Abstract
Background
Research highlights the intense physiological demands of thoroughbred racing on jockeys, with elevated heart rates and substantial oxygen uptake, confirming the rigorous physical nature of the sport, however, the cardiovascular changes resulting from the physical demands of thoroughbred racing remain unexplored in Australian jockeys. Therefore, the objective of this study was to compare measures of cardiac structure and function of professional Australian jockeys to that of the general population and to determine if there are differences in heart structure and function detected using echocardiography.Methods
Forty-six jockeys and thirty-three participants from the general population underwent two-dimensional echocardiography, which included all standard views and measurements. Each measurement was compared between groups using a Mann-Whitney U test.Results
Groups were matched for age (jockeys (35 ± 12 years) and controls (36 ± 13 years)). Jockeys were shorter (1.64 ± 0.07 m vs. 1.75 ± 0.09 m, p < 0.001), lighter (56.5 ± 6.0 kg vs. 74.2 ± 12.9 kg, p < 0.001) and had a lower body surface area (BSA) (1.55 ± 0.17 m2 vs.1.9 ± 0.2 m2, p < 0.001). Jockeys had a larger absolute left ventricular (LV) end diastolic volume than the control group (120 ± 18.2 ml vs. 109.3 ± 29.0 ml, p = 0.05) which had a larger variation when indexed for BSA (78.0 ± 12.2 ml/m2 vs. 57.5 ± 13.3 ml/m2, p < 0.001). Jockeys demonstrated a higher LV mass index (79.4 ± 18.1 g/m2 vs. 64.2 ± 15.4 g/m2, p < 0.001). Left atrial volume index was larger in jockeys (33.4 ± 6.5 mL/m2 vs. 26.3 ± 7.0 mL/m2, p < 0.001). There were no differences in global longitudinal strain (GLS) for either group overall (-19.3 ± 3.0% vs. -19.8 ± 1.6%, p = 0.52), but 17% of the jockey group demonstrated an abnormal GLS.Conclusions
Jockeys have adaptations to their cardiac structure and function compared to the general population. Differences could be attributed to chronic physiological demands of racing and should be considered in future research involving jockeys.Free full text

Cardiac Structure and Function of Elite Australian Jockeys Compared to the General Population: An Observational Cross-Sectional Study
Abstract
Background
Research highlights the intense physiological demands of thoroughbred racing on jockeys, with elevated heart rates and substantial oxygen uptake, confirming the rigorous physical nature of the sport, however, the cardiovascular changes resulting from the physical demands of thoroughbred racing remain unexplored in Australian jockeys. Therefore, the objective of this study was to compare measures of cardiac structure and function of professional Australian jockeys to that of the general population and to determine if there are differences in heart structure and function detected using echocardiography.
Methods
Forty-six jockeys and thirty-three participants from the general population underwent two-dimensional echocardiography, which included all standard views and measurements. Each measurement was compared between groups using a Mann-Whitney U test.
Results
Groups were matched for age (jockeys (35 ±
± 12 years) and controls (36
12 years) and controls (36 ±
± 13 years)). Jockeys were shorter (1.64
13 years)). Jockeys were shorter (1.64 ±
± 0.07 m vs. 1.75
0.07 m vs. 1.75 ±
± 0.09 m, p
0.09 m, p <
< 0.001), lighter (56.5
0.001), lighter (56.5 ±
± 6.0 kg vs. 74.2
6.0 kg vs. 74.2 ±
± 12.9 kg, p
12.9 kg, p <
< 0.001) and had a lower body surface area (BSA) (1.55
0.001) and had a lower body surface area (BSA) (1.55 ±
± 0.17 m2 vs.1.9
0.17 m2 vs.1.9 ±
± 0.2 m2, p
0.2 m2, p <
< 0.001). Jockeys had a larger absolute left ventricular (LV) end diastolic volume than the control group (120
0.001). Jockeys had a larger absolute left ventricular (LV) end diastolic volume than the control group (120 ±
± 18.2 ml vs. 109.3
18.2 ml vs. 109.3 ±
± 29.0 ml, p
29.0 ml, p =
= 0.05) which had a larger variation when indexed for BSA (78.0
0.05) which had a larger variation when indexed for BSA (78.0 ±
± 12.2 ml/m2 vs. 57.5
12.2 ml/m2 vs. 57.5 ±
± 13.3 ml/m2, p
13.3 ml/m2, p <
< 0.001). Jockeys demonstrated a higher LV mass index (79.4
0.001). Jockeys demonstrated a higher LV mass index (79.4 ±
± 18.1 g/m2 vs. 64.2
18.1 g/m2 vs. 64.2 ±
± 15.4 g/m2, p
15.4 g/m2, p <
< 0.001). Left atrial volume index was larger in jockeys (33.4
0.001). Left atrial volume index was larger in jockeys (33.4 ±
± 6.5 mL/m2 vs. 26.3
6.5 mL/m2 vs. 26.3 ±
± 7.0 mL/m2, p
7.0 mL/m2, p <
< 0.001). There were no differences in global longitudinal strain (GLS) for either group overall (-19.3
0.001). There were no differences in global longitudinal strain (GLS) for either group overall (-19.3 ±
± 3.0% vs. -19.8
3.0% vs. -19.8 ±
± 1.6%, p
1.6%, p =
= 0.52), but 17% of the jockey group demonstrated an abnormal GLS.
0.52), but 17% of the jockey group demonstrated an abnormal GLS.
Conclusions
Jockeys have adaptations to their cardiac structure and function compared to the general population. Differences could be attributed to chronic physiological demands of racing and should be considered in future research involving jockeys.
Background
Thoroughbred horse racing is one of the oldest and most lucrative sports in the world [1]. Jockeys are at the forefront of this dynamic and at times perilous competition with their performance having a direct impact on race outcomes [2]. In Australia, there are approximately 844 registered jockeys participating in over 19,000 races annually [3]. Race riding demands physical strength, stamina, and quick decision-making during races [4]. Although there are many studies on the race fitness required by equine athletes, there is little data around jockey participants [4].
A literature review of the physiological demands of racing conducted by Ryan and Brodine (2021) identified only three studies that measured heart rate and mean oxygen uptake (VO2) of jockeys during race conditions. It was demonstrated that jockeys experience elevated heart rates throughout each race (130–180 beats/min) with peak heart rates ranging from 150 to 190 beats/min [5–7]. Substantial oxygen uptake was also demonstrated during races with oxygen uptake values ranging from 42.7 (± 5.6) to 57.5 (±
5.6) to 57.5 (± 4.7) mlO2/kg/min [5–7]. These findings indicate the rigorous physical nature of the sport and demonstrate the need for endurance (isotonic) fitness for this group of athletes [5–7].
4.7) mlO2/kg/min [5–7]. These findings indicate the rigorous physical nature of the sport and demonstrate the need for endurance (isotonic) fitness for this group of athletes [5–7].
On top of endurance (isotonic) fitness, the unique crouched posture that jockeys maintain during races requires strength (isometric) fitness [4]. This posture, often referred to as the “martini glass” position, involves jockeys standing in the stirrups while keeping their knees bent and their bodies forward and parallel to the horse’s body [3]. They must also stay low and close to the horse’s neck [3]. This position ensures better aerodynamics and helps jockeys to absorb the horse’s movements through their legs [3].
Drawing from studies utilizing skin surface electromyography (EMG) and Training Impulse (TRIMP) scores, (used to quantify the load or intensity of an athlete’s training over time and originally defined as the product of training volume, measured in minutes, and training intensity, normally measured as average heart rate) to quantify the muscle activity used by jockeys while maintaining this unique squat posture, it is clear that jockeys experience isometric exercise loads comparable to elite athletes in other sports such as soccer players [4]. In a study by Legg et al. (2022) the TRIMP score for jockeys during 16 professional races was quantified at 292 ±
± 106 arbitrary units (au) [4]. This is noteworthy considering the TRIMP experienced by a professional soccer player during a 1.5-hour match approximates
106 arbitrary units (au) [4]. This is noteworthy considering the TRIMP experienced by a professional soccer player during a 1.5-hour match approximates ~
~ 190 au, whereas that of a world-class marathon runner participating in a 2-hour race reaches approximately 275 au [4]. These findings highlight the quasi-isometric muscle activation that occurs during racing and therefore the athletic requirements while maintaining this position, indicating that jockeys require a unique blend of both endurance and strength fitness [4], and suggest they may experience cardiovascular changes in line with other athletic populations.
190 au, whereas that of a world-class marathon runner participating in a 2-hour race reaches approximately 275 au [4]. These findings highlight the quasi-isometric muscle activation that occurs during racing and therefore the athletic requirements while maintaining this position, indicating that jockeys require a unique blend of both endurance and strength fitness [4], and suggest they may experience cardiovascular changes in line with other athletic populations.
Cardiovascular physiological adaptations can occur due to the unique demands of regular and intense physical activity in the hearts of athletes [8]. Adaptations commonly seen due to chronic and high-intensity exercise include enlargement of all four cardiac chambers, an increase in left ventricular (LV) wall thickness, and enhanced diastolic filling of the LV [9]. This remodelling allows for increased stroke volume (SV) which in turn increases cardiac output when exercising [9]. Whilst these changes have been well demonstrated in many elite athlete groups such as rowers and cyclists [10], the cardiovascular changes resulting from the physical demands of thoroughbred racing remain unexplored in this population of athletes.
The physiological demands of this sport on jockey participants extend further than mere athleticism. Beyond the elite fitness requirements previously mentioned, jockeys face the additional challenge of maintaining a low body mass (50–61 kg) to meet race-related weight requirements [6]. Often this is daily and, as there is no ‘off season’, many attempt to ‘cut weight’ year-round [11]. Weight-loss techniques, including jogging in sweat gear, hot baths, and saunas, coupled severe energy restriction and dehydration, are commonly used by jockeys to maintain a chronically low body mass [5]. There is evidence to suggest that chronic food and fluid restriction can have detrimental effects on cardiac structure and function in the general population as seen in patients with Anorexia Nervosa (AN) [12]. These practices can lead to decreased LV chamber size, left atrial (LA) enlargement, impaired myocardial contractility, altered diastolic function, reduced stroke volume, and decreased cardiac output [12]. Additionally, changes in Global Longitudinal Strain (GLS) may indicate impaired myocardial performance [12]. The combination of these adaptations can potentially increase the risk of cardiac dysfunction, arrhythmias, and other long-term cardiovascular complications [12–14]. However, to the authors’ knowledge, this has yet to be investigated in jockeys.
Therefore, the aim of this study was to determine if there are differences in heart structure and function detected using echocardiography in registered Australian jockeys when compared to the general population. We hypothesised that cardiac remodelling related to athletic training, such as increased cardiac chamber sizes and greater LV mass, would be demonstrated in jockeys when compared to the general population.
Materials and methods
Participant Selection
Professional Australian jockeys holding a current riding license in South Australia and Western Australia were invited to take part in the study. Data were collected from all jockeys on days of rest from race riding. Participants from the general population were invited using social media advertisements. Whilst there were no restrictions on physical activity for participants recruited to the study, they were excluded if they were < 18 years of age or if there was a previously undocumented heart abnormality discovered during the echocardiogram. All participants gave written informed consent, and the study was approved by the University of South Australia Human Research Ethics Committee (project number: 204235).
18 years of age or if there was a previously undocumented heart abnormality discovered during the echocardiogram. All participants gave written informed consent, and the study was approved by the University of South Australia Human Research Ethics Committee (project number: 204235).
Anthropometric Measurements
Immediately prior to their echocardiogram, anthropometric data were collected from all participants. Height (with shoes and socks removed) was assessed to the nearest centimetre using a portable stadiometer (Seca 213, Hamburg, Germany). Body mass and Total body water % (TBW %) were measured in minimal light clothing using portable digital weighing scales (Tanita Innerscan, Body Composition Monitor, BC-541). Body Surface Area (BSA) was calculated according to the formula of Dubois and Dubois as a ratio of weight (kg) to height (m) [15].
Echocardiography
Two-dimensional (2D) echocardiography included all standard views and measurements. These were taken in accordance with American Society of Echocardiography guidelines [16]. All images were acquired from parasternal and apical windows with participants in the left lateral decubitus position and imaging was completed by one member of the research group, a qualified echocardiographer. Commercially available echocardiography systems with either a M55c XD clear Matrix or 4Vc phased array transducer were used for image acquisition (GE E95, Vivid IQ, General Electric, Horten, Norway). Images were recorded digitally in cine-loop format. Advanced image analysis was performed using commercially available analysis software (TOMTEC Image Arena and Autostrain analysis, Unterschleissheim, Germany). As part of a standard echocardiogram, specific images for diastolic function including mitral valve inflow and tissue Doppler imaging (TDI) were taken. Left atrial volume (LAV) was measured from both the apical 4 and 2 chamber views with care to open the LA to its major axis. LV volumes and ejection fraction were measured using the Simpson’s rule of discs from the apical 4 and 2 chamber views where the major axis of the LV was found. Stoke volume was calculated from the LV outflow tract diameter and velocity time integral. LV GLS was calculated from LV focused images from the apical 4-chamber, 2-chamber, and long-axis views. Studies were deemed inadequate for GLS analysis if 2 or more LV segments could not be visualized. A region of interest was automatically applied by the software to the endocardial and epicardial borders, with tracking adjusted to cover the entire myocardium. GLS was calculated as the change in the length of line of the region of interest across all apical view from diastole to systole. To determine intra-rater reliability, the echocardiographic data from 20 jockeys and 20 normal controls was analysed twice with a minimum duration of one month between measurements. Intra-operator variability was determined using intraclass correlation coefficients (ICC) with an ICC <
< 0.5 indicating poor agreement, 0.5 to 0.7 moderate agreement and >
0.5 indicating poor agreement, 0.5 to 0.7 moderate agreement and > 0.8 good agreement [17].
0.8 good agreement [17].
Lifestyle Questionnaire
Jockey participants were also required to complete a lifestyle questionnaire modified from a previous study [18]. The questionnaire collected information on years of jockeying, race riding statistics and weekly exercise activities completed in addition to race riding. Information on previous medical history was also obtained. Where applicable, participants were instructed to select the most appropriate descriptor, select as many options as deemed personally relevant or type extra information. The survey tool Qualtrics (XM, Provo, UT) was used to design and distribute the questionnaire and to record responses.
Statistical Analysis
This study was reported in accordance with the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) guidelines.
All statistical analyses were conducted using Statistical Package for the Social Sciences (SPSS) (v26, IBM, USA) with the level of statistical significance set at p <
< 0.05. Parametric data were presented as mean
0.05. Parametric data were presented as mean ±
± SD and nonparametric data as median (interquartile range) unless otherwise stated. A Mann-Witney U test to determine differences between the specific groups, jockeys, and general population, was used as the data were not normally distributed. Cohen’s effect size [ES] was calculated to determine differences between the two groups with the difference between the means divided by the pooled standard deviation calculated. Effect sizes were calculated (=
SD and nonparametric data as median (interquartile range) unless otherwise stated. A Mann-Witney U test to determine differences between the specific groups, jockeys, and general population, was used as the data were not normally distributed. Cohen’s effect size [ES] was calculated to determine differences between the two groups with the difference between the means divided by the pooled standard deviation calculated. Effect sizes were calculated (= z /sqrt(N)) and interpreted as follows: <0.20
z /sqrt(N)) and interpreted as follows: <0.20 =
= trivial, 0.20–0.49
trivial, 0.20–0.49 =
= small, 0.50–0.79
small, 0.50–0.79 =
= moderate, and >
moderate, and > 0.79
0.79 =
= large [19].
large [19].
Furthermore, as BSA has been suggested to be flawed for indexing cardiac parameters in very small or large people [20], an exploratory analysis was conducted to identify whether any significant differences could be explained by differences in body size, whereby 19 variables were compared between the eight smallest participants in the control group and eight largest participants in the jockey groups using the above statistical methods.
As the groups were not sex matched, and this is a known area of variation in terms of cardiac remodelling [10], subgroup analysis was also performed comparing the females in the jockey group to the females in the control group. Males in the jockey group were also compared with the males in the control group.
Sample size was calculated using the normal value of GLS (-20 ±
± 3%) and the expectation of a 10% clinically relevant change in GLS between the normal population and jockeys. With reference to Diggle et al., (2002) this means that a sample size of 13 participants in both groups was required for 80% power to detect a clinically significant difference between jockeys and controls with a significance level (α) of 0.05 [21]. To account for possible participant dropout, we aimed to recruit a minimum of 20 participants per group.
3%) and the expectation of a 10% clinically relevant change in GLS between the normal population and jockeys. With reference to Diggle et al., (2002) this means that a sample size of 13 participants in both groups was required for 80% power to detect a clinically significant difference between jockeys and controls with a significance level (α) of 0.05 [21]. To account for possible participant dropout, we aimed to recruit a minimum of 20 participants per group.
Results
Forty-six Australian jockeys (age range: 18–51 years of age) and thirty-three participants from the general population (control group) (age range:18–52 years of age) consented to take part in this study. Descriptive and mean anthropometric data for the jockey and control groups are presented in Table 1. There were no significant differences in age (p =
= 0.54) and sex (p
0.54) and sex (p =
= 0.11) between the jockeys and the control group. Jockeys were shorter (p
0.11) between the jockeys and the control group. Jockeys were shorter (p <
< 0.001), weighed less (p
0.001), weighed less (p <
< 0.001) and therefore had a lower BSA than the control group (p
0.001) and therefore had a lower BSA than the control group (p <
< 0.001). Total Body Water % (TBW %) was recorded for the jockey group only. As were minutes of physical activity per week which included aerobic activity not related to race riding.
0.001). Total Body Water % (TBW %) was recorded for the jockey group only. As were minutes of physical activity per week which included aerobic activity not related to race riding.
Table 1
Descriptive data (mean ±
± SD) for controls (N
SD) for controls (N =
= 33) and jockeys (N
33) and jockeys (N =
= 46)
46)
| Value | Control (N = = 33) 33) | Jockey (N = = 46) 46) | U = | p-Value | Effect size |
|---|---|---|---|---|---|
| Age (years) | 36 ± ± 13 13 | 35 ± ± 12 12 | 697.50 | 0.54 | 0.07 |
| Sex (males %) | 17 (52%) | 32 (70%) | 622.0 | 0.11 | 0.19 |
| Weight (kg) | 74.2 ± ± 12.9 12.9 | 56.5 ± ± 6.0* 6.0* | 119.0 |
< 0.001 0.001
| 0.73 |
| Height (m) | 1.75 ± ± 0.09 0.09 | 1.64 ± ± 0.07* 0.07* | 252.5 |
< 0.001 0.001
| 0.58 |
| BSA (m2) | 1.9 ± ± 0.2 0.2 | 1.55 ± ± 0.17* 0.17* | 118.0 |
< 0.001 0.001
| 0.73 |
| TBW (%) | Not recorded | 59.3 ± ± 4.4 4.4 | N/A | N/A | N/A |
| Minutes physical activity per week (mins) | Not recorded | 1182 ± ± 414 414 | N/A | N/A | N/A |
*p <
< 0.05 compared with control group: U - Mann-Witney U test value, BSA – Body surface area, TBW – Total body water %, Values are presented as mean
0.05 compared with control group: U - Mann-Witney U test value, BSA – Body surface area, TBW – Total body water %, Values are presented as mean ±
± standard deviation or number and (percentage). Bold text indicates significant p-values
standard deviation or number and (percentage). Bold text indicates significant p-values
The results of the echocardiographic measurements for the jockey and control groups are presented in Table 2. Even though their height, weight and BSA were less than the control group, jockeys had a larger LV end diastolic volume (LVEDV) (p =
= 0.05), which remained when indexed for BSA (p
0.05), which remained when indexed for BSA (p <
< 0.001) and higher LV ejection fractions (LVEF) (p
0.001) and higher LV ejection fractions (LVEF) (p <
< 0.001). Example images representing the comparison of group averages of these values can be seen in Fig. 1A and B.
0.001). Example images representing the comparison of group averages of these values can be seen in Fig. 1A and B.
Table 2
Echocardiographic measurements (mean ±
± SD) for controls (N
SD) for controls (N =
= 33) and jockeys (N
33) and jockeys (N =
= 46)
46)
| Value | Control (N = = 33) 33) | Jockey (N = = 46) 46) | U = | p-Value | Effect size |
|---|---|---|---|---|---|
| HR (bpm) | 60 ± ± 11 11 | 65 ± ± 13 13 | 577.5 | 0.23 | 0.14 |
| GLS (%) | -19.8 ± ± 1.6 (-17 to -23%) 1.6 (-17 to -23%) | -19.3 ± ± 3.0 (-11 to -25%) 3.0 (-11 to -25%) | 695.0 | 0.52 | 0.07 |
| LVEDV (mL) | 109.3 ± ± 29.0 29.0 | 120.0 ± ± 18.2 18.2 | 547.0 | 0.06 | 0.22 |
| LVESV (mL) | 47.4 ± ± 13.6 13.6 | 48.7 ± ± 12.1 12.1 | 710.5 | 0.8 | 0.03 |
| LVEF (%) | 57 ± ± 3 3 | 60.8 ± ± 5.2* 5.2* | 386.0 |
< 0.001 0.001
| 0.41 |
| Mitral E: A ratio | 1.9 ± ± 0.6 0.6 | 2.1 ± ± 0.9 0.9 | 654.5 | 0.415 | 0.09 |
| E: E’ (avg) | 5.6 ± ± 1.2 1.2 | 6.2 ± ± 1.5 1.5 | 556.0 | 0.09 | 0.19 |
| Stroke Volume (mL) | 75.7 ± ± 20.7 20.7 | 64.1 ± ± 12.6* 12.6* | 473.0 | 0.02 | 0.26 |
| Stroke Volume index (mL/m2) | 39.8 ± ± 9.3 9.3 | 41.7 ± ± 8.2 8.2 | 601.0 | 0.32 | 0.11 |
| Cardiac Output (L/min) | 4.5 ± ± 1.4 1.4 | 4.1 ± ± 0.9 0.9 | 592.0 | 0.298 | 0.12 |
| LA vol (biplane, mL) | 49.7 ± ± 13.8 13.8 | 51.6 ± ± 10.7 10.7 | 593.5 | 0.341 | 0.11 |
| LA vol index (mL/m2) | 26.3 ± ± 7.0 7.0 | 33.4 ± ± 6.5* 6.5* | 311.0 |
< 0.001 0.001
| 0.46 |
| LVEDV index (mL/m2) | 57.5 ± ± 13.3 13.3 | 78.0 ± ± 12.2* 12.2* | 195.0 |
< 0.001 0.001
| 0.63 |
| LV mass (g) | 124.2 ± ± 35.3 35.3 | 123.8 ± ± 36.7 36.7 | 748.5 | 0.917 | 0.01 |
| LV mass index (g/m2) | 64.2 ± ± 15.4 15.4 | 79.4 ± ± 18.1* 18.1* | 429.0 |
< 0.001 0.001
| 0.34 |
*p <
< 0.05 compared with control group: U - Mann-Witney U test value, HR – heart rate, GLS – global longitudinal strain, LVEDV – left ventricular end diastolic volume, LVESV – left ventricular end systolic volume, LVEF – left ventricular ejection fraction, Mitral E: A ratio – ratio of peak velocity blood flow from left ventricular relaxation in early diastole to peak velocity flow in late diastole, E:E’ – mitral inflow early diastolic velocity to tissue Doppler mitral annular early diastolic velocity ratio, LA vol – left atrial volume, LV – left ventricular, Values are presented as mean
0.05 compared with control group: U - Mann-Witney U test value, HR – heart rate, GLS – global longitudinal strain, LVEDV – left ventricular end diastolic volume, LVESV – left ventricular end systolic volume, LVEF – left ventricular ejection fraction, Mitral E: A ratio – ratio of peak velocity blood flow from left ventricular relaxation in early diastole to peak velocity flow in late diastole, E:E’ – mitral inflow early diastolic velocity to tissue Doppler mitral annular early diastolic velocity ratio, LA vol – left atrial volume, LV – left ventricular, Values are presented as mean ±
± standard deviation or number and (percentage). Bold text indicates significant p-values
standard deviation or number and (percentage). Bold text indicates significant p-values
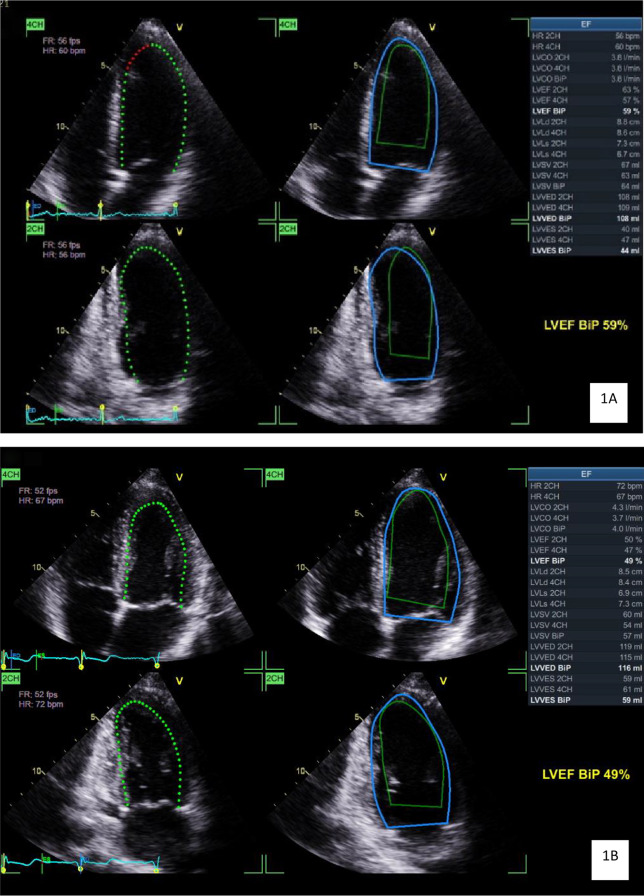
1A and 1B: Echocardiographic images demonstrating the measurement of left ventricular volume and ejection fraction from a general population participant (A) demonstrating a left ventricular end diastolic volume of 109mL and ejection fraction of 59% and in a jockey (B) demonstrating a larger left ventricular end diastolic volume of 116mL and lower ejection fraction of 49%. The dotted green line represents the instantaneous volume, the solid blue line represents end diastolic volume, and the solid green line represents end systolic volume. Abbreviations: 2CH – two chamber view, 4CH – four chamber view, BiP – biplane, bpm – beats per minute, cm – centimetres, CO- cardiac output, EF – ejection fraction, HR – heart rate, L – litres, LV – left ventricular, ml – millilitres, VED – volume at end diastole, VES – volume at end systole
Absolute LV mass did not differ between the two groups (p =
= 0.92); however, the jockey group demonstrated a higher LV mass index than the control group (p
0.92); however, the jockey group demonstrated a higher LV mass index than the control group (p <
< 0.001).
0.001).
Furthermore, LAV values were larger in jockeys, but only when indexed for BSA (p <
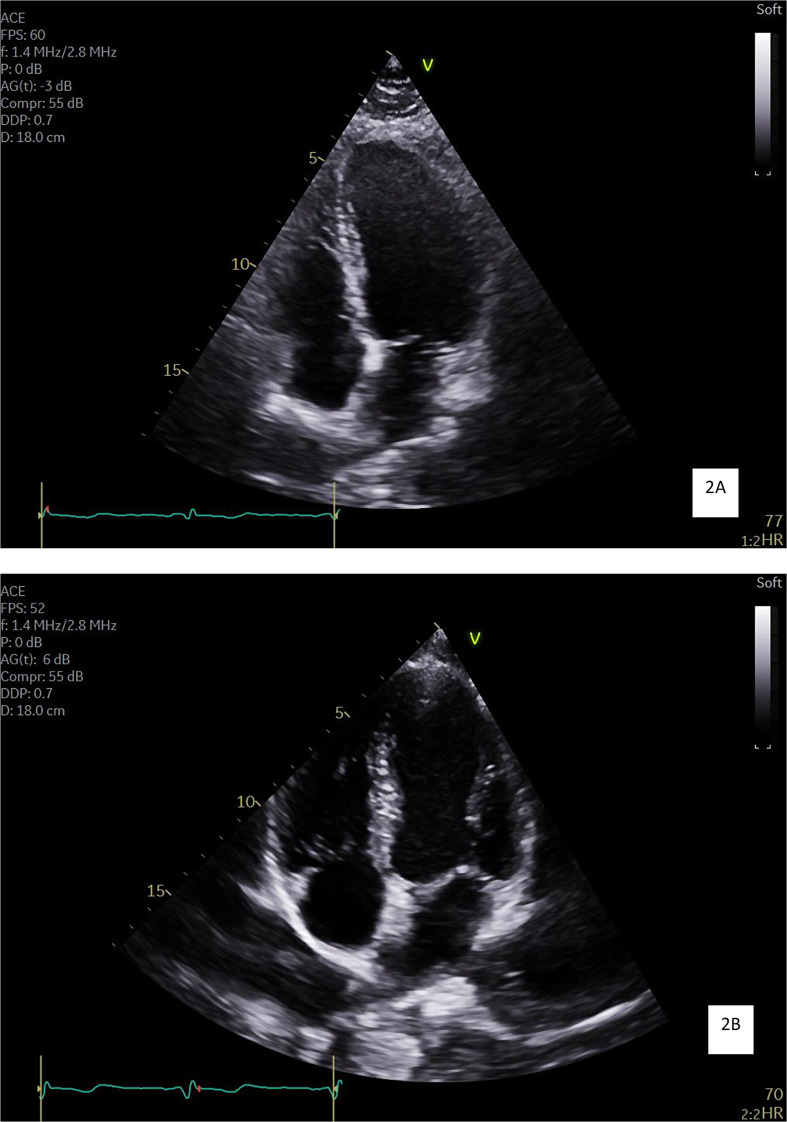
< 0.001). Example images representing the comparison of group averages of the chamber sizes can be seen in Fig. 2A and B.
0.001). Example images representing the comparison of group averages of the chamber sizes can be seen in Fig. 2A and B.
A lower stroke volume (SV) (p =
= 0.021) was also demonstrated by the jockey group. However, when SV was indexed for BSA there was no longer a difference between the groups (p
0.021) was also demonstrated by the jockey group. However, when SV was indexed for BSA there was no longer a difference between the groups (p =
= 0.32).
0.32).
Despite the GLS being similar for both groups, there were 8 (17%) jockeys who had a GLS more impaired than − 16%, whereas all of the control group demonstrated normal GLS. Of these 8 jockeys, 7 had been riding for more than 5 years and 1 had been riding for less than 5 years. 5 were male and 3 were female. 1 was between the ages of 20–30, 3 were between the ages of 30–40 and 4 were between the ages of 40–50.
16%, whereas all of the control group demonstrated normal GLS. Of these 8 jockeys, 7 had been riding for more than 5 years and 1 had been riding for less than 5 years. 5 were male and 3 were female. 1 was between the ages of 20–30, 3 were between the ages of 30–40 and 4 were between the ages of 40–50.
The eight largest jockeys and eight smallest of the control group were also compared. There was no difference in BSA between the 2 groups (p =
= 0.28) suggesting that any cardiac changes observed are independent of body size. LVEDV, LVESV, LAV, LVEDV indexed for BSA, LV mass and LV mass index were all larger in jockeys compared to the control group (p
0.28) suggesting that any cardiac changes observed are independent of body size. LVEDV, LVESV, LAV, LVEDV indexed for BSA, LV mass and LV mass index were all larger in jockeys compared to the control group (p <
< 0.05).
0.05).
Females in the control group and the jockey group, along with males in each group were compared. The results remained significant for weight, height, BSA, LVEDVi, LVEF and LAVi (p <
< 0.05) between groups. However, only female jockeys demonstrated a higher LV mass index compared to the female control group 74.9
0.05) between groups. However, only female jockeys demonstrated a higher LV mass index compared to the female control group 74.9 ±
± 17.9 vs. 56.5
17.9 vs. 56.5 ±
± 12.7 g/m2, p
12.7 g/m2, p =
= 0.004). Whereas male jockeys did not when compared to the male control group (81.3
0.004). Whereas male jockeys did not when compared to the male control group (81.3 ±
± 18.1 vs. 73.3
18.1 vs. 73.3 ±
± 13.4 g/m2, p
13.4 g/m2, p =
= 0.13).
0.13).
ICC of all measurements ranged from 0.85 to 0.95 (all p values <
< 0.0001) indicating good intra-observer agreement.
0.0001) indicating good intra-observer agreement.
Discussion
To the authors’ knowledge, this is the first study to evaluate the cardiac structure and function of jockey athletes, revealing distinctive cardiac adaptations of considerable interest that may be comparable to other athlete groups. A critical finding is the presence of physiological remodelling of cardiac chambers in jockeys, particularly when indexed for BSA. Notably, jockeys exhibited larger LV and LA volumes as well as increased LV mass when indexed for BSA compared to the general population.
It is crucial to emphasize that these alterations in chamber dimensions were not associated with impaired cardiac function, as evidenced by the jockeys demonstrating normal LVEF [23]. It has been demonstrated that athletes generally have LVEF values that are close to the general population [22]. As this is a critical indicator of cardiac systolic function, the preservation of normal LVEF observed in the jockey group, would suggest that despite the increased LA and LV volumes, the contractile capacity of their hearts was not compromised [24].
This observed increase in LV and LA volumes as well as LV mass, when indexed for BSA, could be attributed to the chronic high-intensity physical regimens jockeys complete [6]. According to the World Health Organisation’s 2020 physical activity guidelines, adults are advised to engage in 150–300 min of moderate intensity physical activity, 75–150 min of vigorous intensity physical activity or a combination of both, weekly [25]. Jockey participants in this study, reported performing much more exercise than these recommendations, indicating exposure to considerably elevated levels of physical activity when compared to the general population [25]. Therefore, this extensive physical activity maybe a factor for the structural and functional changes in jockeys’ hearts observed in this study. It has been well documented that an increase in cardiac chamber volumes, particularly LV, occurs in athletes exposed to high levels of physical activity, particularly those that train for endurance sports with both isometric and isotonic exercise, such as rowers and cyclists [10]. The haemodynamic overload and increased cardiac output during exercise helps to better perfuse working muscles [22]. Therefore, giving athletes a competitive edge, particularly those in endurance sports where an increase in the size of LA and RA volumes is seen [10]. Given the increase in both LA and LV volumes observed in the jockey group, this suggests that this population, a group that has not previously been included in athlete heart studies, fit within this demographic of endurance athletes [10, 26, 27]. Whilst it was not a focus of this study, this finding necessitates further research comparing jockeys to other athlete groups to understand the full extent of these adaptations.
Additionally, cardiovascular adaptions seen in athletes do not occur as discrete, all-or-nothing changes, but instead as gradual progression that is proportional with the intensity and duration of training [22]. Perry et al. (2018) examined the physiological changes in 145 Australian Football players over a 6 year period [9]. They demonstrated typical cardiac remodelling that is generally seen in athletes such as increased LV mass and volume in proportion to exercise levels with RV remodelling becoming more apparent with longevity of training and increasing age [9]. They also found that these changes occur over time and are dependent on individual fitness level, physical activity type and age [9]. Therefore, it is plausible to suggest more time spent in the sport could lead to further cardiac adaptations [22]. Thus, examining jockeys over career longevity is another possible avenue for future research.
Jockeys also displayed an increased left ventricular end-diastolic volume (LVEDV), although this was not statistically significant until it was indexed for BSA. This is a common observation in athlete hearts, similar to that seen in endurance athletes such as rowers and cyclists [10, 22, 27]. Regular intensive training, especially endurance training, leads to increased preload due to a higher blood volume and enhanced venous return [28]. This increased preload stretches the ventricular walls, promoting chamber enlargement and increased LVEDV [28]. Physiological remodelling then allows the heart to pump a greater volume of blood per beat, contributing to improved overall cardiovascular efficiency during high-intensity physical activities, such as was observed in the jockey group [28].
Jockeys exhibited significantly lower weight, height, and BSA compared to the control group. This may be attributed to the rigorous weight management requirements they face to meet racing standards, combined with the generally shorter stature of jockeys compared to the general population [29]. These factors could potentially influence cardiac parameters as, whilst their LVEF and GLS were clinically normal as a group, it is important to note that 17% (8) of the jockeys in this study presented with reduced GLS. GLS is a sensitive marker of myocardial deformation and can reveal subclinical changes in cardiac function such as LV dysfunction and hypertrophic myocardiopathy [9, 30]. It has been reported in previous studies, that no difference in GLS has been found in endurance trained rugby players, elite cyclists or rowers when compared to sedentary controls or recreational athletes [31]. This was further supported in a meta-analysis in which no athlete-control differences existed for GLS [31]. Although this study was not designed to delve deeply into specific causes, the finding that 7 of the 8 jockeys with reduced GLS had been riding for more than 5 years, may suggest that certain practices associated with this unique athlete group may lead to undesirable adaptations in cardiac function detected only by GLS. Especially as all age matched general population participants demonstrated GLS within a normal range. One avenue for future investigation may centre on the effects of weight-shedding practices commonly employed by jockeys to meet the rigorous weight requirements of their profession. These practices, which include strategies such as saunas and extreme dietary restrictions, could conceivably exert detrimental effects on myocardial function, similar to those seen in patients with the early stages of AN where altered GLS while systolic function is preserved can indicate early myocardial deformation, thus warranting further exploration [14, 32].
With this in mind, chronic dehydration and calorie deficiencies are known to demonstrate reduced chamber volumes, decreased LVEF and CO [12]. However, the jockey group demonstrated TBW (%) within a normal range for athletes [33–35]. Matais et al. (2016), examined the body composition of 200 athletes from 15 sports including basketball, track and field, soccer, wrestling, tennis, swimming and sailing, demonstrating a mean TBW (%) of ~ 50% [35]. Similar values have been observed in marathon runners (57%) and rowers (54%) [33, 34, 36]. This suggests that the jockey group in the present study were comparable to other athletes and, on the day of data collection, were not dehydrated. This could be due to the measurements being taken on a day of rest when jockeys had not been restricting their diet or sweating in preparation for race day. This also suggests that hydration did not affect preload and LVEDV values of jockeys during echocardiography data collection.
50% [35]. Similar values have been observed in marathon runners (57%) and rowers (54%) [33, 34, 36]. This suggests that the jockey group in the present study were comparable to other athletes and, on the day of data collection, were not dehydrated. This could be due to the measurements being taken on a day of rest when jockeys had not been restricting their diet or sweating in preparation for race day. This also suggests that hydration did not affect preload and LVEDV values of jockeys during echocardiography data collection.
Also, given the findings for height, weight and BSA for the jockey group, it is consistent that jockeys demonstrated a lower absolute stroke volume; however, this was within normal limits and did not differ from the control group when indexed to BSA. This finding aligns with previous studies such as one conducted by De Simone et al., (1997) which observed a relationship between stroke volume and measures of body size [37]. When compared to the general population it would be expected that jockeys also have a lower absolute stroke volume [37].
Limitations
While the study’s results are consistent with the hypothesis of cardiac remodelling in jockeys, it is important to acknowledge the limitations of the cross-sectional design. While the control group used in this study were free of cardiovascular disease, they were not required to meet any physical activity guidelines or report their exercise habits [25]. Therefore, the results may not be replicated in highly active individuals from the general population, and it is unclear whether differences in activity levels were driving the differences seen in cardiac structure and function seen between groups. Also, the sample sizes for both groups were relatively small, which may have affected the statistical power to detect significant differences in some parameters. This may limit the generalisability of these results to other jockey populations. However, the small jockey sample size can be attributed to the low number of jockeys that compete Australia wide, and therefore is a good representation of jockeys competing in Australia. Additionally, while groups were matched for age, given the exploratory nature of the research and the small sample size, they were unable to be matched for sex. There is evidence that differences in sex can influence certain parameters of the heart such as chamber size [16], which may have influenced the results. However, as the data was normalised for BSA, it is unlikely to have had a notable impact.
Conclusions
This study investigated the cardiovascular structure and function of a group that has not previously been examined. It provides insights into the cardiovascular characteristics of registered Australian jockeys compared to the general population. The observed differences in LA volume index, LVEDV index and LV mass index between the two groups may be due to the high level of exercise experienced by the jockey participants. However, as this study was cross-sectional, it cannot be determined if this relationship was causative. These findings highlight the need for further research on this specialised athlete population, especially against other elite athletes. Also, the identification of a subset of jockeys with reduced GLS raises questions about the effects of the physiological demands of racing and is a potential area for future investigation.
Acknowledgements
The authors wish to acknowledge the Australian Jockeys’ Association, Racing South Australia and Racing and Wagering Western Australia for their support throughout this project. The authors would also like to thank GE Healthcare Australia for assistance with equipment and software. The authors would also like to acknowledge the life and enduring contribution to sports science research of the late Professor Roger Eston. Roger was passionate in sharing his knowledge and encouraging the next generation of researchers in this field. He had a great influence on this, his final work, and it would not have happened without him. Rest in peace Roger.
Abbreviations
| EMG | Electromyography |
| TRIMP | Training Impulse |
| au | Arbitrary Units |
| LV | Left ventricular |
| LA | Left atrial |
| GLS | Global Longitudinal Strain |
| AN | Anorexia Nervosa |
| BSA | Body Surface Area |
| 2D | Two-dimensional |
| TDI | Tissue Doppler imaging |
| LAV | Left atrial volume |
| SPSS | Statistical Package for the Social Sciences |
| LVEDV | LV end diastolic volume |
| LVEF | LV ejection fraction |
| SV | Stroke volume |
| E: E’ | Mitral inflow early diastolic velocity to tissue Doppler mitral annular early diastolic velocity ratio |
| HR | Heart rate |
| LVESV | Left ventricular end systolic volume |
| SD | Standard deviation |
| TBW (%) | Total Body Water % |
| 2CH | Two chamber view |
| 4CH | Four chamber view |
| BiP | Biplane |
| bpm | Beats per minute |
| cm | Centimetres |
| CO | Cardiac output |
| EF | Ejection fraction |
| L | Litres |
| ml | Millilitres |
| VED | Volume at end diastole |
| VES | Volume at end systole |
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by AF and RP. The first draft of the manuscript was written by AF. HB, RE and RP edited and provided feedback on further draft versions of the manuscript. AF, RP, HB and RE read and approved the final version of this manuscript for submission to this journal.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data Availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Informed written consent was obtained from all individual participants included in the study. The study was approved by the University of South Australia Human Research Ethics Committee (project number: 204235). The study was also undertaken and performed in accordance and compliance with the standards of ethics outlined in the Declaration of Helsinki and the International Principles governing research with human beings [38].
All participants provided written consent for the use of their images in any publications produced as a result of their participation in this project.
AF, HB, RE, and RP have no declarations of interest or conflicts of interest that are directly relevant to the content of this article and declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Articles from Sports Medicine - Open are provided here courtesy of Springer
Citations & impact
This article has not been cited yet.
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/170188747
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Relationship between Cardiac Remodeling and Exercise Capacity in Elite Athletes: Incremental Value of Left Atrial Morphology and Function Assessed by Three-Dimensional Echocardiography.
J Am Soc Echocardiogr, 33(1):101-109.e1, 01 Oct 2019
Cited by: 13 articles | PMID: 31585830
Workplace Injuries in Thoroughbred Racing: An Analysis of Insurance Payments and Injuries amongst Jockeys in Australia from 2002 to 2010.
Animals (Basel), 5(3):897-909, 08 Sep 2015
Cited by: 7 articles | PMID: 26479392 | PMCID: PMC4598712
Markers of Bone Health, Bone-Specific Physical Activities, Nutritional Intake, and Quality of Life of Professional Jockeys in Hong Kong.
Int J Sport Nutr Exerc Metab, 28(4):440-446, 28 Apr 2018
Cited by: 4 articles | PMID: 28556673
Weight-Making Practices Among Jockeys: An Update and Review of the Emergent Scientific Literature.
Open Access J Sports Med, 12:87-98, 09 Jul 2021
Cited by: 3 articles | PMID: 34267562 | PMCID: PMC8276820
Review Free full text in Europe PMC