepG2 is shown below. To treat HepG2 cells with only uncleaved A110, we used the SN of FurKO#3-βA cells as input, because A70 formation can be inhibited in this clone by culturing it at subconfluent cell density (see supplementary Fig. S2). Error bars, SEM (n =
= 5–7 independent experiments). *p
5–7 independent experiments). *p <
< 0.05, **p
0.05, **p <
< 0.01, ***p
0.01, ***p <
< 0.001, ****p
0.001, ****p <
< 0.0001 ordinary one-way ANOVA with Šídák’s multiple comparisons test. D Analysis of Activin-A processing and signaling after 12
0.0001 ordinary one-way ANOVA with Šídák’s multiple comparisons test. D Analysis of Activin-A processing and signaling after 12 h in cocultures of FurKO-βA cells with HepG2.α1-PDX CAGA-Luc reporter cells that express α1-PDX to inhibit Activin-A cleavage by the signal-receiving HepG2 cells. Where indicated, the cocultures were treated with 100
h in cocultures of FurKO-βA cells with HepG2.α1-PDX CAGA-Luc reporter cells that express α1-PDX to inhibit Activin-A cleavage by the signal-receiving HepG2 cells. Where indicated, the cocultures were treated with 100 µM CMK to also block PCLP activity in the signal-sending FurKO#1-βA melanoma cells. Treatment with 10
µM CMK to also block PCLP activity in the signal-sending FurKO#1-βA melanoma cells. Treatment with 10 ng/ml Activin-A was a control of CAGA-Luc induction. Lack of luciferase activation in control cocultures with FurKO#3-βA cells validated that α1-PDX blocks Activin-A cleavage in trans by HepG2 cells. Error bars, SEM (n
ng/ml Activin-A was a control of CAGA-Luc induction. Lack of luciferase activation in control cocultures with FurKO#3-βA cells validated that α1-PDX blocks Activin-A cleavage in trans by HepG2 cells. Error bars, SEM (n =
= 2 experiments). *p
2 experiments). *p <
< 0.05, Student’s t-test. E As in (D), but in transwell chambers where the FurKO#1-βA cells were co-cultured with the HepG2. α1PDX cells as indicated. Schemas were created using BioRender.com.
0.05, Student’s t-test. E As in (D), but in transwell chambers where the FurKO#1-βA cells were co-cultured with the HepG2. α1PDX cells as indicated. Schemas were created using BioRender.com.
To test the influence of furin-independent processing on Activin-A signaling, we incubated SNs from the βA-transduced FurKO#1-βA or DKO-βA cell lines or from Furin wild-type B16F1-βA cells with HepG2 cells carrying the SMAD3 luciferase reporter CAGA-Luc, together with CMV-Renilla for signal normalization36. Interestingly, SNs from all three cell lines induced CAGA-Luc 150- to 200-fold above baseline within 12 h (Fig. 2B, top). This response is well within the dynamic range of 20- to 400-fold induction by 5–50
h (Fig. 2B, top). This response is well within the dynamic range of 20- to 400-fold induction by 5–50 ng/ml recombinant Activin-A36. SNs from B16-F1 cells lacking Furin alone or both Furin and Pcsk7 also induced CAGA-Luc to a similar extent if βA was only transfected transiently (Fig. 2B, bottom). However, no CAGA-Luc was induced by SN of mS1-transfected cells, confirming that signaling required S1 site cleavage.
ng/ml recombinant Activin-A36. SNs from B16-F1 cells lacking Furin alone or both Furin and Pcsk7 also induced CAGA-Luc to a similar extent if βA was only transfected transiently (Fig. 2B, bottom). However, no CAGA-Luc was induced by SN of mS1-transfected cells, confirming that signaling required S1 site cleavage.
To estimate the contribution of cell non-autonomous Activin-A maturation, we added CMK to the signal-receiving HepG2 reporter cells (Fig. 2C). SNs of Furin wild-type B16F1-βA cells containing A30 and A60 induced CAGA-Luc regardless of the presence of a high dose of CMK (100 µM). By contrast, in reporter cells receiving FurKO#1-βA SNs, CMK administration blocked CAGA-Luc induction or, if traces of A30 still formed in input samples, severely diminished it (Fig. 2C(i), top panel). Western blots of the SNs after the incubation on HepG2 cells showed that CMK treatment stabilized both A110 and A70 (Fig. 2C(i), bottom panel). Collectively, these data suggest that if the melanoma cells lack furin coexpression in cis, signal-receiving cells complete Activin-A processing in trans.
µM). By contrast, in reporter cells receiving FurKO#1-βA SNs, CMK administration blocked CAGA-Luc induction or, if traces of A30 still formed in input samples, severely diminished it (Fig. 2C(i), top panel). Western blots of the SNs after the incubation on HepG2 cells showed that CMK treatment stabilized both A110 and A70 (Fig. 2C(i), bottom panel). Collectively, these data suggest that if the melanoma cells lack furin coexpression in cis, signal-receiving cells complete Activin-A processing in trans.
Signal-receiving cells may cleave proActivin-A extracellularly. To test this, we produced uncleaved A110 in FurKO#3-βA cells, a clone where A70 formation fortuitously can be suppressed by culturing the cells at subconfluent density (Fig. S2A, B). During control incubations at 37 °C without HepG2 reporter cells, A110 from subconfluent FurKO#3-βA cells as well as A70 and A110 from confluent FurKO#1-βA cells remained stable for at least 12 h even in the absence of CMK (Fig. S2C). By contrast, when incubated on HepG2 cells, A110 was converted to A70 and A30 and induced CAGA-Luc as strongly as did treatment with 10
h even in the absence of CMK (Fig. S2C). By contrast, when incubated on HepG2 cells, A110 was converted to A70 and A30 and induced CAGA-Luc as strongly as did treatment with 10 ng/ml Activin-A (Fig. 2C, panel (ii)). Co-treatment of the HepG2 cells with CMK or with the extracellular inhibitor D6R, respectively, blocked or severely inhibited this activity, correlating with the stabilization of A110. Thus, the signal-receiving HepG2 cells can activate even proActivin-A extracellularly.
ng/ml Activin-A (Fig. 2C, panel (ii)). Co-treatment of the HepG2 cells with CMK or with the extracellular inhibitor D6R, respectively, blocked or severely inhibited this activity, correlating with the stabilization of A110. Thus, the signal-receiving HepG2 cells can activate even proActivin-A extracellularly.
Contact of melanoma cells with co-cultured HepG2 reporter cells enables furin-independent signaling by half-processed Activin-A but not by its precursor
Furin is also expressed in HepG2 cells, together with PACE4 and PC737. To test if the signaling activity of half-processed Activin-A or its precursor can be blocked by inhibiting furin and/or related PCs only in the signal-receiving cells, we transduced HepG2 CAGA-Luc reporter cells with the antitrypsin variant α1-PDX38. To induce CAGA-Luc expression, the resulting HepG2.α1-PDX were then co-cultured in contact with FurKO#3-βA as a source of only A110, or with FurKO#1-βA cells which secrete also A70. Alternatively, as a positive control, they were treated with 10 ng/ml Activin-A (Fig. 2D). While the control treatment with Activin-A upregulated CAGA-Luc at least 20-fold, the A110 secreted by co-cultured FurKO#3-βA remained uncleaved and inactive, confirming that the presence of α1-PDX efficiently blocked Activin-A processing in trans. By contrast, in cocultures with FurKO#1-βA cells that secrete A70, the α1-PDX transgene failed to prevent CAGA-Luc induction. In this setting, treatment with CMK was essential to block signaling, correlating with the suppression of A70 formation by the melanoma cells. To test if this signaling activity required cell contacts, cocultures with HepG2.α1-PDX reporter cells were analyzed in transwell chambers (Fig. 2E). FurKO#1-βA melanoma cells again induced CAGA-Luc in HepG2.α1-PDX cells that were in the same compartment. By contrast, if the cells were co-cultured in separate chambers without contact, A70 still formed but failed to signal.
ng/ml Activin-A (Fig. 2D). While the control treatment with Activin-A upregulated CAGA-Luc at least 20-fold, the A110 secreted by co-cultured FurKO#3-βA remained uncleaved and inactive, confirming that the presence of α1-PDX efficiently blocked Activin-A processing in trans. By contrast, in cocultures with FurKO#1-βA cells that secrete A70, the α1-PDX transgene failed to prevent CAGA-Luc induction. In this setting, treatment with CMK was essential to block signaling, correlating with the suppression of A70 formation by the melanoma cells. To test if this signaling activity required cell contacts, cocultures with HepG2.α1-PDX reporter cells were analyzed in transwell chambers (Fig. 2E). FurKO#1-βA melanoma cells again induced CAGA-Luc in HepG2.α1-PDX cells that were in the same compartment. By contrast, if the cells were co-cultured in separate chambers without contact, A70 still formed but failed to signal.
The Activin-A processing intermediates A70 and A60 bind ActR-II despite covalent linkage to their prodomain
Precursor cleavage is essential for Activin-A to bind type I and II activin receptors39. To test if half-processing initiates binding at least to cognate type II receptors, we first injected comparable relative amounts of A110, A70, or A30 from B16F1-βA and FurKO-βA SNs into microfluidic chips that were precoated with ActRIIB-Fc to measure the reduction of plasmonic intensity resulting from ActRIIB-Fc binding (Fig. 3A)40,41. To estimate non-specific background, we injected B16F1-Ctrl or FurKO-Ctrl SNs, and SNs of B16F1-mS1 cells secreting uncleavable proActivin-A. Among FurKO clones, we arbitrarily chose clone #2 which was indistinguishable from clone #1 in vitro (Fig. 1C–E). As expected, SNs from Ctrl or mS1 expressing cells barely diminished the plasmonic intensity. By contrast, injection of B16F1-βA SN resulted in a robust signal intensity drop. A comparable drop in signal intensity ensued upon injection of FurKO#2-βA SN (Fig. 3B). These data suggest that ActRIIB-Fc binds both A30 and A70.
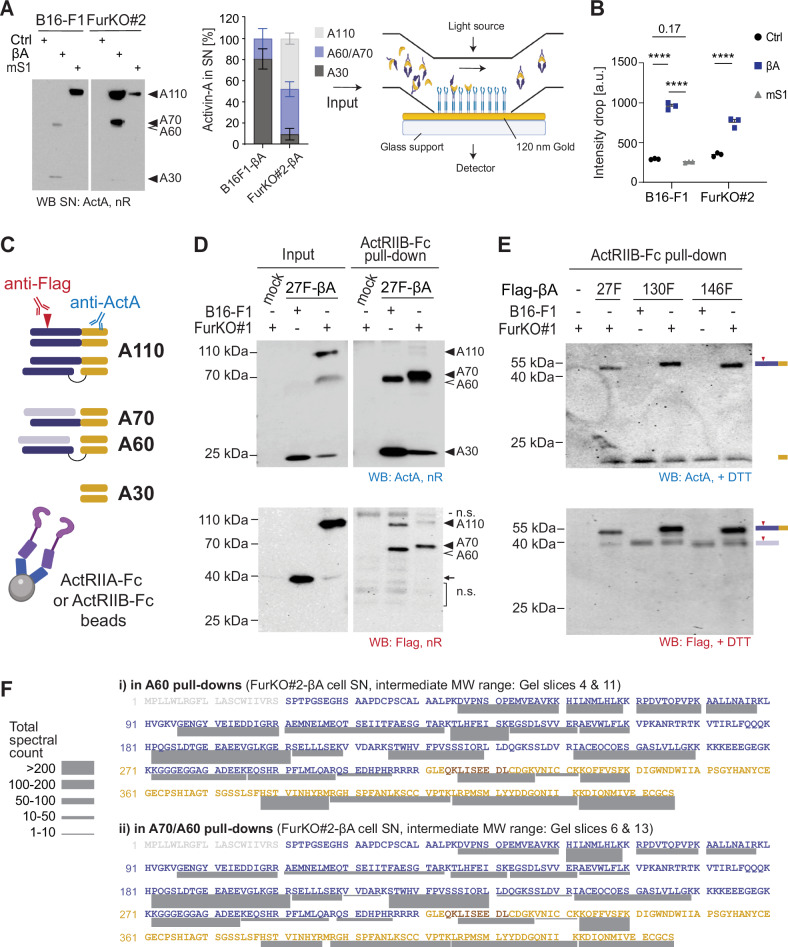
A Western blot of representative B16F1-mS1 and FurKO#2-ctrl or -βA cell SNs, and ImageJ quantification of the proportions of mature, hemicleaved, and uncleaved Activin-A in independent sample preparations from the indicated cell lines used as inputs to test Activin-A binding to immobilized ActRIIB-Fc by plasmon resonance measurements. The microfluidic chamber used to inject cell SNs on a nanoplasmonic chip functionalized with ActRIIB-Fc on gold nanoparticles is depicted on the right. Drops in signal intensity are induced by Activin-A binding to ActRIIB-Fc. B Binding of the different forms of Activin-A was evaluated by injecting the B16F1-βA or FurKO#2-βA cell SNs analyzed in (A). B16F1-mS1, or B16F1-Ctrl cells expressing empty lentivirus served as specificity controls. Error bars, SEM (n =
= 3 independent experiments); *p
3 independent experiments); *p <
< 0.05, **p
0.05, **p <
< 0.01, ***p
0.01, ***p <
< 0.001, ****p
0.001, ****p <
< 0.0001, Student’s t-test. C Schematic of the conversion of hemicleaved Activin-A (A70) to A60 by furin cleavage of the second βA subunit, and strategy to monitor the cleavage using anti-Flag and anti-Activin-A (ActA) antibodies. In this model, an allosteric cystine bond (S–S) links the prodomain (violet) of one βA subunit to the C-terminal region (yellow) in A70 and A60. Prodomain that is not covalently attached (light shading) can be displaced by cognate type II receptors. D Anti-Activin-A (top) or anti-Flag Western blots (bottom) of A70 in SN of FurKO#1 cells expressing Flag-tagged 27F-βA analyzed before (input) and after pull-down by AIIB-Fc. SN of B16-F1 cells, and of mock-transfected FurKO#1 cells served as controls. Arrow, cleaved prodomain; n.s. non-specific. E Anti-Activin-A (top) or anti-Flag (bottom) Western blot of the indicated Flag-tagged Activin-A constructs after pull-down as in (D), but on reducing gels. FurKO#1 and B16-F1 control SNs used to pull down Flag-tagged A70 and A60 encoded by 130F-βA or 146-βA were as in Fig. S3C. F LC–MS analysis and spectral counts of peptides mapping to Activin-A in samples collected in (E). Data represent two pull-down experiments, one using ActRIIA-Fc and the other using AIIB-Fc. Schemas were created using BioRender.com.
0.0001, Student’s t-test. C Schematic of the conversion of hemicleaved Activin-A (A70) to A60 by furin cleavage of the second βA subunit, and strategy to monitor the cleavage using anti-Flag and anti-Activin-A (ActA) antibodies. In this model, an allosteric cystine bond (S–S) links the prodomain (violet) of one βA subunit to the C-terminal region (yellow) in A70 and A60. Prodomain that is not covalently attached (light shading) can be displaced by cognate type II receptors. D Anti-Activin-A (top) or anti-Flag Western blots (bottom) of A70 in SN of FurKO#1 cells expressing Flag-tagged 27F-βA analyzed before (input) and after pull-down by AIIB-Fc. SN of B16-F1 cells, and of mock-transfected FurKO#1 cells served as controls. Arrow, cleaved prodomain; n.s. non-specific. E Anti-Activin-A (top) or anti-Flag (bottom) Western blot of the indicated Flag-tagged Activin-A constructs after pull-down as in (D), but on reducing gels. FurKO#1 and B16-F1 control SNs used to pull down Flag-tagged A70 and A60 encoded by 130F-βA or 146-βA were as in Fig. S3C. F LC–MS analysis and spectral counts of peptides mapping to Activin-A in samples collected in (E). Data represent two pull-down experiments, one using ActRIIA-Fc and the other using AIIB-Fc. Schemas were created using BioRender.com.
A70 and A60 may contain a prodomain that failed to be cleaved, or which remained covalently linked to the mature region via a disulfide bond (Fig. 3C). To distinguish between these scenarios, and to assess the influence of prodomain cleavage on ActR-II binding by an alternative approach, SNs of cells expressing Activin-A were incubated with ActRIIB-Fc on protein A/G beads, followed by Western blot analysis of bound protein (Fig. 3C). To monitor the prodomain, flexible regions after residues 27, 130, or 146 were tagged with a Flag epitope (Fig. S3A). All three constructs were processed as expected both in Furin WT and KO cells (Fig. S3B), and their cleaved prodomains (39 kDa) were detectable by anti-Flag antibody both on reducing and on non-reducing blots (Fig. S3C). However, the binding of anti-Flag antibody to reduced precursor monomers or to non-reduced A70 or A60 was too weak to detect them without prior enrichment. Therefore, we enriched the Flag-Activin-A construct 27F-βA by pull-down using ActRIIB-Fc beads. Non-reducing Western blot analysis of the bead eluates revealed A70 and A60, and that both reacted with the anti-Flag antibody, suggesting that they harbor the prodomain (Fig. 3D). By contrast, binding of ActRIIB-Fc to cleaved prodomain was close to background. Moreover, analysis on reducing gels after ActRIIB-Fc pull-down validated that A70 from all three Flag-tagged constructs contained the cleaved mature region and prodomain together with the uncleaved βA subunit (Fig. 3E, top panel). These results confirm that A70 corresponds to the previously described hemicleaved 70
kDa) were detectable by anti-Flag antibody both on reducing and on non-reducing blots (Fig. S3C). However, the binding of anti-Flag antibody to reduced precursor monomers or to non-reduced A70 or A60 was too weak to detect them without prior enrichment. Therefore, we enriched the Flag-Activin-A construct 27F-βA by pull-down using ActRIIB-Fc beads. Non-reducing Western blot analysis of the bead eluates revealed A70 and A60, and that both reacted with the anti-Flag antibody, suggesting that they harbor the prodomain (Fig. 3D). By contrast, binding of ActRIIB-Fc to cleaved prodomain was close to background. Moreover, analysis on reducing gels after ActRIIB-Fc pull-down validated that A70 from all three Flag-tagged constructs contained the cleaved mature region and prodomain together with the uncleaved βA subunit (Fig. 3E, top panel). These results confirm that A70 corresponds to the previously described hemicleaved 70 kDa processing intermediate of Activin-A27. By contrast, A60 from B16-F1 cell SN retained the prodomain exclusively in its cleaved form (Fig. 3E, bottom), presumably via a disulfide bond (Fig. 3C, and see below).
kDa processing intermediate of Activin-A27. By contrast, A60 from B16-F1 cell SN retained the prodomain exclusively in its cleaved form (Fig. 3E, bottom), presumably via a disulfide bond (Fig. 3C, and see below).
Since A70 and A60 differed in their electrophoretic mobility, we wondered if the A60 prodomain might be truncated. To address this, A70 from FurKO#2-βA SN, and A60 and A30 from B16F1-βA SN were individually excised from non-reducing gels after pull-down by ActRII-Fc fusion proteins and compared to gel-purified A110 from FurKO#2-βA SN using liquid chromatography-mass spectrometry (LC–MS) (Fig. S3D, E). A pull-down from FurKO#2-Ctrl SN served as negative control. To resolve the cystine knot structure of the mature region, immunoprecipitates had to be treated with DTT before tryptic digestion, precluding the detection of potential alternative disulfide bonds. Counting of spectra that match Activin-A revealed that after the first 25 residues, almost all segments of the prodomain were represented both in A70 and A60 samples, and with similar overall coverage as in A110 in two independent experiments using ActRIIA-Fc or ActRIIB-Fc, respectively (Fig. 3F, and Supplementary Table 1). By contrast, >99% of the peptides identified in A30 samples mapped to the mature region, and no Activin-A peptides were detected in the negative control. These results suggest that even though the mature region of Activin-A remains covalently attached to the prodomain of one of its βA subunits in both A70 and A60, this linkage does not prohibit binding to ActR-II.
Furin converts A70 to A60 independently of an N-glycosylation site and of alternative KR or RR motifs in the Activin-A prodomain
To further investigate how furin converts A70 to A60, we tested the role of potential secondary furin sites. In the absence of stronger candidates, we considered only KKR71 and RR110 (Fig. S3A). Alanine substitutions of these motifs did not impair A60 formation by furin, or signaling activity (Fig. S3F, G). As an alternative possibility, we assessed whether A60 and A70 differ in their electrophoretic mobility due to differential N-glycosylation. The only asparagine that can be N-glycosylated in proActivin-A is in the prodomain (N165). Alanine substitution of N165 in proActivin-A (mN) increased the electrophoretic mobility of A60, confirming by yet another approach that A60 contains the prodomain (Fig. S3H). Despite this size shift, the signaling activity of this mN mutant was comparable to WT Activin-A. In addition, analysis on reducing gels confirmed that only cleavage site 1 mutant (mS1) proActivin-A, but neither WT nor mN mutant A60 contained uncleaved βA chains (Fig. S3I). These data show that furin converts A70 to A60 independently of the N-glycosylation site. However, how furin accelerates the electrophoretic mobility of A60 compared to A70 remains to be determined.
Combined mutation of cysteines C35 and C38 inhibits the covalent linkage of proActivin-A to a high molecular weight complex in the ER
The amount of stably accumulating A60 relative to A30 varied considerably among independent experiments (Fig. S4A). We hypothesized that unpredictable fluctuations in the cell culture milieu could stabilize alternative allosteric disulfide bonds42. Activin-A prepro-protein contains nine conserved cysteines in the mature region, four in the prodomain, and one in the signal sequence (Fig. S4B, C). Covalent dimerization of the mature region by C390 is dispensable for the secretion and proteolytic maturation29. By contrast, three intrachain disulfides that form the cystine knot are essential for proActivin-A folding in the ER, including C321-C391, C350-C354, and C423-C42529. An additional intrachain disulfide between C314 and C322 in the mature region adjacent to the S1 cleavage site is predicted to localize within less than 20 Å from cysteines C35 and C38 in a flexible loop of the prodomain (Figs. 4A and S4B). These four cysteines and the arrangement of C35 and C38 in a thioredoxin-like CXXC motif are conserved across species (Fig. S4C) and in all inhibin B isoforms, GDF8, and GDF11 (Fig. S4D)43. To test the influence on Activin-A processing, we substituted each of these four cysteines with alanines. Transient transfection in FurKO#1 or DKO cells confirmed that none of them were essential for the secretion or furin-independent processing of proActivin-A (Fig. 4B, lanes 9-24). However, while mutating C38 exposed A70 to inhomogeneous PCLP cleavage (lanes 11, 19), mutating C35 or C322 markedly diminished A70 (lanes 10, 15, 18, 23). By contrast, mutation of C35 together with C38 restored the proportion of A70 (lanes 12, 20), suggesting that C35 stimulates A70 accumulation indirectly by regulating a function of C38. In particular, we noticed that the C35A and C38A mutants each formed a high molecular weight (HMW) form that was barely visible in SN of cells expressing WT βA (lanes 1, 9, 17 or, after prolonged exposure, 13, 21), and which was absent altogether if C35 and C38 were mutated jointly (lanes 12, 20). Analysis in lysates of cells treated with or without brefeldin A to inhibit ER export confirmed that C35 limits the amount of an Activin-A HMW complex that can be stabilized by C38, and vice versa (Fig. 4C). These results suggest that C35 regulates how much of Activin-A is (or remains) covalently linked to a hitherto unknown HMW complex via C38 or, if C38 is mutated, via C35 itself.
Å from cysteines C35 and C38 in a flexible loop of the prodomain (Figs. 4A and S4B). These four cysteines and the arrangement of C35 and C38 in a thioredoxin-like CXXC motif are conserved across species (Fig. S4C) and in all inhibin B isoforms, GDF8, and GDF11 (Fig. S4D)43. To test the influence on Activin-A processing, we substituted each of these four cysteines with alanines. Transient transfection in FurKO#1 or DKO cells confirmed that none of them were essential for the secretion or furin-independent processing of proActivin-A (Fig. 4B, lanes 9-24). However, while mutating C38 exposed A70 to inhomogeneous PCLP cleavage (lanes 11, 19), mutating C35 or C322 markedly diminished A70 (lanes 10, 15, 18, 23). By contrast, mutation of C35 together with C38 restored the proportion of A70 (lanes 12, 20), suggesting that C35 stimulates A70 accumulation indirectly by regulating a function of C38. In particular, we noticed that the C35A and C38A mutants each formed a high molecular weight (HMW) form that was barely visible in SN of cells expressing WT βA (lanes 1, 9, 17 or, after prolonged exposure, 13, 21), and which was absent altogether if C35 and C38 were mutated jointly (lanes 12, 20). Analysis in lysates of cells treated with or without brefeldin A to inhibit ER export confirmed that C35 limits the amount of an Activin-A HMW complex that can be stabilized by C38, and vice versa (Fig. 4C). These results suggest that C35 regulates how much of Activin-A is (or remains) covalently linked to a hitherto unknown HMW complex via C38 or, if C38 is mutated, via C35 itself.
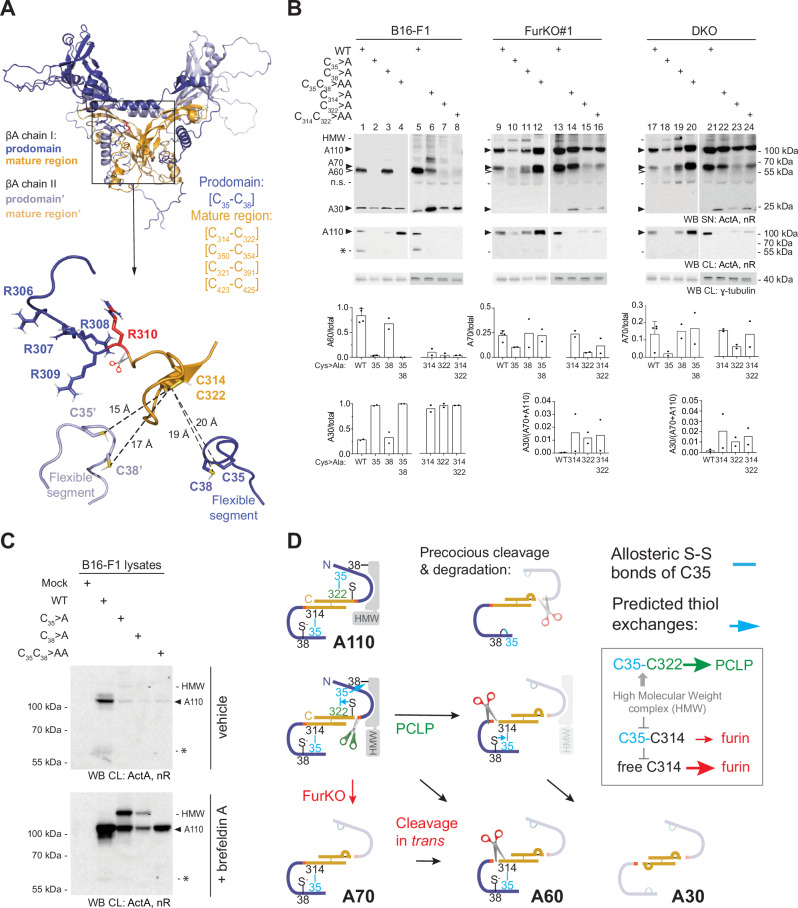
A Quaternary structure of proActivin-A (PDB: 5HLZ), with the mature domains colored ocher and prodomains in violet. Arginine R310 forming the scissile bond is highlighted in red, and thiols of cysteines C35 and C38 in yellow. B Anti-Activin-A Western blots of SNs and extracts (CL) from the B16-F1, FurKO#1, or DKO cell lines that were cultured for 48 h after transfection with wild-type βA (WT) or with the indicated cysteine mutants. Proteins were denaturated with SDS and separated on a non-reducing gel to preserve disulfide bonds. Analysis of γ-tubulin in cell lysates served as a loading control. Data are representative of 2 experiments. Quantifications by Image J are shown below. C Western blot of SN and lysates of transfected B16-F1 cells after treatment with 5
h after transfection with wild-type βA (WT) or with the indicated cysteine mutants. Proteins were denaturated with SDS and separated on a non-reducing gel to preserve disulfide bonds. Analysis of γ-tubulin in cell lysates served as a loading control. Data are representative of 2 experiments. Quantifications by Image J are shown below. C Western blot of SN and lysates of transfected B16-F1 cells after treatment with 5 µg/ml brefeldin A for 12
µg/ml brefeldin A for 12 h. HMW, a high molecular weight form of Activin-A in the ER migrating with an apparent size of 118
h. HMW, a high molecular weight form of Activin-A in the ER migrating with an apparent size of 118 kDa. D Schematic illustration of allosteric disulfide bonds in Activin-A, and their proposed roles in HMW complex formation and S1 site cleavage during transport from the ER (top row) to the more oxidative trans-Golgi network (middle row) and extracellular space (bottom row). The prodomain (violet) and an interacting factor that is predicted to be covalently linked in a HMW complex are shaded in lighter color after they are cleaved off. Blue arrows indicate thiol exchanges predicted to be required to detach the mature region from its disulfide-linked prodomain (see text for details).
kDa. D Schematic illustration of allosteric disulfide bonds in Activin-A, and their proposed roles in HMW complex formation and S1 site cleavage during transport from the ER (top row) to the more oxidative trans-Golgi network (middle row) and extracellular space (bottom row). The prodomain (violet) and an interacting factor that is predicted to be covalently linked in a HMW complex are shaded in lighter color after they are cleaved off. Blue arrows indicate thiol exchanges predicted to be required to detach the mature region from its disulfide-linked prodomain (see text for details).
Covalent linkage of mature Activin-A to its cleaved prodomain in A60 primarily requires cysteines C35 and C322
A disulfide bond of C38 may form at least transiently to stabilize a HMW complex of proActivin-A with a rate-limiting interacting factor (Fig. 4D). A HMW complex of proTGF-β that is stabilized by the homologous cysteine is asymmetric30. In one possible model, an analogous asymmetry in Activin-A precursor dimers enables the free C35 of the HMW-bound βA subunit to link to C322 in the mature region instead of pairing with C314 or C38 (Fig. 4D, top row). In this model, A60 accumulation reflects the perdurance of allosteric disulfide bonds of C35 with C314 or, if C314 is mutated, with C322. In keeping with this model, transient transfection in control B16-F1 cells revealed that the furin-cleaved A60 was absent upon mutating C35 alone or together with C38, but not after mutating only C38 (Fig. 4B, lanes 1–4). Importantly, the furin-cleaved A60 was also depleted or diminished, respectively, if we mutated C322 or C314 (Fig. 4B, lanes 6-8). These results suggest that covalent attachment of a cleaved prodomain to the mature region of A60 involves a disulfide bond of C35 with C322 on one βA subunit, or with C314 on the other.
Allosteric disulfide bonds in proActivin-A prevent its precocious furin-induced degradation
To rule out possible transfection artifacts, cysteine mutant βA constructs were transduced in B16-F1 cells as lentiviral transgenes. Screening by RT-qPCR identified stable cell lines expressing these constructs at comparable levels (Fig. S5A). Western blot analysis confirmed that both C35 and C38 had to be mutated to block the intracellular assembly of an Activin-A HMW complex. In addition, C38 partially compensated for the absence of C35 in stabilizing A60 in cell SN (Fig. S5B). Importantly, in line with our observation in transient transfections described above, the C35C38 >
> AA mutation depleted A60 also in stable cell lines without inducing a corresponding increase of any other cleaved or uncleaved forms of Activin-A (Fig. S5B, lane 5). In sharp contrast, disrupting A60 by the alternative approach of mutating C314 and/or C322 led to a net increase in A30 (Fig. S5B, lanes 6-8). These data support our model that disulfide bond formation between C314 and C322 is inhibited or delayed by C35 and C38 to prevent precocious furin-induced Activin-A clearance (Fig. 4D).
AA mutation depleted A60 also in stable cell lines without inducing a corresponding increase of any other cleaved or uncleaved forms of Activin-A (Fig. S5B, lane 5). In sharp contrast, disrupting A60 by the alternative approach of mutating C314 and/or C322 led to a net increase in A30 (Fig. S5B, lanes 6-8). These data support our model that disulfide bond formation between C314 and C322 is inhibited or delayed by C35 and C38 to prevent precocious furin-induced Activin-A clearance (Fig. 4D).
To estimate the influence of disulfide bonds on Activin-A signaling in reporter cells, equal volumes of SNs from the melanoma cell lines shown in Fig. S5B were administered to HepG2 CAGA-Luc reporter cells with or without the furin inhibitor CMK (Fig. S5C, upper panel). Interestingly, Activin-A increased CAGA-Luc expression in CMK-treated reporter cells 30- to 40-fold regardless of any of the cysteine mutations. Purified mutant proteins are not available to compare their specific activity more accurately. However, normalization to the total signaling activity in reporter cells without CMK revealed that mutation of C314, C322, or both significantly diminished the fraction of Activin-A that remains to be processed by signal-receiving cells relative to how much it was activated by the signal-sending melanoma cells (Fig. S5C, bottom panel). These data suggest that covalent linkage of at least one prodomain to the mature region via an allosteric disulfide bond with C314 or C322 increases the reservoir of Activin-A that can be activated after secretion.
To investigate how the C35C38 >
> AA mutation depleted Activin-A specifically in SN of furin-expressing cells, we considered whether it triggers ER-associated degradation. To test this, B16-F1 cells stably expressing WT or cysteine mutant forms of βA were treated for 12
AA mutation depleted Activin-A specifically in SN of furin-expressing cells, we considered whether it triggers ER-associated degradation. To test this, B16-F1 cells stably expressing WT or cysteine mutant forms of βA were treated for 12 h with the proteasome inhibitor MG-132 with or without brefeldin A (BFA) that blocks ER to Golgi transport. As expected, cells treated with BFA alone accumulated uncleaved precursor in cell lysates instead of secreting it (Fig. S5D). In addition, BFA-treated cells accumulated traces of a 61
h with the proteasome inhibitor MG-132 with or without brefeldin A (BFA) that blocks ER to Golgi transport. As expected, cells treated with BFA alone accumulated uncleaved precursor in cell lysates instead of secreting it (Fig. S5D). In addition, BFA-treated cells accumulated traces of a 61 kDa band of WT Activin-A (Fig. S5D, asterisk). Since this band migrated more slowly than A60 (58
kDa band of WT Activin-A (Fig. S5D, asterisk). Since this band migrated more slowly than A60 (58 kDa) and independently of the cysteines that form A60, it may correspond to incompletely glycosylated hemicleaved Activin-A or a degradation product (Fig. 4D). Importantly, treatment with MG-132 alone or together with BFA neither stabilized A110 nor its HMW complex, regardless of the cysteine mutations examined. These results show that even the combined absence of C35 and C38 does not target Activin-A for ER-associated degradation of unfolded protein.
kDa) and independently of the cysteines that form A60, it may correspond to incompletely glycosylated hemicleaved Activin-A or a degradation product (Fig. 4D). Importantly, treatment with MG-132 alone or together with BFA neither stabilized A110 nor its HMW complex, regardless of the cysteine mutations examined. These results show that even the combined absence of C35 and C38 does not target Activin-A for ER-associated degradation of unfolded protein.
Hemicleaved Activin-A is partially resistant to follistatin inhibition
To test the impact of uncleaved βA subunits on physiological activin antagonists, Activin-A from B16F1-βΑ or FurKO-βΑ cells, respectively, was treated with increasing concentrations of FST, followed by incubation on HepG2 CAGA-Luc reporter cells. To avoid signal saturation, input SNs containing comparable amounts of mature or immature Activin-A, respectively, were each diluted 20-fold to match the activity of 10 ng/ml of recombinant Activin-A (Fig. 5A, B). As expected, CAGA-Luc induction by the SN containing mature Activin-A was inhibited by FST no less than the induction by recombinant Activin-A. By contrast, FST only partially inhibited the signal induced by immature Activin-A from FurKO-βA cells even at the highest dosage examined (Fig. 5C, D). Western blot analysis of the SNs after their incubation on reporter cells validated that FST did not inhibit cleavage of A70 or A110 in the medium (Fig. 5E). These data suggest that the uncleaved prodomain confers partial resistance to inhibition by FST, and that furin-mediated cleavage during secretion sensitizes Activin-A more potently to this inhibitor than cleavage in trans by the signal-receiving cells.
ng/ml of recombinant Activin-A (Fig. 5A, B). As expected, CAGA-Luc induction by the SN containing mature Activin-A was inhibited by FST no less than the induction by recombinant Activin-A. By contrast, FST only partially inhibited the signal induced by immature Activin-A from FurKO-βA cells even at the highest dosage examined (Fig. 5C, D). Western blot analysis of the SNs after their incubation on reporter cells validated that FST did not inhibit cleavage of A70 or A110 in the medium (Fig. 5E). These data suggest that the uncleaved prodomain confers partial resistance to inhibition by FST, and that furin-mediated cleavage during secretion sensitizes Activin-A more potently to this inhibitor than cleavage in trans by the signal-receiving cells.
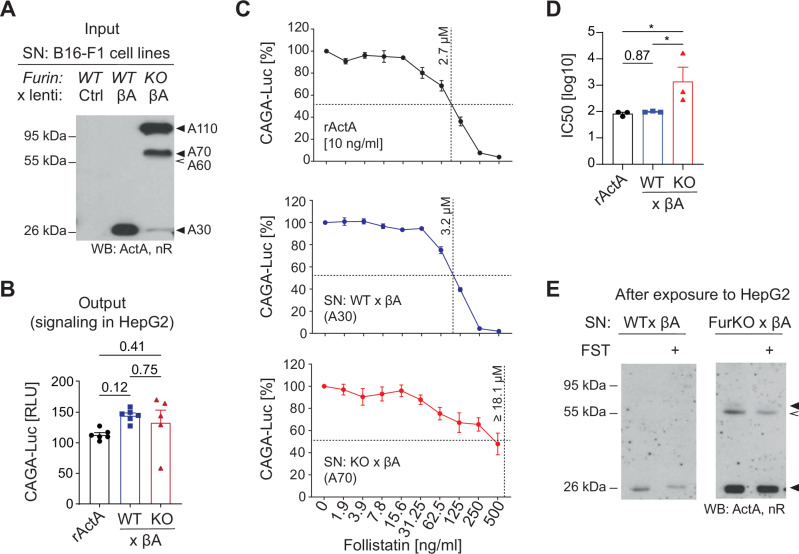
A Anti-Activin-A Western blot of SNs from the indicated Furin wild-type (WT) and knockout (KO) #1 B16F1 cell lines used in (B–D). B CAGA-Luc induction in HepG2 reporter cells after 12 h treatment with Activin-A (10
h treatment with Activin-A (10 ng/ml) (control), or with 1:10 diluted SNs of the indicated Furin WT or KO B16F1 cell lines expressing INHBA (βA). Error bars, SEM (n
ng/ml) (control), or with 1:10 diluted SNs of the indicated Furin WT or KO B16F1 cell lines expressing INHBA (βA). Error bars, SEM (n =
= 5–6 independent experiments). *p
5–6 independent experiments). *p <
< 0.05, **p
0.05, **p <
< 0.01, ***p
0.01, ***p <
< 0.001, ****p
0.001, ****p <
< 0.0001, Student’s t-test. C CAGA-Luc induction in HepG2 reporter cells by 10
0.0001, Student’s t-test. C CAGA-Luc induction in HepG2 reporter cells by 10 ng/ml rActA (up), B16F1-βΑ SN (middle) or FurKO#1-βΑ SN (below) in the presence of increasing doses of FST (up to 500
ng/ml rActA (up), B16F1-βΑ SN (middle) or FurKO#1-βΑ SN (below) in the presence of increasing doses of FST (up to 500 ng/ml). Error bars, SEM (n
ng/ml). Error bars, SEM (n =
= 3 independent experiments). IC50 (μM) values were calculated using nonlinear fit. D Estimation of the half maximal inhibitory concentration (IC50) of FST required to block Activin-A in the samples analyzed in (C). E Representative Western blot analysis of the βA-conditioned SNs shown in (A) after they were incubated for 12
3 independent experiments). IC50 (μM) values were calculated using nonlinear fit. D Estimation of the half maximal inhibitory concentration (IC50) of FST required to block Activin-A in the samples analyzed in (C). E Representative Western blot analysis of the βA-conditioned SNs shown in (A) after they were incubated for 12 h on reporter cells with or without 500
h on reporter cells with or without 500 ng/ml FST.
ng/ml FST.
Both βA subunits must be cleaved for Activin-A to bind and inhibit BMPR-II
Activin-A can also bind BMPR-II receptors, leading to SMAD1/5 signal attenuation5,44 (Fig. 6A). To test whether hemicleaved Activin-A also binds to BMPR-II, cell SNs enriched for mature or hemicleaved Activin-A were incubated with Fc fusions of the extracellular domain of BMPR-II or, as a control, TGFBRII. We found that BMPRII-Fc pulled down both A30 and A60, albeit in low amounts (Fig. 6B, lanes 2, 5), in line with the fact that BMPR-II binds Activin-A with lower affinity than ActR-II4,45. By contrast, binding of BMPRII-Fc to A70 was not detected (Fig. 6B, lane 3). To test the impact on BMP signaling, we supertransfected HepG2 CAGA-Luc cells with BMP-inducible BRE-Luc reporter and then treated them with the ALK4/5/7 inhibitor SB-431542 to block CAGA-Luc induction, followed by quantification of BRE-Luc induction by BMP receptor-activated SMADs (Fig. 6A). Addition of A30 or A70 dose-dependently inhibited the BRE-Luc induction by recombinant BMP-4 (Fig. 6C, top panel). Next, to prevent conversion of A70 to A60 during the assay, BRE-Luc was transfected into HepG2 CAGA-Luc cells expressing α1-PDX. Interestingly, in α1-PDX expressing reporter cells, A70 failed to inhibit BMP-4 signaling (Fig. 6C, bottom panel). These data corroborate the conclusion of our receptor pull-down assay that cleavage of both βA chains is necessary for Activin-A to interfere with BMP receptor signaling.
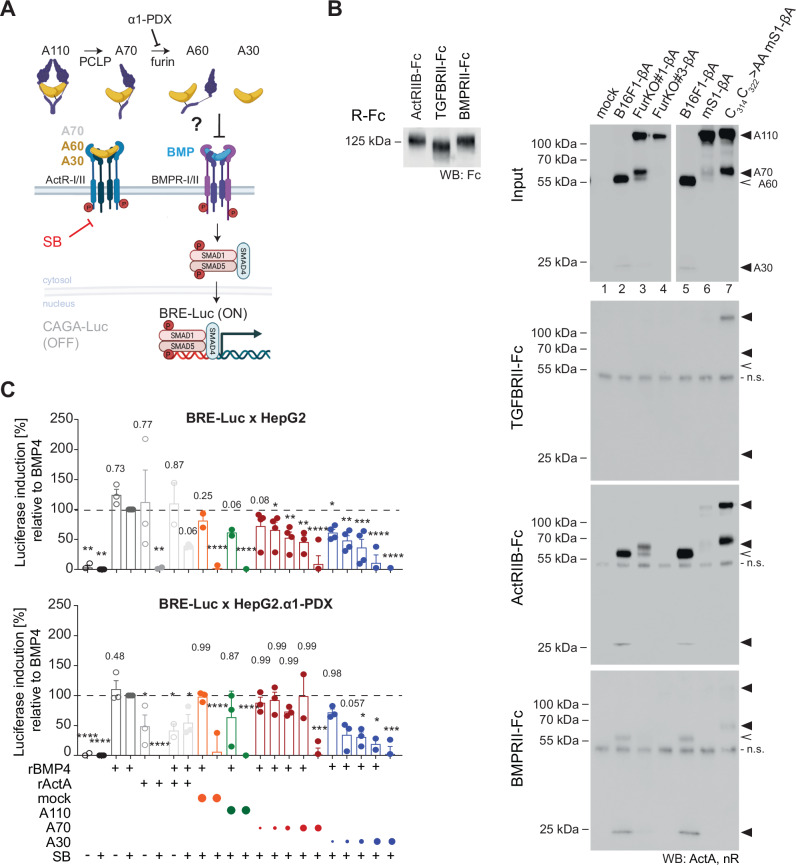
A Experimental strategy to assess which forms of Activin-A can inhibit BMP receptor signaling. HepG2 reporter cells with or without α1-PDX were transfected with the SMAD1/5 reporter BRE-Luc and treated with BMP4 alone or together with A110 in FurKO#3-βA cell SNs, or with A70 (FurKO#1-βA SN), or A30 +
+ A60 (B16F1-βA SN). Co-treatment with the activin type I receptor inhibitor SB-431542 (SB) served to prevent induction of CAGA-Luc. Created using BioRender.com. B Anti-Fc Western blot of Fc fusions of the type II receptors (left) that were used to pull down Activin-A from the concentrated cell SNs indicated on the right (input). C Induction of transiently transfected SMAD1,5 reporter BRE-Luc in HepG2 CAGA-Luc reporter cell lines with or without the α1-PDX transgene within 12
A60 (B16F1-βA SN). Co-treatment with the activin type I receptor inhibitor SB-431542 (SB) served to prevent induction of CAGA-Luc. Created using BioRender.com. B Anti-Fc Western blot of Fc fusions of the type II receptors (left) that were used to pull down Activin-A from the concentrated cell SNs indicated on the right (input). C Induction of transiently transfected SMAD1,5 reporter BRE-Luc in HepG2 CAGA-Luc reporter cell lines with or without the α1-PDX transgene within 12 h after stimulation with 10
h after stimulation with 10 ng/ml BMP4, and its inhibition by 10
ng/ml BMP4, and its inhibition by 10 ng/ml Activin-A (rActA) or by serially diluted SNs from B16F1-βΑ cells (A30) or FurKO#2-βΑ cells (A70), or FurKO-βΑ #3 (A110), or from B16F1-Ctrl cells (mock) cell lines. Cells were co-treated with the type I activin and TGF-β receptor inhibitor SB-431542 (SB, 10
ng/ml Activin-A (rActA) or by serially diluted SNs from B16F1-βΑ cells (A30) or FurKO#2-βΑ cells (A70), or FurKO-βΑ #3 (A110), or from B16F1-Ctrl cells (mock) cell lines. Cells were co-treated with the type I activin and TGF-β receptor inhibitor SB-431542 (SB, 10 µM) to inhibit CAGA-Luc induction, or with empty vehicle. The sizes of colored circles indicate the 10- to 80-fold serial dilutions (from right to left). Induction was normalized to baseline luciferase values in untreated cells and then to treatment with BMP4 and SB-431542 (n
µM) to inhibit CAGA-Luc induction, or with empty vehicle. The sizes of colored circles indicate the 10- to 80-fold serial dilutions (from right to left). Induction was normalized to baseline luciferase values in untreated cells and then to treatment with BMP4 and SB-431542 (n =
= 1–3 independent experiments). Error bars, SEM (ī
1–3 independent experiments). Error bars, SEM (ī =
= 2–4 independent experiments); *p
2–4 independent experiments); *p <
< 0.05, **p
0.05, **p <
< 0.01, ***p
0.01, ***p <
< 0.001, ****p
0.001, ****p <
< 0.0001, ordinary one-way ANOVA with Šídák’s multiple comparisons test.
0.0001, ordinary one-way ANOVA with Šídák’s multiple comparisons test.
Absence of C314 and C322 facilitates access of proActivin-A to type II receptor in pull-down assays
A crystal structure of proActivin-A predicts that the uncleaved furin site S1 prevents type I receptor binding, but how its cleavage facilitates access to type II receptors is unknown39. Since Wang et al. removed cysteines C35 and C38 from the mutant proActivin-A that they crystallized for structural analysis, we wondered if allosteric disulfide linkages of these cysteines with C314 or C322 are important to prevent precocious type II receptor binding. To estimate the influence of disulfides of C35 or C38 with the mature region without disrupting their alternative pairing with each other or the covalent linkage of proActivin-A to a HMW complex, we focused on functional analysis of cysteines C314 and C322. Superimposition of the AlphaFold structure model of monomeric mature Activin-A on the known crystal structure of the closely related GDF11 in a complex with ActR-II and ALK5 indicated that these cysteines and the paralogous C304 and C314 of GDF11 reside too far away from ActR-II to directly influence receptor binding, regardless of their disulfide linkage (Fig. S6). Nevertheless, binding of uncleaved mS1 mutant proActivin-A to cognate type II receptor which normally is barely detectable in pull-down assays dramatically increased upon substitution of C314 and C322 by alanines (Fig. 6B). By contrast, binding of mS1 proActivin-A to TGFBRII-Fc or to BMPRII-Fc remained close to background or below detection even if these cysteines were mutated. In addition, pull-down assays revealed that concentrated input samples after ultrafiltration contained A70-like species that formed even independently of a functional S1 site, and which further increased if C314 and C322 were mutated, indicating significant non-specific breakdown. Although such S1 site-independent processing normally was below detection, these data are consistent with our aforementioned observation that the absence of C314 and 322 increased furin-independent proActivin-A breakdown also in the ER of BFA-treated cells (Fig. S5D).
Discussion
Partial cleavage of one βA subunit of Activin-A by a furin-independent PCLP activity has been described previously 27,32, but its impact on receptor binding and on the function of Activin-A in cancer have remained unknown. Here, deletion of endogenous furin in melanoma grafts suppressed the tumor growth advantage mediated by Activin-A, correlating with increased accumulation of this partially processed hemicleaved A70 form, even though mature Activin-A continued to form in the tumor microenvironment and to accumulate at elevated levels in the circulation of tumor-bearing hosts. Cell non-autonomous Activin-A maturation was recapitulated in vitro by incubating hemicleaved A70 on signal-receiving HepG2 cells, where it was important for cell contact-independent signaling and to facilitate signal inhibition by the secreted antagonist FST. By contrast, furin-independent hemicleavage within melanoma cells by PCLP alone only supported cell contact-dependent juxtacrine signaling. Mechanistically, protein structure modeling combined with functional analysis revealed that Activin-A precursor cleavage by furin promotes cognate type II receptor binding at least in part by overcoming the inhibitory effect of conserved allosteric disulfide bonds between the mature region of Activin-A and its prodomain. Our observations predict that precursor cleavage may be necessary to rearrange the connectivity of these allosteric disulfides. Furthermore, delaying the completion of proteolytic Activin-A maturation until after secretion holds potential to mitigate the associated tumor growth.
27,32, but its impact on receptor binding and on the function of Activin-A in cancer have remained unknown. Here, deletion of endogenous furin in melanoma grafts suppressed the tumor growth advantage mediated by Activin-A, correlating with increased accumulation of this partially processed hemicleaved A70 form, even though mature Activin-A continued to form in the tumor microenvironment and to accumulate at elevated levels in the circulation of tumor-bearing hosts. Cell non-autonomous Activin-A maturation was recapitulated in vitro by incubating hemicleaved A70 on signal-receiving HepG2 cells, where it was important for cell contact-independent signaling and to facilitate signal inhibition by the secreted antagonist FST. By contrast, furin-independent hemicleavage within melanoma cells by PCLP alone only supported cell contact-dependent juxtacrine signaling. Mechanistically, protein structure modeling combined with functional analysis revealed that Activin-A precursor cleavage by furin promotes cognate type II receptor binding at least in part by overcoming the inhibitory effect of conserved allosteric disulfide bonds between the mature region of Activin-A and its prodomain. Our observations predict that precursor cleavage may be necessary to rearrange the connectivity of these allosteric disulfides. Furthermore, delaying the completion of proteolytic Activin-A maturation until after secretion holds potential to mitigate the associated tumor growth.
Furin converts hemicleaved Activin-A to A60 where one cleaved prodomain remains disulfide-linked to the mature region
Using LC–MS analysis and Western blots under reducing conditions, we confirmed that one βA subunit of the furin-independent Activin-A processing intermediate A70 was uncleaved. We named it A70 because hemicleaved Activin-A was first described as a protein of approximately 70 kDa27. Interestingly, further processing by furin after secretion converted A70 to A60, where the mature dimer remained covalently bound to the prodomain by a disulfide bond. This implies that the responsible disulfide bond did not abolish access of furin to either of the two βA subunits, even though it protected Activin-A against precocious furin-induced degradation in the secretory apparatus (Fig. 4D, box). Before testing the existence of such a disulfide bond, we considered whether A60 could correspond to uncleaved monomers. This scenario was ruled out by our findings that A60 was generated by furin and contained no uncleaved subunits on reducing gels. In TGF-β, a disulfide bond between cysteine C33 in the prodomain and a cysteine in the mature region delays cleavage of one subunit at least until after secretion46,47. Cysteine C33 can also be connected to latent TGF-β binding protein (LTBP) in the ECM or to other cell type-specific transporters48,49. Given the known stoichiometry of only one LTBP per TGF-β, this complex must be asymmetric, with C33 of one TGF-β subunit forming a different disulfide bond with LTBP than the other if both subunits are covalently attached30,50. By contrast, the Activin-A prodomain to our knowledge has never been reported to form such disulfide bonds. Here, the apparent molecular weight of a fraction of proActivin-A in extracts of BFA-treated cells was shifted from 104 to 118
kDa27. Interestingly, further processing by furin after secretion converted A70 to A60, where the mature dimer remained covalently bound to the prodomain by a disulfide bond. This implies that the responsible disulfide bond did not abolish access of furin to either of the two βA subunits, even though it protected Activin-A against precocious furin-induced degradation in the secretory apparatus (Fig. 4D, box). Before testing the existence of such a disulfide bond, we considered whether A60 could correspond to uncleaved monomers. This scenario was ruled out by our findings that A60 was generated by furin and contained no uncleaved subunits on reducing gels. In TGF-β, a disulfide bond between cysteine C33 in the prodomain and a cysteine in the mature region delays cleavage of one subunit at least until after secretion46,47. Cysteine C33 can also be connected to latent TGF-β binding protein (LTBP) in the ECM or to other cell type-specific transporters48,49. Given the known stoichiometry of only one LTBP per TGF-β, this complex must be asymmetric, with C33 of one TGF-β subunit forming a different disulfide bond with LTBP than the other if both subunits are covalently attached30,50. By contrast, the Activin-A prodomain to our knowledge has never been reported to form such disulfide bonds. Here, the apparent molecular weight of a fraction of proActivin-A in extracts of BFA-treated cells was shifted from 104 to 118 kDa on non-reducing gels, depending on cysteine C38 or, in its absence, C35. Since the position of C38 corresponds to that of C33 in TGF-β1, the 118
kDa on non-reducing gels, depending on cysteine C38 or, in its absence, C35. Since the position of C38 corresponds to that of C33 in TGF-β1, the 118 kDa form likely relies on an interacting factor that can at least transiently remain disulfide-linked to the prodomain. Reversible covalent attachment to C38, possibly followed by proteolytic breakdown would explain why such a factor has not been identified yet. The spacing and the sequence between C35 and C38 are reminiscent of the CXXC motif of thioredoxins that reduce oxidized cysteines in their substrates to mediate cleavage of disulfide bonds51–53. Thus, the disulfide linkage of proActivin-A via one of these cysteines might be cleaved by a thiol attack from the other. In one possible model which is consistent with our data, the ability of the thiol of C35 to attack C38 is controlled by the connectivity of cysteines C314 and C322, which in turn is regulated by precursor cleavage and by its expected impact on their flexibility (Fig. 4D). Even a non-covalent asymmetric transient association with an interacting factor may change the conformation of one βA subunit of proActivin-A to allow cleavage by PCLP by promoting an allosteric disulfide bond of C35 with C322. On the other subunit, C35 is predicted to instead pair by default with C314, thereby reducing its flexibility and hence the accessibility of the scissile bond of this and the furin recognition motif. Importantly, this model also predicts that allosteric disulfide bonds among these cysteines will at least transiently link the Activin-A prodomain to the mature region.
kDa form likely relies on an interacting factor that can at least transiently remain disulfide-linked to the prodomain. Reversible covalent attachment to C38, possibly followed by proteolytic breakdown would explain why such a factor has not been identified yet. The spacing and the sequence between C35 and C38 are reminiscent of the CXXC motif of thioredoxins that reduce oxidized cysteines in their substrates to mediate cleavage of disulfide bonds51–53. Thus, the disulfide linkage of proActivin-A via one of these cysteines might be cleaved by a thiol attack from the other. In one possible model which is consistent with our data, the ability of the thiol of C35 to attack C38 is controlled by the connectivity of cysteines C314 and C322, which in turn is regulated by precursor cleavage and by its expected impact on their flexibility (Fig. 4D). Even a non-covalent asymmetric transient association with an interacting factor may change the conformation of one βA subunit of proActivin-A to allow cleavage by PCLP by promoting an allosteric disulfide bond of C35 with C322. On the other subunit, C35 is predicted to instead pair by default with C314, thereby reducing its flexibility and hence the accessibility of the scissile bond of this and the furin recognition motif. Importantly, this model also predicts that allosteric disulfide bonds among these cysteines will at least transiently link the Activin-A prodomain to the mature region.
About 40 allosteric disulfide bonds have been identified in various proteins, but existing technologies do not allow to determine their potential to undergo thiol exchanges in proteins containing as many cysteines as Activin-A42. The default connectivity of C35 and C38 cannot be inferred from structural models because these residues had to be removed to determine the existing crystal structure of proActivin-A. Consequently, C314 and C322 paired only with one another39. We propose that C35 by default pairs first with cysteines in the mature region (Fig. 4D). This would explain why A60 was absent when we mutated cysteines C314 and C322, or C35. In stably transduced melanoma cells, C38 partially compensated for C35. The availability of C38 to form A60 may be limited by the expression levels of the interacting factor in the ER. Based on these considerations, we propose that HMW complexes of proActivin-A are stabilized primarily by C38. Accordingly, PCLP cleavage became inhomogeneous in the C38A mutant (Fig. 4B, lanes 11 and 19). In our model, absence of C38 destabilizes C314-C322 disulfides by liberating C35 to attack C322. Alternative mechanisms to account for the regulation of stepwise cleavage of the two βA subunits by furin and PCLP cannot be excluded at this stage. However, they too would have to consider that mutation of one cysteine will influence the connectivity among others. For example, loss of C35 or C38 alone stabilizes traces of HMW complexes even in conditioned medium, as expected if C38 can be attacked by C35 to release a covalently bound factor. Allosteric disulfides may also modulate the fate of the prodomain, including possible alternative and degradative cleavages (Fig. 4D).
Disruption of allosteric disulfide bonds exposes Activin-A to precocious furin-induced depletion
Mutation of C33 impairs TGF-β1 activity in vivo, leading to multiorgan inflammation and digestive tract cancers54. Here, we found that combined mutation of C35 and C38 exposed all forms of Activin-A to precocious furin-induced depletion. Conversely, mutating C314 and/or C322 increased the net accumulation of secreted A30 (Fig. S4B, lanes 6-8). Interestingly, addition of mutant forms to HepG2 reporter cells showed no proportionate increase in signal activity, but rather a decrease in the reservoir of total Activin-A activity that can be activated after secretion. These findings suggest that disulfide bonds among these cysteines serve to restrain furin-induced turnover of immature forms and the predicted associated flux of Activin-A-interacting factors. Previously, the influence of C314 or C322 on Activin-A signaling has been tested by mutating them individually. When added to rat pituitary cells, these mutants labeled fewer cell surface receptors and signaled three- to fivefold less potently than wild-type Activin-A, suggesting that C314 and C322 stabilize ligand/receptor complexes29. Our structural modeling and functional analysis revealed that these cysteines inhibit receptor binding rather than stimulating it (see below), indicating that they likely attenuate receptor-mediated turnover. In keeping with this interpretation, furin cleavage also facilitates endocytosis of the related Nodal precursor34,55. Future studies should test whether precocious cleavage diverts Activin-A to endosomes or lysosomes. In line with a possible role for endosomal furin, blocking furin in endosomes requires a 10-fold higher concentration of the membrane-permeable inhibitor CMK than in the TGN or at the cell surface32, and a similar dosage effect of CMK was observed here when comparing furin-mediated cleavage of one βA subunit compared to the other. As a possible alternative, we considered if the cysteines mediating allosteric disulfide bond formation are important to prevent aberrant Activin-A folding and degradation in the ER. Contrary to this hypothesis, proActivin-A degradation in the ER appeared to be minimal, concurring with the fact that furin normally is inhibited in the ER by its prosegment.
Control of juxtracrine versus paracrine Activin-A signaling by stepwise precursor cleavage
Hemicleavage of Activin-A, defined here as the processing of one βA subunit of the precursor dimer at the S1 site, enabled significant binding to the extracellular domains of type IIA and IIB activin receptors both in plasmonic resonance measurements and in pull-down assays. In proActivin-A, a non-covalent dimer of the prodomain occupies the predicted type I receptor binding surface and several type II receptor-binding epitopes39. The same study confirmed that mature Activin-A remains bound to its prodomains also after precursor cleavage and secretion. However, cognate signaling receptors bind mature Activin-A with higher affinity and readily extract it from this non-covalent complex39,56–58. Our in vitro pull-down experiments suggest that activin type II receptors can also compete away the prodomain if one βA subunit of Activin-A is still uncleaved. By contrast, binding to BMPR-II was only detected after cleavage of both βA subunits, in keeping with its approximately 100-fold lower affinity for Activin-A compared to ActR-II4,45.
An available proActivin-A crystal structure did not explain how furin cleavage facilitates type II receptor binding, possibly because C35 and C38 were removed to ensure that C314 and C322 can only pair with one another39. Indeed, the so-called lasso region adjacent to C35 and C38 of the Activin-A prodomain is known to fold back onto the mature region of the same βA subunit and thus has been hypothesized to help prevent precocious binding of βA monomers to ActR-II already in the ER. We propose that this protective function of the lasso in proActivin-A requires a disulfide bond that links the mature region to C35, but which later can be resolved by a thiol exchange with the nearby C38 or C314. To trigger such an attack, this model predicts that at least the correct positioning of C314 requires prior proteolytic processing at the nearby furin motif for the necessary conformational change to occur (Fig. 4D). In line with this model, we here found that binding of the cleavage mutant proActivin-A to Fc fusions of cognate type II receptors in pull-down assays was inhibited by cysteines C314 and C322. This strongly suggests that before pairing with one another, the cysteines C314 and C322 first form alternative allosteric disulfide bonds that are required to prevent precocious type II receptor binding in the secretory apparatus.
Treatment of various cell types with purified proActivin-A potently stimulates SMAD2/3 signaling58. We found that this involves cell non-autonomous proteolytic processing, and that signal-receiving cells also converted hemicleaved Activin-A to mature form. Without further maturation, hemicleaved Activin-A was inactive in the signal-receiving HepG2 cells, except if the signal-sending melanoma cells were in direct contact. Cell contacts can mechanically unmask receptor-binding sites in latent complexes of TGF-β through integrin αvβ6 or αvβ8 pulling forces59–61. While proActivin-A contains no known integrin binding site, exposure of receptor-binding epitopes by proteolytic processing of the first βA subunit may lead to a receptor-induced conformational change on the uncleaved subunit in a cell contact-dependent manner. Alternatively, cell contacts may facilitate access to a cell surface-bound protease. The cell-non-autonomous precursor processing was blocked by a membrane-impermeable PC inhibitor, suggesting it happens extracellularly. During early embryogenesis, both furin and the related protease PACE4 function cell non-autonomously62,63. Therefore, and since furin is expressed in HepG2 cells37, we did not further investigate how cell non-autonomous cleavage in trans is mediated.
Role of furin in unleashing tumor-promoting Activin-A signaling
Furin and several related PCs have been implicated in mediating pro- or anti-tumor functions, consistent with their known or predicted functions in the processing of numerous growth factors, adhesion molecules, extracellular matrix proteins, and zymogenic forms of matrix-remodeling metalloproteases that can promote or inhibit multiple cancer hallmarks, depending on the context64,65. Importantly, deleting furin in syngeneic mouse melanoma grafts specifically suppressed the growth advantage conferred by βA, coinciding with increased accumulation of uncleaved and half-processed Activin-A. These findings establish a non-redundant essential role for the coexpression of endogenous furin in cis. Interestingly, analysis in tumor extracts and in plasma of tumor-bearing host showed that the completion of Activin-A maturation nevertheless remained efficient and capable of mediating a marked loss of body weight, concurring with our in vitro observation of Activin-A processing in trans by signal-receiving cells. Possible scenarios of how furin expression in cis could alter Activin-A signaling output include the conversion of A70 to A60. Only A60 but not A70 was able to stimulate activin receptor signaling independently of cell contacts, and to interfere with BMP receptors signaling, at least in vitro. Thus, furin-mediated cleavage in the melanoma cells may increase a paracrine signal of Activin-A relative to a tumor-suppressive juxtacrine activity by promoting the formation of A60. Alternatively, or in addition, furin may shift the balance between pro- and anti-tumorigenic Activin-A functions by cleaving an important regulatory co-factor. In the absence of known candidates, we here only considered if it influences the neutralization of Activin-A by antagonists. In contrast to GDF8 and GDF11 where prodomains enhance binding to the secreted antagonist GASP-2/WFIKKN166, binding of Activin-A to FST is slowed by the prodomain39. In keeping with this notion, we found that hemicleaved Activin-A was partially resistant to inhibition by FST. In addition, uncleaved prodomain likely limits Activin-A diffusion by interacting with proteoglycans67. This would explain why we detected only fully mature Activin-A in plasma of tumor-bearing hosts, despite the prominent accumulation of hemicleaved form in tumors. A decrease in diffusivity in turn may favor tumor-suppressive Activin-A signaling at the expense of tumor-promoting paracrine signaling in the TME. In line with this idea, expression of a constitutively active mutant type I receptor within B16-F1 melanoma cells does not stimulate their tumor growth like secreted Activin-A, but instead inhibits it15. Future studies to distinguish between these scenarios will depend on model systems and reagents that are free of the ethical and technical constraints of the present tumor model and hence more suited for high throughput analysis of the dynamics and range of Activin-A signaling.
Methods
Cell lines
B16-F1 cells were purchased from ATCC. CRISPR/Cas9 editing of Furin and Pcsk7 by single guide RNAs and the production of B16F1-FurKO (clones #1 to #3) and DKO cell lines have been described previously32. A375 and C8161 cell lines were provided by Drs. Doug Hanahan (EPFL, Switzerland) or Mary J. C. Hendrix (Feinberg School of Medicine Northwestern University, USA), respectively. HepG2 reporter cells stably expressing Renilla luciferase together with the SMAD3-inducible firefly luciferase reporter CAGA-Luc have been described36. The HepG2.α1-PDX reporter cell line was derived by transducing HepG2 CAGA-Luc cells with α1-PDX-IRES-GFP lentivirus encoding the PC-inhibitory antitrypsin variant α1-PDX38. A clonal cell line was obtained by serial dilution following the sorting of GFP+ cells on a FACSAria Fusion machine (BD Biosciences, San Jose, USA). All cell lines were propagated in DMEM (Gibco) or, in the case of A375 and C8161 cells, RPMI (Gibco), supplemented with 10% fetal bovine serum, 1% GlutaMAX (Gibco), and 50 µg/mL Gentamicin (Gibco). Cells were cultured no longer than 8-10 weeks after de-freezing, and only after testing negative for mycoplasma (Mycospy kit, Biontex). Cell line identities were validated by characteristic cell morphologies, cell pigmentation, and by the presence or absence, respectively, of their distinct transgenes and of furin activity.
µg/mL Gentamicin (Gibco). Cells were cultured no longer than 8-10 weeks after de-freezing, and only after testing negative for mycoplasma (Mycospy kit, Biontex). Cell line identities were validated by characteristic cell morphologies, cell pigmentation, and by the presence or absence, respectively, of their distinct transgenes and of furin activity.
RT-qPCR analysis
Cells were cultured in 6-well plates and total RNA was extracted using the ReliaPrep RNA Cell Miniprep System (Promega, Madison, WI, USA) following the manufacturer’s protocol. For total RNA extractions from tissues, snap-frozen pieces were sonicated or homogenized in 1 mL of QIAzol (Qiagen, Hilden, DE), followed by chloroform extraction. Total RNA was isolated using RNeasy mini kit (Qiagen) according to the manufacturer’s protocol. cDNA was synthesized from 1
mL of QIAzol (Qiagen, Hilden, DE), followed by chloroform extraction. Total RNA was isolated using RNeasy mini kit (Qiagen) according to the manufacturer’s protocol. cDNA was synthesized from 1 μg RNA using the PrimeScript RT-PCR Kit (Takara, Kusatsu, JP). The analysis was performed using the SYBR Green GoTaq Master Mix (Promega) on a QuantStudio 6 instrument (Applied Biosystems, Waltham, MA, USA). qPCR primers are listed in Supplementary Table 1.
μg RNA using the PrimeScript RT-PCR Kit (Takara, Kusatsu, JP). The analysis was performed using the SYBR Green GoTaq Master Mix (Promega) on a QuantStudio 6 instrument (Applied Biosystems, Waltham, MA, USA). qPCR primers are listed in Supplementary Table 1.
Expression vectors and cloning
Lentiviral vector pLenti-EF1alpha-ActA-SV40-puro expressing myc-tagged proActivin-A has been described15. Briefly, nucleotides 218-1609 of the human INHBA reference sequence NM_002192.2 containing a myc epitope (EQKLISEEDL) instead of the glutamic acid residue E311 near the N-terminus of the mature region was subcloned as a Spe I fragment into the Xba I site of pLenti-EF1alpha-MCS-SV40-puro vector. To derive the cleavage site 1 mutant (mS1), the myc-INHBA coding sequence was amplified by overlap extension PCR (OE-PCR) using the forward and reverse primers 5′-GAGATCTAGACTCGAGACGCAAGGC-3′ and 5′-GAGAATCGATACCGTCGACT AGAG-3′, together with the mutagenic internal primers listed in Supplementary Table S2 to thereby replace the wild-type cDNA in pLenti-EF1alpha-ActA-SV40-puro. The KKR71 >
> AAA and RR110
AAA and RR110 >
> AA mutant constructs mS2′ and mS2, as well as the N-glycosylation site mutant N165
AA mutant constructs mS2′ and mS2, as well as the N-glycosylation site mutant N165 >
> A were generated analogously using the indicated internal OE-PCR primers (Supplementary Table S2). To tag the prodomain with a Flag epitope after E27, G130, or D146, the same external OE-PCR primers were used together with the internal primers encoding the amino acid sequence DYKDDDDK. To substitute cysteines 35, 38, 314, and/or 322 in proActivin-A with alanines, the internal mutagenic OE-PCR primers instead encoded the desired mutations (Supplementary Table S2). All mutant constructs were validated by Sanger sequencing of both cDNA strands.
A were generated analogously using the indicated internal OE-PCR primers (Supplementary Table S2). To tag the prodomain with a Flag epitope after E27, G130, or D146, the same external OE-PCR primers were used together with the internal primers encoding the amino acid sequence DYKDDDDK. To substitute cysteines 35, 38, 314, and/or 322 in proActivin-A with alanines, the internal mutagenic OE-PCR primers instead encoded the desired mutations (Supplementary Table S2). All mutant constructs were validated by Sanger sequencing of both cDNA strands.
Lentiviral vector expressing the bicistronic α1-PDX-IRES-GFP cassette was generated by subcloning α1-PDX cDNA (gift of Dr. Gary Thomas, University of Oregon) into attP1 and attP2 sites of pDONR221 vector (Addgene), and from there into the gateway destination vector pDEST between the human EF1α promoter and an IRES-eGFP cassette (Dest-vector IRESeGFP, gift of Dr. Jörg Huelsken, EPFL).
Collection of cell SNs after lentiviral transduction or transient transfection
CRISPR-edited FurKO clones #1 to #3 and a Furin and Pcsk7 double knockout (DKO) clone32 were transduced with βA or empty control lentivirus as described previously for B16F1-Ctrl and -βΑ cell lines15. In short, HEK293T cells were co-transfected with CMVΔR8.74 (Addgene, Watertown, MA, USA 22036), pMD2.VSVg (Addgene 12259), mycINHBA, or empty transfer plasmid. Lentiviral particles were collected from filtered culture supernatant by ultracentrifugation and resuspended in 50 µL sterile PBS and stored at −80
µL sterile PBS and stored at −80 °C or used freshly at a 50-500 dilution to infect cells of interest that were plated at a density of 1 × 105 cells/well in 12-well plates. Non-clonal B16-F1 cell lines stably expressing wild-type or the indicated mutant βA transgenes or empty vector control (Ctrl) were selected using puromycin. FurKO-βA and DKO-βA cell lines and respective empty vector controls were not selected because the parental CRISPR-edited FurKO cell line was already resistant to available antibiotics. Instead, individual stably transduced clones were plated at limiting dilution and expanded. Activin-A secretion was assessed by incubating the SNs on CAGA-Luc reporter cells confirming activity in 21 of 22 clones analyzed. Lentiviral α1-PDX particles to transduce HepG2 CAGA-Luc reporter cells were produced by transfecting HEK293T cells with α1-PDX-IRES-GFP together with the packaging vectors CMVΔR8.74 and pMD2.VSVg. The expression of α1-PDX was validated by flow cytometric sorting of the GFP+ cells.
°C or used freshly at a 50-500 dilution to infect cells of interest that were plated at a density of 1 × 105 cells/well in 12-well plates. Non-clonal B16-F1 cell lines stably expressing wild-type or the indicated mutant βA transgenes or empty vector control (Ctrl) were selected using puromycin. FurKO-βA and DKO-βA cell lines and respective empty vector controls were not selected because the parental CRISPR-edited FurKO cell line was already resistant to available antibiotics. Instead, individual stably transduced clones were plated at limiting dilution and expanded. Activin-A secretion was assessed by incubating the SNs on CAGA-Luc reporter cells confirming activity in 21 of 22 clones analyzed. Lentiviral α1-PDX particles to transduce HepG2 CAGA-Luc reporter cells were produced by transfecting HEK293T cells with α1-PDX-IRES-GFP together with the packaging vectors CMVΔR8.74 and pMD2.VSVg. The expression of α1-PDX was validated by flow cytometric sorting of the GFP+ cells.
For transient transfection, B16-F1, FurKO, or DKO cells were seeded in 24-well or 6-well plates at a density of 2.5 ×
× 105 or 5
105 or 5 ×
× 105 cells/well, respectively. On the following day, the cells were rinsed with PBS and incubated for 4
105 cells/well, respectively. On the following day, the cells were rinsed with PBS and incubated for 4 h in OptiMEM medium (Invitrogen) containing Lipofectamine 3000 (Invitrogen) together with the indicated expression vectors (1
h in OptiMEM medium (Invitrogen) containing Lipofectamine 3000 (Invitrogen) together with the indicated expression vectors (1 µg of plasmid per well in 24-well plates, or 2
µg of plasmid per well in 24-well plates, or 2 µg per well in 6-well plates). After transfection, the cells were rinsed with PBS and cultured for 48–72
µg per well in 6-well plates). After transfection, the cells were rinsed with PBS and cultured for 48–72 h in complete DMEM. Alternatively, to increase the sensitivity of Activin-A Western blots on non-reducing gels, SNs were collected from cells after 48–72
h in complete DMEM. Alternatively, to increase the sensitivity of Activin-A Western blots on non-reducing gels, SNs were collected from cells after 48–72 h in fresh OptiMEM.
h in fresh OptiMEM.
Western blot analysis
To monitor the expression and processing of Activin-A, SNs were prepared as described above either in complete DMEM for analysis on non-reducing gels, or without fetal bovine serum in OptiMEM for reducing gels. Cell lysates were obtained by treating adherent cells with trypsin/EDTA and resuspending them on ice in RIPA buffer (50 mM Tris pH 8.0, 150
mM Tris pH 8.0, 150 mM NaCl, 0.1% SDS, 0.5% Na-Deoxycholate, 1% NP-40, supplemented with protease inhibitor cocktail (A32953, Roche). Conditioned media were cleared of debris by centrifugation for 5
mM NaCl, 0.1% SDS, 0.5% Na-Deoxycholate, 1% NP-40, supplemented with protease inhibitor cocktail (A32953, Roche). Conditioned media were cleared of debris by centrifugation for 5 min at 5000 × g, followed by the addition of ice-cold acetone to precipitate proteins that were pelleted by centrifugation, air-dried, and resuspended in cell extraction buffer PBS containing 1
min at 5000 × g, followed by the addition of ice-cold acetone to precipitate proteins that were pelleted by centrifugation, air-dried, and resuspended in cell extraction buffer PBS containing 1 mM EDTA, 0.5% Triton-X100 and protease inhibitor cocktail. For loading, samples were boiled in Laemmli buffer or, for analysis on reducing gels, in Laemmli buffer containing 50
mM EDTA, 0.5% Triton-X100 and protease inhibitor cocktail. For loading, samples were boiled in Laemmli buffer or, for analysis on reducing gels, in Laemmli buffer containing 50 mM DTT. After size fractionation on 9% SDS-PAGE gels, proteins were transferred to nitrocellulose membranes using the Trans-Blot Turbo transfer system (BioRad). Membranes were blocked using 5% skim milk (Sigma) in PBS containing 0.1% Tween-20 before incubation overnight at 4
mM DTT. After size fractionation on 9% SDS-PAGE gels, proteins were transferred to nitrocellulose membranes using the Trans-Blot Turbo transfer system (BioRad). Membranes were blocked using 5% skim milk (Sigma) in PBS containing 0.1% Tween-20 before incubation overnight at 4 °C or 2
°C or 2 h at room temperature with polyclonal goat anti-Activin-A antibody (AF388, R&D Systems) or, if proteins were separated on non-reducing gels, with mouse monoclonal anti-Activin-A (1:500, Abcam ab89307), anti-myc (1:1000, Santa Cruz biotechnology sc40, clone 9e10), or anti-Flag M2 antibody (1:1000, Sigma F1804) in the same buffer. Alternatively, to detect Activin-A in immunoprecipitates or after receptor-mediated pull-down, or in tumor lysates and in plasma of tumor-bearing mice, Western blots were probed with biotinylated anti-Activin-A antibody (BAM3381, R&D Systems). After washing three times in Tris-buffered saline containing 0.1% Tween-20 to remove unbound primary antibodies, blots were incubated with the HRP-coupled secondary antibodies sheep anti-mouse (GE Healthcare), or with streptavidin-HRP labeled secondary antibody (016-030-084, Jackson Immunosearch). HRP activity was detected using the ChemiDoc imaging system (BioRad) with Super-ECL reagent to reveal Activin-A, or with ECL for all other Western blots (ThermoFisher Scientific). While all effects of furin and of conserved cysteines on Activin-A processing reported in this study were highly reproducible, the actual proportion of A60 and A30 detected in SNs of βA expressing cells fluctuated among different series of experiments conducted over the course of several years. Possible factors contributing to this variability include the cell culture medium (DMEM versus OptiMEM), duration of the medium conditioning, concentration of the sample and amount of protein loaded for SDS-PAGE, as well as the availability and use of different primary antibodies and mode of chemiluminescence detection. However, none of the conclusions of this study require that absolute amounts of stably accumulating A30 or A60 or their proportion have to be invariable across independent series of experiments.
h at room temperature with polyclonal goat anti-Activin-A antibody (AF388, R&D Systems) or, if proteins were separated on non-reducing gels, with mouse monoclonal anti-Activin-A (1:500, Abcam ab89307), anti-myc (1:1000, Santa Cruz biotechnology sc40, clone 9e10), or anti-Flag M2 antibody (1:1000, Sigma F1804) in the same buffer. Alternatively, to detect Activin-A in immunoprecipitates or after receptor-mediated pull-down, or in tumor lysates and in plasma of tumor-bearing mice, Western blots were probed with biotinylated anti-Activin-A antibody (BAM3381, R&D Systems). After washing three times in Tris-buffered saline containing 0.1% Tween-20 to remove unbound primary antibodies, blots were incubated with the HRP-coupled secondary antibodies sheep anti-mouse (GE Healthcare), or with streptavidin-HRP labeled secondary antibody (016-030-084, Jackson Immunosearch). HRP activity was detected using the ChemiDoc imaging system (BioRad) with Super-ECL reagent to reveal Activin-A, or with ECL for all other Western blots (ThermoFisher Scientific). While all effects of furin and of conserved cysteines on Activin-A processing reported in this study were highly reproducible, the actual proportion of A60 and A30 detected in SNs of βA expressing cells fluctuated among different series of experiments conducted over the course of several years. Possible factors contributing to this variability include the cell culture medium (DMEM versus OptiMEM), duration of the medium conditioning, concentration of the sample and amount of protein loaded for SDS-PAGE, as well as the availability and use of different primary antibodies and mode of chemiluminescence detection. However, none of the conclusions of this study require that absolute amounts of stably accumulating A30 or A60 or their proportion have to be invariable across independent series of experiments.
CAGA-Luciferase assay
3 × 104 HepG2 CAGA-Luc reporter cells were seeded into 96-well plates in complete DMEM and incubated for 12 h with fresh medium containing 20
h with fresh medium containing 20 ng/mL recombinant ActA (R&D Systems), or with 3- to 10-fold diluted control melanoma cell SNs, which induces CAGA-Luc expression within its dynamic range (20- to 200-fold increase above baseline) without reaching signal saturation. Where indicated, the reporter cells were co-treated with the pan-PC inhibitor CMK (100
ng/mL recombinant ActA (R&D Systems), or with 3- to 10-fold diluted control melanoma cell SNs, which induces CAGA-Luc expression within its dynamic range (20- to 200-fold increase above baseline) without reaching signal saturation. Where indicated, the reporter cells were co-treated with the pan-PC inhibitor CMK (100 µM) or empty vehicle (H2O). Cells were lysed in 50
µM) or empty vehicle (H2O). Cells were lysed in 50 μL potassium buffer (10
μL potassium buffer (10 mM) containing 0.2% Triton-X100. Five microliters of each lysate were transferred into white Nunc 96-well plates. After adding 50
mM) containing 0.2% Triton-X100. Five microliters of each lysate were transferred into white Nunc 96-well plates. After adding 50 μL
μL P/R A (Firefly) and P/R B (Renilla) reagents68, Firefly and Renilla luciferase luminescence were measured using a Centro XS3 LB960 luminometer (Berthold Technologies). Firefly luciferase measurements were normalized to Renilla values in each well on two technical replicates per experiment for each sample.
P/R A (Firefly) and P/R B (Renilla) reagents68, Firefly and Renilla luciferase luminescence were measured using a Centro XS3 LB960 luminometer (Berthold Technologies). Firefly luciferase measurements were normalized to Renilla values in each well on two technical replicates per experiment for each sample.
To monitor the inhibition of hemicleaved Activin-A by recombinant FST, SNs from 1 × 106 B16F1-βA or FurKO-βA cells were collected after 72 h and cleared of debris by centrifugation. Titration on CAGA-Luc reporter cells determined that SNs of FurKO#1-βA or control B16F1-βA had to be diluted 10-fold to match the induction of luciferase expression by 10
h and cleared of debris by centrifugation. Titration on CAGA-Luc reporter cells determined that SNs of FurKO#1-βA or control B16F1-βA had to be diluted 10-fold to match the induction of luciferase expression by 10 ng/ml of recombinant Activin-A. To compare the IC50 of the inhibition of mature versus hemicleaved Activin-A by specific inhibitors, 10× diluted SNs were incubated on HepG2 CAGA-Luc reporter cells in 96-well plates after addition of 1–500
ng/ml of recombinant Activin-A. To compare the IC50 of the inhibition of mature versus hemicleaved Activin-A by specific inhibitors, 10× diluted SNs were incubated on HepG2 CAGA-Luc reporter cells in 96-well plates after addition of 1–500 ng/ml of FST-300 (R&D Systems).
ng/ml of FST-300 (R&D Systems).
To assess BMP receptor signaling, HepG2 CAGA-Luc reporter cells were transfected with the BMP-inducible SMAD1/5 luciferase reporter BRE-Luc69. Briefly, one day after plating, HepG2 CAGA-Luc cells at a density of 5 x
x 105 cells/well in 6-well dishes were incubated for 4
105 cells/well in 6-well dishes were incubated for 4 h in OptiMEM containing 2
h in OptiMEM containing 2 µg of BRE-Luc in Lipofectamine 3000, followed by overnight culture in complete DMEM. The following day, cells were collected by trypsin/EDTA treatment and re-seeded in 96-well plates and incubated for 12
µg of BRE-Luc in Lipofectamine 3000, followed by overnight culture in complete DMEM. The following day, cells were collected by trypsin/EDTA treatment and re-seeded in 96-well plates and incubated for 12 h with 10
h with 10 ng/ml of recombinant BMP-4 (Biotechne) or Activin-A, or with 10- to 80-fold diluted B16F1- or FurKO-βA SN from 1 × 106 cells that had been cultured for 72
ng/ml of recombinant BMP-4 (Biotechne) or Activin-A, or with 10- to 80-fold diluted B16F1- or FurKO-βA SN from 1 × 106 cells that had been cultured for 72 h in complete DMEM. In addition, the reporter cells were co-treated with 100
h in complete DMEM. In addition, the reporter cells were co-treated with 100 μM CMK and, where indicated, with 10
μM CMK and, where indicated, with 10 μM SB-431542 (S4317, Sigma-Aldrich).
μM SB-431542 (S4317, Sigma-Aldrich).
For co-culture experiments, 5 × 104 B16F1 FurKO#1-βA cells were seeded in the upper or lower chamber of 24-well plates with 0.4 µm transwell inserts (Millicell). The following day, 4 × 104 HepG2-CAGA reporter cells were seeded in the other compartment. After cell lysis in 100
µm transwell inserts (Millicell). The following day, 4 × 104 HepG2-CAGA reporter cells were seeded in the other compartment. After cell lysis in 100 µL 50 μL10 mM potassium buffer containing 0.2% Triton-X100, 5
µL 50 μL10 mM potassium buffer containing 0.2% Triton-X100, 5 µL of each extract was used to measure Firefly and Renilla luciferase luminescence.
µL of each extract was used to measure Firefly and Renilla luciferase luminescence.
Analysis of ER-associated degradation
To assess ERAD, 2 × 105 cells were cultured for 12 h in 24-well plates in complete DMEM and treated with 5
h in 24-well plates in complete DMEM and treated with 5 µg/mL (17.83
µg/mL (17.83 µM) BFA (LubioScience, S7046) and/or 10
µM) BFA (LubioScience, S7046) and/or 10 µM MG-132 (Sigma-Aldrich, C2211), or empty vehicle control. Effects of BFA and MG-132 on the accumulation of Activin-A in cell lysates and SNs were analyzed by Western blot.
µM MG-132 (Sigma-Aldrich, C2211), or empty vehicle control. Effects of BFA and MG-132 on the accumulation of Activin-A in cell lysates and SNs were analyzed by Western blot.
Receptor-mediated pull-down
Lentivirally transduced HEK293T cell lines secreting AIIB-Fc, AIIA-Fc, TGFBRII-Fc, or BMPRII-Fc36, were cultured at a density of 1 × 106 cells/plate in 10 cm tissue culture dishes during 72
cm tissue culture dishes during 72 h in complete DMEM. SNs were collected, cleared from cell debris by centrifugation, and concentrated 10-fold using 4
h in complete DMEM. SNs were collected, cleared from cell debris by centrifugation, and concentrated 10-fold using 4 kDa cut-off ultrafiltration tubes (Merk Millipore). SNs containing the indicated Fc receptor fusion proteins were loaded on Pierce magnetic protein A/G beads (88802, Thermo Ficher Scientific) by overnight incubation at 4
kDa cut-off ultrafiltration tubes (Merk Millipore). SNs containing the indicated Fc receptor fusion proteins were loaded on Pierce magnetic protein A/G beads (88802, Thermo Ficher Scientific) by overnight incubation at 4 °C on a rotating wheel. The next day, beads and bound receptors were washed twice and incubated with 10-fold concentrated SNs of Ctrl- or βA-transduced B16-F1 and FurKO cells on a rotor at 4
°C on a rotating wheel. The next day, beads and bound receptors were washed twice and incubated with 10-fold concentrated SNs of Ctrl- or βA-transduced B16-F1 and FurKO cells on a rotor at 4 °C for 4
°C for 4 h. Alternatively, the protein A/G beads were incubated with concentrated SNs from transiently transfected B16-F1 and FurKO cells expressing E27 Flag-tagged βA, or GFP (control). After magnetic pull-down, beads were washed three times in buffer containing 30
h. Alternatively, the protein A/G beads were incubated with concentrated SNs from transiently transfected B16-F1 and FurKO cells expressing E27 Flag-tagged βA, or GFP (control). After magnetic pull-down, beads were washed three times in buffer containing 30 nM Tris pH 7.4, 150
nM Tris pH 7.4, 150 nM NaCl and 0.05% Nonidet P-40 to remove unbound proteins, resuspended in 50
nM NaCl and 0.05% Nonidet P-40 to remove unbound proteins, resuspended in 50 µL of 4× Laemmli buffer and boiled before loading on a non-reducing 9% SDS-PAGE gel for Activin-A Western blot analysis.
µL of 4× Laemmli buffer and boiled before loading on a non-reducing 9% SDS-PAGE gel for Activin-A Western blot analysis.
Liquid chromatography and tandem mass spectrometry (LC–MS/MS)
Samples for LC–MS/MS analysis were separated on preparative non-reducing 9% SDS-polyacrylamide gels that were stained by colloidal coomassie blue (1610803, Bio-Rad) overnight and extensively washed in Milly-Q H2O for 1 h. The indicated gel pieces were excised and washed twice with 50% ethanol in 50
h. The indicated gel pieces were excised and washed twice with 50% ethanol in 50 mM ammonium bicarbonate (AB, Sigma-Aldrich) for 20
mM ammonium bicarbonate (AB, Sigma-Aldrich) for 20 min and dried by vacuum centrifugation. Proteins were reduced with 10
min and dried by vacuum centrifugation. Proteins were reduced with 10 mM dithioerythritol (Merck-Millipore) for 1
mM dithioerythritol (Merck-Millipore) for 1 h at 56
h at 56 °C, washed and dried as described above, before alkylation with 55
°C, washed and dried as described above, before alkylation with 55 mM Iodoacetamide (Sigma-Aldrich) for 45
mM Iodoacetamide (Sigma-Aldrich) for 45 min at 37
min at 37 °C in the dark. After another wash-and-dry cycle, proteins were digested overnight at 37
°C in the dark. After another wash-and-dry cycle, proteins were digested overnight at 37 °C using MS Grade Trypsin or Chymotrypsin (Pierce) at a concentration of 12.5
°C using MS Grade Trypsin or Chymotrypsin (Pierce) at a concentration of 12.5 ng/µl in 50
ng/µl in 50 mM ammonium bicarbonate supplemented with 10
mM ammonium bicarbonate supplemented with 10 mM CaCl2. The resulting peptides were extracted for 20
mM CaCl2. The resulting peptides were extracted for 20 min twice in 70% ethanol containing 5% formic acid (Merck-Millipore), dried by vacuum centrifugation, and finally desalted on C18 Stage Tips (Pierce) as described70. For LC–MS/MS analysis, each sample was resuspended in 2% acetonitrile; 0.1% formic acid and nano-flow separations were performed on a Dionex Ultimate 3000 RSLC nano UPLC system connected online with an Exploris 480 Orbitrap Mass Spectrometer. A capillary precolumn (Acclaim Pepmap C18; 3 μm-100 Å; 2
min twice in 70% ethanol containing 5% formic acid (Merck-Millipore), dried by vacuum centrifugation, and finally desalted on C18 Stage Tips (Pierce) as described70. For LC–MS/MS analysis, each sample was resuspended in 2% acetonitrile; 0.1% formic acid and nano-flow separations were performed on a Dionex Ultimate 3000 RSLC nano UPLC system connected online with an Exploris 480 Orbitrap Mass Spectrometer. A capillary precolumn (Acclaim Pepmap C18; 3 μm-100 Å; 2 cm × 75 μm internal diameter) was used for sample trapping and cleaning. Analytical separations were performed at 250 nL/min over a 90
cm × 75 μm internal diameter) was used for sample trapping and cleaning. Analytical separations were performed at 250 nL/min over a 90 min biphasic gradient on a 50
min biphasic gradient on a 50 cm long in-house packed capillary column (75 μm ID; ReproSil-Pur C18-AQ 1.9 μm silica beads). Acquisitions were performed through Top Speed Data-Dependent acquisition mode with 2
cm long in-house packed capillary column (75 μm ID; ReproSil-Pur C18-AQ 1.9 μm silica beads). Acquisitions were performed through Top Speed Data-Dependent acquisition mode with 2 seconds cycle time and using an inclusion list of expected masses of human INHBA chain (Uniprot entry sequence: P08476). Initial MS scans were acquired at a resolution of 120,000 (200
seconds cycle time and using an inclusion list of expected masses of human INHBA chain (Uniprot entry sequence: P08476). Initial MS scans were acquired at a resolution of 120,000 (200 m/z). The most intense parent ions were selected and fragmented by High energy Collision Dissociation (HCD) with a Normalized Collision Energy (NCE) of 30%, using an isolation window of 1.7
m/z). The most intense parent ions were selected and fragmented by High energy Collision Dissociation (HCD) with a Normalized Collision Energy (NCE) of 30%, using an isolation window of 1.7 m/z. Fragmented ion scans were acquired with a resolution of 30,000 (200
m/z. Fragmented ion scans were acquired with a resolution of 30,000 (200 m/z). Selected ions were then excluded for the following 20
m/z). Selected ions were then excluded for the following 20 s. The resulting raw data were processed using SequestHT, Mascot and MS Fragger in Proteome Discoverer v.2.5 against the Uniprot Human reference proteome database (i.e., 79,052 sequences). Enzyme specificity was set to Trypsin or Chymotrypsin. Peptides were identified using a minimum of six amino acids. Up to two missed cleavages were allowed, and a 1% FDR cut-off was applied both at peptide and protein identification levels. For the database search, oxidation (M), phosphorylation (S, T, Y), acetylation (N-term Prot) and Gln to pyro Glu were considered as variable modifications, whereas carbamidomethylation (C) was set as invariable. Final data visualization used Scaffold Viewer (v. 5).
s. The resulting raw data were processed using SequestHT, Mascot and MS Fragger in Proteome Discoverer v.2.5 against the Uniprot Human reference proteome database (i.e., 79,052 sequences). Enzyme specificity was set to Trypsin or Chymotrypsin. Peptides were identified using a minimum of six amino acids. Up to two missed cleavages were allowed, and a 1% FDR cut-off was applied both at peptide and protein identification levels. For the database search, oxidation (M), phosphorylation (S, T, Y), acetylation (N-term Prot) and Gln to pyro Glu were considered as variable modifications, whereas carbamidomethylation (C) was set as invariable. Final data visualization used Scaffold Viewer (v. 5).
Fabrication and functionalization of the plasmonic biosensor
Ctrl-, βA-, or mS1-transduced B16-F1 and FurKO cell lines were cultured at a density of 1 × 106 cells/58 cm2 for 72 h in OptiMEM. Aliquots of SNs were acetone precipitated and analyzed on non-reducing gels by anti-Activin-A Western blot as described above. For plasmon resonance measurements, gold nanohole array plasmonic chips fabricated at EPFL40,41 were functionalized by precoating with anti-Activin-A antibody (BAM050, R&D Systems), or with AIIB-Fc (339-RBB-100). Plasmonic chips were manufactured using deep ultraviolet (DUV) lithography to achieve a wafer-scale and robust fabrication. In brief, after Radio Corporation of America (RCA) cleaning of a fused silica wafer, an e-beam evaporator (Alliance Concept EVA 760) was used to deposit Ti (10
h in OptiMEM. Aliquots of SNs were acetone precipitated and analyzed on non-reducing gels by anti-Activin-A Western blot as described above. For plasmon resonance measurements, gold nanohole array plasmonic chips fabricated at EPFL40,41 were functionalized by precoating with anti-Activin-A antibody (BAM050, R&D Systems), or with AIIB-Fc (339-RBB-100). Plasmonic chips were manufactured using deep ultraviolet (DUV) lithography to achieve a wafer-scale and robust fabrication. In brief, after Radio Corporation of America (RCA) cleaning of a fused silica wafer, an e-beam evaporator (Alliance Concept EVA 760) was used to deposit Ti (10 nm thickness) and Au (120
nm thickness) and Au (120 nm thickness) on the wafer. Thereafter, the wafer was coated with a photoresist, and an array of nanoholes was patterned on the surface using a DUV stepper (ASML PAS5500/300). The pattern was developed and transferred to the Au and Ti layers by ion beam etching (Oxford Instruments PlasmaLab 300), followed by a final resist removal step using oxygen plasma.
nm thickness) on the wafer. Thereafter, the wafer was coated with a photoresist, and an array of nanoholes was patterned on the surface using a DUV stepper (ASML PAS5500/300). The pattern was developed and transferred to the Au and Ti layers by ion beam etching (Oxford Instruments PlasmaLab 300), followed by a final resist removal step using oxygen plasma.
For coating with AIIB-Fc, the plasmonic biosensor surface was pre-cleaned by sequential washing with acetone, isopropanol, and Milly-Q water (5 min each) and treated with a UV-ozone cleaner for 20
min each) and treated with a UV-ozone cleaner for 20 min, followed by immersion in a 1:4 mixture of COOH/OH-functional PEGylated alkanethiols (ProChimia) in absolute ethanol that coat the surface with a conventional self-assembled monolayer (SAM). After overnight incubation, the carboxylic group on the chip surface was activated by incubation for 20
min, followed by immersion in a 1:4 mixture of COOH/OH-functional PEGylated alkanethiols (ProChimia) in absolute ethanol that coat the surface with a conventional self-assembled monolayer (SAM). After overnight incubation, the carboxylic group on the chip surface was activated by incubation for 20 min at room temperature (RT) in MES buffer (pH 6) containing 200 mM N-3-dimethylaminopropyl-N′-ethylcarbodiimide-hydrochloride and 50
min at room temperature (RT) in MES buffer (pH 6) containing 200 mM N-3-dimethylaminopropyl-N′-ethylcarbodiimide-hydrochloride and 50 mM sulfo-N-hydroxysuccinimide and then immersed with protein A/G (50
mM sulfo-N-hydroxysuccinimide and then immersed with protein A/G (50 µg/mL, Thermo Fisher Scientific) in acetate buffer (pH 4.5) for 2
µg/mL, Thermo Fisher Scientific) in acetate buffer (pH 4.5) for 2 h at RT. Unreacted carboxyl groups in the resulting covalently linked biorecognition layer were inactivated by immersion in 1
h at RT. Unreacted carboxyl groups in the resulting covalently linked biorecognition layer were inactivated by immersion in 1 M ethanolamine solution in Milli-Q water for 5
M ethanolamine solution in Milli-Q water for 5 min at RT. Thereafter, the surface of the chip was loaded with recombinant AIIB-Fc receptor (50
min at RT. Thereafter, the surface of the chip was loaded with recombinant AIIB-Fc receptor (50 µg/mL, R&D Systems, 339-RBB-100) for 2
µg/mL, R&D Systems, 339-RBB-100) for 2 h at RT on a thermomixer, followed by incubation at RT for 1
h at RT on a thermomixer, followed by incubation at RT for 1 h with 1% bovine serum albumin (BSA) as a blocking agent to reduce non-specific protein binding.
h with 1% bovine serum albumin (BSA) as a blocking agent to reduce non-specific protein binding.
For coating with Activin-A antibody, the biosensor surface was first pre-cleaned and UV treated as described above. A SAM layer was formed by immersing the chip in biotin/OH-functional PEGylated alkanethiols (ProChimia) at a molar ratio of 1:9 (2 mM final concentration) in absolute ethanol overnight at RT. Thereafter, the surface was washed for 5
mM final concentration) in absolute ethanol overnight at RT. Thereafter, the surface was washed for 5 min with ethanol three times and once with Milly-Q water, dried using pressurized nitrogen, and then incubated with streptavidin in PBS for 2
min with ethanol three times and once with Milly-Q water, dried using pressurized nitrogen, and then incubated with streptavidin in PBS for 2 h at RT. After washing three times with PBS for 5
h at RT. After washing three times with PBS for 5 min each to remove unbound streptavidin, the surface was incubated with biotinylated Activin-A antibody (40
min each to remove unbound streptavidin, the surface was incubated with biotinylated Activin-A antibody (40 µg/mL) in PBS for 2
µg/mL) in PBS for 2 h at RT on a thermomixer. After washing the surface three times with PBS (5
h at RT on a thermomixer. After washing the surface three times with PBS (5 min each), it was incubated at RT for 1
min each), it was incubated at RT for 1 h with 1% BSA solution as a blocking agent.
h with 1% BSA solution as a blocking agent.
Monitoring of surface plasmon resonance by nanoplasmonic platform
After immobilizing the desired capturing agents on the surface, the plasmonic biosensor was mounted in a polydimethylsiloxane (PDMS) microfluidic device (microchannels of 500 μm width and 180 μm height) and connected to a syringe pump for sample injection. Samples were injected into microchannels over the surface at a flow rate of 5 µL/min. For each measurement, one channel was used for the sample and one for referencing with a constant flow of PBS. Ligand binding was monitored on an imaging-based nanoplasmonic platform comprising a gold nanohole array biosensor, a narrow-band illumination source, a camera, and a microfluidic device41. In brief, after sample injections, interactions between Activin-A and the capturing agents changed the refractive index on the biosensor surface leading to redshifts in the chip transmission spectrum. The camera translated such spectral variations into intensity drops over time. The platform had a large dynamic range covering up to 12,000 arbitrary units for intensity changes without requiring prism-coupling configurations. Signals from three different regions of interest in the field of view were measured every 30
µL/min. For each measurement, one channel was used for the sample and one for referencing with a constant flow of PBS. Ligand binding was monitored on an imaging-based nanoplasmonic platform comprising a gold nanohole array biosensor, a narrow-band illumination source, a camera, and a microfluidic device41. In brief, after sample injections, interactions between Activin-A and the capturing agents changed the refractive index on the biosensor surface leading to redshifts in the chip transmission spectrum. The camera translated such spectral variations into intensity drops over time. The platform had a large dynamic range covering up to 12,000 arbitrary units for intensity changes without requiring prism-coupling configurations. Signals from three different regions of interest in the field of view were measured every 30 s and averaged to obtain the final sensograms.
s and averaged to obtain the final sensograms.
Syngeneic grafting and measurement of tumor growth
B16-F1, FurKO, or DKO cells stably expressing βA or empty control vector were injected intradermally either into the right flank of 8–12-week-old female C57BL/6J or Rag1-/- mice (Charles River laboratories) or on both sides in contralateral grafts (105 cells each). Tumors were measured with a caliper every second day. Tumor volumes were calculated using the formula V = [1.58π × (length × width)3/2]/671.
Quantification of Activin-A plasma levels by ELISA
To determine the influence of proteolytic maturation on the reactivity of Activin-A in ELISA assays, SNs from B16F1-βA or FurKO-βA cell were analyzed before and after treatment with recombinant furin. In brief, 3 μL (6 units) of soluble furin (P8077L, Biolabs) were incubated for 5
μL (6 units) of soluble furin (P8077L, Biolabs) were incubated for 5 h at 37
h at 37 °C with 300
°C with 300 μL aliquots of SNs from 1 × 106 cells that had been cultured for 72
μL aliquots of SNs from 1 × 106 cells that had been cultured for 72 h in complete DMEM. Thereafter, furin-treated and control SNs were analyzed using an anti-Activin-A ELISA kit (R&D Systems, DAC00B). To quantify circulating levels of mature Activin-A in tumor-bearing hosts, their blood was collected at the end of the experiment in heparin-coated tubes (Microvette 500 LH, SARSTEDT AG & Co.). Plasma was separated by centrifugation and aliquoted into new Eppendorf tubes and analyzed by ELISA.
h in complete DMEM. Thereafter, furin-treated and control SNs were analyzed using an anti-Activin-A ELISA kit (R&D Systems, DAC00B). To quantify circulating levels of mature Activin-A in tumor-bearing hosts, their blood was collected at the end of the experiment in heparin-coated tubes (Microvette 500 LH, SARSTEDT AG & Co.). Plasma was separated by centrifugation and aliquoted into new Eppendorf tubes and analyzed by ELISA.
Molecular modeling
To generate a model of proActivin-A dimer including all protein segments, the structure model of full-length proActivin-A was retrieved from the AlphaFold database72 (AF-A4D1W7-F1-model_v4_human Activin beta-A chain). After duplication, two single chains of the proActivin-A model were docked using the PyMOL program (Schrödinger). Briefly, the crystal structure of the mature Activin homodimer39 (PDB: 5HLZ) was used as a template to align the mature domains from the proActivin-A models in a homodimeric fashion. The resulting proActivin-A dimer model was subjected to an additional step of energy minimization based on discrete molecular dynamics using the automated Chiron protocol provided on the Dokhlab webserver73 (https://dokhlab.med.psu.edu/chiron/login.php). To generate a model of an artificial heterodimer GDF11:Activin-A bound to ActR-II and ALK5 receptor heterodimer, the known crystal structure of human GDF11 bound to ActR-II and ALK5 (PDB: 6MAC)74 was aligned with one mature subunit (residues 311-426) of our proActivin-A dimer model (Fig. S6A). After alignment, the mature subunit superimposed with GDF11, as well as the two prodomains were removed (Fig. S6B). For all the figures, the final views were rendered on PyMOL.
Statistical analysis
In all experiments that were repeated at least three time, statistical tests were performed using the Prism software (GraphPad) to show the mean ±
± SEM or SD as indicated in the figure legends. When comparing two groups, normal distributions were analyzed by the Shapiro-Wilk normality test. P-values were calculated by Student’s t-test (normal distribution) or Mann–Whitney’s test (non-parametric test). One-way ANOVA was used to compare several groups of unpaired values. Data points identified as outliers by the regression and outlier (ROUT) removal method in Prism 9 with a false discovery rate ≤1% were excluded. Power Analysis was waved by the animal experimentation authorities due to pre-existing data about the effect sizes of Activin-A on tumor growth and on the associated loss of body weight. Tumor volumes at the endpoint were compared by ANOVA or Student’s t-test, as indicated in the figure legends.
SEM or SD as indicated in the figure legends. When comparing two groups, normal distributions were analyzed by the Shapiro-Wilk normality test. P-values were calculated by Student’s t-test (normal distribution) or Mann–Whitney’s test (non-parametric test). One-way ANOVA was used to compare several groups of unpaired values. Data points identified as outliers by the regression and outlier (ROUT) removal method in Prism 9 with a false discovery rate ≤1% were excluded. Power Analysis was waved by the animal experimentation authorities due to pre-existing data about the effect sizes of Activin-A on tumor growth and on the associated loss of body weight. Tumor volumes at the endpoint were compared by ANOVA or Student’s t-test, as indicated in the figure legends.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Acknowledgements
The authors are grateful to Drs. Diego Chiappe and Maria Pavlou of the EPFL Proteomics core facility for their support with the analysis and identification of Activin-A processing intermediates. We also would like to thank the members of the Center of PhenoGenomics for the animal housing. This work was supported by grants 31003A_179330 of the Swiss National Science foundation and KFS-4454-02-2018 from the Swiss Cancer League to DBC, and by services of the Center of PhenoGenomics and Flow Cytometry Research Core Facilities at the School of Life Sciences of EPFL.
Author contributions
Conceptualization and methodology: K.P., M.B., P.G., H.A., D.B.C.; formal analysis: K.P., M.B, B.R., S.A., Y.L., C.S.; validation: K.P., M.B.; visualization and writing: K.P., M.B., B.R., D.B.C; review and editing, K.P., M.B., S.A., P.G., C.S., D.B.C.; supervision, project administration, funding acquisition: H.A., D.B.C.; guarantor: D.B.C.
Peer review
Peer review information
Communications Biology thanks Michael Grusch and the other, anonymous, reviewer for their contribution to the peer review of this work. Primary Handling Editor: Christina Karlsson Rosenthal. A peer review file is available.
Data availability
All the data are made available in the main figures and supplementary materials. Uncropped images of all Western blots in this paper are shown in Supplementary Fig. S7. The source data behind the graphs can be found in Supplementary Data 1.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Katarina Pinjusic, Manon Bulliard.
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-024-07053-0.
References
Articles from Communications Biology are provided here courtesy of Nature Publishing Group
Citations & impact
This article has not been cited yet.
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/169630637
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Recombinant BMP4 and BMP7 increase activin A production by up-regulating inhibin βA subunit and furin expression in human granulosa-lutein cells.
J Clin Endocrinol Metab, 100(3):E375-86, 06 Jan 2015
Cited by: 15 articles | PMID: 25562508
An activin/furin regulatory loop modulates the processing and secretion of inhibin alpha- and betaB-subunit dimers in pituitary gonadotrope cells.
J Biol Chem, 283(48):33059-33068, 30 Sep 2008
Cited by: 19 articles | PMID: 18826955 | PMCID: PMC2586270
Notch1 Autoactivation via Transcriptional Regulation of Furin, Which Sustains Notch1 Signaling by Processing Notch1-Activating Proteases ADAM10 and Membrane Type 1 Matrix Metalloproteinase.
Mol Cell Biol, 35(21):3622-3632, 17 Aug 2015
Cited by: 28 articles | PMID: 26283728 | PMCID: PMC4589600
New insights into the mechanisms of activin action and inhibition.
Mol Cell Endocrinol, 359(1-2):2-12, 08 Jul 2011
Cited by: 59 articles | PMID: 21763751
Review
Funding
Funders who supported this work.
Swiss National Science Foundation (1)
Grant ID: 179330

 1
1

