Abstract
Background
Worldwide, mild traumatic brain injury, synonymous with concussion, affects more than 30-50 million each year. The incidence of concussion in Denmark is estimated to be about 20,000 yearly. Although complete resolution normally occurs within a few weeks, up to a third develop persistent post-concussion symptoms (PPCS) beyond 3 months. Evidence for effective treatment strategies is scarce. The objective of this study is to evaluate the efficacy of the novel intervention GAIN Lite added to enhanced usual care (EUC) for adults with mild-to-moderate PPCS compared to EUC only.Methods
An open-label, parallel-group, two-arm randomised controlled superiority trial (RCT) with 1:1 allocation ratio. Potential participants will be identified through the hospital's Business Intelligence portal of the Central Denmark Region or referred by general practitioners within 2-4 months post-concussion. Participants with mild-to-moderate PPCS will be randomly assigned to either (1) EUC or (2) GAIN Lite added to EUC. GAIN Lite is characterised as a complex intervention and has been developed, feasibility-tested and process evaluated before effect evaluation in the RCT. GAIN Lite contains an initial remote interview, self-administrated e-learning videos and voluntary remote counselling with an allocated occupational- or physiotherapist. Sixty-six participants will be recruited to each group. Primary outcomes are mean changes in PPCS and limitations in daily life from baseline to 24 weeks after baseline.Discussion
GAIN Lite is a low-intensity intervention for adults with mild-to-moderate PPCS. Offering a remote intervention may improve access to rehabilitation and prevent chronification for individuals with mild-to-moderate PPCS. Moreover, GAIN Lite will facilitate access to healthcare, especially for those with transportation barriers. Overall, GAIN Lite may provide an accessible, flexible and convenient way to receive treatment based on sound theories and previous evidence of effective interventions for adults with mild-to-moderate PPCS.Trial registration
ClinicalTrials.gov NCT05233475. Registered on February 10, 2022.Free full text

A remotely delivered intervention targeting adults with persisting mild-to-moderate post-concussion symptoms (GAIN Lite): a study protocol for a parallel group randomised trial
Abstract
Background
Worldwide, mild traumatic brain injury, synonymous with concussion, affects more than 30–50 million each year. The incidence of concussion in Denmark is estimated to be about 20,000 yearly. Although complete resolution normally occurs within a few weeks, up to a third develop persistent post-concussion symptoms (PPCS) beyond 3 months. Evidence for effective treatment strategies is scarce. The objective of this study is to evaluate the efficacy of the novel intervention GAIN Lite added to enhanced usual care (EUC) for adults with mild-to-moderate PPCS compared to EUC only.
Methods
An open-label, parallel-group, two-arm randomised controlled superiority trial (RCT) with 1:1 allocation ratio. Potential participants will be identified through the hospital’s Business Intelligence portal of the Central Denmark Region or referred by general practitioners within 2–4 months post-concussion. Participants with mild-to-moderate PPCS will be randomly assigned to either (1) EUC or (2) GAIN Lite added to EUC. GAIN Lite is characterised as a complex intervention and has been developed, feasibility-tested and process evaluated before effect evaluation in the RCT. GAIN Lite contains an initial remote interview, self-administrated e-learning videos and voluntary remote counselling with an allocated occupational- or physiotherapist. Sixty-six participants will be recruited to each group. Primary outcomes are mean changes in PPCS and limitations in daily life from baseline to 24 weeks after baseline.
Discussion
GAIN Lite is a low-intensity intervention for adults with mild-to-moderate PPCS. Offering a remote intervention may improve access to rehabilitation and prevent chronification for individuals with mild-to-moderate PPCS. Moreover, GAIN Lite will facilitate access to healthcare, especially for those with transportation barriers. Overall, GAIN Lite may provide an accessible, flexible and convenient way to receive treatment based on sound theories and previous evidence of effective interventions for adults with mild-to-moderate PPCS.
Trial registration
ClinicalTrials.gov NCT05233475. Registered on February 10, 2022.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13063-024-08546-3.
Introduction
Background and rationale {6a}
Worldwide, mild traumatic brain injury, synonymous with concussion, affects more than 30–50 million each year [1, 2]. Based on hospital data, the incidence of concussion in Denmark is estimated to be about 20,000 yearly [3], although the true number may be higher.
Although complete resolution normally occurs within a few weeks, up to a third develop persistent post-concussion symptoms (PPCS) beyond 3 months [4]. PPCS is a term used to describe a constellation of ongoing physical, cognitive and emotional sequelae associated with concussion [5]. Concussion is associated with substantial ongoing disability, distress and limitations for the individual in daily life, as well as with high societal costs in general and high healthcare costs in particular [6–10].
Recovery from concussion is influenced by various biological, psychological and social factors [11–14]. The individual’s beliefs about their injury and behavioural responses can play important roles in the development of PPCS, suggesting these may be potential targets for early preventive interventions [14]. Evidence for effective treatment strategies is scarce, resulting in only weak recommendations for most treatment approaches, as reflected in the recently published Danish National Guidelines for Non-Pharmacological Treatment for PPCS [15, 16].
Wasteful costs in the healthcare system due to overtreatment could be avoided by substituting cheaper alternatives with equivalent or stronger benefits [17]. As the role of technology in healthcare delivery is rapidly expanding [18], remote delivery could be a pragmatic way to match an intervention to the severity of symptoms. Telehealth interventions to prevent PPCS have demonstrated promising results and are likely to be cost-effective with regard to healthcare usage and return to work [19–21]. Furthermore, telehealth interventions may improve access to appropriate rehabilitation for people residing in remote and rural areas [22]. Yet, many opportunities remain unexplored; among others, the use of technology as an intervention modality across the continuum of concussion care [23].
Thastum et al. developed the ‘Get going After concussIoN’ (GAIN), an 8-week intervention for people with PPCS, based on principles from cognitive behavioural therapy and graded exercise therapy [24]. They evaluated GAIN in in a randomised controlled trial and found a significantly larger reduction in post-concussion symptoms 3 months post-intervention compared to participants receiving enhanced usual care (EUC) only. Based on these encouraging findings, a large research initiative was established in part to evaluate the community-based implementation of GAIN and to develop a low-resource, remote-delivered, cost-effective version of GAIN for adults with relatively mild symptoms, termed GAIN Lite [25, 26].
Objectives {7}
The objective of this study is to evaluate the efficacy of GAIN Lite added to EUC for adults with mild-to-moderate PPCS compared to EUC only. It is hypothesised that GAIN Lite added to EUC is superior to EUC only. The primary outcomes are self-reported PPCS and self-reported limitations in daily life from baseline to 24 weeks after baseline.
Trial design {8}
The present study is an open-label, parallel-group, two-arm randomised controlled superiority trial (RCT) with 1:1 allocation ratio. Participants will be randomly assigned to either (1) EUC or (2) GAIN Lite added to EUC. GAIN Lite is characterised as a complex intervention and has been developed, feasibility-tested and process evaluated before effect evaluation in a RCT [27, 28].
Methods: participants, interventions and outcomes
Study setting {9}
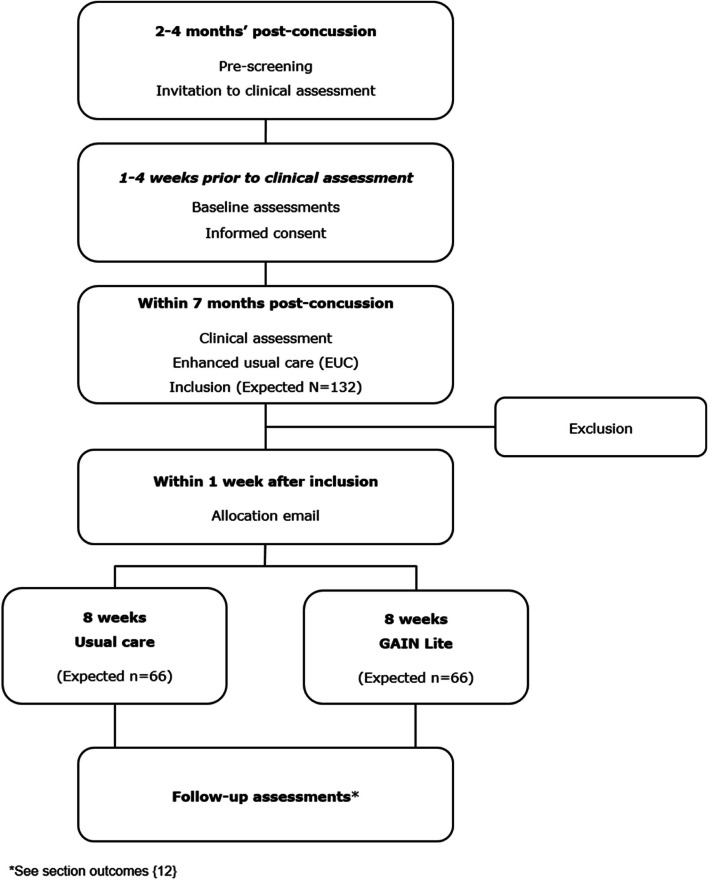
The present study is conducted in Central Denmark Region (population 1.3 million) and led by Hammel Neurorehabilitation Centre and University Research Clinic (HNC). GAIN Lite will be embedded in an epidemiologic cohort study which includes adults diagnosed with concussion at emergency wards in the Central Denmark Region and adults referred to the project by general practitioners (GPs). The cohort is followed by means of questionnaires and health registers. From January 2023 to December 2024, a sub-sample of adults with PPCS will be recruited from the cohort to the present study.
Eligibility criteria {10}
At enrolment, a clinical assessment of all participants is performed by physicians at HNC to verify the diagnosis and to ensure eligibility criteria are met. The clinical assessment consists of a neurological examination and a short, standardised medical history interview. Non-eligible participants are recommended to contact their GP for further advice if needed. Participants are recruited based on the following inclusion criteria: (1) Concussion caused by a head trauma according to the diagnostic criteria recommended by the Danish Consensus Report on Commotio Cerebri [29]. The criteria are based on recommendations by the World Health Organization (WHO) Task Force [2], but with the amendment that there must have been direct contact between the head and an object to rule out acceleration–deceleration traumas; (2) Age 18 to 60 years at the time of the trauma; (3) Mild-to-moderate PPCS defined by a total score of 10–30 on the Rivermead Post-Concussion Symptom Questionnaire (RPQ) [30] within 1 week before enrolment in the study. As there is no universal sub-division of PPCS, the definition emerged after several discussions in the research group. The definition is roughly consistent with the mild-to-moderate categories by Potter et al. [31]; (4) Able to understand, speak and read Danish; (5) Living in Central Denmark Region and (6) Identified from registers of the emergency departments or referred by GPs within 2–4 months after a concussion. Exclusion criteria are (1) Objective neurological findings; (2) Previous concussion within the last 2 years with PPCS at the time of the present concussion; (3) Severe misuse of alcohol, prescription drugs and/or illegal drugs and (4) Severe psychiatric co-morbidity (e.g. bipolar disorder, autism, psychotic disorder (lifetime)) or severe neurological disease (e.g. multiple sclerosis) that impedes participation in the programme.
Who will take informed consent {26a}
Prior to the clinical assessment and inclusion in GAIN Lite, written informed consent will be obtained from each participant. Informed consent is based on written information about GAIN Lite, which is emailed to potential participants prior to the clinical assessment. Furthermore, a physician will provide verbal information on the project through a video or telephone consultation 1–4 weeks before the clinical assessment. In case of no prior consultation, verbal information will be provided by the designated physician just before the clinical assessment. The physicians have no role in delivering EUC or GAIN Lite.
Additional consent provisions for collection and use of participant data and biological specimens {26b}
No biological specimens will be collected during the present study.
Explanation for the choice of comparators {6b}
Evidence-based recommendations for effective non-pharmacological treatment for people with PPCS are sparse [15]. However, early written or oral information, reassurance and advice are recommended [15, 32]. Hence, providing EUC as standard treatment to all participants is considered most ethically sound for current participants, although potentially limiting the possibility to demonstrate a potential treatment effect of GAIN Lite for future people with mild to moderate PPCS.
Intervention description {11a}
Enhanced usual care—EUC
Immediately after the clinical assessment, all participants are shortly introduced to a biopsychosocial understanding of PPCS [33], advised to gradually resume premorbid activities and are introduced to balance activity and rest [34, 35]. Furthermore, reassurance that a good prognosis is expected is given. The short introduction and advice are considered to be EUC and is performed by a health professional with knowledge about concussion, the development of PPCS and the principles of GAIN Lite. EUC has a duration of maximum 30 min. For a summary of EUC see Fig. Fig.11.
GAIN Lite
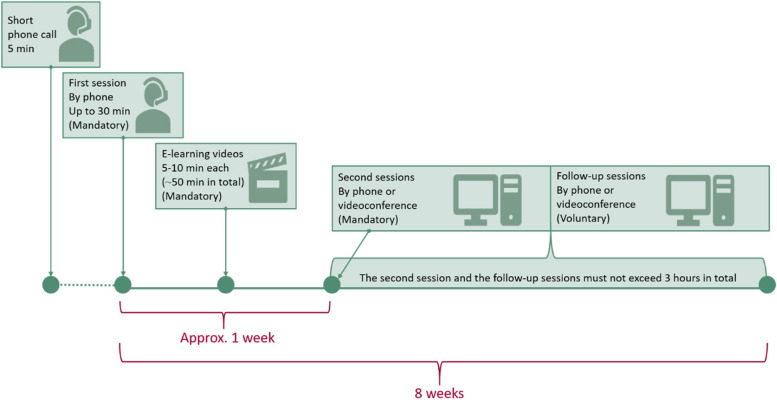
The add-on GAIN Lite intervention is a remote programme based on the treatment principles and content from GAIN with a duration of 8 weeks [24]. The intervention is overall intended to reduce PPCS and decrease limitations in daily life by modifying symptom-perpetuating illness cognition and behaviours. The intervention contains an initial remote interview, self-administrated e-learning videos and voluntary remote counselling with an allocated occupational- or physiotherapist. The timeline of GAIN Lite is illustrated in Fig. Fig.22.
Within a week after randomisation, the allocated therapist makes a short phone call to arrange the programme start. Typically, the first session (by phone) takes place within 2 weeks after the short phone call and aims to facilitate therapeutic alliance and introduce the e-learning videos. The e-learning videos take the participants through a 6-stage programme. Each video has a duration of 5–10 min including reflective questions. The link to the videos is emailed immediately after the first session and can be accessed by the participants at any time throughout the 8-week intervention period. The second session (by phone or videoconference) takes place about a week after the link has been emailed and aims to link the participant’s reflections from the videos to the participant’s daily life, including illumination of individually important goal(s). The voluntary subsequent follow-up sessions (by phone or videoconference) are targeted to change symptom perpetuating illness cognition and behaviour in relation to the individual’s context and goal(s). The number and content of the individual sessions are based on shared decision-making, but it is recommended that each session has a maximum duration of 45 min. Furthermore, the upper limit of the individual sessions is 3 h during the 8-week intervention period. For a summary of the content in GAIN Lite see Fig. Fig.33.
Therapists experienced in health promotion, health education and/or in the facilitation of health behaviour change will facilitate the individual sessions. Furthermore, the therapists will be trained in basic principles of a cognitive behavioural approach according to the treatment manual of GAIN Lite [36–40].
To ensure fidelity and adherence to the intervention principles, a treatment manual has been developed. Furthermore, the therapists will receive 1 h of monthly supervision by a neuropsychologist who is an expert on PPCS and trained in supervision. Therapist meetings to share experiences and discuss practical issues are also planned monthly. Furthermore, the therapists are encouraged to reflect on their feedback and guidance after each session using a worksheet designed specifically for the present study. The treatment manual and the e-learning videos are available upon reasonable request to the corresponding author.
Criteria for discontinuing or modifying allocated interventions {11b}
There will be no specific criteria for discontinuing or modifying allocated interventions. However, participants will be informed that they have the right to withdraw their consent and reject further participation in GAIN Lite at any time without any consequences.
Strategies to improve adherence to interventions {11c}
At the first session, participants are encouraged to watch the videos before the second session. Adherence to the videos is logged, and the therapists can see if the participants played the videos. Moreover, the allocated therapist will send reminders by SMS or email to participants 1–2 days before each session. SMS is the preferred method. However, reminders are sent by email in advance of videoconferences since a conference link is included.
Relevant concomitant care permitted or prohibited during the trial {11d}
Participants are free to continue or seek other additional treatment during the period. A questionnaire on other treatments is emailed to participants at 24- and 36-week follow-up.
Provisions for post-trial care {30}
Based on feasibility tests of GAIN Lite and the original GAIN study, there is no anticipated harm. No compensation is provided for trial participation.
Outcomes {12}
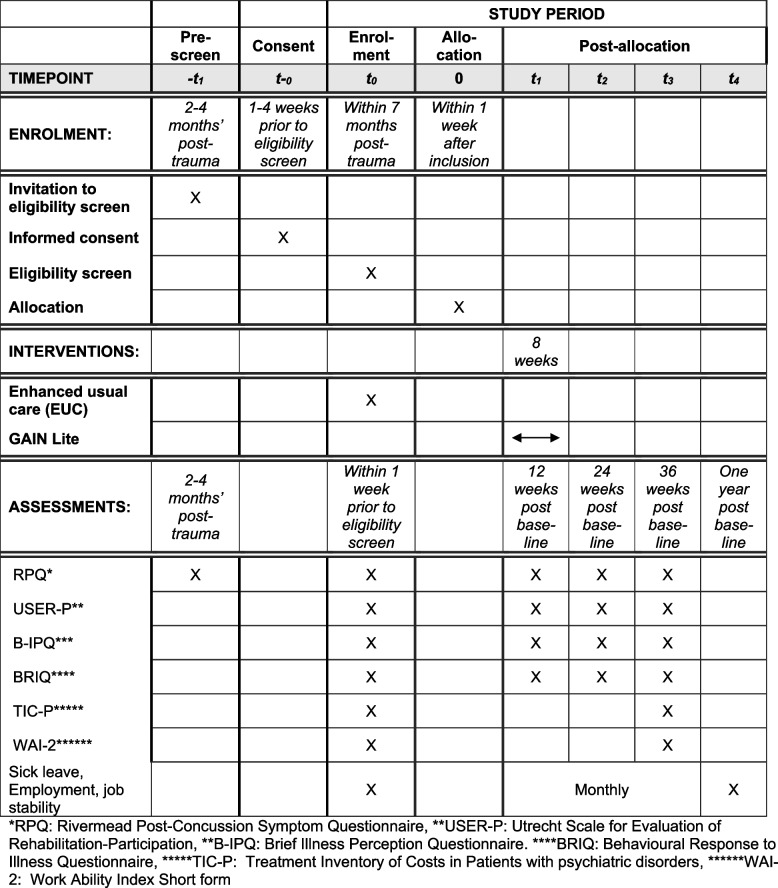
The primary outcomes are mean changes in PPCS and limitations in daily life from baseline to 24 weeks after baseline (time frame: 6 months). Secondary outcomes are mean changes in illness cognition and illness behaviour from baseline to 12 weeks after baseline (time frame: 3 months) and in sickness absence and work ability from baseline to 36 weeks after baseline (time frame: 9 months). PPCS, limitation in daily life, illness cognition and illness behaviour are measured at four time points: at baseline, 12 (end of intervention), 24 and 36 weeks after baseline. Additionally, sickness absence and work ability will be measured at baseline and 36 weeks after baseline. Furthermore, register-based data will provide additional measures during the first year after baseline.
The register-based data include monthly proportion of participants on sick leave (defined as public assistance benefits related to illness), monthly proportion of employed participants (defined as receiving no public assistance benefits except from state education fund grants monthly) and monthly degree of job stability (based on whether labour market contributions have been paid). All outcome measures and data collection time points are shown in Table Table11.
Table 1
Data collection time points
| Data source | Outcome | T-0 | T0 | T1 | T2 | T3 | T4 | |
|---|---|---|---|---|---|---|---|---|
| Pre-screening | Baseline assessments | Follow-up assessments | ||||||
| 2-4 months after the trauma | 1-4 weeks prior to clinical assessment/EUC | 12 weeks post baseline | 24 weeks post baseline | 36 weeks post baseline | One year post baseline | |||
| Primary outcome | Questionnaire | • PPCS is measured by the Rivermead Post-Concussion Symptom Questionnaire (RPQ/DK) (16 items) | X | X | X | Xa | X | |
| Questionnaire | • Limitation in daily life is measured by the Utrecht Scale for Evaluation of Rehabilitation-Participation (USER-P/DK), part 2 (11 items) | X | X | Xa | X | |||
| Secondary outcomes | Questionnaire | • Illness cognition is measured by the Brief Illness Perception Questionnaire (B-IPQ/DK) (8 items) | X | Xb | X | X | ||
| Questionnaire | • Illness behaviour is measured by the Behavioural Response to Illness Questionnaire (BRIQ/DK), part 1 & 2 (13 items) | X | Xb | X | X | |||
| Questionnaire | • Sickness absence is measured by the Treatment Inventory of Costs in Patients with psychiatric disorders (TiC-P) (2 items) | X | Xb | |||||
| Questionnaire | • Work ability is measured by Work Ability Index Short form (WAI-2) (1 items) | X | Xb | |||||
| Additional outcomes | DREAM data | • Sick leave | X | Monthly | Xc | |||
| DREAM data | • Employment | X | Monthly | Xc | ||||
| DREAM data | • Job stability | X | Monthly | Xc | ||||
aPrimary outcomes: mean changes in PPCS and limitations in daily life from baseline to 24 weeks after baseline (time frame: 6 months)
bSecondary outcomes: mean changes in illness cognition and illness behaviour from baseline to 12 weeks after baseline (time frame: 3 months) and in sickness absence and work ability from baseline to 36 weeks after baseline (time frame: 9 months)
cMonthly the first year post-baseline
Participant timeline {13}
Potential participants will be identified through the hospital’s Business Intelligence (BI) portal of the Central Denmark Region or referred by GPs within 2–4 months post-concussion. Baseline questionnaires will be answered within 1 week before the clinical assessment. The clinical assessment is performed as soon as possible after informed consent and no later than 7 months after the trauma. Immediately after the clinical assessment, participants receive EUC. Allocation is emailed to participants a week after the clinical assessment. The participant timeline is illustrated in Fig. Fig.44.
Sample size {14}
In the present study, RPQ and the Utrecht Scale for Evaluation of Rehabilitation-Participation (USER-P) are used to measure the primary outcomes. The sample size calculation is based on two studies: the original GAIN study [24] and a study by van der Zee et al. [41]. To account for having to primary outcomes, we set a 2.5% threshold for significance level to adjust for multiple testing (Bonferroni’s correction) [42]. In the original GAIN study, the control group had a mean of 37.4 (SD 7.4) on the RPQ total score at baseline. Furthermore, a mean difference of 7.6 points between treatment groups (EUC and EUC +
+ GAIN) was found on the RPQ total score 3 months after end of treatment. Since the spectrum of symptoms in this present study is lower compared to the original GAIN study, the best guess is that we will be able to detect a difference of 4 points in RPQ total score 24 weeks after baseline. For the same reason, the sample size estimated in the present study is based on the SD from the control group at baseline in the original GAIN study. To detect a mean difference in RPQ total score of 4 points (SD
GAIN) was found on the RPQ total score 3 months after end of treatment. Since the spectrum of symptoms in this present study is lower compared to the original GAIN study, the best guess is that we will be able to detect a difference of 4 points in RPQ total score 24 weeks after baseline. For the same reason, the sample size estimated in the present study is based on the SD from the control group at baseline in the original GAIN study. To detect a mean difference in RPQ total score of 4 points (SD =
= 7) at 24-week follow-up with a two-sided significance level of 2.5% and power of 80% with equal allocation to two arms would require 60 participants in each arm. To allow for 10% dropout, 66 will be recruited per arm, i.e. 132 in total. The scientific literature on which the sample size calculation of USER-P could be based on is sparse. However, in the study by van der Zee et al. [41], a mean difference of 5.4 (SD 17.2) was found on the restriction scale from baseline to 4-month follow-up in a population with neurological diseases receiving a rehabilitation programme. Based on the study by van der Zee et al., but taking into consideration that the sample included in this present study is less impaired and without degenerative diseases, it is assumed that it is possible to detect a difference in USER-P total score of 10 points. To detect a mean difference in USER-P total score of 10 points (SD
7) at 24-week follow-up with a two-sided significance level of 2.5% and power of 80% with equal allocation to two arms would require 60 participants in each arm. To allow for 10% dropout, 66 will be recruited per arm, i.e. 132 in total. The scientific literature on which the sample size calculation of USER-P could be based on is sparse. However, in the study by van der Zee et al. [41], a mean difference of 5.4 (SD 17.2) was found on the restriction scale from baseline to 4-month follow-up in a population with neurological diseases receiving a rehabilitation programme. Based on the study by van der Zee et al., but taking into consideration that the sample included in this present study is less impaired and without degenerative diseases, it is assumed that it is possible to detect a difference in USER-P total score of 10 points. To detect a mean difference in USER-P total score of 10 points (SD =
= 17) at 24-week follow-up with a two-sided significance level of 2.5% and power of 80% with equal allocation to two arms would require 57 participants in each arm. To allow for 10% dropout, 63 will be recruited per arm, i.e. 126 in total. In sum, 66 participants in each arm are recruited to ensure both sample size calculations are met.
17) at 24-week follow-up with a two-sided significance level of 2.5% and power of 80% with equal allocation to two arms would require 57 participants in each arm. To allow for 10% dropout, 63 will be recruited per arm, i.e. 126 in total. In sum, 66 participants in each arm are recruited to ensure both sample size calculations are met.
Recruitment {15}
Citizens who are diagnosed with an ICD-10 diagnosis of concussion (DS060) at emergency wards in the Central Denmark Region will be identified for the epidemiologic cohort study through the hospital’s Business Intelligence (BI) portal of the Central Denmark Region. In order to recruit participants with concussion without hospital contact, GPs in the region are invited to refer participants to the project. Information concerning the study will be shared on the GPs’ internet platform, through the GPs’ regional coordinator and at the HNC’s website. Based on experience from the original and the up-scaled GAIN studies [24, 25], it is expected that half of the study population for the present study will be recruited from a hospital and half referred by GPs. Data from May 2021 to October 2022 in the epidemiologic cohort study revealed 290 citizens with an RPQ between 10 and 30 who accepted to participate in other research studies. Thus, there was an average of 16 potential participants each month. However, about one third did not meet the inclusion criteria and were excluded in the original and the up-scaled GAIN studies [24, 25], which is also expected to be the case in GAIN Lite. This leaves about 10 potential participants each month which supports the expectation that at least 132 participants may be recruited during the 2-year recruitment period.
Assignment of intervention: allocation
Sequence generation {16a}
A Research Electronic Data Capture (REDCap) administrator (not involved in any other study procedures) is creating a block randomisation with varying block sizes in REDCap Randomisation Module. The allocation ratio will be 1:1 without stratification, and the block sizes will not be disclosed, to ensure concealment.
Concealment mechanism {16b}
To conceal allocation, the randomisation table uploaded in REDCap is only accessible to the REDCap administrator throughout the study. Allocation concealment will be ensured, as randomisation will not take place until the participant has been included in the study after all baseline measurements, the clinical assessment and EUC have been completed.
Implementation {16c}
Within a week after inclusion in the study, the principal investigator (PI) will randomise the participants to either (1) EUC or (2) GAIN Lite added to EUC by the generated randomisation button in REDCap. Subsequently, REDCap will send an auto-generated allocation email to the participants, and the PI informs the allocated therapist.
Assignment of interventions: blinding
Who will be blinded {17a}
The physicians assessing for eligibility and the health professionals performing EUC are blinded to the randomisation sequence. Only the REDCap administrator and the project data manager have full access to the collected data and will not be involved in primary outcome analysis. The primary data analyser will be masked to whether a specific participant received the intervention but will not be masked during post hoc analyses. Due to the nature of the intervention, neither participants nor providers can be blinded to allocation.
Procedure for unblinding if needed {17b}
Not relevant in the present study.
Data collection and management
Plans for assessment and collection of outcomes {18a}
REDCap will send auto-generated emails with a link to all the self-reported follow-up questionnaires. Data collection time points are shown in Table Table11.
Primary outcomes
PPCS will be measured by the Rivermead Post-Concussion Symptom Questionnaire (RPQ/DK) [30] which is a widely used self-report inventory of post-concussion symptoms. RPQ/DK measures the severity of current symptoms covering physical, cognitive and emotional symptoms with 16 items on a five-point ordinal scale from 0 (‘not experienced at all’) to 4 (‘a severe problem’). In accordance with the standard scoring method, a score of 1 corresponding to ‘no more of a problem’ will be assigned the value 0. The RPQ total score range from 0 to 64. The validity and reliability for the RPQ total score have been found to be adequate to excellent [30, 43–45].
Limitation in daily living is measured by the Utrecht Scale for Evaluation of Rehabilitation-Participation (USER-P/DK) [46] which is designed to measure aspects of functional independence. USER-P/DK is a self-report questionnaire that covers aspects of participation with three separate scales: frequency, restrictions and satisfaction. The restrictions subscale will be used. It contains 11 items that are based on whether the participant experiences any participation limitations in vocational, leisure and social activities as a result of the person’s health or disability. Each item score ranges from 0 (not possible at all) to 3 (independent without difficulty). A ‘not applicable’ option is available for each item and can be used in case the item is not relevant to the person or if experienced restrictions are not related to the person’s health status or disability. A sum score is converted to a 0–100 scale. A higher score indicates less restriction in participation. The restriction scale has been found to be a valid measure in people with physical disabilities [46].
Secondary outcomes
Illness cognition is measured on the Brief Illness Perception Questionnaire (B-IPQ/DK) [47] which consists of eight self-reported items on an individual’s cognitive and emotional representation of one’s health condition on a 0-to-10 numerical rating scale. Using the sum score of the items, the total score ranges from 0 to 80. A higher score reflects more symptom-perpetuating illness perception. B-IPQ has demonstrated good psychometric properties in different illness groups [47–49].
Illness behaviour is measured on the Behavioural Response to Illness Questionnaire (BRIQ/DK) [50] which is designed to measure self-imposed general activity following illness. Two subscales were applied: the 7-item subscale ‘all-or-nothing behaviour’ and the 6-item subscale ‘limiting behaviour’. The total range is 0–65 on a 0–5 response scale. A higher score indicates that the participant engaged in the behaviour more frequently. BRIQ is a valid and reliable measure that can predict the development of medically unexplained syndrome after acute infection [50].
Sickness absence is measured on the Treatment Inventory of Costs in Patients with psychiatric disorders (TiC-P/DK), part II, question 4 [51]. TiC-P/DK is a self-reported measure designed to assess direct and indirect costs associated with mental health. Question 4 contains 2 items on respondents’ workplace absenteeism and/or reduction in productivity in paid or unpaid work due to mental health illnesses in the past month. Both the reliability and validity of TiC-P have been found to be fair to good in people with mild to moderate mental health problems [52].
Work ability is measured on Work Ability Index Short form (WAI-2/DK) [53] which is designed to describe how capable an employee is of doing his/her job. The first question (WAI 1) ‘Current work ability compared with lifetime best’ is used. The question it to be answered on a 0–10 scale. WAI-2 is found to be suitable measure for work ability [54].
Additional outcomes
Register-based data will be assessed using the Danish Register for Evaluation of Marginalisation (DREAM) [55]. The register contains weekly information on social transfer payments for all residents of Denmark (since 1996). Access to the register-based data requires separate approval from Statistics Denmark according to the legislation in Denmark.
Plans to promote participant retention and complete follow-up {18b}
Responses to the self-reported follow-up questionnaires will be observed closely by the project data manager and the PI. If no or incomplete response to the questionnaires, participants will be contacted twice by email.
Data management {19}
Study data are collected and managed using REDCap, which is a secure web-based software platform designed to support data capture for research studies hosted by Aarhus University [52]. REDCap provides (1) an intuitive interface for validated data capture; (2) audit trails for tracking data manipulation and export procedures; (3) automated export procedures for seamless data downloads to common statistical packages and (4) procedures for data integration and interoperability with external sources. The validation features (e.g. range checks for data values) in REDCap will be used in all questionnaire set-ups. The project data manager administers REDCap access within the REDCap system during the whole project.
Confidentiality {27}
The present study will be carried out in accordance with the rules of the Helsinki Declaration II and adhere to the Danish Data Protection Agency. All data collection and management are in compliance with the General Data Protection Regulation guidelines. Handling of data will be conducted according to general guidelines for encryption and anonymisation. Data to be analysed locally will be extracted from REDCap and immediately uploaded to MidtX which is a secure digital collaboration platform designed by Central Denmark Region to ensure secure storage of data from research and quality projects. Data to be merged with register-based data will be extracted from REDCap and immediately uploaded to the research computers at Statistics Denmark using the Statistics Denmark encrypted upload function.
Plans for collection, laboratory evaluation and storage of biological specimens for genetic or molecular analyses in this trial/future use {33}
The present study does not involve biological specimens.
Statistical methods
Statistical methods for primary and secondary outcomes {20a}
Descriptive statistics of baseline characteristics will be displayed by treatment groups. The average treatment effect will be evaluated with-in and between-groups (GAIN Lite against the EUC group) on intention-to-treat basis. The confidence intervals will be set to 95%, and the significance level will be set to 2.5% for the primary outcomes. For the secondary outcomes, the significance level of 5% will be divided by the number of independent tests. A statistician will be continuously involved in the project.
Primary outcomes
The primary outcomes are mean changes in total scores on RPQ and USER-P from baseline to 24 weeks after baseline. By adhering to the recommendations for primary analysis of continuous endpoints in longitudinal clinical trials, a mixed model for repeated measures (MMRM) will be used [56]. In details, mean changes from baseline will be analysed using a restricted maximum likelihood (REML) based on a repeated measures approach. The linear mixed model includes the fixed, categorical effects of assigned treatment group (EUC +
+ GAIN Lite vs EUC) and measure (pre-screening (only RPQ), baseline assessment, 12 weeks post-baseline, 24 weeks post-baseline and 36 weeks post-baseline) and treatment group by measure interaction (basic model). The random part of the model included a random intercept of participant (unstructured covariance structure within participants). The primary treatment comparison will be the contrast between treatment groups 24 weeks post-baseline and baseline. The treatment effect will be analysed using both basic and adjusted linear mixed models.
GAIN Lite vs EUC) and measure (pre-screening (only RPQ), baseline assessment, 12 weeks post-baseline, 24 weeks post-baseline and 36 weeks post-baseline) and treatment group by measure interaction (basic model). The random part of the model included a random intercept of participant (unstructured covariance structure within participants). The primary treatment comparison will be the contrast between treatment groups 24 weeks post-baseline and baseline. The treatment effect will be analysed using both basic and adjusted linear mixed models.
Secondary outcomes
Secondary outcomes are mean changes in total scores on B-IPQ and BRIQ from baseline to 12 weeks after baseline; sickness absence (no/full-time/part-time) and mean change in WAI-2 (subscale 1) from baseline to 36 weeks after baseline. B-IPQ and BRIQ will be analysed using the MMRM as described above. Ordinary outcomes (TiC-P and WAI-2) will be analysed using a mixed model for repeated measures, but using a logit link function and multinomial distributed response.
Additional outcomes
Binary outcomes (sick leave, employment, job stability) will be analysed using mixed model for repeated measures, but using a logit link function and binomial distributed response.
Interim analysis {21b}
Not applicable. Interim analysis will not be performed in the present study.
Methods for additional analysis (e.g. subgroup analysis) {20b}
Within the MMRM, the treatment effect measured by RPQ, USER-P, B-IPQ and BRIQ will be subdivided by age, sex and baseline assessment. Additionally, mediation analysis is planned to examine whether change in illness cognition and illness behaviour mediate change in PPCS and limitations in daily living.
Methods in analysis to handle protocol non-adherence and any statistical methods to handle missing data {20c}
Data will be analysed according to the intention-to treat principles. Data will be analysed using linear mixed models for repeated measures providing unbiased estimates when data are missing at random or missing completely at random [56]. Furthermore, per-protocol analysis for completers defined as participants who received the first and second session and watched the e-learning videos will be performed.
Plans to give access to the full protocol, participant-level data and statistical code {31c}
The present study includes sensitive personal data. The data cannot be shared publicly due to existing data protection laws in Denmark imposed by the Danish Data Protection Agency. The access may be granted on pseudo anonymised data and case-by-case basis by approval from the project group who has the legal responsibility as owner and data collector. Access will be granted to the extent permissible by the General Data Protection Regulation. In this case, the head of the research unit at HNC (Jørgen Feldbæk Nielsen, email: [email protected]) will make data available for the investigator through his affiliated research institution in Denmark with approved authority to access the data. The General Data Protection Regulation and regulations prohibit all other forms of data sharing.
Oversight and monitoring
Composition of the coordination centre and trial steering committee {5d}
The PI is responsible for leading and coordinating the overall project. Challenges and reflections of the process will be shared with the core research group to ensure sustainability of recruitment and to support the PI throughout the project. Furthermore, the core research group will continuously review the progress of study and any necessary changes to the protocol to facilitate the smooth running of the study.
Composition of the data monitoring committee, its role and reporting structure {21a}
Data monitoring committee will not be included in the present study as sponsors are not involved in the study design, data collection or analysis. Furthermore, data are collected using public administered systems (REDCap and Statistics Denmark). The research group will be in charge of reporting immediately to the PI about any adverse incidence.
Adverse event reporting and harms {22}
No adverse effect of the intervention is expected, as neither previous results from the original or the up-scaled GAIN studies nor the finding from the development of GAIN Lite showed any adverse effects. A transient and harmless increase of some symptoms can be expected in some cases because of the gradual return to activity. However, the intervention will be adjusted to individual tolerance. Nevertheless, any related or unrelated adverse events will be reported to the Ethics Committee in the Central Denmark Region once yearly.
Frequency and plans for auditing trial conduct {23}
The Ethics Committee in the Central Denmark Region is free to make an audit at any time during the trial. Access to the source data and study-related files is granted on such occasions. All documents and data analyses will be available for auditing for at least 5 years after the end of the study.
Plans for communicating important protocol amendments to relevant parties (e.g. trial participants, trial ethical committees) {25}
The PI will be responsible for reporting any important protocol modification to the Ethics Committee and to the ClinicalTrials.gov (Identifier: NCT05233475).
Dissemination plans {31a}
The scientific dissemination will be performed by publishing results in international peer-reviewed journals. The intention is to publish positive as well as negative or inconclusive results to add scientific knowledge concerning treatment of people with PPCS. Furthermore, the results will be presented at national and international conferences. By the end of the study period, it is planned to host a seminar to enable the transfer of knowledge to municipalities nationally and patient organisations. In collaboration with the press offices at HNC, it is planned to execute a media strategy, including dissemination of results through social media, webpages, press releases and feature articles. Furthermore, to inform the public it is also aimed at publishing popular featured articles in relevant Danish professional magazines.
Discussion
GAIN Lite is a low-intensity intervention for adults with mild-to-moderate severity PPCS. Offering a remote intervention may improve access to rehabilitation and prevent chronification for individuals with mild-to-moderate PPCS. Moreover, GAIN Lite will facilitate access to healthcare, especially for those with transportation barriers. We hypothesise that GAIN Lite will decrease PPCS and increase participation in daily activities. These improvements may reduce demands on the social and healthcare systems. The results of the present study could add to the empirical evidence of the efficacy of cognitive and behavioural-based interventions. Overall, GAIN Lite may provide an accessible, flexible and convenient way to receive treatment based on sound theories and previous evidence of effective interventions for adults with mild-to-moderate PPCS.
Trial status
This is the first version of the protocol dated 7 February 2024. The second version is dated 30 September 2024. Enrolment of participants began in January 2023. Recruitment, follow-up assessments and data analyses are expected to be completed by the end of 2025. See Fig. Fig.55 for a template for the schedule of enrolment, interventions and assessments and the Trials populated SPIRIT checklist in Additional file 3.
Abbreviations
| BI | Business Intelligence (portal) |
| B-IPQ | Brief Illness Perception Questionnaire |
| BRIQ | Behavioural Response to Illness Questionnaire |
| DREAM | Danish Register for Evaluation of Marginalisation |
| EUC | Enhanced usual care |
| GAIN | Get going After concussIoN |
| GAIN Lite | Get going After concussIoN Lite |
| GDPR | General Data Protection Regulation |
| GP | General practitioners |
| HNC | Hammel Neurorehabilitation Centre and University Research Clinic |
| ICD-10 | International Classification of Diseases, Tenth Revision |
| MMRM | Mixed model for repeated measures |
| PPCS | Persistent post-concussion symptoms |
| PI | Principal investigator |
| RCT | Randomised controlled trial |
| REDCap | Research Electronic Data Capture |
| REML | Restricted maximum likelihood |
| RPQ | Rivermead Post-Concussion Symptoms Questionnaire |
| SD | Standard deviation |
| TiC-P | Treatment Inventory of Costs in Patients with psychiatric disorders |
| USER-P | Utrecht Scale for Evaluation of Rehabilitation-Participation |
| WAI-2 | Work Ability Index Short form |
| WHO | World Health Organization |
Authors’ contributions {31b}
Authors’ contribution using Contributor Roles Taxonomy (CRediT) [57]: SKSP performed writing—original draft, conceptualisation, methodology, investigation,visualisation, project administration and funding acquisition. MMT performed writing—review and editing, conceptualisation, investigation and supervision. LO performed writing—review and editing and investigation. ETNS performed writing—review and editing. CBP performed writing—review and editing, formal analysis and visualisation. CN performed writing—review and editing and investigation. HP performed writing—review and editing. NS performed writing—review and editing and supervision. IR performed writing—review and editing, conceptualisation, supervision and funding acquisition.
Funding {4}
The project is funded by the research unit at Hammel Neurorehabilitation Centre and University Research Clinic and by external funds from the Health Research Foundation of Central Denmark Region plus the Department of Clinical Medicine at Aarhus University. The funders have not been involved in the design of the study; collection, analysis or interpretation of data; and the writing of the manuscript.
Data availability{29}
The PI and the project data manager will have access to the final trial dataset. Requests of access to the data will be reviewed on an individual basis and approved by the project group after publication of the final trial results. Data in the present study is defined as sensitive personal data. The final dataset cannot be shared publicly due to existing data protection laws in Denmark imposed by the Danish Data Protection Agency. See {27} and {31c} for details.
Declarations
Approval has been obtained from the National Committee on Health Research Ethics: 1–16-02–69-21. Furthermore, approval has been obtained from the Central Denmark Region Committee on Health Research Ethics regarding processing of questionnaire data from the epidemiologic cohort study to the present study and the passing on of data from the BI portal to the present study, the intervention programme and the process of informed consent. Written and verbal information of the study will be given to participants before obtaining written informed consent. Also, participants will be informed that they are able to decline participation in the project at any time.
Written consent form (in Danish) can be found in Additional file 4.
The authors declare that they have no competing interests.
Footnotes
Publisher’ s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Articles from Trials are provided here courtesy of BMC
Citations & impact
This article has not been cited yet.
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/169759804
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
Clinical Trials
- (1 citation) ClinicalTrials.gov - NCT05233475
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Sub-symptom threshold aerobic exercise for patients with persisting post-concussion symptoms and exercise intolerance after mild traumatic brain injury - a study protocol with a nested feasibility study for a randomized controlled trial.
BMC Neurol, 23(1):179, 03 May 2023
Cited by: 1 article | PMID: 37138202 | PMCID: PMC10155435
Interdisciplinary intervention (GAIN) for adults with post-concussion symptoms: a study protocol for a stepped-wedge cluster randomised trial.
Trials, 23(1):613, 29 Jul 2022
Cited by: 2 articles | PMID: 35906645 | PMCID: PMC9338593
Novel interdisciplinary intervention, GAIN, vs. enhanced usual care to reduce high levels of post-concussion symptoms in adolescents and young adults 2-6 months post-injury: A randomised trial.
EClinicalMedicine, 17:100214, 16 Dec 2019
Cited by: 12 articles | PMID: 31891145 | PMCID: PMC6933237
[Mild traumatic brain injury and postconcussive syndrome: a re-emergent questioning].
Encephale, 38(4):329-335, 31 Aug 2011
Cited by: 8 articles | PMID: 22980474
Review

 1
1