Abstract
Free full text

Clinical update on acute cholecystitis and biliary pancreatitis: between certainties and grey areas
Summary
Acute calculous cholecystitis (ACC) and acute biliary pancreatitis (ABP) are significant complications of gallstone disease. This review aims to provide a comprehensive analysis of current management practices for ACC and ABP. The Tokyo Guidelines (TG) and World Society of Emergency Surgery (WSES) guidelines recommend early laparoscopic cholecystectomy (ELC) as the treatment of choice for ACC. High-risk patients may benefit from alternative treatments like biliary drainage, with emerging techniques such as endoscopic drainage showing promise. ABP requires prompt diagnosis and intervention. The Revised Atlanta Classification (RAC) criteria are used for diagnosis, with endoscopic retrograde cholangiopancreatography (ERCP) and cholecystectomy as primary treatments. Minimally invasive approaches are preferred for managing complications like infected pancreatic necrosis, with the endoscopic step-up method showing superior outcomes. The management of ACC and ABP continues to evolve. Future research is needed to refine guidelines further and address existing controversies, ultimately improving patient outcomes in these acute biliary conditions.
Introduction
10–15% of the general population is affected by cholelithiasis and 20–40% of them will develop complications.1 In most patients, the index presentation of gallstone-related complications is Acute Calculous Cholecystitis (ACC), accounting for 10–15% of symptomatic patients.1,2 The most widely used guidelines worldwide for the management of ACC are the Tokyo Guidelines (TG) and the World Society of Emergency Surgery (WSES) guidelines.3, 4, 5, 6, 7 In many aspects, the TG were in line with the recommendations of the WSES.4,6, 7, 8, 9 However, there are still some differences between the TG and the WSES guidelines. Our review, herein, will particularly focus on the grey and controversial areas in the management of ACC and acute biliary pancreatitis (ABP). These areas still remain under-addressed despite the breadth of prior published literature.
Acute calculous cholecystitis
Pathogenesis and pathophysiology
ACC is the most common complication of gallstones, and 95% of cases are caused by an obstruction of the cystic duct. The obstruction of bile outflow causes biliary stasis which promotes chemical irritation of the mucosa and inflammation of the gallbladder. Histological examination of ACC specimens reveals the presence of arteriole obstruction due to thrombus formation around the necrotic lesions, suggesting that this lesion has an element of ischemic necrosis.10 The first phase of ACC, also known as “edematous”, lasts between 2 and 4 days, during which congestion and edema are evident. Then, over the next 3–5 days, ACC progresses to the necrotizing phase, characterized by bleeding and necrosis. Approximately 7–10 days after symptom onset, the disease progresses to its purulent phase, also known as suppurative ACC. If the disease is left untreated, it progresses to subacute cholecystitis and it eventually becomes chronic cholecystitis.10, 11, 12 Possible complications of ACC include perforation of the gallbladder, peri-gallbladder abscess, internal biliary fistula, biliary peritonitis, acute cholangitis. Contained perforation can lead to the formation of a walled-off pericholecystic abscess, while a chronic perforation can lead to the formation of a fistulous connection between the gallbladder and other viscera in 2–3% of cases10 (Supplementary Material Fig. S1).
Diagnosis
According to TG, the diagnosis of ACC can be made when all three of the following criteria are met: signs of local infection, signs of systemic infection and radiological signs (Supplementary Material Table S1).7 Using the pathological examination of the gallbladder as the gold standard, the diagnostic accuracy of the TG criteria ranges from 60.4% to 94.0%. For this reason, according to WSES guidelines, only the integration of a detailed medical history, a complete clinical examination, laboratory tests and imaging investigations can lead to the diagnosis of ACC, although the best combination of these findings is not yet known.1,8 Ultrasound (US) of the abdomen is unanimously recognized as the first choice for the diagnosis of ACC, with a sensitivity of 81% and a specificity of 83% (Fig. 1a).1,7,13 Contrast-enhanced computed tomography (CT) is less accurate than US in diagnosing ACC but may be useful for the diagnosis of gangrenous ACC (Fig. 1b). Magnetic resonance imaging (MRI) has a diagnostic accuracy comparable to US and it is useful in cases where US does not arrive at a definitive diagnosis, although it is rarely available in an urgent setting (Fig. 1c).
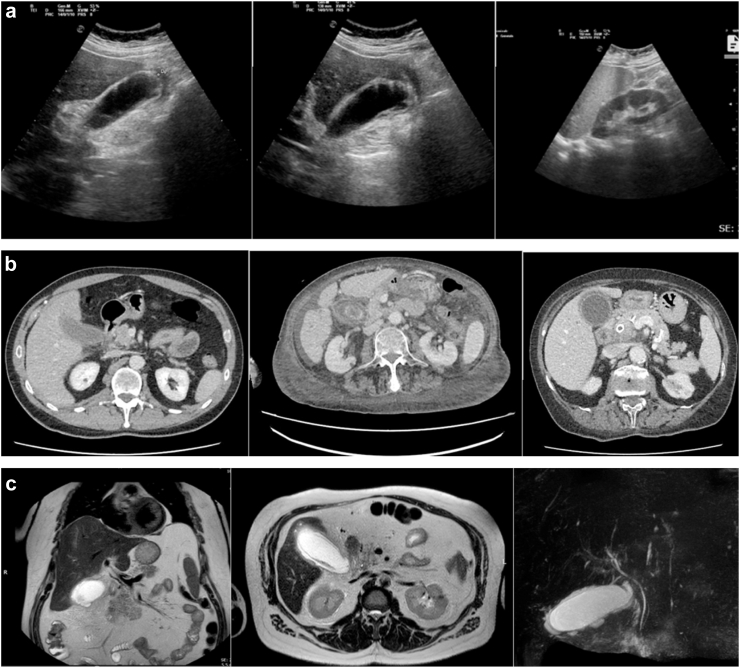
Radiologic images of Acute Calculous Cholecystitis: (a) Ultrasound images of Acute Calculous Cholecystitis; (b) Computed tomography images of Acute Calculous Cholecystitis; (c) Magnetic resonance images of Acute Calculous Cholecystitis.
TG suggest a classification for ACC, structured on three different levels of severity according to the characteristics of the acute inflammatory process7: Grade III, severe ACC—presence of ACC, associated with organ dysfunction; Grade II, Moderate ACC—associated with local and systemic inflammatory conditions; and Grade I, Mild ACC–ACC that does not meet the criteria of “Grade III” and “Grade II”. This stratification was the first attempt to create a shared classification system to standardize patient data and characteristics. The classification of TG has been validated by some studies in terms of mortality, length of hospital stay, conversion to open surgery and costs. However, a recent prospective international study showed only moderate diagnostic accuracy of this classification in predicting 30-day mortality with an AUC (Area Under the Curve) of 0.76.7,14,15
Treatment
The treatment of choice for ACC is Early Laparoscopic Cholecystectomy (ELC), performed during the index hospitalization. The WSES guidelines broadly recommend ELC for most patients, including those at high surgical risk, unless absolute contraindications exist.1,8 The TG offer a more detailed framework with explicit stratification of ACC severity and corresponding treatment pathways. While both guidelines advocate for ELC as the cornerstone of ACC treatment, they also acknowledge the necessity of alternative approaches in high-risk patients or when immediate surgery is not feasible.
Contraindications to ELC and selection of high-risk patients
After establishing the severity of ACC in accordance to the TG, there are some contraindications to ELC. These include the patient’s general conditions and comorbidities, which are stratified with the Charlson Comorbidity Index (CCI) and the Physical Status of the American Society of Anesthesiologists (ASA-PS).
According to the WSES guidelines,1,8 the only contraindications to ELC are the patient’s refusal of surgery or absolute anesthesiological contraindications. ELC is also recommended for patients at high surgical risk. In 2020, a systematic review reported on the ability of various prognostic factors and risk prediction models to predict the outcome of patients with ACC. The review showed that despite the multiple studies, there were still no reliable predictive models.1,16,17 Recently, the SPRIMACC multicenter prospective study15 found that the most accurate risk prediction model for postoperative 30-day mortality after ELC in patients with ACC is the POSSUM-Physiological Score (PS). The authors defined the term “CHOLE-POSSUM” as the POSSUM-PS with a cut-off of 25. The CHOLE-POSSUM was demonstrated to be an accurate and reliable tool with a sensitivity and a negative predictive value of 100% for the postoperative mortality of these patients.
Timing of ELC
The definition of ELC in terms of timing is still debated. The WSES defined ELC as cholecystectomy performed within 7 days of hospitalization and 10 days of symptom onset. The Japanese guidelines, instead, defined ELC more vaguely, as a cholecystectomy performed as soon as possible, preferably within 72 h of the onset of symptoms, but also after this time interval.1,18 Several studies and meta-analyses comparing different timings for ELC revealed no significant association between operation timing and postoperative mortality or morbidity; but demonstrated longer post-operative stays in the patient groups with longer time intervals between hospitalization and surgery. Other observational studies showed a significantly lower rate of biliary complications and 30-day mortality and morbidity in patients who underwent cholecystectomy within 3 days of admission, showing that the number of days from admission to ELC was an independent predictor of mortality.19, 20, 21, 22, 23, 24 A post hoc analysis of the SPRIMACC study25 showed that delaying ELC for up to ten days after symptoms onset did not affect postoperative complications and mortality. However, ACC is an evolutive inflammatory process, and as the days go by, local and systemic inflammation increases, which makes surgery more complex and difficult with a higher risk of intraoperative complications. The authors therefore recommended performing ELC as early as possible within the first ten days of symptom onset.
Stratification of common bile duct stones (CBDSs) risk
CBDSs are associated with ACC with a rate ranging from 8.7% to 25%.26 Endoscopic Retrograde CholangioPancreatography (ERCP) is very effective in diagnosing and treating CBDSs, but it carries the risk of perforation, infection, anesthesia-related adverse events, post-ERCP pancreatitis and bleeding.27 For this reason, ERCP should not be used for diagnostic purposes. Endoscopic UltraSound (EUS) or Magnetic Resonance CholangioPancreatography (MRCP) are very accurate and low-risk options for second-level assessment when the diagnosis of CBDSs is uncertain, allowing patients to be selected for ERCP. These second-level examinations, however, are expensive and not readily available and they could delay surgical therapy for ACC patients, worsening outcomes.20,23,24,28, 29, 30 The WSES did not recommend basing the diagnosis of CBDSs solely on liver function tests, because the latter can be altered due to the inflammatory processes affecting the gallbladder and the biliary tree.1 The American Society of Gastrointestinal Endoscopy (ASGE) and the Society of American Gastrointestinal Endoscopic Surgeons (SAGES) have proposed a risk stratification for CBDSs based on moderate, strong, and very strong predictors. In light of this, the WSES suggested stratifying the risk of CBDSs according to a modified version of the ASGE and SAGES guidelines.1,20,23,24,27, 28, 29, 30, 31 However, some studies have shown that this tool works sub-optimally for the prediction of choledocholithiasis in the specific population with ACC.27,32 For these reasons in 2020 in Israel, Khoury et al. created a new score for the prediction of CBDSs, specifically for patients with ACC.33 The Israeli Score (IS) takes into account age, the diameter of the CBD on US and the value of total bilirubin (Supplementary Material Table S2). This score was prospectively validated by a post-hoc analysis of the SPRIMACC study.34 Only patients with an IS of three should directly undergo ERCP or intraoperative CBD treatment. Patients with an IS of two should be considered as intermediate risk and undergo second-level investigations such as EUS or MRCP, intraoperative cholangiography or laparoscopic ultrasound, depending on local expertise and availability. Patients with an IS of zero or one should be considered at low risk for CBDSs and undergo ELC directly.27,32,33
Alternative approaches to ELC in high-risk patients
Although the treatment of choice for ACC is ELC performed during the index hospitalization, real-world scenarios, including the COVID-19 pandemic, have revealed limitations to the universal implementation of ELC. For high-risk patients or those where ELC is contraindicated, alternative treatments like percutaneous gallbladder drainage (PGBD) and endoscopic techniques such as Endoscopic Transpapillary GallBladder Drainage (ETGBD) and Endoscopic UltraSound-guided GallBladder transmural Drainage (EUS-GBD) have emerged.35, 36, 37 Traditionally, PGBD has been used to manage high-risk patients who are not immediate candidates for surgery. PGBD is usually readily available and does not raise concerns about airway management in severely ill patients. Despite its utility, PGBD has significant drawbacks, including pain at the insertion site, risk of infection, and the need for re-intervention, especially when the catheter is left in place for over three months.1 The CHOCOLATE randomized controlled trial38 compared ELC and PGBD in high-risk patients with ACC and showed a higher rate of major complications and recurrent biliary events in patients undergoing PGBD. Some recent meta-analyses compared emergency cholecystostomy as a bridge to DLC, and cholecystectomy alone in patients with ACC.39, 40, 41, 42 Cirocchi et al.40 found that the incidence of postoperative complications were lower in the group of patients treated with cholecystostomy as a bridge to cholecystectomy. However, the comparator group included both patients treated with ELC and patients treated with conservative therapy and elective cholecystectomy. In the meta-analysis by Cai et al.,39 the authors found that, compared with the ELC group, the DLC after the PGBD group had significantly better outcomes in terms of and postoperative complications. However, one of the inclusion criteria in the meta-analysis was that the PGBD group had no severe complications, so the study did not consider the complications and mortality derived from the PGBD. The meta-analysis by Nassar et al.,41 analyzed separately the outcomes of ELC compared to PGBD, and the outcomes of ELC compared to DLC. They found that patients who had ELC were at higher risk of bile duct injury and post-operative complications, compared to DLC. On the other hand, patients who had PGBD were at more than three times the risk of mortality compared to the ELC group; furthermore, for patients aged 65 years or older and for high-risk surgical patients, overall post-procedural complications were significantly higher in patients who had PGBD compared to ELC group patients. In summary, probably the most objective approach to compare ELC to PGBD and delayed cholecystectomy should consider not only the outcome of the DLC compared to ELC (that is probably better due to the improved inflammatory conditions) but also the outcome of the PGBD, which seems to be worse than that of the ELC. Recently, new endoscopic gallbladder drainage techniques have been introduced: ETGBD during ERCP and EUS-GBD.1 ETGBD involves placing a stent via ERCP to ensure gallbladder drainage. One of the primary advantages of ETGBD is that it does not create a fistula or significant adhesions, which simplifies subsequent cholecystectomy. The effectiveness of ETGBD generally lasts for at least three months, making it suitable for patients who need to wait for cholecystectomy, including those whose condition may improve sufficiently to tolerate surgery later. EUS-GBD, particularly with the placement of lumen-apposing metal stents (LAMS), has shown excellent results in managing ACC in patients who are poor surgical candidates. In fact, EUS-GBD can complicate future cholecystectomy due to the formation of a fistula that may need to be closed surgically. Therefore, EUS-GBD is suggested primarily for the definitive treatment of patients with high ECOG scores or limited life expectancy, who are considered unsuitable for any future surgical intervention.35 The recent DRAC 1 randomized controlled trial35 and some meta-analyses,43, 44, 45, 46, 47 compared EUS-GBD with cholecystostomy drainage in high-risk patients with ACC, finding better results in EUS-GBD. The EUS-GBD with LAMS placement showed promising results when compared to ELC: a recent retrospective propensity-score matched study showed that technical and clinical success rates, length of stay, 30-day adverse events and mortality rates in patients treated with EUS-GBD with LAMS (because they were considered high-risk for surgery) were comparable to those of patients considered “fit for surgery” and subjected to ELC. The rates of recurrent biliary events, reoperations and unplanned hospital readmissions over one year were also similar. In light of these data, the best treatment for patients with ACC with high surgical and anesthesiological risk may be endoscopic BD, but randomized trials demonstrating the superiority of EUS-GBD with LAMS over ELC in high-risk patients are still lacking.36
Antibiotic therapy
The use of perioperative antibiotics in ACC has been a matter of discussion for a long time. On the one hand, according to recent guidelines48,49 in patients with uncomplicated ACC, post-operative antibiotics are unnecessary if source control is adequate. On the other side, instead, there are still current guidelines supporting the use of pre-operative antibiotics for patients undergoing ELC in ACC,3,50 but the evidence used in these guidelines appears to be scarce, because recently published randomized controlled trials are conflicting.51, 52, 53, 54 A cost-effectiveness analysis suggests only a modest cost-effectiveness of antibiotic prophylaxis in ELC for ACC, being marginally less costly and more effective than no prophylaxis.55 For these reasons, Colling et al. in the recent Surgical Infection Society Guidelines still also recommend the use of antibiotic prophylaxis for patients undergoing ELC for ACC.48 In cases of complicated ACC, a 4-day postoperative antibiotic therapy is suggested. For empiric antibiotic therapy of ACC in not-critically ill patients, guidelines56 suggest Amoxicillin/Clavulanate. However, surgeons should avoid its use if the local Enterobacteriaceae resistance rate is higher than 20%. For critically ill patients the antibiotic of choice is Piperacillin/Tazobactam. In patients with a beta-lactam allergy the association of Ciprofloxacin or Amikacin with Metronidazole is suggested. Patients at high risk for infection with community-acquired ESBL-producing Enterobacteriaceae deserve a separate discussion. In this case guidelines suggest the use of Tigecycline or Ertapenem. Meropenem should be used only in case of patients with septic shock and high risk for infection with community-acquired ESBL-producing Enterobacteriaceae. In patients at high risk for infection from Enterococci, including immunocompromised patients or patients with recent antibiotic exposure, Ampicillin, if patients have not been treated with Piperacillin/Tazobactam or Imipenem/Cilastatin (active against ampicillin-susceptible enterococci) or Tigecycline should be considered.
Fig. 2 reports a flowchart for ACC management with the “grey areas” highlighted and a proposed algorithm based on updated evidence. Grey areas in ACC and ABP management are listed in Supplementary Material Table S3.
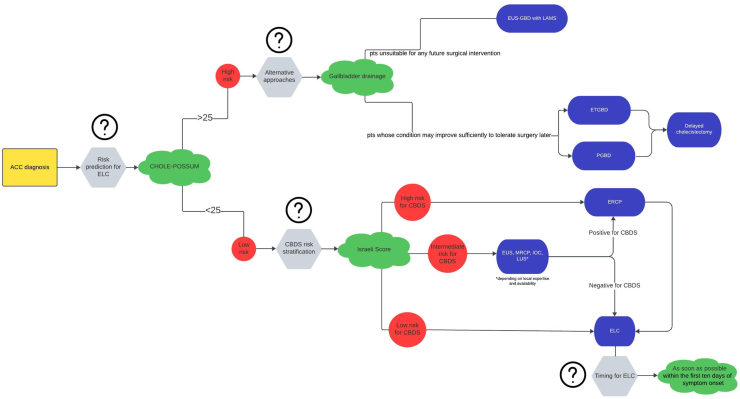
Flowchart for Acute Calculous Cholecystitis management with a proposed algorithm based on updated evidence. The question marks indicate the “grey areas” (ACC, Acute Calculous Cholecystitis; ELC, Early Laparoscopic Cholecystectomy; EUS-GBD, UltraSound-guided GallBladder transmural Drainage; LAMS, Lumen Apposing Metal Stent; ETGBD, Endoscopic Transpapillary GallBladder Drainage; PGBD, Percutaneous Gallbladder Drainage; CBDS, Common Bile Duct Stones; EUS, Endoscopic UltraSound; MRCP, Magnetic Resonance CholangioPancreatography; IOC, Intraoperative Cholangiography; LUS, Laparoscopic UltraSound; ERCP, Endoscopic Retrograde Cholangiopancreatography).
Acute biliary pancreatitis
Pathogenesis and pathophysiology
ABP is an inflammatory condition of the pancreas caused by the obstruction of the bile ducts, most commonly by gallstones. This obstruction leads to the activation of pancreatic enzymes within the pancreas, causing autodigestion, inflammation, and damage to the pancreatic tissue. Early diagnosis and intervention, including appropriate fluid resuscitation, pain management, and timely cholecystectomy, play a vital role in reducing the occurrence of adverse outcomes of ABP. Long-term management strategies are essential to minimize the risk of recurrence and associated complications.57 The incidence of ABP is about 40 per 100,000 people annually in the United States.58, 59, 60
Diagnosis
According to the Revised Atlanta Classification (RAC), the diagnosis of acute pancreatitis, including ABP, requires at least two of the following three criteria57: acute onset of persistent, severe, epigastric pain often radiating to the back; serum lipase or amylase activity at least three times greater than the upper limit of normal; and characteristic findings of acute pancreatitis on imaging—Computed Tomography (CT), Magnetic Resonance Imaging (MRI), or Ultrasound (US).
Abdominal US is the initial imaging modality of choice to detect gallstones, biliary sludge, and bile duct dilatation. It is non-invasive and readily available but may be limited in patients with excessive bowel gas or obesity. Contrast-enhanced CT scan is essential for assessing the severity of acute pancreatitis (Supplementary Material Fig. S2). CT is particularly useful in detecting complications such as necrosis, infected necrotizing pancreatitis, or pseudocysts, and in ruling out other intra-abdominal conditions. MRCP is a non-invasive imaging technique that provides detailed images of the biliary and pancreatic ducts. MRCP is useful for detecting choledocholithiasis and assessing the biliary tree. Endoscopic Ultrasound (EUS) offers high-resolution images of the pancreas and biliary tree, allowing for the detection of small stones and assessment of the biliary tree. It can also facilitate therapeutic interventions.
Severity assessment
The RAC further categorizes the severity of AP into three levels based on the presence and duration of organ failure and the presence of local or systemic complications.57 Mild acute pancreatitis is characterized by the absence of organ failure and local or systemic complications. Moderately-Severe acute pancreatitis is defined by transient organ failure (lasting less than 48 h) and/or local complications such as acute peripancreatic fluid collections, sterile pancreatic necrosis, or exacerbation of comorbid conditions. Severe acute pancreatitis is characterized by persistent organ failure (lasting more than 48 h). Patients with severe forms are at high risk of complications and increased mortality.
Treatment
The treatment of ABP involves supportive care and specific interventions to address the underlying cause and prevent complications. Severe cases with systemic complications and organ failure should be managed in an intensive care unit (ICU) whenever possible.61 Supportive care includes fluid resuscitation, pain management, and nutritional support. Early and adequate intravenous fluid resuscitation is critical to maintain perfusion and prevent complications such as acute kidney injury. Moderate fluid resuscitation (e.g., a bolus of 10 mL/kg followed by 1.5 mL/kg per hour) is preferred to aggressive hydration to reduce the risk of fluid overload, respiratory complications, and abdominal compartment syndrome.62 Analgesics, including opioids and nonsteroidal anti-inflammatory drugs (NSAIDs), are used to manage severe pain.63 Enteral nutrition is preferred over parenteral nutrition to maintain gut integrity and prevent infections61 for patients who are not able to tolerate a full oral diet. Routine use of antibiotics is not recommended unless there is evidence of infected pancreatic necrosis or cholangitis.64
Specific interventions
ERCP plays a crucial role in the management of ABP. For patients with acute cholangitis or persistent bile duct obstruction, early ERCP within 48–72 h is recommended.65 In patients who cannot undergo ELC due to acute comorbidities, ERCP with empiric biliary sphincterotomy is a viable alternative. This procedure can effectively reduce the recurrence of ABP by facilitating bile drainage and preventing future episodes of biliary obstruction.66, 67, 68
Cholecystectomy
Cholecystectomy is recommended to prevent recurrent episodes of ABP. For patients with mild ABP, ELC should be performed during the same hospitalization, ideally within 48 h of admission.62 In cases of severe ABP, cholecystectomy should be delayed until the patient stabilizes and the acute inflammation resolves, typically within 6–8 weeks.69 Intraoperative cholangiography (IOC) can be performed during cholecystectomy to identify any remaining bile duct stones. If stones are detected, options include laparoscopic common bile duct exploration or postoperative ERCP.70
Management of complications
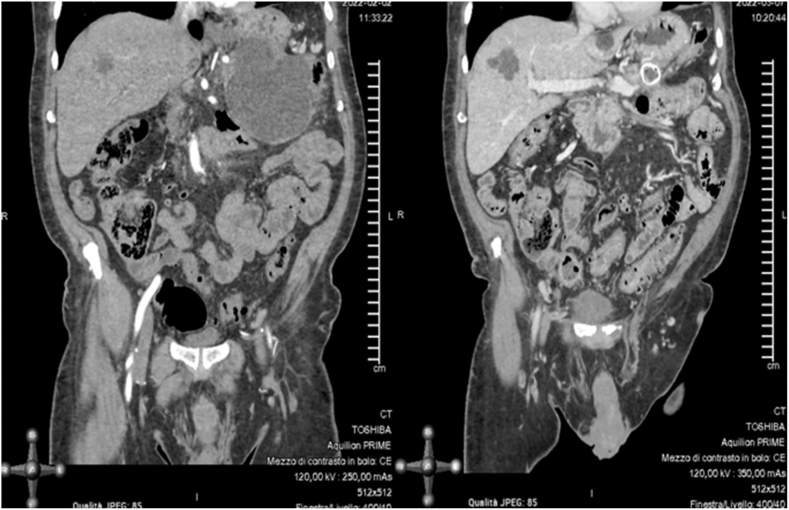
Sterile pancreatic necrosis is managed conservatively with supportive care, whereas infected necrosis requires antibiotics and may necessitate drainage procedures, either percutaneous, endoscopic, or as a last resort, surgical68,71 (Fig. 3). Minimally invasive surgical step-up (Fig. 4) and endoscopic step-up (Fig. 5) approaches are associated with fewer adverse events, lower organ failure rates, and reduced hospital costs compared to open surgical necrosectomy.72,73 While both approaches have similar clinical success, the endoscopic step-up method has a lower risk of pancreatic fistula.72,73 However, while endoscopic procedures are preferred, the anatomical location is crucial, and sometimes interventional radiology procedures may be considered. Walled-off necrosis (WON) is a common complication of severe ABP that develops typically four weeks after the onset of pancreatitis. WON is characterized by a well-defined inflammatory encapsulation of necrotic pancreatic tissue and fluid. The management of WON has evolved significantly with the advent of endoscopic techniques, particularly the use of LAMS. LAMS has emerged as a highly effective tool for the drainage of WON. These stents create a direct conduit between the gastrointestinal lumen and the necrotic collection, allowing for efficient drainage and promoting resolution of the necrosis. Recent studies have shown promising results with the use of LAMS, including high technical and clinical success rates, reduced need for surgical necrosectomy, and lower complication rates compared to traditional percutaneous and surgical approaches.37,72,73 The step-up approach to managing infected WON involves initial percutaneous or endoscopic drainage, followed by minimally invasive necrosectomy if necessary. This strategy has been associated with better outcomes compared to open surgery.68,71, 72, 73 Pancreatic pseudocysts are another potential complication that may resolve spontaneously. However, symptomatic or persistent pseudocysts causing upper gastrointestinal outlet obstruction or other complications may require drainage. Endoscopic drainage using LAMS is increasingly preferred due to its minimally invasive nature and high success rates.68,74
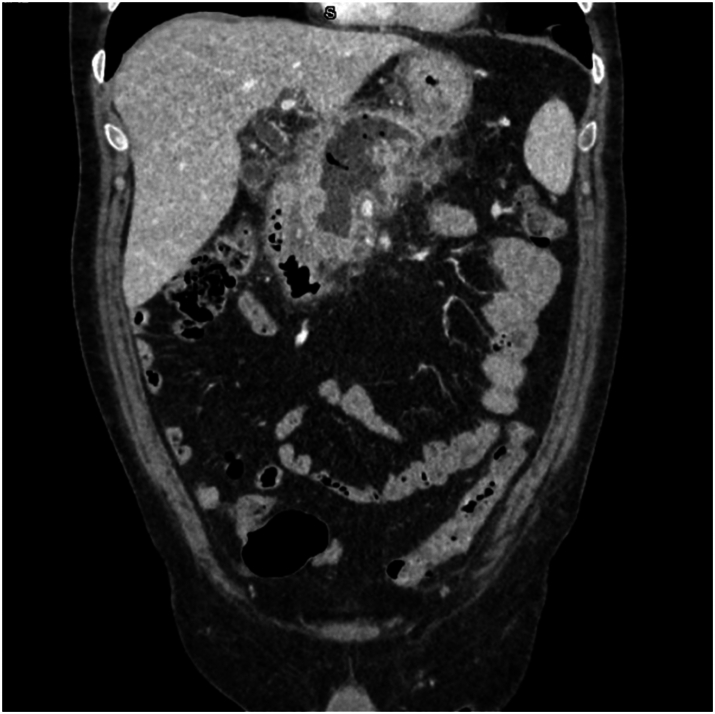
CT scan performed four weeks after the onset of symptoms, demonstrating intra-pancreatic walled-off necrosis with signs of infection (intra-necrotic air bubbles).
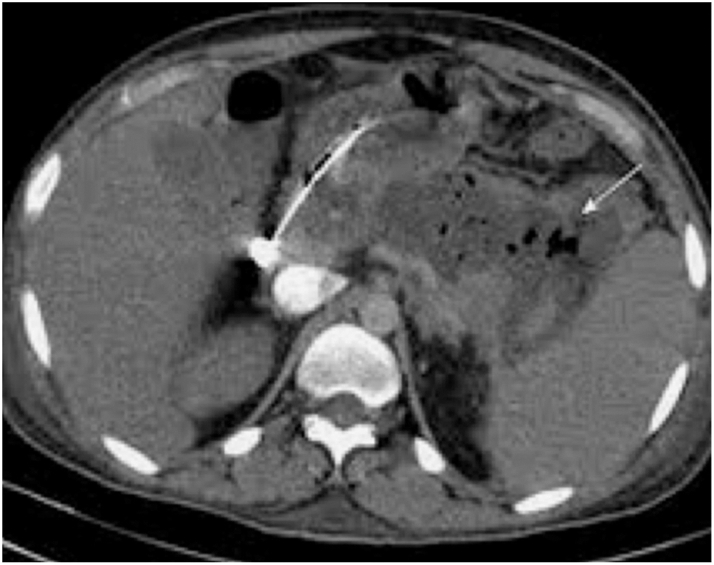
CT scan showing the positioning of a percutaneous retroperitoneal drain inside a large infected necrotic collection, as the first step of a step-up surgical approach.
Prognosis
The prognosis of ABP depends on the severity of the initial episode and the presence of complications. Most patients with mild ABP recover fully with appropriate treatment. The mortality rate for mild ABP is low, around 1–2%. However, severe cases are associated with a higher mortality rate, ranging from 10 to 30%, especially in the presence of complications such as pancreatic necrosis, infected necrotizing pancreatitis, and multiorgan failure.75 Factors influencing prognosis include severity of ABP according with the RAC system, timeliness of treatment, comorbid conditions and age.
Discussion
This review aimed to consolidate existing knowledge and address uncertain clinical issues in the management of ACC and ABP. For ACC, the TG and the WSES guidelines provide comprehensive frameworks for care, though differences in severity stratification and management persist. ELC is the preferred treatment, with alternative methods like percutaneous or endoscopic biliary drainage for high-risk patients. Endoscopic techniques such as EUS-GBD with LAMS show promising outcomes. Studies suggest that delaying ELC beyond 10 days can increase complications, yet performing ELC within 3 days of admission significantly reduces morbidity and mortality. This underscores the need for tailored decision-making, considering individual patient risk factors and hospital capabilities. CBDSs should be managed using a stratified approach, with second-level diagnostics like EUS or MRCP for intermediate-risk patients. For ABP, early diagnosis using clinical evaluation and imaging is crucial, guided by the RAC. Supportive care, particularly fluid resuscitation and pain management, remains fundamental in ABP treatment. Recent evidence favors moderate fluid resuscitation to avoid complications associated with fluid overload. Enteral nutrition is preferred to maintain gut integrity. Managing complications such as infected pancreatic necrosis favors minimally invasive approaches, with the endoscopic step-up method emerging as the preferred option due to its lower risk and better patient outcomes. ERCP and timely cholecystectomy are key interventions, with cholecystectomy ideally performed during the same hospitalization for mild cases and delayed for severe cases until stabilization.
Contributors
Paola Fugazzola: conceptualization, data collection and curation, formal analysis, investigation, methodology; Mauro Podda: data collection and curation, formal analysis, investigation, methodology; Brian Wca Tian: conceptualization, investigation, methodology, project administration, supervision, validation, original draft preparation, reviewing & editing; Lorenzo Cobianchi: data collection and curation, formal analysis, investigation, methodology; Luca Ansaloni: conceptualization, methodology, project administration, supervision, validation, original draft preparation, reviewing & editing; Fausto Catena: conceptualization, methodology, project administration, supervision, validation, original draft preparation, reviewing & editing. All the authors read and approved the final version of the manuscript.
Declaration of interests
Paola Fugazzola, Mauro Podda, Brian Wca Tian, Lorenzo Cobianchi, Luca Ansaloni and Fausto Catena declare that they have no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This reasearch was published under the University of Cagliari (Italy), Department of Surgical Science, Italian Ministry of University and Research (Ministero dell'Università e della Ricerca Italiano), PRIN (Progetti di Ricerca di Rilevante Interesse Nazionale) 2022, ID 202273A4YP, grant number F53D23006530006.
Footnotes
Appendix ASupplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102880.
Appendix A. Supplementary data
References
Articles from eClinicalMedicine are provided here courtesy of Elsevier
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The impact of empiric endoscopic biliary sphincterotomy on future gallstone-related complications in patients with non-severe acute biliary pancreatitis whose cholecystectomy was deferred or not performed.
Surg Endosc, 33(10):3325-3333, 07 Dec 2018
Cited by: 6 articles | PMID: 30535937
Early routine endoscopic retrograde cholangiopancreatography strategy versus early conservative management strategy in acute gallstone pancreatitis.
Cochrane Database Syst Rev, (5):CD009779, 16 May 2012
Cited by: 65 articles | PMID: 22592743 | PMCID: PMC11491195
Review Free full text in Europe PMC
Acute biliary pancreatitis: focus on recurrence rate and costs when current guidelines are not complied.
Scand J Gastroenterol, 52(3):264-269, 09 Dec 2016
Cited by: 5 articles | PMID: 27700180
Small Gallstone Size and Delayed Cholecystectomy Increase the Risk of Recurrent Pancreatobiliary Complications After Resolved Acute Biliary Pancreatitis.
Dig Dis Sci, 62(3):777-783, 29 Dec 2016
Cited by: 12 articles | PMID: 28035552
Funding
Funders who supported this work.