Abstract
Aim
To investigate clinical outcomes in adults with type 2 diabetes (T2D) after insulin degludec/insulin aspart (IDegAsp) treatment in a real-world setting.Methods
The 26 weeks study involved 1102 adults with T2D who were either initiated with or switched to IDegAsp according to local practice in six countries. It was an open-label, non-interventional study. The primary endpoint was the change in glycosylated haemoglobin (HbA1c) levels from baseline to the end of study (EOS).Results
From India, 185 adults participated in this study with mean age of 58.1 (10.3) years and 14.4 (8.1) years of mean duration of T2D. Mean HbA1c decreased from 9.8% (1.8) at baseline to 8.2% (0.1) at the EOS; change in HbA1c from baseline [95% CI]: -1.6% (0.1) [-1.8; -1.4], P < 0.0001. There was a significant reduction in mean fasting plasma glucose (FPG) level from 190.0 (65.8) mg/dl at baseline to 141.9 (4.3) mg/dl at EOS; change in FPG from baseline [95% CI]: -52.2 (4.3) mg/dl [-60.7; -43.7], P < 0.0001. There was a numerical reduction in resource utilization related to diabetes and its complications and hypoglycaemic episodes. From baseline to EOS, the participants with outpatient visits (72 to 32) and workdays missed (2 to 0) decreased. Additionally, the number of patient-reported non-severe hypoglycaemic (47 to 8) and severe hypoglycaemic (4 to 1) episodes decreased as well.Conclusion
Initiation or switching to IDegAsp led to improvement in glycaemic control in real-world population of Indian adults with T2D. This was accompanied by a numerical reduction in resource utilization and patient-reported hypoglycaemia. Clinical trial registration: NCT04042441.Free full text

Initiation or switch to insulin degludec/insulin aspart in adults with type 2 diabetes in India: Results from a prospective, non-interventional, real-world study
Associated Data
ABSTRACT
Aim:
To investigate clinical outcomes in adults with type 2 diabetes (T2D) after insulin degludec/insulin aspart (IDegAsp) treatment in a real-world setting.
Methods:
The 26 weeks study involved 1102 adults with T2D who were either initiated with or switched to IDegAsp according to local practice in six countries. It was an open-label, non-interventional study. The primary endpoint was the change in glycosylated haemoglobin (HbA1c) levels from baseline to the end of study (EOS).
Results:
From India, 185 adults participated in this study with mean age of 58.1 (10.3) years and 14.4 (8.1) years of mean duration of T2D. Mean HbA1c decreased from 9.8% (1.8) at baseline to 8.2% (0.1) at the EOS; change in HbA1c from baseline [95% CI]: -1.6% (0.1) [-1.8; -1.4], P < 0.0001. There was a significant reduction in mean fasting plasma glucose (FPG) level from 190.0 (65.8) mg/dl at baseline to 141.9 (4.3) mg/dl at EOS; change in FPG from baseline [95% CI]: -52.2 (4.3) mg/dl [-60.7; -43.7], P < 0.0001. There was a numerical reduction in resource utilization related to diabetes and its complications and hypoglycaemic episodes. From baseline to EOS, the participants with outpatient visits (72 to 32) and workdays missed (2 to 0) decreased. Additionally, the number of patient-reported non-severe hypoglycaemic (47 to 8) and severe hypoglycaemic (4 to 1) episodes decreased as well.
Conclusion:
Initiation or switching to IDegAsp led to improvement in glycaemic control in real-world population of Indian adults with T2D. This was accompanied by a numerical reduction in resource utilization and patient-reported hypoglycaemia. Clinical trial registration: NCT04042441
Introduction
India has the second-highest prevalence of diabetes globally, with 74.2 million adult Indians living with diabetes in 2021. This number is predicted to increase to 124.9 million by 2045, with the majority of cases being type 2 diabetes (T2D).[1] While Indians represent a large proportion of T2D patients, popular treatment guidelines tend to originate in Western regions, such as Europe or North America. Consequently, several medical societies and organizations in India have also formulated guidelines suited to local needs.[2]
Guidelines constituted by the American Diabetes Association (ADA), the European Association for the Study of Diabetes (EASD), and the American Association of Clinical Endocrinology (AACE) recommend that, in people with T2D uncontrolled with oral antidiabetic drug (OAD) treatment, basal insulin should be the first choice for insulin initiation.[3,4,5] However, due to the high carbohydrate diet in much of the Indian population, a prandial component to an insulin regimen is often required and basal insulin plus OAD therapy may be insufficient.[2] This represents a major challenge for people with T2D in India, and there is a need for insulin treatments that provide more effective control of both fasting and post-prandial glucose (PPG) in these people.
Insulin degludec/insulin aspart (IDegAsp) is a 70/30 fixed ratio co-formulation of ultra-long acting basal insulin, insulin degludec and the rapid-acting insulin, insulin aspart. IDegAsp has previously been shown to lower both basal and prandial glucose levels with a low risk of hypoglycaemia.[6] IDegAsp is recommended as an option for insulin initiation in people with T2D uncontrolled on three OADs by the Research Society for the Study of Diabetes in India—Endocrine Society of India (RSSDI-ESI) clinical practice guidelines for the management of T2D. IDegAsp has also been recommended as an intensification option for people with uncontrolled T2D on other insulin regimens.[7]
The BOOST® program consisted of a series of randomized controlled trials (RCTs) aimed to assess the efficacy, and safety of IDegAsp treatment (initiation and intensification) in participants with T2D.[8,9,10,11,12] The superiority of IDegAsp over insulin glargine U100 in terms of glycaemic control was demonstrated in the phase 3 BOOST® Japan trial in insulin-naïve participants aged ≥20 years with T2D. IDegAsp also resulted in numerically lower rates of overall and nocturnal non-severe hypoglycaemia and comparable fasting plasma glucose (FPG) values.[6] Results from a trial comparing IDegAsp to a combination of insulin glargine 100 units/ml (U100) and insulin aspart in participants aged ≥18 years with T2D indicated non-inferiority with IDegAsp for glycaemic control.[13] IDegAsp was also associated with a significantly reduced rate of nocturnal confirmed symptomatic hypoglycaemic episodes.[13]
Due to the scarcity of prospective real-world studies, despite compelling data from the RCTs, further data is needed regarding the effectiveness and safety of IDegAsp. A Ryzodeg Initiation and Switch Effectiveness (ARISE) study was conducted to investigate the effects of IDegAsp on glycaemic control and clinical outcomes in participants with T2D. The rate of hypoglycaemia and healthcare resource utilisation (HRU) with IDegAsp was also explored further. Conducted among participants from six countries, including India, Australia, Philippines, Malaysia, South Africa, and Saudi Arabia; the study subjects were the patients initiated with IDegAsp or the ones who were, as per the regional clinical practice, switched to IDegAsp from other insulins.[14] This sub-analysis of ARISE investigates glycaemic control, rate of hypoglycaemia, and HRU in participants with T2D treated with IDegAsp in India.
Methods
Study design and population
The ARISE study design has been described and published previously.[15] Briefly, this was a 26-weeks, multi-centre, prospective, open-label, non-interventional study investigating clinical outcomes in patients suffering from T2D after initiating or switching to IDegAsp according to the decision of the physician based on approved label and local clinical practice (ClinicalTrials.gov, NCT04042441). The study consisted of a baseline visit (which included treatment initiation after the informed consent), intermediate visits conducted according to clinical practice, and an end-of-study visit (EOS), which took place between weeks 26 and 36 [Figure 1]. The decision to initiate or switch to IDegAsp was made prior to the baseline visit and was independent of the participant’s recruitment into the study. The physician determined the starting dose and frequency of IDegAsp and any subsequent adjustments. During the study period, dose adjustments or discontinuation of other glucose-lowering medication/s were allowed. However, the study did not include monitoring or diagnostic activities beyond the standard clinical practice. Between July 2019 and December 2020, data for participants were gathered from ten different sites located within India. ARISE was conducted in accordance with the Declaration of Helsinki, with a protocol duly approved by the Independent Review Boards (IRB)/Independent Ethics Committee (IEC) for all the study sites [Table S1]. Before the start of the study, informed consent was obtained from each of the participants.
Table S1
List of investigators, research ethics boards/institutional review boards from India
| Investigator name and address | Name of research ethics board/institutional review board |
|---|---|
| Dr Abhijit Bhograj Columbia Asia Hospital, Kirloskar Business Park, Bellary Rd, Bengaluru, Karnataka 560024 | Institutional Ethics Committee Columbia Asia Hospital, Columbia Asia Referral Hospital – Yeshwanthpur, #26/4, Brigade Gateway, Beside Metro, Malleshwaram West, Bangalore – 560055. India |
| Dr Ajay Aggarwal Fortis Hospital, A Block, Shaheed Udham Singh Marg, Poorbi Shalimar Bag, Shalimar Bagh, New Delhi, Delhi 110088 | Institutional Ethics Committee - Fortis Hospital Shalimar Bagh, Fortis Hospital, Upper Basement, A - Block, Shalimar Bagh, New Delhi -110088. India |
| Dr Deepaklal Madhavdas R.M Education & Research Foundation, AA2, 2nd Avenue, Anna Nagar, Chennai, 600040 | Universal Ethics Committee, #180/109, G-2, R.R. Vila, Rangarajapuram Main Road, Kodambakkam Chennai – 600024, Tamil Nadu, India. |
| Dr G M Prasad Pace Clinical Research, Unit of Pranav Diabetes Center, 57/1, Nanda Complex Rammurthynagar, Banaswadi, Bangalore, 560043 | People tree Hospital Ethics committee, People Tree Hospitals, No. 2 Tumkur Road, Opp. Taj Vivanta, Goraguntepalya, Yeshwanthpur, Bengaluru – 560022, Karnataka. India |
| Dr Kiran Pal Singh Fortis Hospital, Sec 62, Phase 8, Mohali 160062 | Institutional Ethics Committee, Fortis Hospital, Sector 62, Phase-VIII, Mohali -160062 |
| Dr Manash P Baruah Excelcare Hospital, NH-37 Highway, near Ganesh Mandir, Paschim Boragaon, Guwahati, Assam 781033, India | Institutional Ethics Committee, Excelcare Hospital, NH-37 Highway, near Ganesh Mandir, Paschim Boragaon, Guwahati, Assam 781033, India |
| Dr Pankaj Aneja Max Hospital, C and D Block, Shalimar Place Site, Shalimar Bagh, New Delhi, Delhi 110088 | Max Healthcare Ethics Committee, Max Super Specialty Hospital, 6th floor, 2, press Enclave Saket, New Delhi, 110017, India |
| Dr Ritesh Kumar Agrawala AMRI Hospital, Plot no 1, Near Jayadev Vatika Park, Khandagiri, Bhubaneshwar, Orissa 751019 | Institutional Ethics Committee, AMRI Hospital Ltd., Bhubaneswar, Plot No. 1, Khata No. 276, Satyasai Enclave, Beside Jaydev Vatika, Khandagiri, Bhubaneswar-751030, Odisha, India |
| Dr Sanjay Shah Narayana Superspeciality Hospital, 120, 1, Andul Rd, Near Nabanna, Shibpur, Howrah, West Bengal 711103, India | NSH Ethics Committee, Narayana Superspeciality Hospital, 120/1 Andul Road, Howrah, Howrah West Bengal – 711103, India |
| Dr Shailesh Pitale DEW Medicare & Trinity Hospital, 8-81, Hindustan colony, Wardha Road, Nagpur 440015 | DEW and Trinity Institutional Ethics Committee, DEW Medicare and Trinity Hospital, Plot no 80, 81 Hindustan Colony, Wardha road, Nagpur, Maharashtra – 440015. India |
Participants selected were sourced from the participating physicians’ databases. Eligible participants were male or female with a diagnosis of T2D, aged ≥18 years, receiving treatment with antidiabetic medications other than IDegAsp for a minimum of 26 weeks, with available HbA1c value ≤12 weeks prior to providing informed consent. Participants who had previously been treated with IDegAsp were excluded.
Study objectives and endpoints
The main objectives of this sub-study were to evaluate glycaemic control and rates of hypoglycaemia after initiating or switching to IDegAsp. The secondary objectives were to define the clinical use case of IDegAsp in a real-world setting, consisting of reasons for starting or stopping the treatment, and to evaluate the treatment effect with IDegAsp on rates of HRU.
The study’s primary endpoint was the change in HbA1c from baseline to EOS. Secondary endpoints included determining the proportion of participants accomplishing HbA1c level <7.0%, the proportion of participants who achieved HbA1c levels below a pre-defined individualized treatment target, and the change in total, basal, and prandial insulin dose, FPG and body weight from baseline to EOS. Additional endpoints included assessing patient-reported non-severe hypoglycaemic episodes (overall and nocturnal) manifesting within 4 weeks prior to the starting of IDegAsp and 4 weeks prior to the EOS. Non-severe hypoglycaemia was defined as an episode with symptoms and/or self-measured blood glucose value ≤3.9 mmol/L wherein the patient was able to self-manage. Nocturnal non-severe hypoglycaemia was defined depending on whether the patient perceived the event to have occurred at night. Occurrence of severe hypoglycaemic episodes (needing help from another person to take corrective measures to alleviate neuropsychological symptoms) within 26 weeks before starting of IDegAsp and during the 26-weeks study duration were also assessed.
HRU was assessed in relation to diabetes and complications observed within 12 weeks before the starting of IDegAsp and within 12 weeks before the EOS/discontinuation. Moreover, HRU related to severe hypoglycaemia, manifested within 26 weeks before starting of IDegAsp and within 26 weeks before the EOS/discontinuation was recorded. The study also collected data on the rationale for starting IDegAsp treatment at baseline, the rationale for discontinuing IDegAsp treatment, and the percentage of study participants who discontinued treatment during the study period.
Statistical methods
With the assumption of a mean difference in HbA1c of 0.5% (standard deviation, 1.8%) and a missing HbA1c value at the EOS in 25% of them, enrolment of 1112 participants, with a minimum of 139 participants in each country was planned to detect HbA1c difference at 90% power. The statistical power was sufficient for the analysis of the primary endpoint overall and in each of the six individual participating countries.
The full analysis set (FAS) comprised all eligible participants who provided written informed consent and started treatment with IDegAsp. The primary endpoint analysis involved the use of crude and adjusted mixed models for repeated measurements (MMRM). The analysis included all participants in the FAS who had at least one HbA1c measurement post-baseline assessment using the ‘in-study’ observation period. This observation period encompassed the period during which participants were part of the study, regardless of whether they discontinued IDegAsp treatment. The crude model consisted of baseline HbA1c and time of HbA1c as covariates. The adjusted model comprised of baseline age of the participants, sex, HbA1c, time of HbA1c, body mass index (BMI), study site, and earlier antidiabetic treatment as covariates. Secondary analyses of the primary endpoint were based on the ‘on-treatment’ observation period. This observation period was regarded as the period during which participants were administered IDegAsp, and thus, values measured after treatment discontinuation were neglected.
Primary and secondary analyses were again performed to investigate the difference from baseline to the EOS in FPG, insulin doses, and body weight, with the baseline values of the suitable endpoint incorporated as a covariate.
The results of the primary analyses were presented by performing adjusted MMRM for the in-study observation period. However, for the analysis of HRU, an on-treatment observation period was considered. Glycaemic control endpoints analysed using the on-treatment observation period are listed in Table S2.
Table S2
Glycaemic control endpoints analysed using the on-treatment observation period
| Observed mean (SD) at baseline | Estimated mean (SE) at EOS | Estimated difference (baseline to EOS) [95% CI] | |
|---|---|---|---|
| HbA1c, % | 9.8 (1.8) | 8.2 (0.1) | –1.6 [–1.8; –1.4], p<0.0001 |
| FPG, mg/dL | 190.0 (65.8) | 141.9 (4.3) | –52.2 [–60.7; –43.7], p<0.0001 |
Data based on the FAS. EOS, end of study
Results
Study population demographics and clinical characteristics
Of 186 eligible participants, 185 were initiated or switched to IDegAsp [Figure S1]. Of these, 92.4% completed the study. The demographic characteristics and clinical attributes of the participants at baseline are presented in Table 1. Within the FAS, consisting of 185 participants, the average (SD) age was 58.1 (10.3) years with the duration of diabetes being 14.4 (8.1) years and the mean HbA1c level at baseline as 9.8% (1.8). Before initiating or switching to IDegAsp, 180 participants had received previous anti-hyperglycaemic treatment, 50.0% of whom were receiving OADs only, 19.4% received premix insulin, 17.8% received basal–bolus insulin, 10.6% received basal insulin, and 2.2% received GLP-1RA ± insulin.
Table 1
Demographic and clinical characteristics at baseline
| India N=185 | |
|---|---|
| Age (years), mean (SD) | 58.1 (10.3) |
| Male, n (%) | 106 (57.3) |
| Duration of diabetes (years), mean (SD) | 14.4 (8.1) |
| Body weight (kg), mean (SD) | 70.8 (11.5) |
| BMI (kg/m2), mean (SD) | 26.5 (3.9) |
| HbA1c (%), mean (SD) | 9.8 (1.8) |
| FPG (mg/dL), mean (SD) | 190.0 (65.8) |
| Antidiabetic treatment, n (%) | n=180 |
 OADs only OADs only | 90 (50.0) |
 Premix insulin±bolus insulin (± OADs) Premix insulin±bolus insulin (± OADs) | 35 (19.4) |
 Basal–bolus insulin (± OADs) Basal–bolus insulin (± OADs) | 32 (17.8) |
 Basal insulin only (± OADs) Basal insulin only (± OADs) | 19 (10.6) |
 GLP-RA ± insulin (± OADs) GLP-RA ± insulin (± OADs) | 4 (2.2) |
| Dose of previous prandial insulin (U), mean (SD) | 26.2 (19.2) |
| Diabetes complications, n (%) | |
 Diabetic neuropathy Diabetic neuropathy | 33 (21.2) |
 Cardiovascular disease Cardiovascular disease | 23 (14.7) |
 Diabetic nephropathy Diabetic nephropathy | 17 (10.9) |
 Diabetic retinopathy Diabetic retinopathy | 6 (3.8) |
BMI, body mass index; FPG, fasting plasma glucose; OAD, oral antidiabetic drug; N, number of participants in the full analysis set; n, number of participants; U, unit
43.8% of participants (n = 81) were prescribed IDegAsp once daily (OD) while 56.2% of participants (n = 104) received IDegAsp twice daily (BID). The mean (SD) starting total daily dose of IDegAsp was 30.3 (16.2) U, 19.8 (9.5) U in participants receiving IDegAsp OD, and 38.5 (15.8) U in participants receiving IDegAsp BID. The reasons for initiating IDegAsp from physicians’ perspectives are summarized in Table 2. The most commonly reported reason was the need for improvement in the patient’s glycaemic control (98.4%). No participant discontinued IDegAsp during the treatment period.
Table 2
Physicians’ reasons for initiating or switching to IDegAsp
| India N=185 | |
|---|---|
| To improve the patient’s glycaemic control | 182 (98.4) |
| To lower the risk of hypoglycaemia | 48 (25.9) |
| Flexibility in the dosing regimen | 50 (27.0) |
| Fewer injections than basal and bolus therapy | 54 (29.2) |
| No reconstitution needed | 40 (21.6) |
| Change in coverage status favouring IDegAsp | 36 (19.5) |
| Other | 3 (1.6) |
Physicians could select more than one reason for each patient. A change in coverage status favouring IDegAsp refers to a change in healthcare insurance or reimbursement requirements that led to better access to the drug. N, number of participants in the full analysis set
Glycaemic control
The observed mean (SD) HbA1c at baseline was 9.8% (1.8) and the estimated mean (SD) at EOS was 8.2% (0.1). HbA1c was significantly lower at the end of study compared with baseline (estimated difference: –1.6 (0.1) % [95%CI: –1.8; –1.4]; P < 0.0001). Results were consistent when analysed using the on-treatment observation period as well [Table S2].
A reduction in HbA1c was observed by all prior treatment subgroups [Figure 2]. The mean reduction was numerically greatest in the basal-only subgroup (–2.1% (1.8)) and smallest in the premix insulin subgroup (–0.9% (2.5)).
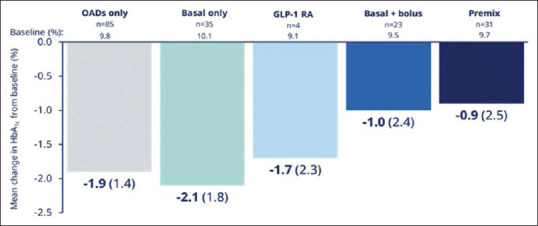
Mean change in HbA1c from baseline to EOS across prior treatment subgroups. Overall mean change in HbA1c was calculated using a fully adjusted model including baseline value, time, time-squared for HbA1c measure, age, sex, BMI, previous antidiabetic regimen and study site. Changes in HbA1c according to prior treatment subgroups were investigated in an exploratory analysis and are descriptive only. CI, confidence interval; EOS, end of study; GLP-1 RA, glucagon-like peptide-1 receptor agonist; OAD, oral antidiabetic drug
At EOS, 8.9% of participants in the Indian cohort achieved an HbA1c level <7.0% compared with 4.3% of participants at baseline. Likewise, 9.5% of participants achieved an HbA1c level below their pre-defined individualized target by EOS compared with 3.2% of participants at baseline.
The observed mean (SD) FPG (mg/dL) at baseline was 190.0 (65.8) mg/Dl, and the estimated mean (SD) at EOS was 141.9 (4.3) mg/dL. The estimated mean (SD) change in FPG (mg/dL) from baseline to EOS was -52.2 (4.3) mg/dL [-60.7; -43.7] 95% CI, and it was statistically significant (P value: <0.0001). Results were consistent when analysed using the on-treatment observation period as well [Table S2].
Insulin dose
For participants with experience of insulin (prior basal insulin only, basal–bolus insulin, and premix insulin users), the reported mean total daily insulin dose at baseline was 43.9 (28.8) U and the estimated total daily insulin dose at EOS was 42.4 (2.6) U; the change was not statistically significant. There was no significant change in basal insulin dose from baseline (observed mean 24.4 (19.0) U) to EOS (estimated mean 23.4 (1.0) U). Likewise, there was no significant change in daily prandial insulin dose from baseline (observed mean 19.6 (19.6) U) to EOS (estimated mean 18.9 (2.1) U).
Body weight
The mean body weight changed from a baseline of 70.8 (11.5) kg to 70.5 (0.4) kg at EOS. The estimated mean (SD) change was not significant, 0.3 (0.4) kg [-0.6; 1.1] 95% CI (P value = 0.5449).
Hypoglycaemia
More participants experienced non-severe hypoglycaemic episodes during the 4-week period prior to starting of IDegAsp compared with the 4-week period before EOS or discontinuation (27 vs 4) (not statistically analysed) [Table 3]. Nocturnal non-severe hypoglycaemic episodes and severe hypoglycaemic episodes were also experienced by more participants in the period before starting IDegAsp compared with the period before EOS or discontinuation (15 vs 2 participants and 2 vs 1 participant/s, respectively). Results were identical when analysed using the on-treatment observation period.
Table 3
Hypoglycaemic episodes occurring prior to initiation of IDegAsp (baseline) and prior to EOS or discontinuation
| Events, n (% of total events) | Participants, n (% of participants) | |
|---|---|---|
| Non-severe | 55 | 29 |
 Within 4 weeks of initiation Within 4 weeks of initiation | 47 (85.5) | 27 (93.1) |
 Within 4 weeks prior to EOS or discontinuation Within 4 weeks prior to EOS or discontinuation | 8 (14.5) | 4 (13.8) |
| Nocturnal non-severe | 26 | 17 |
 Within 4 weeks of initiation Within 4 weeks of initiation | 23 (88.5) | 15 (88.2) |
 Within 4 weeks prior to EOS or discontinuation Within 4 weeks prior to EOS or discontinuation | 3 (11.5) | 2 (11.8) |
| Severe | 5 | 3 |
 Within 26 weeks of initiation Within 26 weeks of initiation | 4 (80.0) | 2 (66.7) |
 Within 26 weeks prior to EOS Within 26 weeks prior to EOS | 1 (20.0) | 1 (33.3) |
Hypoglycaemic episodes according to country were investigated in an exploratory analysis and are descriptive only. EOS, end of study
Healthcare resource utilization
Of the 173 self-reported outpatient visits associated with diabetes and its complications during the on-treatment observation period, the majority occurred in the 12-week period prior to starting of IDegAsp compared with the 12-week period before EOS or discontinuation (131 vs 42) (not statistically analysed) [Table 4]. The number of participants with self-reported workdays missed reduced from 2 at baseline to 0 at the EOS as well.
Table 4
Healthcare resource utilization
| Events, n (% of total events) | Participants, n (% of participants) | |
|---|---|---|
| Self-reported outpatient visits associated with diabetes and its complications | ||
| All participants | 173 | 93 |
 Within 12 weeks prior to initiation Within 12 weeks prior to initiation | 131 (75.7) | 72 (77.4) |
 Within 12 weeks prior to EOS or discontinuation Within 12 weeks prior to EOS or discontinuation | 42 (24.3) | 32 (34.4) |
| Self-reported emergency room visits associated with diabetes and its complications | ||
| All participants | 0 | 0 |
 Within 12 weeks prior to initiation Within 12 weeks prior to initiation | 0 (0.0) | 0 (0.0) |
 Within 12 weeks prior to EOS or discontinuation Within 12 weeks prior to EOS or discontinuation | 0 (0.0) | 0 (0.0) |
| Self-reported in-patient hospitalizations associated with diabetes and its complications | ||
| All participants | 4 | 3 |
 Within 12 weeks prior to initiation Within 12 weeks prior to initiation | 4 (100.0) | 3 (100.0) |
 Within 12 weeks prior to EOS or discontinuation Within 12 weeks prior to EOS or discontinuation | 0 (0.0) | 0 (0) |
| Self-reported other healthcare provider visits and contacts associated with diabetes and its complications | ||
| All participants | 33 | 24 |
 Within 12 weeks prior to initiation Within 12 weeks prior to initiation | 10 (30.3) | 7 (29.2) |
 Within 12 weeks prior to EOS or discontinuation Within 12 weeks prior to EOS or discontinuation | 23 (69.7) | 17 (70.8) |
| Self-reported workdays missed associated with diabetes and its complications | ||
| All participants | 9 | 2 |
 Within 12 weeks prior to initiation Within 12 weeks prior to initiation | 9 (100.0) | 2 (100.0) |
 Within 12 weeks prior to EOS or discontinuation Within 12 weeks prior to EOS or discontinuation | 0 (0.0) | 0 (0.0) |
Data based on FAS, on-treatment observation period. Healthcare resource utilization according to country was investigated in an exploratory analysis and is descriptive only. EOS, end of study
Adverse events
In total, 21 adverse events (AEs) were reported in 13 Indian participants [Table S3]. Of these, five serious AEs were reported in three participants, with two resulting in death. All serious AEs were classed as ‘unlikely related’ to IDegAsp treatment.
Table S3
Adverse events in the Indian cohort
| Serious | Non-serious | |||||
|---|---|---|---|---|---|---|
|
|
| |||||
| n | % | E | n | % | E | |
| Adverse events | 3 | 1.6 | 5 | 10 | 5.4 | 16 |
| Severity | ||||||
 Mild Mild | 0 | 8 | 4.3 | 13 | ||
 Moderate Moderate | 1 | 0.5 | 1 | 2 | 1.1 | 3 |
 Severe Severe | 3 | 1.6 | 4 | 0 | ||
| Causality | ||||||
 Probable Probable | 0 | 3 | 1.6 | 4 | ||
 Possible Possible | 0 | 2 | 1.1 | 3 | ||
 Unlikely Unlikely | 3 | 1.6 | 5 | 6 | 3.2 | 9 |
Data based on FAS. %, percentage of patients; E, number of events; FAS, full analysis set; n, number of participants
Discussion
In this real-world, prospective, non-interventional sub-study of ARISE, participants with T2D were either initiated or switched to IDegAsp from previous antidiabetic treatment as part of routine clinical practice in India. Use of IDegAsp for 26–36 weeks was associated with a significant reduction in HbA1c and FPG compared with baseline. Rates of hypoglycaemia and outpatient visits were also numerically lower at EOS than at baseline. A minority of participants experienced AEs, and these were mostly non-serious; serious AEs reported were all deemed unlikely to be related to IDegAsp treatment.
Although a pronounced improvement was reported in the basal insulin users in the study, the improvement in HbA1c was reported to be similar among the preceding treatment subgroups. It is to be noted that the basal insulin users were also reported with the highest mean HbA1c at baseline. Few physicians treating their patients to achieve the target of HbA1c <7.0% can be a contributing factor in the explanation of how a low percentage (8.9%) of participants were reported to have an HbA1c of <7.0%. Similarly, comparable percentage (9.5%) of participants showed achievement of HbA1c lower than the target as set by treating physician. On the other hand, in the overall cohort study, a total of 14.9% participants reported achievement of HbA1c level <7% at the EOS as compared to 4.3% of participants at the baseline and 14.9% participants showed achievement of pre-defined target at the EOS as compared to 2.5% at the baseline.[14]
ARISE was conducted in parallel with the COVID-19 global pandemic and associated lockdowns, and it is likely that these may have prevented clinic visits and in turn reduced the number of participants achieving treatment targets.
A significant reduction in FPG mirrored the reduction in HbA1c. Crucially, observed improvements in glycaemic control did not have associated negative impacts on rates of hypoglycaemia. The basal component of IDegAsp has very low pharmacodynamic variability compared to other basal insulins.[16] This should alleviate concerns from clinicians regarding the safety of IDegAsp while prescribing.
Basal and prandial components of IDegAsp also show much clearer pharmacokinetic/pharmacodynamic separation compared with premix insulins.[16] This means the prandial effect of treatment with IDegAsp is able to match physiological needs after a major meal which can be particularly advantageous in the Indian context, where most of the people with T2D consume a diet rich in carbohydrates, with the resultant need for effective control of PPG.[2] It should be noted that capturing data related to PPG in a real-world study is difficult due to the non-interventional nature. As improving glycaemic control was one of the common reasons provided by physicians for initiating or switching to treatment with IDegAsp, these properties should be reassuring.
In contrast to the ARISE study, which reported a significant decrease in mean daily basal insulin with a significant increase in mean daily prandial insulin dose, the present study reported no statistically significant difference while observing changes in mean total daily insulin dose, mean daily basal insulin dose, or mean daily prandial insulin dose from baseline to the end of study.[14] The reason behind this inconsistency is unclear although the smaller sample size in the Indian cohort precludes any conclusion in this regard.
Following the initiation or switch to IDegAsp, exploratory analyses suggest that there was a reduction in the number of outpatient visits for diabetes and its complications and in workdays missed. This may reflect ease of use, safety, and improvement in glycaemic control. The reduction in HRU suggests the possibility of reduced economic costs associated with the use of IDegAsp; however, the movement restrictions and social distancing imposed during the COVID-19 lockdown would also have impacted HRU during the study period.
The overall ARISE study results, reporting an improved glycaemic control with a reduced rate of hypoglycaemia, after initiating or switching to IDegAsp were found to be reflected in the present sub-study. However, no significant difference was reported in the body weight in the present sub-study, which contrasts with the ARISE study results. The difference in bodyweight results may be due to the reason that the ARISE study participants were reported with a higher mean BMI of 29.2 kg/m2 at baseline, as compared to the mean BMI of 26.5 kg/m2 in the present sub-study.[14]
The current findings are also consistent with the improvements in glycaemic control and low risk of hypoglycaemia reported in the BOOST® clinical trial programme.[6,9,10,11,12,13] Similar HbA1c results, a significant reduction in FPG, and reduced rates of overall and nocturnal hypoglycaemia with IDegAsp were observed across a range of baseline characteristics (HbA1c, duration of diabetes or BMI) in a meta-analysis of 5-phase III, 26-weeks, treat-to-target trials which compared IDegAsp BID with basal–bolus insulin or premix insulin.[17] Our safety findings also reflect previous results from the India-specific Study of Management of Diabetes with Ryzodeg™ Treatment (SMART) study of IDegAsp, with no new safety signals observed.[18]
Failure to adhere to complicated treatment regimens is one known explanation for discrepancies between results observed in RCTs and real-life clinical practice.[19] However, the potential advantages of treatment with IDegAsp over other insulin regimens are indicated by the qualitative alignment between the results of ARISE and the BOOST clinical trial programme.
This sub-study provides useful insights into the clinical outcomes associated with initiating or switching to the IDegAsp in a real-world setting among the Indian population. The broad inclusion and exclusion criteria make the results generalizable to a wide population of participants with T2D in India and the sample size provides sufficient power for the country-level result of the primary endpoint. In terms of study limitations, analyses of hypoglycaemia and HRU were secondary and exploratory, respectively, and differences before and after treatment were not analysed statistically. The open-label nature of the study design allows the potential for bias in reporting, especially as participants were recruited to ARISE expecting a benefit as a result of a regimen change to treatment with IDegAsp. As ARISE was also a non-interventional study, the study sponsor had no control over baseline parameter ranges, insulin titration methods, or insulin titration frequency.
Conclusion
In this real-world, prospective, non-interventional study in participants with T2D in India, initiating or switching to IDegAsp was associated with improved glycaemic control and numerically lower rates of hypoglycaemia and HRU in comparison with baseline.
Ethical consideration
ARISE was conducted in accordance with the Declaration of Helsinki, with protocol and participant consent forms approved by research ethics boards/institutional review boards for all sites [Table S1]. Every participant was provided with an informed consent form in writing.
Financial support and sponsorship
Novo Nordisk funded this study along with medical writing, editorial support and publication. Medical Writing support was provided by Mediception Science Pvt Ltd.
Conflicts of interest
There are no conflicts of interest. Muzammil Khan. A. Pathan, Manjunatha Revanna and Manu Chandrappa are employees of Novo Nordisk.
References
Articles from Journal of Family Medicine and Primary Care are provided here courtesy of Wolters Kluwer -- Medknow Publications
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
Clinical Trials
- (1 citation) ClinicalTrials.gov - NCT04042441
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
A Real-World, Prospective, Non-interventional Study of Adults with T2D Switching to IDegAsp from Glargine U100 or U300 in Japan.
Diabetes Ther, 12(9):2405-2421, 25 Jul 2021
Cited by: 4 articles | PMID: 34304385 | PMCID: PMC8385001
Initiating or Switching to Insulin Degludec/Insulin Aspart in Adults with Type 2 Diabetes: A Real-World, Prospective, Non-interventional Study Across Six Countries.
Adv Ther, 39(8):3735-3748, 25 Jun 2022
Cited by: 5 articles | PMID: 35752730 | PMCID: PMC9244059
Initiating or switching to insulin degludec/insulin aspart in a real-world population of adults with type 2 diabetes in Australia: results from a prospective, non-interventional study.
Intern Med J, 54(10):1626-1633, 22 Aug 2024
Cited by: 0 articles | PMID: 39171857
Overview of Clinical Trial Program and Applicability of Insulin Degludec/Insulin Aspart in Diabetes Management.
J Assoc Physicians India, 63(5 suppl):21-28, 01 May 2015
Cited by: 0 articles | PMID: 26548031
Review




