Abstract
Objectives
This study aimed to investigate the potential roles of proprotein convertase subtilisin/ kexin type 9 (PCSK9) and apelin in the etiology of fibromyalgia syndrome (FS).Patients and methods
The retrospective study was conducted between May 2022 and February 2023. Fifty-eight female FS patients (mean age: 45.2±9.9 years; range, 25 to 66 years) and 30 age- and body mass index-matched control subjects (mean age: 43.1±9.9 years; range, 26 to 67 years) were included in the study. Apelin and PCSK9 levels of all individuals were measured using appropriate methods.Results
The levels of PCSK9 (173.2±62.2 vs. 75.1±44.1, p<0.001) and apelin (354.6±195.5 vs. 229.0±83.2, p<0.001) were significantly higher in patients with FS compared to the control group. A positive correlation was found between PCSK9 and apelin levels and various measures, including the Fibromyalgia Impact Questionnaire (FIQ), Symptom Severity Scale (SSS), Pittsburgh Sleep Quality Index (PSQI), and Beck Depression Inventory (BDI). Additionally, there was a positive correlation between apelin levels and FIQ, SSS, PSQI, Beck Anxiety Inventory, and BDI scores. The optimal cutoff value for PCSK9 in predicting FS was 110.0 ng/mL, with a sensitivity of 84.5% and specificity of 83.9% (area under the curve [AUC]=0.920, 95% confidence interval [CI]: 0.852-0.987, p<0.001). For apelin, the optimal cutoff value for predicting FS was 258.8 ng/L, with a sensitivity of 63.8% and specificity of 64.5% (AUC=0.732, 95% CI: 0.623-0.840, p<0.001).Conclusion
Our findings suggest that PCSK9 may play a role in FS etiology and potentially contribute to oxidative stress. Increased apelin levels may be a compensatory response to high oxidative stress, possibly leading to hyperalgesia. Both PCSK9 and apelin can be predictive markers for FS.Free full text

Proprotein convertase subtilisin/kexin type 9 and apelin in fibromyalgia syndrome
Abstract
Objectives
This study aimed to investigate the potential roles of proprotein convertase subtilisin/ kexin type 9 (PCSK9) and apelin in the etiology of fibromyalgia syndrome (FS).
Patients and methods
The retrospective study was conducted between May 2022 and February 2023. Fifty-eight female FS patients (mean age: 45.2±9.9 years; range, 25 to 66 years) and 30 age- and body mass index-matched control subjects (mean age: 43.1±9.9 years; range, 26 to 67 years) were included in the study. Apelin and PCSK9 levels of all individuals were measured using appropriate methods.
Results
The levels of PCSK9 (173.2±62.2 vs. 75.1±44.1, p<0.001) and apelin (354.6±195.5 vs. 229.0±83.2, p<0.001) were significantly higher in patients with FS compared to the control group. A positive correlation was found between PCSK9 and apelin levels and various measures, including the Fibromyalgia Impact Questionnaire (FIQ), Symptom Severity Scale (SSS), Pittsburgh Sleep Quality Index (PSQI), and Beck Depression Inventory (BDI). Additionally, there was a positive correlation between apelin levels and FIQ, SSS, PSQI, Beck Anxiety Inventory, and BDI scores. The optimal cutoff value for PCSK9 in predicting FS was 110.0 ng/mL, with a sensitivity of 84.5% and specificity of 83.9% (area under the curve [AUC]=0.920, 95% confidence interval [CI]: 0.852-0.987, p<0.001). For apelin, the optimal cutoff value for predicting FS was 258.8 ng/L, with a sensitivity of 63.8% and specificity of 64.5% (AUC=0.732, 95% CI: 0.623–0.840, p<0.001).
Conclusion
Our findings suggest that PCSK9 may play a role in FS etiology and potentially contribute to oxidative stress. Increased apelin levels may be a compensatory response to high oxidative stress, possibly leading to hyperalgesia. Both PCSK9 and apelin can be predictive markers for FS.
Introduction
Fibromyalgia syndrome (FS) is characterized by chronic musculoskeletal pain, fatigue, and functional and is often accompanied by psychological symptoms or sleep, memory, and mood issues. Its prevalence ranges from 0.2% to 6.6% in the general population and 2.4% to 6.8% in women.[1] Predisposing factors for FS include exposure to stressful environments, adverse life events, and experiences of physical and emotional trauma.[2] Common comorbidities with FS are irritable bowel syndrome, headache, temporomandibular joint disorder, rheumatoid arthritis, and endometriosis.[3] Despite advances in laboratory tests and imaging techniques, the etiology of FS remains unclear. Multiple factors are believed to contribute to its development, including central sensitization, genetic predisposition, immunological factors, hormonal imbalances, and environmental influences.[4] Among these factors, the dopaminergic pathway and iron deficiency are emphasized more frequently.[5]
Lipid peroxidation and oxidative stress may also play a vital role in the etiology of FS.[6] Prooxidants cause increased superoxide activity in mitochondrial membrane potential and excessive synthesis of lipid peroxidation products.[6] Cell mitophagy converts the balanced oxidant-antioxidant capacity in favor of oxidation.[7]
Increased levels of prooxidative factors, such as nitric oxide, lipid peroxidation, and mitophagy can cause pain sensitivity in fibromyalgia.[8] FS symptoms appear in a wide range from pain to sleep disturbance. These symptoms have a significant impact on patients' quality of life. As a result, research has focused on establishing the relationship between clinical and biochemical parameters.[9] Preventing the specified oxidative stress, which has an important role in FS pathophysiology, is also important in treatment management. Effective treatment of FS is important to reduce pain and fatigue and improve quality of life.[6]
Proprotein convertase subtilisin/kexin type 9 (PCSK9) is one of nine members of the family of proprotein converters and is a serine protease in glycoprotein structure consisting of 692 amino acids.[10] Although predominantly in the liver, it is also expressed in the kidneys, small intestine, and central nervous system.[11] PCSK9 cleaves low-density lipoprotein (LDL) receptors (LDLRs), thus preventing the clearance of LDL from the blood.[11] Increased LDL levels can lead to lipid peroxidation, converting LDL into toxic metabolites harmful to the organism.[12] Additionally, PCSK9 promotes the release of proinflammatory cytokines and induces oxidative stress independently of LDL.[13]
Apelin is an endogenous peptide ligand for the apelin receptor (APJ), which is an extensively expressed G protein-coupled receptor.[14] Apelin is expressed in various tissues, such as adipose tissue, liver, heart, lung, kidney, adrenal glands, gastrointestinal tract, and brain.[15] Apelin serves multiple functions, such as blood pressure regulation, vascular angiogenesis, fluid and water intake, and effects on pituitary functions.[16] Additionally, apelin is known for its antioxidant and anti-inflammatory effects.[17]
Although oxidative stress is known to play a role in the etiology of FS, to date, oxidative stress-related markers PCSK9 and apelin have not been investigated in patients with FS. This study aimed to evaluate the effects of apelin and PCSK9 levels associated with oxidative stress on FS. If apelin and PCSK9 were found to have predictive values on FS, it was intended to shed light on further studies to evaluate their effects in their treatment.
Patients and Methods
This prospective case-control study included 58 female patients (mean age: 45.2±9.9 years; range, 25 to 66 years) who applied to the physical therapy and rehabilitation outpatient clinic of the Health Sciences University, Elazığ Fethi Sekin City Health Application and Research Center and were diagnosed with FS according to the 2010 American College of Rheumatology (ACR) criteria between May 2022 and February 2023.[18] For the control group, 30 female volunteers (mean age: 43.1±9.9 years; range, 26 to 67 years) who applied to the clinic without a diagnosis of FS according to the 2010 ACR criteria and who were matched for age and body mass index were included. Individuals with comorbid conditions, such as diabetes mellitus, hypertension, cardiovascular disease, chronic renal failure, hypothyroidism, malignancy, and psychiatric diseases, were excluded from the study. Subjects taking medication for sleep disorders and those who were pregnant or breastfeeding were not included in the study.
Baseline demographic variables (sex, age, and body mass index) and clinical data (disease duration, Symptom Severity Scale [SSS]) were collected. The severity of FS was assessed using the Fibromyalgia Impact Questionnaire (FIQ). Pain severity was evaluated using the Visual Analog Scale (VAS). Pain and fatigue were evaluated with VAS fatigue. Sleep and depression were assessed with Pittsburgh Sleep Quality Index (PSQI), Beck Depression Inventory (BDI), and Beck Anxiety Inventory (BAI).
Symptoms were assessed with the SSS in four sections: fatigue, waking up tired, cognitive problems, and somatic symptoms. Symptom severity was evaluated as follows: 0=no issues, 1=mild/occasional issue, 2=moderate/frequent issues, 3=severe/continuous issues. Somatic symptoms of the patients included muscle pain, irritable bowel, memory problems, fatigue, muscle weakness, headache, abdominal pain/cramps, numbness/tingling, dizziness, insomnia, depression, nausea, constipation, irritability, blurred vision, chest pain, hot flashes, hair loss, painful urination, frequent urination, diarrhea, dry mouth, itching, Raynaud's phenomenon, tinnitus, urticaria, vomiting, heartburn, burning, oral ulcer, taste perversion, dry eyes, shortness of breath, loss of appetite, and rash. The common symptoms of fibromyalgia, such as sun sensitivity, hearing difficulty, and easy bruising, were questioned, and the patient scored between 0 and 3 according to the number of symptoms: no symptoms (0 points), one to 10 signs (few symptoms, 1 point), 11 to 20 signs (moderate symptoms, 2 points), 21 to 37 signs (many symptoms, 3 points). The total score can be between 0 and 12 points. An SSS ≥5 is significant in terms of FS.[19]
Fibromyalgia Impact Questionnaire is a 20-item tool that assesses the impact of FS on patients. The total score is between 0 and 100. Higher scores indicate greater health deterioration.[20]
Beck Anxiety Inventory is a 21-item multiple-choice self-reported instrument used to measure the severity of anxiety in adults. The questions in this assessment inquire about common anxiety symptoms experienced by the subject over the past week. Each question on the BAI is scored on a scale from 0 (never) to 3 (severe), and higher total scores indicate more severe anxiety symptoms.[21]
Beck Depression Inventory is a widely used scale for assessing depression in patients. It contains 21 items, each graded between 0 and 3 points, and all are added to give a total score.[22]
Visual Analog Scale is a simple tool for assessing various symptoms and has been validated in many chronic diseases. It employs a scoring system from 0 to 10, where 0 represents the absence of symptoms and 10 indicates severe symptoms.[23]
Pittsburgh Sleep Quality Index is a self-reported questionnaire designed to assess sleep quality. It comprises seven components: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, sleep medication use, and daytime dysfunction. An overall PSQI score of 5 or higher indicates clinically significant sleep disorders.[24]
Blood samples were taken from the individuals and placed into gel separator (serum) tubes in the morning after an overnight fast. After collection, the serum tubes were kept at room temperature for 30 min, coagulated, and then centrifuged at 1,200× g for 10 min. After centrifugation, serum samples were carefully separated from the tubes and transferred to microvolume Eppendorf tubes. The serum samples were then stored at –80°C until biochemical analysis was conducted using the enzyme-linked immunosorbent assay (ELISA).
For the analysis of PCSK9, a commercial sandwich ELISA kit utilizing two antibodies (catalog no: SEE189Hu; Cloud Clone Corp., Houston, TX, USA) was employed according to the manufacturer's instructions. The absorbance of the samples were determined using a microplate reader adjusted to 450-nm wavelength. PCSK9 results were reported as ng/mL. For apelin analysis, a commercial ELISA kit (catalog no: E2014Hu; Bioassay Technology Laboratory, Shanghai, China) was used following the manufacturer's instructions. The absorbance of the samples was also measured at a wavelength of 450 nm, and apelin results were reported in ng/L.
Statistical analysis
The minimum sample size required to detect a significant difference was determined to be 17 in each group (34 in total), considering an alpha of 0.05, power of 0.8, effect size of 0.99, and two-sided alternative hypothesis.[15]
Data were analyzed using IBM SPSS version 20.0 software (IBM Corp., Armonk, NY, USA). The results were presented as frequency percentage) or mean ± standard deviation (SD). The distribution of the data was analyzed using the Kolmogorov-Smirnov test. Homogeneous data were analyzed using Student's t-test. Categorical data were analyzed using the chi-square test. Correlation analysis was performed using the Pearson correlation or Spearman rank test according to the distribution of the data. The diagnostic utility of PCSK9 and apelin in predicting the FS was evaluated through the receiver operating characteristic (ROC) curve analysis. The optimal cutoff points of PCSK9 and apelin for FS were determined based on Youden's J statistic. A p-value <0.05 was considered statistically significant.
Results
PCSK9 (173.2±62.2 vs. 75.1±44.1 ng/mL, p<0.001), apelin (354.6±195.5 vs. 229.0±83.2 ng/L, p<0.001), and C-reactive protein (p=0.020) values of the patients were higher than the controls. Patients' FIQ, PSQI, BAI, BDI, somatic symptoms, SSS, duration of diffuse pain, and VAS scores were higher than the controls (all p<0.001). The biochemical and sociodemographic characteristics of the patients are given in Tables 1 and 2.
Table 1
| FS (n=58) | Control (n=30) | ||
| Parameters | Mean±SD | Mean±SD | p |
| Age (years) | 45.2±9.9 | 43.1±9.9 | 0.346 |
| Body mass index (kg/m2) | 27.2±4.2 | 28.5±3.5 | 0.132 |
| Visual Analog Scale fatigue | 5.1±2.0 | 1.1±0.3 | <0.001 |
| Fibromyalgia impact questionnaire | 47±17.5 | 20.7±6.4 | <0.001 |
| Beck anxiety inventory | 26.1±11.0 | 10.7±4.8 | <0.001 |
| Beck depression inventory | 29.5±12.9 | 12.4±4.2 | <0.001 |
| Pittsburgh Sleep Quality Index | 12.4±5.3 | 5.3±2.3 | <0.001 |
| Somatic symptoms | 16.7±5.6 | 5.3±1.7 | <0.001 |
| Symptom severity scale | 7.1±1.9 | 2.1±1.1 | <0.001 |
| FS: Fibromyalgia syndrome; SD: Standard deviation. | |||
Table 2
| FS (n=58) | Control (n=30) | ||
| Parameters | Mean±SD | Mean±SD | p |
| PCSK9 (ng/mL) | 173.20±62.20 | 75.10±44.10 | <0.001 |
| Apelin (ng/L) | 354.60±195.50 | 229.00±83.20 | <0.001 |
| C-reactive protein (mg/dL) | 4.30±2.60 | 3.20±1.50 | 0.020 |
| Erythrocyte sedimentation rate (mm/h) | 10.10±5.60 | 9.30±5.20 | 0.503 |
| Total cholesterol (mg/dL) | 206.10±40.10 | 197.00±47.80 | 0.373 |
| Triglyceride (mg/dL) | 140.50±74.80 | 160.80±89.40 | 0.287 |
| High-density lipoprotein (mg/dL) | 49.70±10.90 | 51.00±10.90 | 0.591 |
| Low-density lipoprotein (mg/dL) | 126.60±30.00 | 117.80±40.30 | 0.290 |
| FS: Fibromyalgia syndrome; SD: Standard deviation; PCSK9: Proprotein convertase subtilisin/kexin type 9. | |||
There was a positive correlation between PCSK9 and apelin, FIQ, SSS, PSQI, BDI, somatic symptoms, generalized pain duration, and VAS scores. There was a positive correlation between apelin and FIQ, SSS, PSQI, BAI, BDI, and VAS fatigue. All correlation analysis results are given in Table 3.
Table 3
| PCSK9 | Apelin | |||
| r | p | r | p | |
| PCSK9 | 0.549 | <0.001 | ||
| Apelin | 0.549 | <0.001 | ||
| Age | 0.099 | 0.462 | 0.144 | 0.282 |
| Body mass index | 0.179 | 0.178 | 0.059 | 0.660 |
| C-reactive protein | 0.073 | 0.589 | 0.145 | 0.278 |
| Erythrocyte sedimentation rate | 0.008 | 0.952 | 0.090 | 0.501 |
| Pain duration | 0.282 | 0.032 | 0.194 | 0.145 |
| Visual Analog Scale pain | 0.333 | 0.011 | 0.175 | 0.188 |
| Visual Analog Scale fatigue | 0.273 | 0.038 | 0.278 | 0.034 |
| Fibromyalgia impact questionnaire | 0.461 | <0.001 | 0.435 | 0.001 |
| Beck anxiety inventory | 0.245 | 0.064 | 0.303 | 0.021 |
| Beck depression inventory | 0.303 | 0.021 | 0.342 | 0.009 |
| Pittsburgh Sleep Quality Index | 0.349 | 0.007 | 0.299 | 0.022 |
| Somatic symptoms | 0.298 | 0.023 | 0.270 | 0.040 |
| Symptom severity scale | 0.379 | 0.003 | 0.346 | 0.008 |
| Total cholesterol | 0.032 | 0.765 | 0.097 | 0.365 |
| Triglyceride | 0.156 | 0.145 | 0.144 | 0.177 |
| High-density lipoprotein | -0.017 | 0.878 | -0.068 | 0.525 |
| Low-density lipoprotein | 0.112 | 0.297 | 0.001 | 0.997 |
| FS: Fibromyalgia syndrome; PCSK9: Proprotein convertase subtilisin/kexin type 9. | ||||
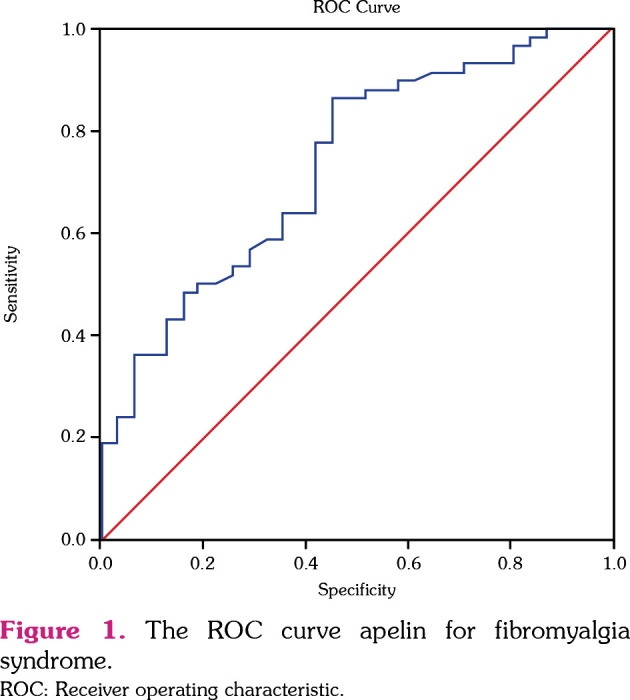
Based on the ROC curve analysis, the optimal PCSK9 cutoff value for FS was 110.0 ng/mL, with a sensitivity of 84.5% and specificity of 83.9% (area under the curve [AUC]=0.920, 95% confidence interval [CI]: 0.852-0.987, p<0.001). The optimal apelin cutoff value for FS was 258.8, with a sensitivity of 63.8% and a specificity of 64.5% (AUC=0.732, 95% CI: 0.623-0.840, p<0.001). The ROC results are presented in Figure 1.
Discussion
PCSK9 and apelin levels were higher in FS patients than in controls. A positive association was detected between PCSK9 and apelin in this study. There was a correlation between PCSK9 and apelin and FIQ and SSS. In ROC analysis, we found PCSK9 and apelin to be predictive markers for FS with high sensitivity and specificity. For FS, PCSK9 had higher sensitivity and specificity than apelin.
The relationship between FS and oxidative stress is well known. Tender points in the muscles may be affected by local hypoxia,[25] and abnormal oxygen pressure on the muscle surface above the trigger points may play a role in the etiology of FS.[26] Free radicals can cause changes in nociception.[27] Superoxide radicals may play a significant role in developing pain by increasing peripheral and central nervous system sensitivity.[28] Additionally, nitric oxide can trigger pain in patients with FS.[27] Previous studies reported that total oxidant capacity, oxidative stress index, oxidized LDL (ox-LDL), and proinflammatory cytokine levels were higher in patients with FS than in controls.[29,30] LDL levels may also be higher in patients with FS,[24] and a positive relationship was reported between SSS and ox-LDL.[29] An increase in malondialdehyde, one of the final products of lipid peroxidation, and a decrease in antioxidant enzyme levels, such as superoxide dismutase, catalase, and glutathione peroxidase, have been detected in patients with FS.[31,32]
PCSK9 is a peptide released from many tissues, and its primary task is the degradation of LDLR. The degradation of LDLR leads to increased lipid peroxidation by raising serum LDL levels. Moreover, it contributes to arterial plaque formation, inflammation, and oxidative stress development independently of LDL. PCSK9 increases oxidative stress and superoxide radicals by activating ox-LDL/LOX-1 (lectinlike ox-LDL receptor 1), NADPH (nicotinamide adenine dinucleotide phosphate hydrogen), and nuclear factor-kappa B.[13,33] While ox-LDL increases PCSK9 expression, PCSK9 can also stimulate Nox2 (NADPH oxidase 2)-mediated ox-LDL formation.[33] PCSK9 inhibitors have been reported to reduce oxidative stress by preventing lipid peroxidation.[34] In our study, PCSK9 levels were higher in patients with FS than in controls. Elevated ox-LDL and peroxide levels have been reported in patients with major depression,[35] and PCSK9 has been linked to LDL and depression.[36,37] Furthermore, PCSK9 may play a role in the etiology of the disease by increasing oxidative stress in patients with FS.
Ozgoçmen and Ardicoglu[31] reported high LDL levels in patients with FS. Additionally, Cordero et al.[38] found that approximately 58% of patients with FS had elevated LDL. While the LDL levels of our patients were slightly higher than those of the control group, the difference was not statistically significant. Although it is known that PCSK9 level increases LDL, some studies did not find a relationship between PCSK9 and LDL.[39,40] Since PCSK9 causes oxidative stress independent of the LDL level, it may also play a role in the etiology of the disease independent of the LDL level in patients with FS.
Apelin is formed by the cleavage of the precursor peptide released as prepro-apelin with 77 amino acids. As the number of amino acids decreases, the biological effect of apelin increases. The most effective form of apelin is apelin-13.[41] PCSK3 cleaves pro-apelin and converts it to apelin-13.[42] On the other hand, PCSK1 and PCSK7 cannot degrade pro-apelin to apelin-13.[42] The effect of PCSK9 on the formation of apelin is not clear. However, few studies have reported an indirect relationship between PCSK9 and apelin in the literature.[42,43] Apelin has been shown to reduce inflammation in LDLR knockout rats.[43] Plasma apelin levels were low in individuals with high LDL cholesterol.[44] It has been shown that apelin reduces LDL levels by inhibiting lipid synthesis.[45] A high-fat diet or insulin resistance can cause a compensable increase in apelin gene expression.[45] In our patients, the increase in apelin might have prevented an increase in LDL levels.
Apelin, released by various tissues, has a multitude of functions and is known for its free radical scavenging effect. Apelin suppresses the formation of superoxide and hydrogen peroxide radicals by upregulating antioxidant enzymes such as superoxide dismutase, catalase, and glutathione peroxidase.[46] It also reduces the creation of peroxynitrite radicals by suppressing the expression of inducible nitric oxide synthase.[47] While apelin was found to be low in the presence of inflammation, one study reported the opposite.[47] Apelin has a reducing effect on the formation of reactive oxygen species. Increased reactive oxygen species and inflammation may elevate apelin levels.[46] Apelin could be considered a marker of chronic oxidative stress.[48] Our study suggests that there may be a relationship between PCSK9 and apelin. PCSK9 may have increased the level of apelin-13 by playing a role in apelin cleavage. Additionally, PCSK9 may be related to increased oxidative stress and LDL, and the level of apelin may be elevated as a compensatory rebound.[45]
Similar to apelin's effects on inflammation, its effects on pain and depression are also controversial. Intraperitoneal injections of apelin have been reported to reduce chronic and acute pain.[49] APJ can cause an analgesic effect by forming a heterodimer with the kappa opioid receptor.[50] Apelin also reduces depression by increasing brain-derived neurotrophic factors and possibly by correcting hippocampal glucocorticoid receptor dysfunction and the hypothamalic-pituitary-adrenal axis.[51] However, apelin may cause hyperalgesia through gamma-aminobutyric acid receptor type A.[50] It may also increase pain sensitivity via intracellular calcium signaling pathways.[52] Likewise, the apelin level was high in patients with depression and anxiety.[53] The results of our study suggest that PCSK9 and apelin have a potent effect on FIQ and SSS and that PCSK9 and apelin may play a role in the etiology of FS. In addition to the impacts of PCSK9 through oxidative stress, increased apelin due to a response to increased PCSK9 may cause hyperalgesia and depression.
The relatively small size of the study population may be considered the primary limitation of this study. Additionally, this study did not evaluate oxidative stress markers and antioxidant markers. The current study is the first to examine PCSK9 and apelin levels in patients with FS. While this study offers valuable insights, we believe that randomized controlled studies with a larger cohort of patients are necessary to validate our findings.
In conclusion, serum PCSK9 and apelin levels were found to be higher in patients with FS compared to the controls. The elevation of PCSK9 may contribute to the etiology of FS by promoting increased oxidative stress. Apelin levels may increase in response to oxidative stress and elevated LDL levels as a compensatory mechanism. Elevated apelin levels may lead to hyperalgesia. Both PCSK9 and apelin have the potential to serve as predictive markers for the presence of FS.
Footnotes
Ethics Committee Approval: The study protocol was approved by the Firat University Non-interventional Research Ethics Committee (date: 28.04.2022, no: 2022/175-260). The study was conducted in accordance with the principles of the Declaration of Helsinki.
Informed Consent: A written informed consent was obtained from each patient.
Author Contributions: Idea/concept: N.P.T., R.A.B.; Design: N.P.T., T.T.Y.; Control/supervision: M.C., A.K., M.E.; Data collection and/or processing: N.P.T., A.G., R.A.B.; Analysis and/or interpretation: E.C., M.C., M.E.; Literature review: N.P.T., R.A.B., A.K., A.G., E.C., M.C., M.E., T.T.Y.; Writing the article: N.P.T., R.A.B.; Critical review: A.K., E.C., M.C.; References and fundings: N.P.T., R.A.B., A.G., A.K., E.C., M.C., M.E., T.T.Y.; Materials: N.P.T., R.A.B., A.G.
Conflict of Interest: The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Financial Disclosure: The authors received no financial support for the research and/or authorship of this article.
Citation: Pihtili Taş N, Aydogan Baykara R, Kamanli A, Gürbüz A, Cure E, Cumhur Cüre M, et al. Proprotein convertase subtilisin/kexin type 9 and apelin in fibromyalgia syndrome. Arch Rheumatol 2024;39(3):375-383. 10.46497/ArchRheumatol.2024.10462.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Articles from Archives of Rheumatology are provided here courtesy of Turkish League Against Rheumatism
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Proprotein convertase subtilisin/kexin type 9 is associated with atherosclerosis in patients with Behcet's disease.
Clin Exp Hypertens, 44(5):480-486, 03 May 2022
Cited by: 1 article | PMID: 35502687
Proprotein Convertase Subtilisin/Kexin Type 9 Loss-of-Function Is Detrimental to the Juvenile Host With Septic Shock.
Crit Care Med, 48(10):1513-1520, 01 Oct 2020
Cited by: 11 articles | PMID: 32769621 | PMCID: PMC8477606
Circulating Furin-Cleaved Proprotein Convertase Subtilisin/Kexin Type 9 Concentration Predicts Future Coronary Events in Japanese Subjects.
JACC Asia, 1(3):360-368, 23 Nov 2021
Cited by: 5 articles | PMID: 36341208 | PMCID: PMC9627806

 1
1