Abstract
Objective
To explore changing trends in circulating immune indicators of hepatocellular carcinoma (HCC) undergoing TACE plus immune checkpoint inhibitors (ICIs) and anti-VEGF antibodies/TKIs and to elucidate the relationship between immune response and tumor prognosis.Materials
This single-center retrospective study included patients with unresectable HCC undergoing TACE plus ICIs and anti-VEGF antibodies/TKIs from March 11, 2019, to February 15, 2024. Peripheral blood samples were collected at baseline and every cycle, from which blood cell counts and immune indicators were analyzed. The primary outcome was the objective response rate (ORR) at the first evaluation. According to the first evaluation based on mRECIST, patients were classified into PD, SD, and OR groups for analysis. Further subgroup analysis was performed on the OR group based on whether experiencing progression after the first evaluation. Lymphocyte subsets were measured by flow cytometry. Immunoglobulins were measured using the immune turbidimetric method. The neutrophil-to-lymphocyte ratio (NLR) was measured by the complete blood count. Simple linear regression was employed to examine the dynamic trends.Results
A total of 63 patients were enrolled, with an ORR of 55.6% and a disease control rate (DCR) of 87.3% at the first evaluation. The median overall survival (mOS) was 27.5 months (95% CI: 22.5-32.5 months). In the OR group (n=35), more active immune responses, expressed in a decrease in CD3-CD19+ (p=0.004), CFB (p=0.027), NLR (p<0.001) and an increase in Ig λ (p=0.010), Ig κ (p=0.037), Ig A (p=0.005), Ig G (p=0.006), were related to better prognosis, while similar patterns seen in the OR-nPD subgroup. Concurrently, no significant differences were noted in the PD group (n=8).Conclusion
The combination therapy may modify the tumor microenvironment of HCC. Changing trends in circulating immune indicators and NLR can serve as potential biomarkers for predicting tumor response and guiding clinical treatment.Free full text

Immune Indicator Changes in Hepatocellular Carcinoma Undergoing TACE Plus ICIs and Anti-VEGF Antibodies/TKIs: A Prognostic Biomarker Analysis
Abstract
Objective
To explore changing trends in circulating immune indicators of hepatocellular carcinoma (HCC) undergoing TACE plus immune checkpoint inhibitors (ICIs) and anti-VEGF antibodies/TKIs and to elucidate the relationship between immune response and tumor prognosis.
Materials
This single-center retrospective study included patients with unresectable HCC undergoing TACE plus ICIs and anti-VEGF antibodies/TKIs from March 11, 2019, to February 15, 2024. Peripheral blood samples were collected at baseline and every cycle, from which blood cell counts and immune indicators were analyzed. The primary outcome was the objective response rate (ORR) at the first evaluation. According to the first evaluation based on mRECIST, patients were classified into PD, SD, and OR groups for analysis. Further subgroup analysis was performed on the OR group based on whether experiencing progression after the first evaluation. Lymphocyte subsets were measured by flow cytometry. Immunoglobulins were measured using the immune turbidimetric method. The neutrophil-to-lymphocyte ratio (NLR) was measured by the complete blood count. Simple linear regression was employed to examine the dynamic trends.
Results
A total of 63 patients were enrolled, with an ORR of 55.6% and a disease control rate (DCR) of 87.3% at the first evaluation. The median overall survival (mOS) was 27.5 months (95% CI: 22.5–32.5 months). In the OR group (n=35), more active immune responses, expressed in a decrease in CD3−CD19+ (p=0.004), CFB (p=0.027), NLR (p<0.001) and an increase in Ig λ (p=0.010), Ig κ (p=0.037), Ig A (p=0.005), Ig G (p=0.006), were related to better prognosis, while similar patterns seen in the OR-nPD subgroup. Concurrently, no significant differences were noted in the PD group (n=8).
Conclusion
The combination therapy may modify the tumor microenvironment of HCC. Changing trends in circulating immune indicators and NLR can serve as potential biomarkers for predicting tumor response and guiding clinical treatment.
Introduction
Hepatocellular carcinoma (HCC) is characterized by high incidence and mortality rates, with approximately half of the global new cases and deaths occurring in China each year.1 HCC often presents insidiously, and over 80% of patients are diagnosed at an advanced stage, resulting in a low curative resection rate and poor prognosis.2
According to the Barcelona Clinic Liver Cancer (BCLC) staging system, transarterial chemoembolization (TACE) is the recommended standard treatment for patients with intermediate HCC and is also widely used for advanced HCC.3,4 TACE works by embolizing the tumor blood supply, leading to tumor ischemia and hypoxia, thereby inhibiting tumor progression while chemotherapy drugs can be administered locally and accurately.5 However, the resultant hypoxic environment can stimulate angiogenic pathways, such as vascular endothelial growth factor (VEGF), reducing the number and function of immune cells. Combining immune checkpoint inhibitors (ICIs) and tyrosine kinase inhibitors (TKIs) can improve the hypoxic and immunosuppressive tumor microenvironment (TME) and inhibit tumor angiogenesis.6 Anti-VEGF plays its role as an anti-vascular, anti-angiogenic, or anti-permeability factor in preventing tumor growth and metastasis.7 Therefore, the combination therapy of TACE plus ICIs and anti-VEGF antibodies/TKIs holds the potential for further improving the efficacy of treatment for unresectable HCC (uHCC).8,9 Some studies, such as EMERALD-1, CHANCE001, and CHANCE2201, have further demonstrated that this combination therapy significantly improves the prognosis of patients with uHCC.10–12
The liver, being an immune-privileged organ, often exhibits a lack or even absence of tumor-infiltrating lymphocytes in both the HCC tumor nests and surrounding stroma, making it difficult to generate functional tumor-specific T cells and thus an effective anti-tumor immune response.13–15 TACE can reduce the density of immune-exhausted effector cells and regulatory T cells within the tumor.16 The combination of ICIs and TKIs can further activate T cells.17 Through TACE plus ICIs and anti-VEGF antibodies/TKIs, a non-immunogenic tumor, lacking immune effector cells, can be transformed into an immunogenic tumor with immune effector cell infiltration.8 There are few reports on how various humoral and cellular immune indicators in the peripheral circulation change in highly heterogeneous HCC patients undergoing this combination therapy.
In this study, we aim to explore the changes in immune indicators in the peripheral circulation of HCC patients undergoing this combination therapy and attempt to elucidate the relationship between immune response and tumor prognosis.
Materials and Methods
Patient Selection
This retrospective study was approved by the Institutional Review Board and the Ethics Committee. Since the study was retrospective, informed consent was not required. From March 11, 2019, to February 15, 2024, patients with uHCC who underwent TACE plus ICIs and anti-VEGF antibodies/TKIs were included. The inclusion criteria were as follows: (1) histologically or clinically diagnosed with HCC according to the American Association for the Study of Liver Diseases (AASLD) guidelines;18 (2) BCLC stage B or C; (3) Child-Pugh class A or B, without uncontrollable ascites or hepatic encephalopathy; (4) ECOG performance status of 0 or 1; (5) treatment with TACE plus ICIs and anti-VEGF antibodies/TKIs. The criteria for the timing of combination therapy were defined as simultaneous use of the initial TACE and the first ICI treatment or within a 3-month interval, with TKIs used simultaneously with TACE or ICIs. At least one cycle of ICI treatment should be used after TACE. (6) having complete blood cell count and immune indicators before combination therapy and at the first evaluation, and at least one serological assessment within 4 cycles after combination therapy. The first evaluation is often conducted in the fourth cycle or the third month.
Exclusion criteria were: (1) histologically or clinically confirmed intrahepatic cholangiocarcinoma (ICC), mixed hepatocellular carcinoma, sarcomatoid hepatocellular carcinoma, or fibrolamellar hepatocellular carcinoma; (2) concomitant other malignant tumors; (3) prior systemic treatment with ICIs, anti-VEGF antibodies /TKIs or chemotherapy; (4) incomplete blood or immune indicators that preclude data analysis; (5) follow up time less than 3 months after the combination therapy.
TACE Procedure
The TACE procedures were performed by two interventional radiologists with at least 10 years of experience. Precision TACE was used to reduce the heterogeneity and improve the embolization effect.19 The decision to perform “on-demand” TACE was based on the results of tumor markers and radiological examinations. “On-demand” TACE was discontinued if any of the following conditions occurred: (1) Child-Pugh class C (uncontrollable ascites, severe jaundice, overt hepatic encephalopathy, or hepatorenal syndrome); (2) ECOG score >2; (3) continuous progression of target lesions after three TACE sessions according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST).20
ICIs and Anti-VEGF Antibodies/TKIs Administration
All patients included in the study received ICIs and anti-VEGF antibodies/TKIs combination therapy, all of which were approved by the National Medical Products Administration and available in China. Anti-VEGF antibodies, like bevacizumab, were administered concurrently with ICIs. All oral TKIs were interrupted for two or three days before and after TACE. All drugs were administered based on the guidelines and availability in China following their standard dose and frequency. ICIs and anti-VEGF antibodies/TKIs treatment continued until disease progression or unacceptable toxic effects.
Follow-Up
The primary outcome was the objective response rate (ORR) at the first evaluation. After combination therapy, follow-up evaluations were conducted every 6–9 weeks, including imaging examinations (enhanced abdominal MRI and chest-abdomen plain CT scans) and laboratory examinations (complete blood cell count, comprehensive biochemical analysis, tumor markers, HBV-DNA load analysis, cytokines, immunity index, chest pain panel, and comprehensive thyroid function tests). ICIs were administered every three weeks, with laboratory tests (complete blood cell count, comprehensive biochemical analysis, immunity index, chest pain panel, and comprehensive thyroid function tests) conducted before each cycle. Tumor response was assessed according to mRECIST. The last follow-up date was May 15, 2024.
Explore Systemic Immune Response in Peripheral Blood
Peripheral blood samples were collected before TACE and every three weeks before ICI treatment. Immune indicators include the percentage of CD3+ cells, CD3+CD4+ cells, CD3+CD8+ cells, CD3−CD (16+56)+ cells, and CD3−CD19+ cells; the CD4+/CD8+ ratio; and the counts of immunoglobulin λ (Ig λ), immunoglobulin κ (Ig κ), complement 4 (C4), complement 3 (C3), immunoglobulin M (Ig M), immunoglobulin A (Ig A), immunoglobulin G (Ig G), and complement B factors (CFB). The neutrophil-to-lymphocyte ratio (NLR) is also included. Immune and peripheral blood indicators were completely collected before combination treatment and each cycle.
The peripheral blood mononuclear cells (PBMCs) were isolated using the Ficoll-Paque method and resuspended at a concentration of 1×10^6 cells/mL. PBMCs were collected and analyzed using flow cytometry (FACScan Caliber, Becton Dickinson, Franklin Lakes, NJ, USA). Immunoglobulins and complements were measured by the immune turbidimetric method. NLR was measured by the complete blood count.
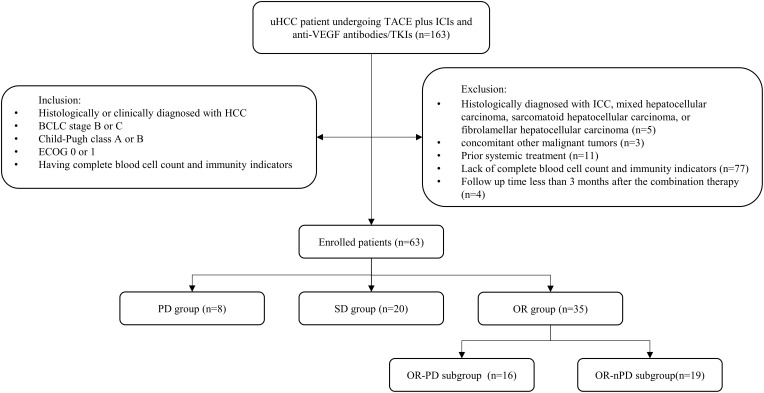
The initial treatment efficacy and tumor response were first assessed at the fourth cycle or the end of the third month according to mRECIST. Compare the baseline indicators of all patients with those at the first evaluation. Classify all patients into PD, SD, and OR (PR+CR) groups based on the first evaluation. Do inter-group and intra-group comparisons for each group. Within the OR group, further classify patients into OR-PD and OR-non-PD (OR-nPD) subgroups based on whether experiencing progression after the first evaluation. Collect immune and peripheral blood indicators at the time of progression. Compare the indicators of both subgroups at the different time points respectively. (Figure 1).
Statistical Analysis
Continuous variables are expressed as means with 95% confidence intervals. For categorical variables, counts and percentages are presented. Shapiro–Wilk tests were performed to determine the normality of the data distribution. For data that followed a normal distribution, significance testing for differences was conducted using either the chi-square test or the two-tailed paired Student’s t-test. For data that did not follow a normal distribution, the Kruskal–Wallis test or the nonparametric Mann–Whitney U-test was used to test for significant differences. The dynamic changes in the indicators were examined using simple linear regression analysis. Statistics were judged to be significant when p values were less than 0.05. All statistical analyses were performed using SPSS (version 26.0; IBM, Somers, NY) and GraphPad Prism software (version 9.0.2; GraphPad Software, CA, https://www.graphpad.com/).
Results
Patient Characteristics
From March 11, 2019, to February 15, 2024, a total of 163 uHCC patients undergoing TACE plus ICIs and anti-VEGF antibodies/TKIs were screened. Finally, 63 patients with complete follow-up data who met the inclusion criteria were enrolled. Based on the first evaluation, patients were categorized into three groups: PD group (8/63), SD group (20/63), and OR group (35/63), whereas 1 case of CR and 34 cases of PR were included in the OR group. The characteristics of each group were summarized in Table 1.
Table 1
Patient Baseline Characteristics of Each Groups
| Characteristics | Overall (n=63) | PD (n=8) | SD (n=20) | OR (n=35) | p value |
|---|---|---|---|---|---|
| Age(years) | 60(57,62) | 55(43,67) | 60(55,65) | 60(57,64) | 0.38 |
| Gender | 0.03 | ||||
 Male Male | 52(82.5) | 8(100.0) | 13(65.0) | 31(88.6) | |
 Female Female | 11(17.5) | 0(0.0) | 7(35.0) | 4(11.4) | |
| HBV | 0.89 | ||||
 Yes Yes | 51(81.0) | 7(87.5) | 16(80.0) | 28(80.0) | |
 No No | 12(19.0) | 1(12.5) | 4(20.0) | 7(20.0) | |
| Cirrhosis | 0.34 | ||||
 Yes Yes | 32(50.8) | 6(75.0) | 10(50.0) | 16(45.7) | |
 No No | 31(49.2) | 2(25.0) | 10(50.0) | 19(54.3) | |
| AFP | 0.23 | ||||
 <400ng/mL <400ng/mL | 43(68.3) | 5(62.5) | 11(55.0) | 27(77.1) | |
 ≥400ng/mL ≥400ng/mL | 20(31.7) | 3(37.5) | 9(45.0) | 8(22.9) | |
| PIVKA-II (mAU/mL) | 8475(4765,12,186) | 14,277(−1848,30,402) | 5180(−100,10,460) | 9033(3783,14,282) | 0.32 |
| Child-Pugh stage | 0.33 | ||||
 A A | 51(81.0) | 7(87.5) | 14(70.0) | 30(77.1) | |
 B B | 12(19.0) | 1(12.5) | 6(30.0) | 8(22.9) | |
| BCLC stage | 0.72 | ||||
 B B | 30(47.6) | 4(50.0) | 8(40.0) | 18(51.4) | |
 C C | 33(52.4) | 4(50.0) | 12(60.0) | 17(48.6) | |
| ECOG score | 0.86 | ||||
 0 0 | 34(54.0) | 4(50.0) | 10(50.0) | 20(57.1) | |
 1 1 | 29(46.0) | 4(50.0) | 10(50.0) | 15(42.9) | |
| MELD | 3.63(2.93,4.35) | 4.07(1.35,6.79) | 3.29(1.96,4.63) | 3.74(2.79,4.69) | 0.77 |
| ALBI | −2.29(−2.38,-2.18) | −2.27(−2.68,-1.87) | −2.25(−2.44,-2.05) | −2.31(−2.44,-2.17) | 0.85 |
| Extrahepatic spread | 0.64 | ||||
 Yes Yes | 16(25.4) | 1(12.5) | 6(30.0) | 9(25.7) | |
 No No | 47(74.6) | 7(87.5) | 14(70.0) | 26(74.3) | |
| Vascular invasion | 0.61 | ||||
 Yes Yes | 23(36.5) | 3(37.5) | 9(45.0) | 11(31.4) | |
 No No | 42(63.5) | 5(62.5) | 11(55.0) | 24(68.6) | |
| Up-to-seven criteria | 0.34 | ||||
 Within Within | 9(14.3) | 2(25.0) | 4(20.0) | 3(8.6) | |
 Beyond Beyond | 54(85.7) | 6(75.0) | 16(80.0) | 32(91.4) |
Abbreviations: HBV, hepatitis B virus; AFP, Alpha-fetoprotein; PIVKA-II, protein induced by vitamin K absence or antagonist-II; BLCL, Barcelona Clinic Liver Cancer; ECOG, Eastern Cooperative Oncology Group; MELD, model for end-stage liver disease score; ALBI, albumin bilirubin score.
Efficacy and Safety
The ORR was 55.6% at the first evaluation, while the disease control rate (DCR) was 87.3%. As of the last follow-up on May 15, 2024, the median follow-up time for the entire cohort was 16.3 months (interquartile range [IQR]: 10.6–25.5 months). The median overall survival (mOS) was 27.5 months (95% confidence interval [CI]: 22.5–32.5 months), and the median progression-free survival (mPFS) was 15.9 months (95% CI: 12.6–19.3 months).
All reported adverse events were mild and well-tolerated, with no treatment-related fatalities during the study. The incidence of adverse events was 61.9% (39/63), including primary fever (59.0%), liver function impairment (20.5%), abdominal pain (48.7%), hand-foot skin reaction (23.1%), hypertension (15.4%), thrombocytopenia (10.3%), proteinuria (17.9%), and immune myocarditis (2.6%).
Systemic Immune Response in Peripheral Blood
Comparisons of Pre-Treatment and Post-Treatment Indicators in the Whole Group
A comparison of baseline indicators with those at the first evaluation revealed significant differences, with a decrease in CD3−CD19+ (p=0.009), C4 (p=0.039), CFB (p=0.002), NLR (p=0.006) and an increase in Ig λ (p=0.002), Ig κ (p=0.005), Ig A (p<0.001), Ig G (p=0.001) (Table 2).
Table 2
Comparation Between Baseline and First Evaluation Indicators for All Patients
| Baseline-Overall | At First Evaluation-Overall | p value | |
|---|---|---|---|
| CD3+ (%) | 72.88(70.46,75.30) | 73.44(70.44,76.44) | 0.571 |
| CD3+CD4+ (%) | 41.74(39.52,43.96) | 42.01(39.31,44.71) | 0.750 |
| CD3+CD8+ (%) | 27.02(24.68,29.36) | 26.92(24.52,29.33) | 0.885 |
| CD4+/CD8+ | 1.82(1.55,2.09) | 1.83(1.58,2.09) | 0.904 |
| CD3−CD(16+56)+ (%) | 16.67(14.54,18.81) | 17.72(15.01,20.42) | 0.419 |
| CD3−CD19+ (%) | 9.80(8.29,11.31) | 8.20(6.59,9.80) | 0.009 |
| Ig λ (mg/dl) | 672.86(611.27,734.44) | 740.59(684.81,796.37) | 0.002 |
| Ig κ (mg/dl) | 1212.59(1120.41,1304.76) | 1335.95(1246.17,1425.74) | 0.005 |
| C4 (g/l) | 0.22(0.20,0.25) | 0.21(0.19,0.23) | 0.039 |
| C3 (g/l) | 0.92(0.86,0.97) | 0.90(0.85,0.94) | 0.653 |
| Ig M (g/l) | 1.23(1.05,1.40) | 1.26(1.08,1.45) | 0.584 |
| Ig A (g/l) | 3.34(2.98,3.69) | 3.75(3.35,4.16) | <0.001 |
| Ig G (g/l) | 14.15(13.07,15.24) | 15.61(14.62,16.60) | 0.001 |
| CFB (mg/dl) | 45.10(41.90,48.31) | 40.66(38.17,43.15) | 0.002 |
| NLR | 5.72(4.62,6.83) | 3.78(2.72,4.83) | 0.006 |
Inter-Group and Intra-Group Comparisons for Each Group
When comparing among PD, SD, and OR groups, only CD3−CD19+ showed a significant difference at baseline (p=0.019). Further intra-group comparisons revealed no significant differences in the PD group, while the SD group showed significant differences in CFB (p=0.008) and NLR (p=0.037). The OR group exhibited significant differences, similar to those in the whole group, with a decrease in CD3−CD19+ (p=0.004), CFB (p=0.027), NLR (p<0.001), and an increase in Ig λ (p=0.010), Ig κ (p=0.037), Ig A (p=0.005), Ig G (p=0.006) (Table 3).
Table 3
Inter-Group and Intra-Group Comparisons for Each Group
| Baseline | p1 value | At First Evaluation | p2 value | p3 value | |
|---|---|---|---|---|---|
| CD3+ (%) | 0.916 | 0.647 | |||
 PD PD | 73.75(66.53,80.98) | 74.13(64.26,84.01) | 0.875 | ||
 SD SD | 73.31(68.31,78.31) | 71.36(64.91,77.82) | 0.307 | ||
 OR OR | 72.44(69.20,75.67) | 74.47(70.70,78.24) | 0.064 | ||
| CD3+CD4+ (%) | 0.468 | 0.303 | |||
 PD PD | 39.14(34.27,44.02) | 39.56(32.94,46.19) | 0.851 | ||
 SD SD | 40.76(36.73,44.78) | 39.70(34.79,44.61) | 0.439 | ||
 OR OR | 42.89(39.65,46.14) | 43.89(40.02,47.77) | 0.425 | ||
| CD3+CD8+ (%) | 0.148 | 0.298 | |||
 PD PD | 32.32(23.65,41.00) | 31.82(24.69,38.96) | 0.744 | ||
 SD SD | 27.76(23.73,31.78) | 26.54(21.90,31.18) | 0.332 | ||
 OR OR | 25.38(22.24,28.53) | 26.03(22.78,29.27) | 0.465 | ||
| CD4+/CD8+ | 0.141 | 0.255 | |||
 PD PD | 1.35(0.89,1.80) | 1.32(0.94,1.70) | 0.777 | ||
 SD SD | 1.64(1.28,2.01) | 1.72(1.30,2.13) | 0.313 | ||
 OR OR | 2.04(1.61,2.47) | 2.02(1.62,2.41) | 0.572 | ||
| CD3−CD(16+56)+ (%) | 0.328 | 0.485 | |||
 PD PD | 18.04(12.64,23.43) | 19.66(11.07,28.25) | 0.499 | ||
 SD SD | 18.62(14.19,23.06) | 19.22(14.23,24.21) | 0.765 | ||
 OR OR | 15.24(12.42,18.07) | 16.42(12.63,20.20) | 0.342 | ||
| CD3−CD19+ (%) | 0.019 | 0.484 | |||
 PD PD | 7.53(4.59,10.46) | 5.67(3.53,7.81) | 0.143 | ||
 SD SD | 7.44(5.49,9.38) | 4.28(1.36,7.19) | 0.412 | ||
 OR OR | 11.67(9.36,13.99) | 8.48(6.57,10.40) | 0.004 | ||
| Ig λ (mg/dl) | 0.064 | 0.095 | |||
 PD PD | 531.13(382.36,679.89) | 610.38(437.51,783.24) | 0.139 | ||
 SD SD | 773.60(644.22,902.98) | 808.25(690.03,926.47) | 0.319 | ||
 OR OR | 647.69(572.05,723.32) | 731.69(664.52,798.85) | 0.010 | ||
| Ig κ (mg/dl) | 0.567 | 0.586 | |||
 PD PD | 1141.75(852.18,1431.32) | 1234.13(999.70,1468.55) | 0.441 | ||
 SD SD | 1286.80(1113.61,1459.99) | 1396.75(1217.72,1575.78) | 0.080 | ||
 OR OR | 1186.37(1058.80,1313.94) | 1324.49(1201.08,1447.89) | 0.037 | ||
| C4 (g/l) | 0.329 | 0.052 | |||
 PD PD | 0.26(0.17,0.36) | 0.30(0.16,0.43) | 0.388 | ||
 SD SD | 0.22(0.19,0.25) | 0.21(0.18,0.23) | 0.144 | ||
 OR OR | 0.22(0.18,0.26) | 0.19(0.17,0.22) | 0.067 | ||
| C3 (g/l) | 0.977 | 0.528 | |||
 PD PD | 0.90(0.72,1.07) | 0.86(0.68,1.04) | 0.594 | ||
 SD SD | 0.92(0.82,1.02) | 0.87(0.78,0.96) | 0.146 | ||
 OR OR | 0.92(0.83,1.00) | 0.92(0.85,0.99) | 0.590 | ||
| Ig M (g/l) | 0.686 | 0.389 | |||
 PD PD | 1.49(0.40,2.58) | 1.43(0.35,2.51) | 0.310 | ||
 SD SD | 1.08(0.85,1.30) | 1.09(0.85,1.33) | 0.533 | ||
 OR OR | 1.26(1.05,1.47) | 1.33(1.10,1.55) | 0.416 | ||
| Ig A (g/l) | 0.415 | 0.944 | |||
 PD PD | 3.10(2.38,3.82) | 3.61(2.85,4.37) | 0.065 | ||
 SD SD | 3.78(2.96,4.59) | 4.01(3.04,4.97) | 0.268 | ||
 OR OR | 3.14(2.70,3.58) | 3.64(3.15,4.14) | 0.005 | ||
| Ig G (g/l) | 0.295 | 0.252 | |||
 PD PD | 12.70(9.01,16.39) | 13.92(10.36,17.48) | 0.255 | ||
 SD SD | 15.40(13.29,17.50) | 16.59(14.65,18.53) | 0.058 | ||
 OR OR | 13.77(12.36,15.19) | 15.44(14.18,16.71) | 0.006 | ||
| CFB (mg/dl) | 0.480 | 0.426 | |||
 PD PD | 41.00(30.32,51.68) | 41.79(34.22,49.35) | 0.838 | ||
 SD SD | 44.02(38.90,49.14) | 38.25(33.84,42.66) | 0.008 | ||
 OR OR | 46.66(41.95,51.37) | 41.77(38.22,45.33) | 0.027 | ||
| NLR | 0.822 | 0.075 | |||
 PD PD | 4.56(2.33,6.79) | 6.10(1.99,10.21) | 0.327 | ||
 SD SD | 5.77(4.02,7.53) | 4.28(1.36,7.19) | 0.037 | ||
 OR OR | 5.96(4.23,7.69) | 2.96(2.34,3.58) | <0.001 |
Subgroup Analysis Within the OR Group
The OR group was further divided into OR-PD (16/35) and OR-nPD (19/35) subgroups. Subsequent indicator comparisons in the OR-PD subgroup showed no significant differences among baseline, first evaluation, and progression points (Figure 2, Tables S1 and S2).
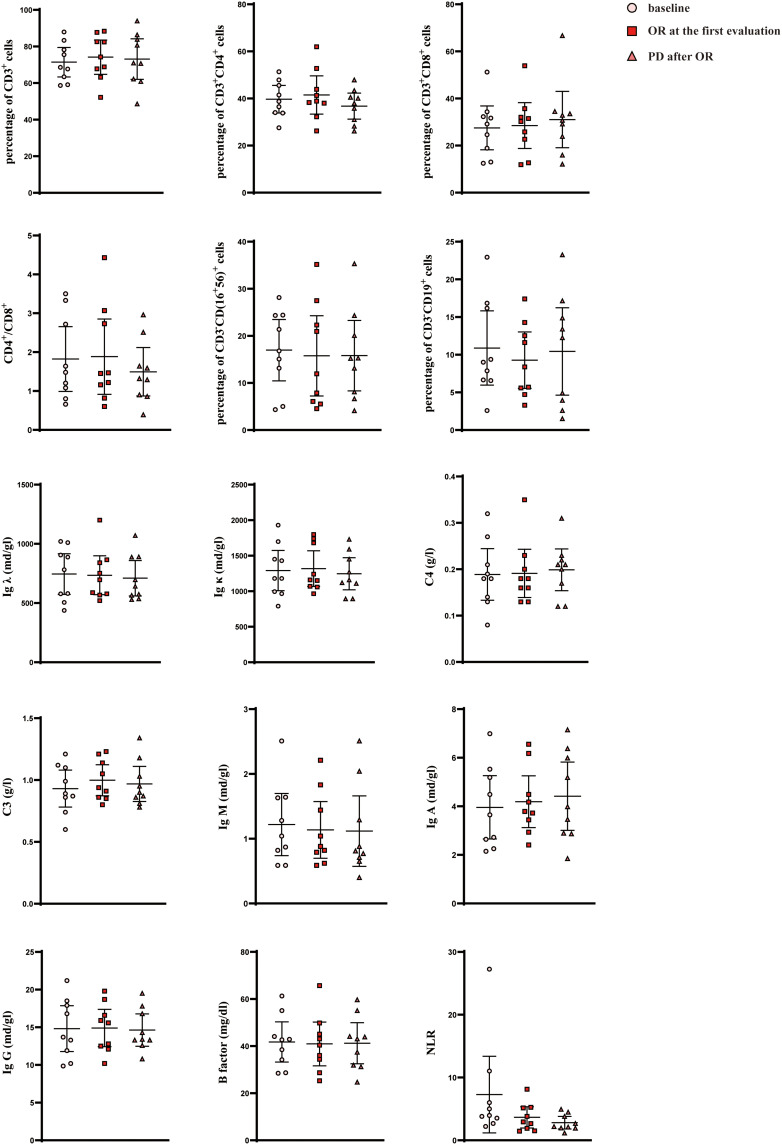
The distribution of indicators at different timepoints in the OR-PD subgroup.
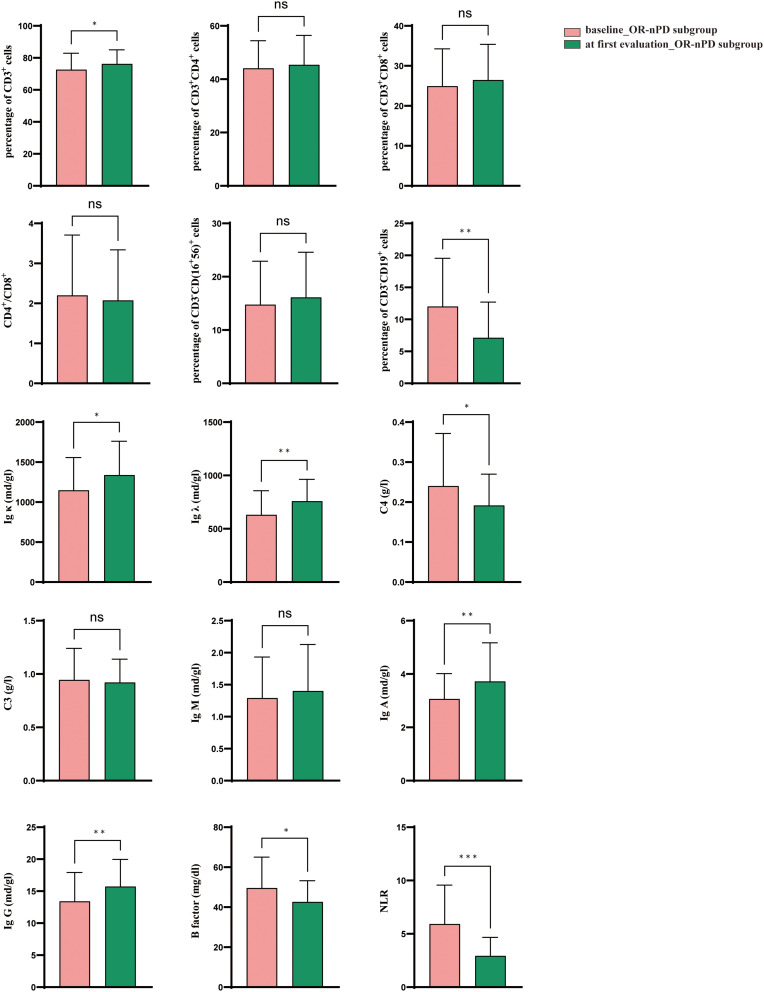
Intriguingly, similar trends were observed in the OR-nPD subgroup with a decrease in CD3−CD19+ (p=0.001), C4 (p=0.040), CFB (p=0.029), NLR (p=0.001), and an increase in CD3+ (p=0.013), Ig λ (p=0.004), Ig κ (p=0.021), Ig A (p=0.005), Ig G (p=0.003). In contrast, the OR-PD subgroup showed no significant differences (Figure 3, Table S2).
Simple Linear Regression Analysis
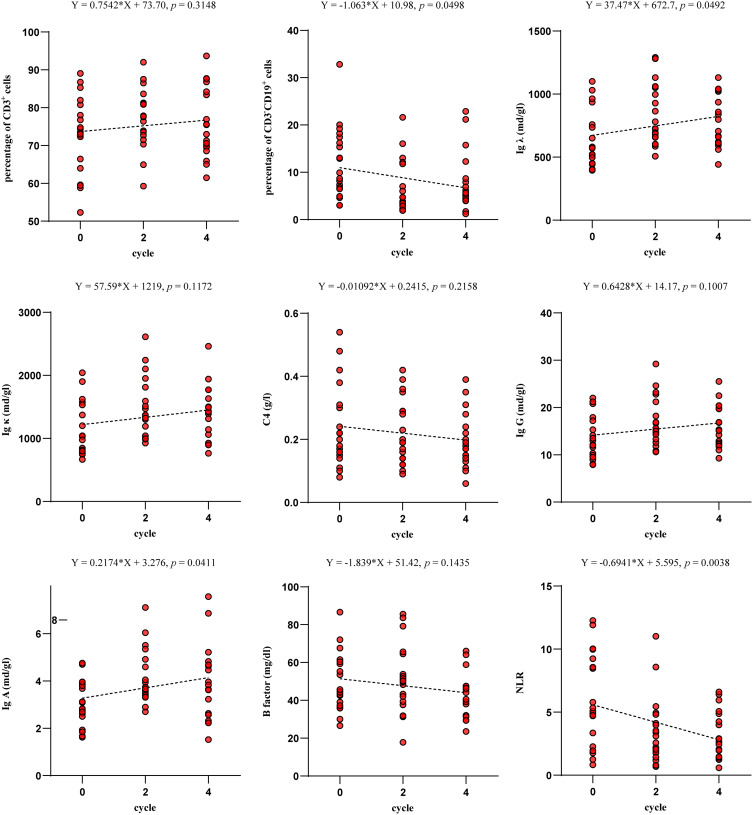
For indicators showing significant differences in the OR-nPD subgroup, simple linear regression analysis revealed dynamic changes, including CD3−CD19+ (Y = −1.063*X + 10.98, p=0.0498), Ig λ (Y = 37.47*X + 672.7, p=0.0492), Ig A (Y = 0.2174*X + 3.276, p=0.0411) and NLR (Y = −0.6941*X + 5.595, p=0.0038) (Figure 4).
Discussion
This retrospective study demonstrated the safety and efficacy of the combination therapy of TACE plus ICIs and anti-VEGF antibodies/TKIs for uHCC patients, with an ORR of 55.6% and a DCR of 87.3% at the first evaluation. This combination therapy may enhance systemic immune responses, with changes in circulating immune indicators linked to prognosis. It indicated that more active immune responses may correlate with better prognosis, expressed in a decrease in CD3−CD19+, C4, CFB, NLR and an increase in CD3+, Ig λ, Ig κ, Ig A, Ig G. These changes can potentially guide personalized treatment strategies by identifying patients who are more likely to benefit from this combined therapy.
In previous studies, low levels of CD3+ cells have been confirmed as an adverse prognostic factor.21–24 In the OR group, especially in the OR-nPD subgroup, a similar trend can be observed: higher levels of CD3+ and CD4+ cells suggest improved immune status and better prognosis. Conversely, during recurrence or metastasis, CD3+ and CD4+ cell levels are significantly reduced, although not statistically significant. Unlike previous studies, changes in CD4/CD8 ratio and CD8+ cells did not show specific patterns related to prognosis.25–27 Rather than solely the frequency of CD3+, CD8+, and CD4+ lymphocytes, biomarkers preferentially expressed on tumor-reactive lymphocytes, such as PD-1hi, CD39, CXCL13, CD103, or the co-expression of PD-1hi and CXCL13, have more predictive value.28 Nonetheless, it is clear that the frequency of CD3+ was positively correlated with prognosis.
In this study, the frequency of B cells was also associated with the progression of HCC patients undergoing combination therapy. Consistent with previous findings, the number of B cells in HCC patients achieving disease remission was significantly reduced compared to those with disease progression (p=0.004).29 Although not statistically significant, circulating B cell counts tended to increase at the time of progression in the OR-PD subgroup. In other words, B cell is possibly another negative regulatory factor.30 The antibodies secreted by B cells can independently activate innate immune responses and induce the cancer immunity cycle.31 B cells migrate from the bone marrow to secondary lymphoid organs, where they undergo activation upon encountering antigens. This activation process includes class switching, during which B cells change the type of immunoglobulin.32 Therefore, in this study, a decrease in circulating B cells was observed to be accompanied by an increase in IgG, IgA, Ig κ, and Ig λ.
Previous studies have used RNA sequencing (RNA-seq) to analyze the whole transcriptome of shCFB (knockdown) cells and shControl (mock) cells, concluding that CFB itself is an early initiator of tumorigenesis and suggesting that increased CFB expression may be an indicator of pancreatic cancer development.33,34 In this study, both the OR group (p=0.027) and OR-nPD subgroup (p=0.029) showed a decreasing trend in CFB expression. This indicates that a decrease in CFB was associated with a better prognosis. Besides CFB, complement factors like C3 and C5 are involved in tumor development.35,36 However, the role of C4 in tumor progression is less studied, likely due to its early involvement in the classical complement pathway. Markiewski et al injected TC-1 tumor cells subcutaneously into mice and found that mice lacking C3 or C4 showed significantly reduced tumor proliferation compared to mice with adequate levels of C3 and C4.37 This suggests that C4 can infect tumor progression.
Elevated NLR, resulting from increased neutrophil count and decreased lymphocyte count, has been associated with poor prognosis in certain malignant tumors.38–41 The TME, involving inflammatory cells, plays a crucial role in tumor formation while NLR serves as an effective indicator of systemic inflammatory response.42 Wu et al reported that NLR ≥ 5 is an independent predictor of poor prognosis for uHCC treated with atezolizumab plus bevacizumab.43 In this study, the PD group exhibited an increase in NLR compared to baseline, while the SD and OR groups showed a decrease. Additionally, the OR-nPD group demonstrated a continuous decrease in NLR (p=0.0038). Zhou et al reported that tumor-associated neutrophils promote HCC cell growth.44 Increased circulating neutrophil levels may elevate levels of proteases, growth factors, VEGF, and intercellular adhesion molecule-1 (ICAM-1), contributing to cancer progression by modulating angiogenesis, cell growth, or inflammation, ultimately leading to lower survival rates in HCC patients.45,46
Additionally, this study included exploratory subgroup analyses based on three different immune checkpoint inhibitors: atezolizumab, camrelizumab, and sintilimab. Consistent with the results mentioned earlier, the atezolizumab and sintilimab subgroups, which had a higher ORR at the first assessment, demonstrated more active immune responses compared to the camrelizumab subgroup, which had a lower ORR (Tables S3 and S4). In this study, atezolizumab and sintilimab were primarily combined with bevacizumab, whereas camrelizumab was more paired with TKIs. In the short term, bevacizumab’s anti-angiogenic effect may be more effective against the ischemic and hypoxic environment caused by TACE. This finding provides a basis for clinical decision-making regarding combined immunotherapy regimens.
This study has some limitations. Firstly, it is a single-center retrospective study, which may introduce selection bias. Secondly, incomplete follow-up data resulted in a small sample size. Future studies should standardize and extend monitoring periods to collect more comprehensive data on dynamic changes in these immune indicators. Lastly, integrating pathological immunohistochemistry results could further investigate reasons for the differences in these immune indicators within the TME and in circulation.
In conclusion, the combination therapy of TACE plus ICIs and anti-VEGF antibodies/TKIs may modify the TME of HCC. Decreases in CD3−CD19+, C4, CFB, NLR, along with increases in Ig λ, Ig κ, Ig A, Ig G, were associated with better tumor response, serving as potential biomarkers. Monitoring these trends can help tailor treatments individually and enhance efficacy.
Acknowledgments
Xiao-Yang Xu, Ze Wang and Chen-You Liu contributed equally to this work and share first authorship.We wish to acknowledge the support from all the investigators in this study.
Funding Statement
This work is supported by the Foundation of 2023 Jiangsu Province Natural Science Foundation Project (SBK2023022210), the 2023 Clinical Research Project of the First Affiliated Hospital of Soochow University (BXLC010), and the 2023 Zhou Nursing Research Project of the First Affiliated Hospital of Soochow University (HLYJ-Z-2023-02).
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding authors upon reasonable request.
Ethics Statement
This retrospective study was approved by the Institutional Review Board and Human Ethics Committee of the First Affiliated Hospital of Soochow University (Approval Number: 2024352). The need for informed consent was waived by the IRB due to the retrospective nature of this study. However, all participating patients provided written informed consent at each admission. The data collected were used solely for the purposes of this study. The study was performed in accordance with the ethical guidelines of the 1975 Declaration of Helsinki.
Disclosure
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. The authors report no conflicts of interest in this work.
References
Articles from Journal of Hepatocellular Carcinoma are provided here courtesy of Dove Press
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Transarterial Chemoembolization Combined With Immune Checkpoint Inhibitors and Tyrosine Kinase Inhibitors for Unresectable Hepatocellular Carcinoma: Efficacy and Systemic Immune Response.
Front Immunol, 13:847601, 18 Feb 2022
Cited by: 24 articles | PMID: 35300339 | PMCID: PMC8922415
Immune checkpoint inhibitors and anti-vascular endothelial growth factor antibody/tyrosine kinase inhibitors with or without transarterial chemoembolization as first-line treatment for advanced hepatocellular carcinoma (CHANCE2201): a target trial emulation study.
EClinicalMedicine, 72:102622, 06 May 2024
Cited by: 9 articles | PMID: 38745965 | PMCID: PMC11090892
Transarterial Chemoembolization Combined with Immune Checkpoint Inhibitors Plus Tyrosine Kinase Inhibitors versus Immune Checkpoint Inhibitors Plus Tyrosine Kinase Inhibitors for Advanced Hepatocellular Carcinoma.
J Hepatocell Carcinoma, 9:1217-1228, 30 Nov 2022
Cited by: 14 articles | PMID: 36474670 | PMCID: PMC9719708
TACE plus tyrosine kinase inhibitors and immune checkpoint inhibitors versus TACE plus tyrosine kinase inhibitors for the treatment of patients with hepatocellular carcinoma: a meta-analysis and trial sequential analysis.
Hepatol Int, 18(2):595-609, 16 Oct 2023
Cited by: 5 articles | PMID: 37843788
Review