Abstract
Objective
Efficacy of thoracic endovascular aortic repair (TEVAR) for chronic type B aortic dissection (CTBAD) is dependent on eliminating retrograde false lumen perfusion and remodeling the aorta. We describe the efficacy of a novel transcatheter electrosurgical technique to fenestrate the dissection flap and create a distal seal zone for TEVAR in CTBAD.Methods
A retrospective review of the Emory Aortic Database from 2016 to 2023 identified 33 patients who underwent TEVAR with intentional endovascular rupture of the dissection flap (Knickerbocker; KNICK) for CTBAD. In 11 patients, we performed transcatheter electrosurgical aortic septostomy (TECSAS) before KNICK. The technical aspects of TECSAS + KNICK are described and results compared with TEVAR + KNICK alone.Results
Dissection chronicity, aortic size, and preoperative demographics were similar between groups. Technical success was 100%, with zero stroke or paraplegia in both groups. Thirty-day mortality for TECSAS versus KNICK was 0% versus 13.6% (P = .199). Median follow-up was shorter after TECSAS versus KNICK, although not statistically significant (14.6 months vs 21.9 months; P = .065). Elimination of retrograde false lumen perfusion (TECSAS 100% vs KNICK 68.2%; P = .035) and complete false lumen thrombosis or obliteration (TECSAS 91.9% vs KNICK 54.6%; P = .037) were more frequent after the TECSAS procedure. Aortic reinterventions were less frequent after TECSAS versus KNICK (0% vs 13.6%, P = .199), although not statistically significant.Conclusions
The addition of TECSAS to intentional endovascular rupture of the dissection flap in CTBAD improves distal seal, eliminating retrograde false lumen perfusion. This technique is a safe and precise method to fenestrate a dissection flap and optimize TEVAR in CTBAD.Free full text

Transcatheter electrosurgical aortic septostomy optimizes distal landing zone in chronic dissection
Associated Data
Abstract
Objective
Efficacy of thoracic endovascular aortic repair (TEVAR) for chronic type B aortic dissection (CTBAD) is dependent on eliminating retrograde false lumen perfusion and remodeling the aorta. We describe the efficacy of a novel transcatheter electrosurgical technique to fenestrate the dissection flap and create a distal seal zone for TEVAR in CTBAD.
Methods
A retrospective review of the Emory Aortic Database from 2016 to 2023 identified 33 patients who underwent TEVAR with intentional endovascular rupture of the dissection flap (Knickerbocker; KNICK) for CTBAD. In 11 patients, we performed transcatheter electrosurgical aortic septostomy (TECSAS) before KNICK. The technical aspects of TECSAS + KNICK are described and results compared with TEVAR + KNICK alone.
Results
Dissection chronicity, aortic size, and preoperative demographics were similar between groups. Technical success was 100%, with zero stroke or paraplegia in both groups. Thirty-day mortality for TECSAS versus KNICK was 0% versus 13.6% (P = .199). Median follow-up was shorter after TECSAS versus KNICK, although not statistically significant (14.6 months vs 21.9 months; P = .065). Elimination of retrograde false lumen perfusion (TECSAS 100% vs KNICK 68.2%; P = .035) and complete false lumen thrombosis or obliteration (TECSAS 91.9% vs KNICK 54.6%; P = .037) were more frequent after the TECSAS procedure. Aortic reinterventions were less frequent after TECSAS versus KNICK (0% vs 13.6%, P = .199), although not statistically significant.
Conclusions
The addition of TECSAS to intentional endovascular rupture of the dissection flap in CTBAD improves distal seal, eliminating retrograde false lumen perfusion. This technique is a safe and precise method to fenestrate a dissection flap and optimize TEVAR in CTBAD.
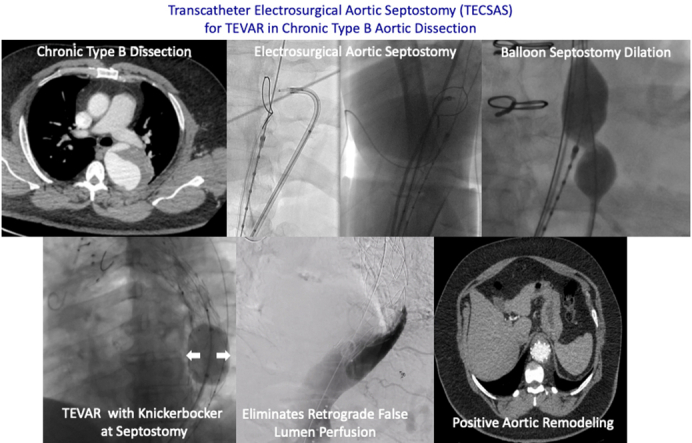
Thoracic endovascular aortic repair (TEVAR) in chronic type B aortic dissection (CTBAD) has been shown to be technically feasible with low short-term morbidity and mortality.1, 2, 3 However, the long-term efficacy of TEVAR for chronic dissection is contingent on positive aortic remodeling. In CTBAD, the presence of a rigid aortic septum presents a significant challenge for complete relamination of the aortic wall with TEVAR, resulting in persistent retrograde false lumen perfusion and pressurization from distal aortic fenestrations. Retrograde false lumen perfusion remains the “Achilles’ heel” of endovascular therapy for CTBAD and represents the most problematic mechanism of persistent false lumen pressurization, expansion, and rupture.
The 2 main strategies that have been developed to address persistent retrograde false lumen perfusion are false lumen embolization and “uni-lumenization.” False lumen embolization is a nonspecific treatment strategy that involves the deployment of coils, plugs, stent grafts, and/or glue into the false lumen to induce false lumen thrombosis.4 “Uni-lumenization” describes the creation of a single lumen at the distal landing zone in order to achieve complete apposition between the stent graft and outer aortic wall, thereby obliterating the false lumen. Kölbel and colleagues5 described the initial technique to achieve a single lumen, the “Knickerbocker” (KNICK), in which an aortic balloon is inflated to intentionally rupture the dissection flap and expand the stent graft to the outer aortic wall after TEVAR. The efficacy of this technique is variable, as the rigid dissection flap frequently prevents complete TEVAR expansion and relamination of the aortic wall, even with aggressive balloon dilation of the septum. Furthermore, there is a lack of control with respect to the extent of septal rupture both proximal and distal to the segment of aorta that is undergoing aortoplasty. This can result in a stent graft induced new entry tear at the distal aspect of the endograft which can lead to increased false lumen perfusion and expansion.
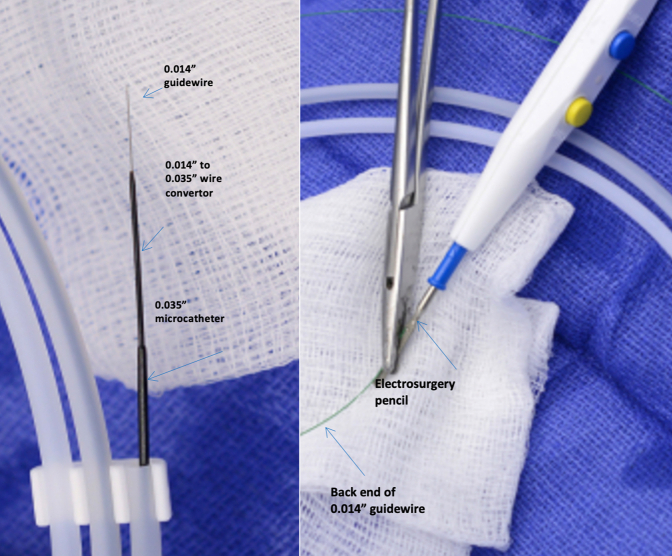
In an attempt to limit the uncontrolled propagation of the septal rupture and improve stent graft apposition during the KNICK procedure, we developed the transcatheter electrosurgical aortic septostomy (TECSAS) technique. Transcatheter electrosurgery relies on application of an external source of electric current to “electrify” a guidewire inside the body for the purpose of perforating tissue (Figure 1).6, 7, 8, 9 The use of transcatheter electrosurgical techniques is well-described in the management of structural heart disease.6,10,11 This report describes the technical details and clinical and radiographic outcomes of the TECSAS technique as an adjunct to TEVAR with KNICK to treat patients with CTBAD.
Methods
Patient Cohort
A retrospective review of the Emory TEVAR database identified 33 patients who underwent TEVAR with a KNICK procedure to treat patients with descending or thoracoabdominal aortic aneurysms secondary to chronic distal aortic dissection from 2016 to 2023. The institutional review board approved this study with waiver of signed informed consent due to the retrospective nature (institutional review board number: IRB00022795, approval date October 5, 2018). The cohort of 33 patients was divided into 2 groups: 11 patients underwent a TECSAS procedure as an adjunct to TEVAR + KNICK and were compared with 22 patients who underwent TEVAR + KNICK alone. Perioperative outcomes and postoperative imaging were analyzed to assess the safety and efficacy of the TECSAS procedure.
Definitions
Technical success was defined as successful endovascular electrosurgical aortic septostomy, followed by TEVAR deployment at predetermined landing zones, without endograft-related complications, aortic perforation, or evidence of residual antegrade or retrograde false lumen perfusion. Aortic landing zones are reported in accordance with conventional reporting standards.12
TECSAS Procedure
Video 1 outlines the TECSAS technique and rationale. Preoperatively, all patients underwent electrocardiogram-gated computed tomography (CT) angiogram of the chest, abdomen, and pelvis for full imaging evaluation. All CT images were uploaded onto an Aquarius iNtuition (TeraRecon) workstation. Semiautomated algorithms were used to generate multiplanar reformatted images and aortic centerlines. Proximal and distal landing zones were determined by the aortic centerline-based diameters in zones 2 and 5, respectively. The level of the aortic septostomy was typically 4 to 8 cm cephalad to the celiac artery and chosen on the basis of an aortic diameter <44 mm, typically translating into the T8-12 level. The vertebral body level was subsequently used as the landmark for septostomy. Biplane imaging angles were obtained from the preoperative CT angiography imaging using the Aquarius iNtuition software by positioning the anterior-posterior camera angle to be aligned in the same exact plane as the dissection flap, and the lateral camera angle to be orthogonal to the dissection flap.
All cases are performed in a hybrid operating suite with the patient under general anesthesia. Left subclavian artery revascularization via carotid-subclavian bypass is performed selectively on the basis of the need to optimize the proximal landing zone. Ultrasound-guided percutaneous bilateral femoral access is obtained, and intravenous heparin is administered to achieve an activated clotting time goal of >250 seconds. Intravascular ultrasound (IVUS) is used to confirm both true and false lumen access. A 7-French deflectable guiding sheath (Oscor Inc) is advanced into the false lumen to the level of the planned aortic septostomy and pointed toward the septum. Through this catheter, a coaxial system composed of a 0.014" stiff guidewire (300-cm Astato XS 20; Asahi Intec) inside a 0.014" to 0.035" hubless microcatheter (145-cm PiggyBack; Teleflex) inside a 0.035" microcatheter (90-cm NaviCross; Terumo Medical Corporation) is advanced. This platform allows conversion from a 0.014" to a 0.035" wire system after septal perforation. The distal end of the 0.014" stiff guidewire is denuded of any external coating and clamped to a monopolar electrocautery outside the body (Figure 1). In the true lumen, a 25-mm single-loop snare (Amplatz Goose Neck Snare; Medtronic) is advanced inside a 6-French JR4 guide catheter and opened at the level of the planned aortic septostomy. Using biplane imaging, slight adjustments of the true and false lumen catheter systems are performed to achieve the proper alignment. The optimal anterior-posterior camera view of the snare should be a side profile, which appears as a single wire, and the lateral camera “en face” view should be a “bull’s eye” (Figure 2).
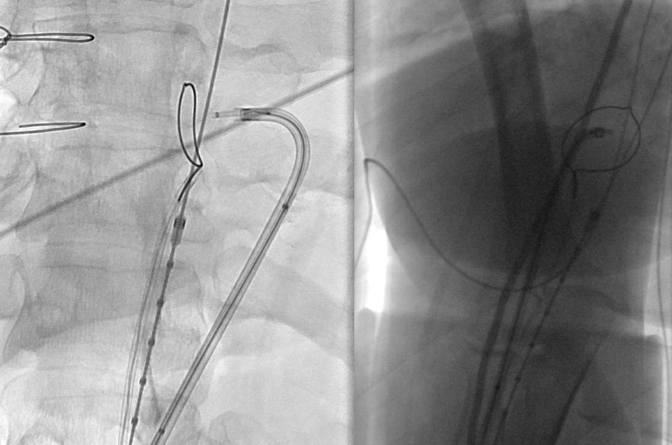
Biplane imaging confirms orthogonal alignment of wire and snare in preparation for electrosurgical puncture from false to true lumen.
Once the biplane imaging confirms the proper catheter and snare positions, a brief electrosurgery pulse is applied on 50 watts pure cut for 1 to 3 seconds while the 0.014" wire is advanced across the dissection flap and through the center of the snare. The snare is closed and advanced into the proximal descending thoracic aortic true lumen. The PiggyBack wire converter followed by the 0.035" Navicross support catheter are advanced across the dissection flap and into the proximal descending thoracic aorta. The 0.014" wire is exchanged for a 0.035" Amplatz (Medtronic) wire, and IVUS is performed to confirm that the wire crosses from the false lumen into the true lumen (Video 1). The IVUS is subsequently exchanged for a 28- × 40-mm balloon (Z-Med; Braun Interventional Systems), which is advanced across the septostomy and inflated to create a large fenestration. TEVAR is performed with total thoracic coverage from zone 2 or 3 to zone 5. After TEVAR, a Coda balloon (Cook Medical) is inflated at the level of the septostomy to intentionally rupture the dissection flap and expand the stent graft to the outer aortic wall. IVUS is used to evaluate the stent graft apposition to the outer aortic wall, and repeat ballooning is performed as necessary (Video 1). Completion aortography of the true and false lumen is used to confirm the absence of antegrade and retrograde false lumen perfusion (Figure 3). All devices and sheaths are removed, and the access sites are closed using previously placed closure sutures (Perclose; Abbott Cardiovascular).
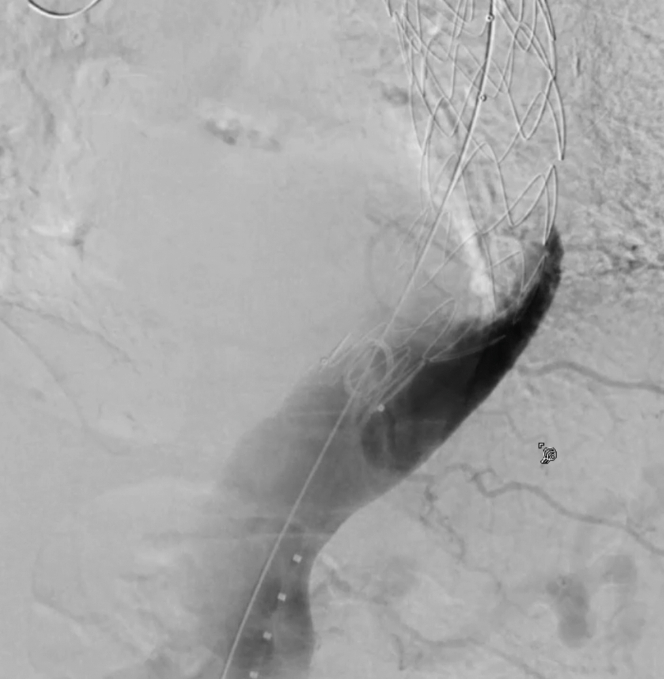
Retrograde false lumen angiogram confirms successful thoracic endovascular aortic repair apposition and obliteration of retrograde false lumen flow.
Patients are extubated in the operating room and a neurologic examination is performed. Patients demonstrating evidence of lower-extremity weakness undergo lumbar drain placement before leaving the operating room. Postoperative intensive care unit management consists of maintenance of systolic blood pressure in the range of 160 to 200 mm Hg, including use of vasopressors as necessary for 24 hours. Patients are discharged from the hospital with permissive hypertension, and blood pressure control is gradually optimized over a 3-month period. Postoperative CT angiograms with delayed venous phase imaging are obtained at 1, 6, 12 months and annually thereafter and analyzed for metrics of aortic remodeling, including true lumen expansion, false lumen thrombosis and shrinkage, total aortic diameter, and presence of retrograde false lumen perfusion proximal to the Knickerbocker segment (Figure 4, A-C).
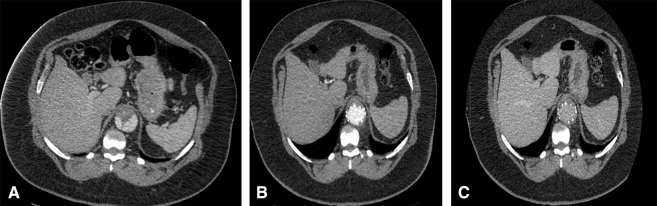
Preoperative (A), postoperative arterial phase (B), and postoperative (C) venous-phase computed tomography showing level of planned TECSAS TEVAR with preoperative false lumen flow that is obliterated with TEVAR apposition to the outer aortic wall after TECSAS technique at the distal landing zone. TECSAS, Transcatheter electrosurgical aortic septostomy; TEVAR, thoracic endovascular aortic repair.
Statistical Analysis
Univariate tests for categorical variables were conducted using the χ2 test, and continuous variables were compared using either the Student t test or Mann-Whitney U test on the basis of distributional assumptions, tested with the Shapiro-Wilk test. Aortic remodeling was assessed using a paired t test. Time-to-event analyses were performed with Kaplan-Meier method and log-rank test. All calculations were performed using Stata18 software (StataCorp LLC).
Results
Preoperative Demographics
Preoperative demographics are outlined in Table 1. All patients were high risk for open repair as the result of frailty and/or other comorbidities, with a similar incidence of coronary artery disease, smoking, cerebrovascular disease, and chronic renal disease in both groups. Preoperative aortic characteristics were also similar between TECSAS versus KNICK groups, including maximal aortic diameter (6.4 cm [5.7-7.1] vs 6.1 cm [5.4-7.2], P = .144), dissection chronicity (6.5 years [1.5-11] vs 4.7 years [1.0-8.5], P = .342), and septal thickness (3 mm [2-4] vs 2.5 mm [2-4], P = .113). Previous cardiovascular operations were common in both groups (54.6% vs 68.2%, P = .443), many of them in combination, as outlined in Table 1.
Table 1
Patient demographics
| Variable | TECSAS n = 11 | Knickerbocker n = 22 | P value |
|---|---|---|---|
| Age, y | 62 [51-70] | 60 [50-69] | .483 |
| Male sex | 8 (72.7%) | 17 (77.3%) | .774 |
| Hypertension | 11 (100%) | 21 (95.5%) | .864 |
| Hyperlipidemia | 9 (81.8%) | 20 (90.9%) | .451 |
| Diabetes mellitus | 4 (36.4%) | 5 (22.7%) | .407 |
| Coronary artery disease | 1 (9.1%) | 2 (9.1%) | 1.000 |
| Active/previous smoking | 5 (45.5%) | 9 (40.9%) | .803 |
| Cerebrovascular disease | 2 (18.2%) | 3 (13.6%) | .731 |
| Left ventricular ejection fraction | 55% [50-60] | 50% [45-55] | .664 |
| Chronic kidney disease | 7 (63.6%) | 12 (54.6%) | .618 |
| Preoperative dialysis | 2 (18.2%) | 2 (9.1%) | |
| Previous acute type A aortic dissection | 6 (54.6%) | 12 (54.6%) | 1.000 |
| Maximum aortic diameter, cm | 6.4 [5.7-7.1] | 6.1 [5.4-7.2] | .144 |
| Septal thickness, mm | 3 [2-4] | 2.5 [2-4] | .113 |
| Time from dissection to TECSAS, y | 6.5 [1.5-11] | 4.7 [1.0-8.5] | .342 |
| Previous cardiovascular surgery | 6 (54.6%) | 15 (68.2%) | .443 |
| Previous cardiovascular surgery details | N/A | ||
| Aortic valve replacement | 1 (9.1%) | 3 (13.6%) | |
| Aortic root replacement | 1 (9.1%) | 3 (13.6%) | |
| Ascending/hemiarch replacement | 4 (36.4%) | 10 (45.5%) | |
| Total arch replacement | 2 (18.2%) | 2 (9.1) | |
| Total arch/frozen elephant trunk | 1 (9.1%) | 1 (4.5%) | |
| TEVAR | 3 (27.3%) | 1 (4.5%) | |
| Carotid-subclavian bypass | 1 (9.1%) | 1 (4.5%) | |
| Axillary-bifemoral bypass | 0 | 1 (4.5%) |
Categorical variables displayed as n (%); continuous variables presented as median [interquartile range]. TECSAS, Transcatheter electrosurgical aortic septostomy; NA, not available; TEVAR, thoracic endovascular aortic repair.
Operative Results
Technical success for both TECSAS as an adjunct to TEVAR + KNICK and KNICK alone in CTBAD was 100%. The median level of aortic septostomy in the TECSAS group was at T10 (range, T8-L1). All patients in the TECSAS group were extubated in the operating room versus 95.5% of the KNICK group (P = .864), with the single remaining patient extubated later in the intensive care unit on the day of surgery. Intraoperative data are listed in Table 2.
Table 2
Intraoperative data
| Variable | TECSAS N = 11 | Knickerbocker N = 22 | P value |
|---|---|---|---|
| Technical success | 11 (100%) | 22 (100%) | 1.000 |
| Number of TEVAR endografts | 2 [2-3] | 2 [2-3] | .192 |
| TEVAR devices | N/A | ||
| Medtronic Valiant | 7 | 12 | |
| Cook Zenith | 2 | 4 | |
| Gore CTAG | 1 | 2 | |
| Gore TBE and CTAG | 1 | 2 | |
| Artivion NEXUS Arch Endograft | 0 | 2 | |
| Fenestration level | T10 [T8-L1] | N/A | N/A |
| Proximal landing zone | 3 [2-4] | 3 [2-4] | .689 |
| Subclavian revascularization | 3 (27.3%) | 10 (45.5%) | .216 |
| Carotid-subclavian bypass | 2 (18.2%) | 8 (36.4) | |
| Branched endovascular device | 1 (9.1%) | 2 (9.1%) | |
| Endovascular subclavian occlusion | 1 (9.1%) | 0 (0%) | .484 |
| Endoleak requiring immediate treatment (type 1 or 3) | 0 (0%) | 1 (4.5%) | .473 |
Categorical variables displayed as n (%); continuous variables presented as median [interquartile range]. TECSAS, Transcatheter electrosurgical aortic septostomy; TEVAR, thoracic endovascular aortic repair; NA, not available; CTAG, conformable thoracic aortic graft; TBE, thoracic branched endograft.
Postoperative Outcomes and Aortic Remodeling
Median follow-up was shorter after TECSAS versus KNICK, with a trend toward significance (14.6 months [5-23] vs 21.9 months [4-48], P = .065), and was 100% complete for both groups. Postoperative clinical outcomes are listed in Table 3. There were no operative mortalities after TECSAS versus 3 of 22 (13.6%) after KNICK alone (P = .199), and no cases of stroke, or paraplegia in either group. Temporary lower-extremity weakness was infrequent (1/11 [9.1%] vs 1/22 [4.5%], P = .684) and resolved in all cases with blood pressure augmentation and cerebrospinal fluid drainage. Late reinterventions were less frequent after TECSAS versus KNICK (0% vs 13.6%, log-rank P = .199, Figure E1). Late all-cause mortality (0% vs 22.7%, log-rank P = .086, Figure E2) and aortic-specific mortality (0% vs 13.6%, log-rank P = .244, Figure E3) were also less frequent after TECSAS versus KNICK, although neither comparison was statistically significant.
Table 3
Postoperative results
| Variable | TECSAS N = 11 | Knickerbocker N = 22 | P value |
|---|---|---|---|
| 30-d mortality | 0 (0%) | 3 (13.6%) | .199 |
| Hospital length of stay | 3 [3-5] | 4 [4-6] | .198 |
| Permanent stroke | 0 (0%) | 0 (0%) | N/A |
| Paraplegia | 0 (0%) | 0 (0%) | N/A |
| Temporary lower-extremity weakness | 1 (9.1%) | 1 (4.5%) | .684 |
| Acute kidney injury | 0 (0%) | 3 (13.6%) | .199 |
Categorical variables displayed as n (%); continuous variables presented as median [interquartile range]. TECSAS, Transcatheter electrosurgical aortic septostomy; NA, not available.
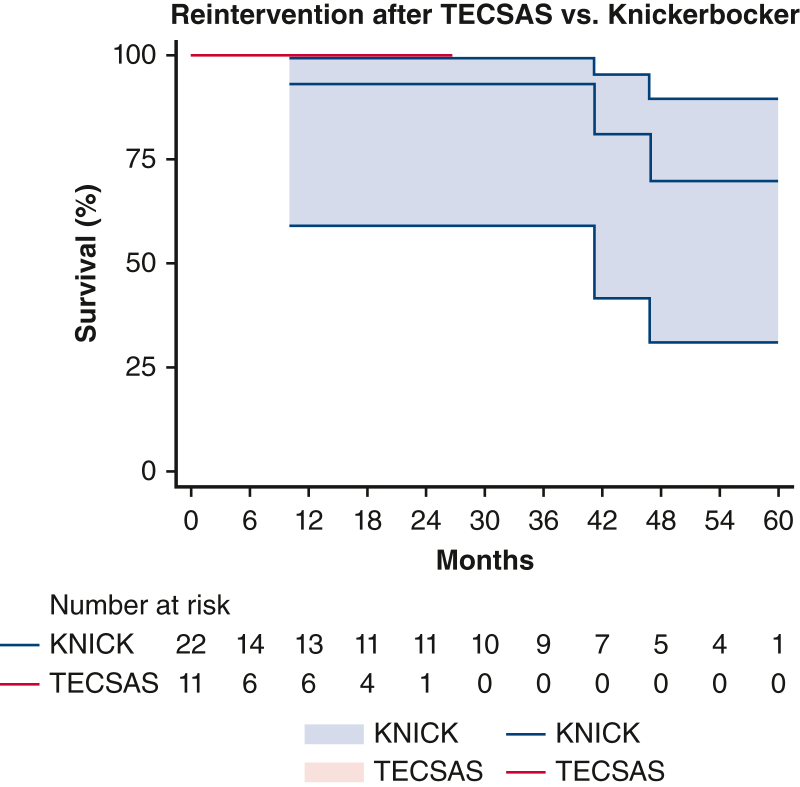
Freedom from late reintervention after TECSAS versus Knickerbocker (KNICK). TECSAS: 0% (95% confidence interval, 0%-9.1%); KNICK: 13.6% (95% confidence interval, 9.3%-43.1%). Log-rank P = .199. TECSAS, Transcatheter electrosurgical aortic septostomy; TEVAR, thoracic endovascular aortic repair.
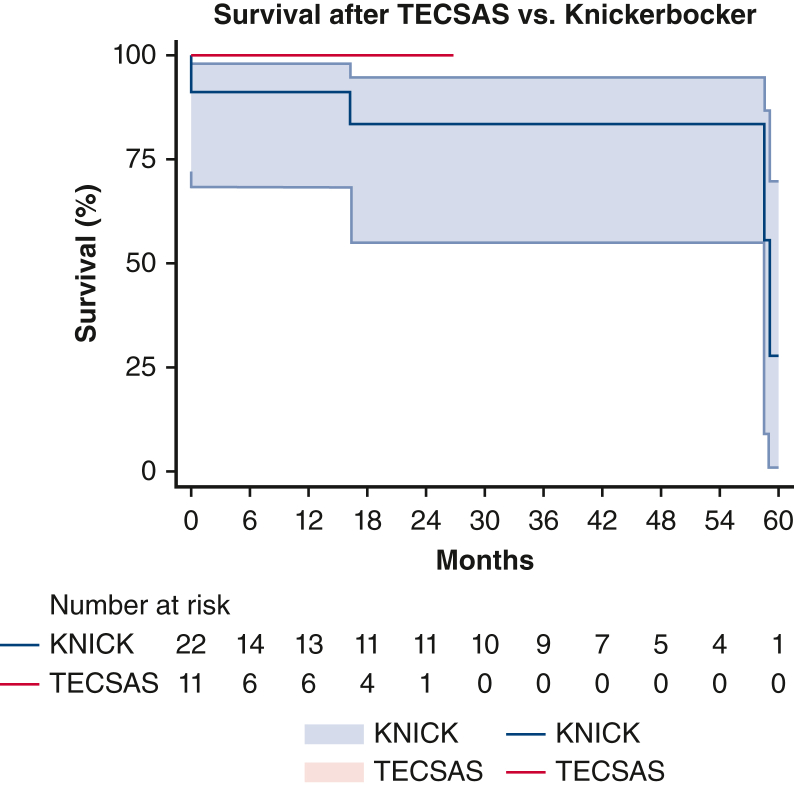
Overall mortality after TECSAS versus Knickerbocker (KNICK). TECSAS: 0% (95% confidence interval, 0%-9.1%); KNICK: 22.7% (95% confidence interval, 7.7%-46.3%). Log-rank P = .086. TECSAS, Transcatheter electrosurgical aortic septostomy; TEVAR, thoracic endovascular aortic repair.
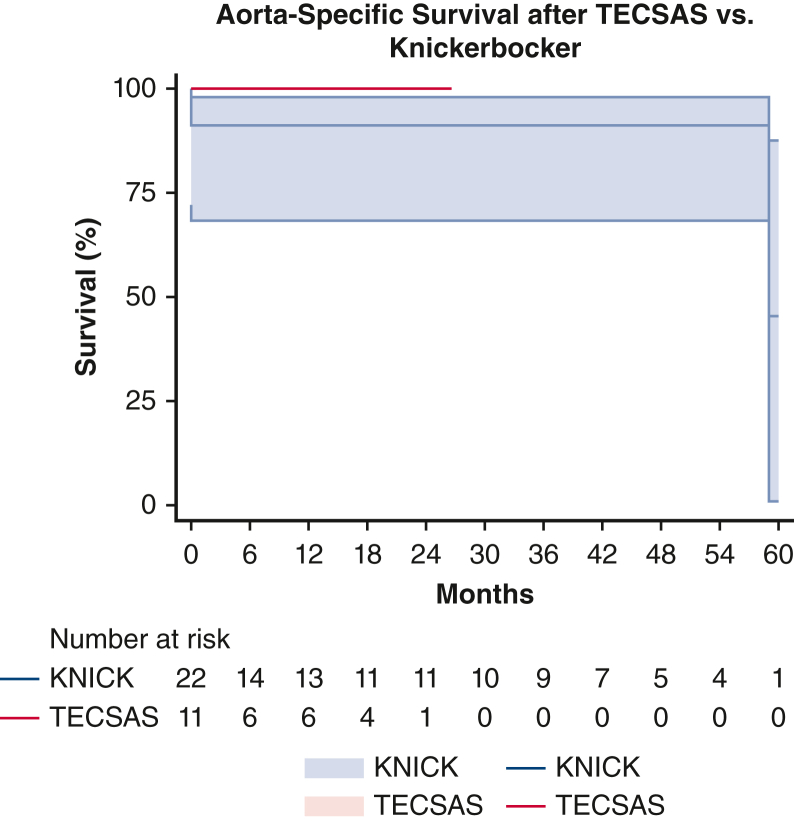
Aorta-specific mortality after TECSAS versus Knickerbocker (KNICK). TECSAS: 0% (95% confidence interval, 0%-9.1%); KNICK: 13.6% (95% confidence interval, 6.7%-32.6%). Log-rank P = .244. TECSAS, Transcatheter electrosurgical aortic septostomy; TEVAR, thoracic endovascular aortic repair.
Postoperative aortic remodeling data for the 11 TECSAS patients are shown in Table E1. There have been no cases of aortic expansion during surveillance. Median maximal aortic diameter decreased from 65 mm [58-72] to 56 mm [48-65] (P = .004) during follow-up. Simultaneously, true lumen expansion has been observed at the proximal landing zone, mid-descending thoracic aorta (measured at T6), the level of aortic septostomy, and distal landing zone (P < .05 for all comparisons). Cessation of retrograde false lumen perfusion was observed in 100% of patients treated with TECSAS. One patient remains under additional surveillance for a type 2 endoleak from an intercostal artery without evidence of false lumen expansion. The remaining 10 of 11 (91.9%) have demonstrated complete false lumen thrombosis in 8 (72.7%) patients or obliteration in 2 (18.2%) patients.
Comparison of aortic remodeling outcomes between the 11 patients undergoing TECSAS and 22 patients undergoing KNICK is shown in Table E2. Both groups demonstrated reductions in maximal aortic size, although this was more pronounced in the TECSAS group (TECSAS –9 mm [–14 to −2] vs KNICK –3 mm [–10 to +3], P = .042). Maximal abdominal aortic diameter demonstrated minimal change in both the TECSAS and KNICK groups (−2 mm [–5 to +3] vs +1 mm [–2 to +4], P = .097) Elimination of retrograde false lumen perfusion occurred more frequently after TECSAS versus KNICK (100% vs 68.2%, P = .035), as did complete false lumen thrombosis or obliteration (91.9% vs 54.6%, P = .037).
Discussion
Open descending/thoracoabdominal aortic replacement remains the definitive therapy for the treatment of aneurysms secondary to CTBAD. However, many patients are poor candidates for open repair as the result of their age and medical comorbidities. TEVAR can be performed safely for CTBAD with minimal short-term morbidity and mortality.1, 2, 3 However, its long-term efficacy has been limited by its inability to eliminate retrograde false perfusion from distal abdominal fenestrations. Various strategies to optimize the distal landing zone in particular have been employed, each carrying their own individual limitations.
In the current article, we present our initial experience using transcatheter electrosurgical techniques for distal landing zone optimization in TEVAR for CTBAD. The unique aspects of TECSAS include (1) the creation of a precise, well-controlled, focal aortic septostomy at a predetermined location; (2) safety with minimal risk of aortic injury using a single 3-second period of intra-aortic electrosurgical wire activation with a 0.014" coronary wire; and (3) widespread applicability to aortic specialists as the technique is performed using commercially available off-the-shelf, devices.
Our belief is that the addition of the TECSAS technique to the KNICK procedure significantly improves the ability to attain complete apposition of the stent graft to the aortic wall, thereby achieving a distal seal, and complete elimination of retrograde false lumen perfusion from abdominal aortic fenestrations. In our comparison of the TECSAS procedure with KNICK alone, the addition of the electrosurgical fenestration improved the ability of the stent graft to oppose the outer aortic wall. This resulted in elimination of retrograde false lumen perfusion and an increased incidence of complete false lumen thrombosis or obliteration. As a result, there was a greater reduction in maximal thoracic aortic diameter (TECSAS –9 mm vs KNICK –3 mm, P = .042) with the TECSAS procedure, despite a shorter length of follow-up. In contrast to the uncontrolled, unpredictable outcomes with balloon fracture fenestration alone of the thickened, rigid dissection septum, electrosurgical septostomy with the TECSAS technique is precise and affords the ability to create a large, controlled balloon septostomy. This significantly reduces the risk of creating at new stent graft induced re-entry tear at the distal end of the stent, which can result in increased false lumen flow and rapid abdominal false lumen expansion (Video 1). Importantly, the abdominal aorta does not appear to be compromised with either technique.
Although the differences did not achieve statistical significance, an important decrease in late aortic reinterventions was also seen after TECSAS, likely driven by improvements in early aortic remodeling, without downstream growth in the downstream abdominal aorta. Reduced rates of overall and aorta-specific mortality in the TECSAS cohort are also encouraging.
Recently, aortic septotomy using both the laser and “cheese wire” techniques were described by Fukuhara and colleagues13 at the University of Michigan to optimize both proximal and distal landing zones in patients undergoing TEVAR for CTBAD. These authors reported significantly improved aortic remodeling and a reduction in aortic reinterventions in patients undergoing septotomy compared with patients who did not undergo septotomy. Indeed, their report sparked our interest in endovascular “uni-lumenization” to create a distal seal zone in patients with CTBAD. Given our institutional experience with transcatheter electrosurgical techniques in structural heart disease, we developed the TECSAS procedure as an alternative to laser fenestration.10 In our opinion, the use of biplane imaging and the “bulls-eye” technique may allow for a greater degree of safety and precision in fenestrating the narrow aortic septum and gaining access to a small true lumen in CTBAD. By imaging the aorta in 2 planes simultaneously, the operator gains a complete orientation of the catheters in the anterior-posterior and lateral planes, which prevents inadvertent perforation of the aorta.
In comparison with the false lumen embolization strategy using the candy plug technique, TECSAS does not require use of any physician-modified endografting, making it less costly.14 In addition, even after successful candy plug deployment, additional gutters may persist with resultant endoleaks and false lumen expansion that may be difficult to manage.15 Finally, when compared with false lumen embolization, TECSAS does not have this same limitation, offers greater precision, and does not leave behind any coils, glue, or other embolization material that may cause radiographic artifacts that limit the ability to obtain accurate long-term aortic surveillance imaging.4
Limitations
Our study has limitations inherent to its small sample size and retrospective nature. Limitations in sample size and the need for additional experience at other institutions make it difficult to draw definitive conclusions regarding the remaining learning curve for TECSAS optimization, patient selection, and mid- and long-term results.
Conclusions
The efficacy of TEVAR in CTBAD remains dependent on elimination of false lumen perfusion to induce complete false lumen thrombosis or obliteration. We believe the TECSAS technique offers improved safety and precision when creating a single lumen at the distal landing zone. These early results demonstrate a high rate of technical success, minimal short-term morbidity, and increased frequency of eliminating retrograde false lumen perfusion using a reproducible technique that can be applied to a wide range of complex aortic anatomy and dissection chronicity. The next step will be to apply the TECSAS technique to optimize the proximal landing zone in patients with chronic distal aortic dissection. This would provide endovascular options for patients who underwent hemiarch replacement for a DeBakey I dissection and have a residual flap throughout the arch. Septal fenestration with techniques such as laser fenestration or TECSAS may ultimately allow for the treatment of these descending/thoracoabdominal aortic aneurysms without the attendant morbidity of conventional open repair.
Webcast
You can watch a Webcast of this AATS meeting presentation by going to: https://www.aats.org/resources/transcatheter-electrosurgical-7081.
Conflict of Interest Statement
Y.M.D. serves as a consultant for Cook Medical. W.D.J. serves as a consultant for Gore, Medtronic, Cook Medical, and Endologix. V.C.B. serves as a consultant for Edwards, Abbott, Medtronic, Boston Scientific, and Transmural Systems, Inc. B.G.L. serves as a consultant for Endospan and as a Speaker's Bureau for Medtronic. All other authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Supplementary Data
Accompanying video demonstrating TECSAS technique and rationale. Video available at: https://www.jtcvs.org/article/S2666-2507(24)00280-3/fulltext.
Appendix E1
Table E1
Aortic remodeling after TECSAS
| Variable | Preoperative | Postoperative | P value |
|---|---|---|---|
| Maximum aortic diameter, mm | 65 [58-72] | 56.2 [48-65] | .004 |
| True lumen diameter at proximal landing zone, mm | 28 [24-32] | 32 [28-34] | .049 |
| True lumen diameter at T6, mm | 17 [12-21] | 34 [28-38] | .002 |
| True lumen diameter at aortic septostomy, mm | 13 [11-17] | 40 [36-42] | <.001 |
| True lumen diameter at distal landing zone, mm | 14 [10-16] | 32 [26-38] | <.001 |
| False lumen status | N/A | ||
| Obliterated | 0 (0%) | 2 (18.2%) | |
| Completely thrombosed | 0 (0%) | 8 (72.7%) | |
| Partially thrombosed | 3 (27.3%) | 1 (9.1%) | |
| Patent | 8 (72.7%) | 0 (0%) |
Categorical variables displayed as n (%); continuous variables presented as median [interquartile range]. NA, Not available; TECSAS, transcatheter electrosurgical aortic septostomy.
Table E2
Aortic remodeling after TECSAS versus Knickerbocker alone
| Variable | TECSAS N = 11 | Knickerbocker N = 22 | P value |
|---|---|---|---|
| Maximum aortic diameter size change, mm | −9 [–14 to −2] | −3 [–10 to +3] | .0423 |
| True lumen diameter increase at proximal landing zone, mm | 4 [1-8] | 6 [2-10] | .087 |
| True lumen diameter increase at T6, mm | 17 [8-23] | 15 [9-21] | .327 |
| True lumen diameter increase at distal landing zone, mm | 17 [11-23] | 16 [9-23] | .898 |
| Abdominal aortic diameter size change, mm | −2 [–5 to +3] | +1 [–2 to +4] | .097 |
| Elimination of retrograde false lumen perfusion | 11 (100%) | 15 (68.2%) | .035 |
| False lumen obliteration or complete thrombosis | 10 (90.9%) | 12 (54.6%) | .037 |
| Postoperative false lumen status | N/A | ||
| Obliterated | 2 (18.2%) | 2 (9.1%) | |
| Completely thrombosed | 8 (72.7%) | 10 (45.5%) | |
| Partially thrombosed | 1 (9.1%) | 10 (45.5%) | |
| Patent | 0 (0%) | 0 (0%) |
Categorical variables displayed as n (%); continuous variables presented as median [interquartile range]. TECSAS, Transcatheter electrosurgical aortic septostomy; NA, not available.
References
Articles from JTCVS Techniques are provided here courtesy of Elsevier
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Initial Single-Center Experience With the Knickerbocker Technique During Thoracic Endovascular Aortic Repair to Block Retrograde False Lumen Flow in Patients With Type B Aortic Dissection.
J Endovasc Ther, 31(4):597-605, 07 Nov 2022
Cited by: 1 article | PMID: 36342189
Laser-assisted "Scissor" Technique to Facilitate Thoracic Endovascular Aortic Repair for Chronic Type B Aortic Dissection.
Ann Vasc Surg, 77:347.e7-347.e11, 26 Jun 2021
Cited by: 2 articles | PMID: 34182117
Impact of proximal seal zone length and intramural hematoma on clinical outcomes and aortic remodeling after thoracic endovascular aortic repair for aortic dissections.
J Vasc Surg, 69(4):987-995, 24 Oct 2018
Cited by: 13 articles | PMID: 30528404
Techniques and outcomes of false lumen embolization in chronic type B aortic dissection.
J Cardiovasc Surg (Torino), 59(6):784-788, 26 Jun 2018
Cited by: 4 articles | PMID: 29943961
Review