Abstract
Background
Acute kidney injury is an acute and reversible increment in serum creatinine (SCr) levels with a reduction in urine output oliguria, or anuria. Acute kidney injury is a major cause of neonatal morbidity and mortality worldwide; it is a serious problem in low and middle-income countries, particularly in sub-Saharan Africa such as Ethiopia. Moreover, there are few studies in developing countries. This study aimed to investigate the incidence and predictors of acute kidney injury in neonates admitted to the neonatal intensive care unit of some specialized hospitals in the Amhara region of northwestern Ethiopia.Methods
A facility-based retrospective follow-up study was conducted in the Northwest Amhara Region's comprehensive specialized hospitals with 634 neonates from January 2020 to December 2022. Data were collected by reviewing patient charts using simple random sampling with a pretested checklist, entered using Epi-data 4.6, and analyzed using STATA 14. Median survival time, Kaplan-Meier survival curve, and log-rank test were calculated. Bivariable and multivariable Cox hazard models were used to determine the determinants of acute kidney injury. A hazard ratio with a 95% confidence interval was calculated. Variables with p-values less than 0.05 were considered statistically significant.Results
The incidence of Acute Kidney Injury among Neonates admitted to Neonatal Intensive Care Unit was 14.9 per 1000 (95% CI: 12.5-17.7) with the proportion of acute kidney injury (20.19%) (95% CI: 17.23-23.50) neonates with sepsis (AHR: 2.59; 95%CI: 1.21-5.56), neonates with perinatal asphyxia [(AHR: 2.70; 95%CI: 1.29-5.65) were taking gentamicin drugs [(AHR = 1.74; 95%CI: 1.03-2.94], neonates were preterm [(AHR; 1.77: 95%CI: 1.05 -2.98], and neonatal-hyponatremia [(AHR: 2.14; 95%CI: (1.00 -4.9)] and hyperkalemia [(AHR: 2.64; 95 CI: (1.11- 6.2)] were statistically found to be significant predictors of acute kidney injury.Conclusions
The incidence of acute kidney injury in neonates was high. Premature infants, neonates with sepsis, who suffered perinatal asphyxia, who received gentamicin drugs, whose sodium levels decreased and potassium levels increased were at higher risk of developing acute kidney injury. All concerned agencies should work to prevent acute kidney injury and pay special attention to multifactorial causes. Therefore, strategies need to be developed and/or strengthened to prevent the occurrence of acute kidney injury in infants with sepsis, neonates who suffered perinatal asphyxia, and preterm infants whose sodium levels decreased and potassium levels increased.Free full text

Incidence of acute kidney injury and its predictors among neonates admitted at neonatal intensive care unit of, Northwest Ethiopia comprehensive specialized hospitals, 2023
Abstract
Background
Acute kidney injury is an acute and reversible increment in serum creatinine (SCr) levels with a reduction in urine output oliguria, or anuria. Acute kidney injury is a major cause of neonatal morbidity and mortality worldwide; it is a serious problem in low and middle-income countries, particularly in sub-Saharan Africa such as Ethiopia. Moreover, there are few studies in developing countries. This study aimed to investigate the incidence and predictors of acute kidney injury in neonates admitted to the neonatal intensive care unit of some specialized hospitals in the Amhara region of northwestern Ethiopia.
Methods
A facility-based retrospective follow-up study was conducted in the Northwest Amhara Region’s comprehensive specialized hospitals with 634 neonates from January 2020 to December 2022. Data were collected by reviewing patient charts using simple random sampling with a pretested checklist, entered using Epi-data 4.6, and analyzed using STATA 14. Median survival time, Kaplan–Meier survival curve, and log-rank test were calculated. Bivariable and multivariable Cox hazard models were used to determine the determinants of acute kidney injury. A hazard ratio with a 95% confidence interval was calculated. Variables with p-values less than 0.05 were considered statistically significant.
Results
The incidence of Acute Kidney Injury among Neonates admitted to Neonatal Intensive Care Unit was 14.9 per 1000 (95% CI: 12.5–17.7) with the proportion of acute kidney injury (20.19%) (95% CI: 17.23–23.50) neonates with sepsis (AHR: 2.59; 95%CI: 1.21–5.56), neonates with perinatal asphyxia [(AHR: 2.70; 95%CI: 1.29–5.65) were taking gentamicin drugs [(AHR =
= 1.74; 95%CI: 1.03–2.94], neonates were preterm [(AHR; 1.77: 95%CI: 1.05 -2.98], and neonatal-hyponatremia [(AHR: 2.14; 95%CI: (1.00 -4.9)] and hyperkalemia [(AHR: 2.64; 95 CI: (1.11- 6.2)] were statistically found to be significant predictors of acute kidney injury.
1.74; 95%CI: 1.03–2.94], neonates were preterm [(AHR; 1.77: 95%CI: 1.05 -2.98], and neonatal-hyponatremia [(AHR: 2.14; 95%CI: (1.00 -4.9)] and hyperkalemia [(AHR: 2.64; 95 CI: (1.11- 6.2)] were statistically found to be significant predictors of acute kidney injury.
Conclusions
The incidence of acute kidney injury in neonates was high. Premature infants, neonates with sepsis, who suffered perinatal asphyxia, who received gentamicin drugs, whose sodium levels decreased and potassium levels increased were at higher risk of developing acute kidney injury. All concerned agencies should work to prevent acute kidney injury and pay special attention to multifactorial causes. Therefore, strategies need to be developed and/or strengthened to prevent the occurrence of acute kidney injury in infants with sepsis, neonates who suffered perinatal asphyxia, and preterm infants whose sodium levels decreased and potassium levels increased.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12887-024-05147-6.
Introduction
Acute kidney injury (AKI), is an acute and reversible increment in serum creatinine (SCr) levels with a reduction in urine output (UO) oliguria, or anuria [1], AKI imposes a high risk of morbidity and mortality in critically ill neonates [2], with a major economic effect on the health-care expenditure of countries, particularly in low-income and middle-income countries (LMIC) [3, 4].
Perico and Remuzzi [3] Globally, the incidence of AKI among neonates has fluctuated from 8.4% to 63.3% [5], similarly a multi-national multicenter (24 centers) study showed that 30% of neonates have developed AKI in Neonatal Intensive Care Unit (NICU).
The fact that AKI survivors have an increased risk of developing chronic kidney disease(CKD)or end-stage renal disease (ESRD), the poor prognosis of many patients after an episode of AKI, the public health ramifications of progression to late-stage chronic kidney disease (CKD), and the need to identify clinical risk factors for progression in patients with and without pre-existing chronic kidney disease (CKD) who have survived an episode of AKI are urgently needed [6].
As different studies indicate, the magnitude of AKI among neonates remains high, presenting a significant public health problem, For instance, studies in Sudan 54.1% [7], in Kenya 36.1% [8], in Tanzania 31.5% [9] in Egypt 10.8% [10] in Saudi Arabia 56% [11] in Taiwan 56% [12] Serbia 44% [13], in turkey 20% [14] of neonates developed AKI. The magnitude of AKI in Ethiopia is 18.27%, and it is a major public health concern [15].
These studies documented that different factors contribute to the development of AKI in neonates, such as perinatal asphyxia, respiratory distress syndrome (RDS), prematurity, sepsis, umbilical catheterization, early drug administration, especially aminoglycosides, necrotizing enterocolitis (NEC), hypovolemia, placental insufficiency, intrauterine growth restriction (IUGR), maternal medication, hemodynamically significant patent ductus arteriosus, and urogenital abnormalities [11, 16, 17]. However the sodium and potassium derangement also another factor of acute kidney injury.
To alleviate these problems different strategies and interventions were designed and implemented like the Kidney Disease Improving Global Outcome (KDIGO) guideline, and the Ethiopia Federal Ministry of Health has given due attention to the expansion of high-impact quality neonatal care to reduce neonatal morbidity like AKI [18].
Implementation of this program in low- and middle-income countries faces serious obstacles due to lack of resources, lack of information on the epidemiology, cause-and-effect relationships, and determinants of AKI in our countries, especially in neonatal intensive care unit guidelines, lack of resources for diagnosis and treatment of AKI, and lack of knowledge on the impact of AKI and treatment outcomes. a result, the increase in regular health care costs for newborns in the first month of life remains a major community health problem in a low-income country such as Ethiopia. These high medical costs can burden parents, their families, and the community at large [19, 20]. Esethere is limited evidence regarding AKI and its associated factors. Therefore, this study aims to assess the incidence and predictors of acute kidney injury among newborns admitted to Northwest Ethiopia comprehensive specialized hospitals, 2023.
Methods
Study design, period, and area
An institution-based retrospective follow-up study design was conducted among a cohort of neonates in the previous two consecutive years, from January 1, 2020, to December 30, 2022.
The study was conducted in the Northwest Amhara region's comprehensive specialized hospitals. In the Northwest Amhara region, there are five comprehensive specialized hospitals (CSH), and three comprehensive specialized hospitals were selected. Those are the University of Gondar CSH, Tibebe Ghion CSH, and Debre Tabor CSH. Each hospital is far from Addis Ababa UoGCSH 727 KM, Tibebe Ghion CSH 552 km, and Debre Tabor CSH 645KM. Averagely admission to the NICU 4560, 1920, and1560 in UoGCSH, Tibebe Ghion CSH, and Debre Tabor CSH respectively. These hospitals are the final referral choice for other health institutions. Those hospitals have NICUs staffed with a mix of health professionals, including neonatal and comprehensive nurses, general practitioners, pediatricians, and other support staff. The major services provided in the NICU include general neonatal care, blood and exchange transfusions, phototherapy, and ventilation support, such as continuous positive airway pressure.
Source and study population
Source populations and study populations
The source population is all neonates admitted to the NICUs of the three Amhara Region hospitals, Comprehensive Specialized Hospitals Northwest Ethiopia. From January 1, 2020, to December 30, 2022, all neonates were admitted to the three selected comprehensive specialized hospitals in Amhara Region, Northwest Ethiopia.
Eligibility criteria
Inclusion criteria
All neonates were admitted to the neonatal intensive care units of the Northwest Amhara Region Comprehensive Specialized Hospitals from October 1, 2020, to September 30, 2022.
Exclusion criteria
All neonates’ medical cards documented in the previous two years from the study period were recruited and incomplete cards were excluded.
Sample size determination and sampling procedures
Sample size determination
The sample size was calculated using the single population proportion formula, considering the following factors: a 95% confidence interval (CI), a 50% proportion of neonates with acute kidney injury, and a 5% margin of error. The required sample size was determined using the following formula.
P=proportion kidney injury=50%
d = margin of error 5%
Z α/2= the corresponding Z score of 95% CI=1.96
N = Sample size
Consider design effect 1.5
By adding 10% chart attrition, a total of 634 charts were included in the study.
Sampling technique and procedures
There are three comprehensive specialized hospitals in the Amhara region. Based on the data, UoGCSH has admitted 9120 newborns over 2 years, TGCSH has admitted 3,840 newborns over 2 years, and DTCSH has admitted 3,120 newborns over 2 years, in total 16,080 newborns were admitted to these hospitals from January 1, 2020, to December 30, 2022.
A sample of 634 charts of neonates was selected from 16,080 neonate charts admitted to Northwest Amhara neonatal intensive care units. The chart numbers were extracted from the registration file using a computer-generated random number technique. The estimated number of neonates admitted to these hospitals over the previous two years was as follows: UoGCSH had 9,120 admission, TGCSH had 3,840 admissions, and DTCSH had 3,120 admissions, all between January 1, 2020, and December 30, 2022.
Since the final sample size was 634, proportional allocation was performed for each hospital. The sampling frame was prepared by collecting the identification numbers of neonatal charts from the registration book. After identifying the patients who met the inclusion criteria, study subjects were selected using a simple random sampling technique with computer-generated random numbers.
Study variables
Dependent variable
Incidence of Neonatal Acute Kidney Injury.
Independent variables
Socio-demographic maternal and neonatal
Age, sex, gestational age, Age of the mother, birth weight, and weight for Gestation.
Maternally related factors
Placental hemorrhage, prenatal steroids, Prolonged rupture of membranes (PROM), antenatal care (ANC), Placental hemorrhage, Polyhydramnios, oligohydramnios, Place of delivery, preeclampsia, gestational hypertension, Diabetes mellitus (DM), Prolonged labor, and Parity),
Clinical factors
Neonatal Sepsis, necrotizing enterocolitis, meconium aspiration syndrome (MAS), Shock, respiratory distress syndrome (RDS), intraventricular hemorrhage (IVH), Low APGAR, hypoxic-ischemic encephalopathy (HIE) Congenital heart disease (CHD), and Persistent pulmonary hypertension (PPH)Sodium level, potassium level.
Operational definition
Acute kidney injury: is defined as a serum creatinine level of 1.5 mg/dl or an increase in serum creatinine of 0.2 to 0.3 mg/dl within 48 h [21].
Censored: newborn who did not develop the outcome of interest (AKI) until the end of the follow-up period or lost to follow-up, recovered from illness, discharged against medical advice, or transferred out to other health institutions without knowing the outcome.
Necrotizing Enterocolitis (NEC): in this study, a medical diagnosis of the neonate’ is stated as stage II or stage III NEC by the physician in the neonate’s medical record chart, or NEC diagnosed with confirmed evidence through abdominal X-ray findings that suggest necrotizing enterocolitis.
Meconium aspiration syndrome (MAS): is a clinical condition characterized by respiratory failure occurring in neonates born through meconium-stained amniotic fluid whose symptoms cannot be otherwise explained and have typical radiological characteristics.
Neonatal sepsis: neonates who are diagnosed with sepsis by their attending physician and fulfill sepsis criteria by clinical or laboratory investigation within 0–28 days of life.
HIE (Hypoxic ischemic encephalopathy): it was determined based on Saranat classifications of the clinical diagnosis made by a health care provider (stage I, stage II, and stage III) [22].
Birth asphyxia: Diagnosed based on the World Health Organization's (WHO) definition of "the inability to initiate and maintain breathing at birth and the 5-min APGAR score of 7" [23].
Neonatal Respiratory Distress Syndrome: is diagnosed based on the presence of one or more of the following signs: abnormal respiratory rate, expiratory grunting, nasal flaring, chest wall recessions, and thoracoabdominal asynchrony with or without cyanosis [24].
Incomplete charts: A card that did not fulfill neonatal age, medical diagnosis of the neonate, and absence of creatinine level.
Outcome: newborn who developed the outcome during the study period.
Data collection tools
A pretested checklist was used to collect the required data from patients' medical records, and consistency was verified. Data were gathered by reviewing the complete patient charts for the last two consecutive years of the study period. AKI was confirmed through a thorough review of the medical records.
Data quality control
Data quality was ensured through development of appropriate data abstraction tools. The data collection instrument was pretested on 5% of the sample size. To maintain the validity of the tool, its content was reviewed by senior pediatric and child health specialist nurses as well as medical nurses. Both data collectors and supervisors were neonatal nurses with training at the in BSc and MSc levels respectively, in the data collection checklist and process. During data collection, strict monitoring and supervision were conducted by the supervisors and the investigator. Data entry was performed using Epi Data 4.6 software.
Data processing, analysis, and presentation
After data collection, the data were cleaned, edited, and coded. Errors identified at this stage were corrected after reviewing of the original data using the code numbers. Data were entered using Epi-Data version 4.6 and analyzed using STATA 14 statistical software. Descriptive statistics were calculated using mean, frequency, percentage, tables, and accounts. Additionally, a log-rank test was employed to compare AKI survival among different categorical predictor variables. The variance inflation factor (VIF =
= 1.83) was used to test for multicollinearity, demonstrating the association between predictor variables. Follow up data were reported per 100person—days. Kaplan–Meier was used to estimate mean survival.
1.83) was used to test for multicollinearity, demonstrating the association between predictor variables. Follow up data were reported per 100person—days. Kaplan–Meier was used to estimate mean survival.
Based on the Cox proportional hazards assumption test using Schoenfeld residuals and a log–log plot, all covariates met the assumption, and the overall model also satisfied the proportional hazards assumption (global test, p =
= 0.6638). The Cox-Snell residual test was employed to assess goodness of fit. Variables with a P value below 0.25 were considered candidates. The crude hazard ratio and the 95% confidence interval (CI) for the adjusted hazard ratio (AHR) were estimated to determine the strength of the association. Statistical significance between predictors and AKI was determined with a p- value of 0.05.
0.6638). The Cox-Snell residual test was employed to assess goodness of fit. Variables with a P value below 0.25 were considered candidates. The crude hazard ratio and the 95% confidence interval (CI) for the adjusted hazard ratio (AHR) were estimated to determine the strength of the association. Statistical significance between predictors and AKI was determined with a p- value of 0.05.
Result
A total of 634 neonates who were admitted from January 2020 to December 2022 in selected Amhara comprehensive specialized hospitals were included.
Socio-demographic characteristics of mothers and neonates
In the neonatal socio-demographics, the majority of patients at admission 575 (90.69%) were between the ages of the first seven days. Out of 634 neonates, 392 (61.83%) were male neonates. The majority of the neonates, 567 (89.43%), were born in health institutions with skilled health professionals. The mean maternal age of the neonates was 29.15 +
+ 6.457 SD years, with 425 (67.03) being between the ages of 20 and 34. More than half of the mothers were urban residents (53%) (Table 1).
6.457 SD years, with 425 (67.03) being between the ages of 20 and 34. More than half of the mothers were urban residents (53%) (Table 1).
Table 1
Socio-demographic characteristics of mothers and neonates at NICU in Amhara region, Ethiopia, 2023(n =
= 634)
634)
| Variables | Frequency(n) | Percentage (%) |
|---|---|---|
| Age_ group of the mother | ||
 < < 20 20 | 65 | 10.25 |
 20–34 20–34 | 425 | 67.03 |
 ≥ ≥ 35 35 | 144 | 22.72 |
| Residency | ||
 Urban Urban | 336 | 53 |
 Rural Rural | 298 | 47 |
| Age at the admission of the newborn | ||
 0 0 < < 7 7 | 575 | 90.69 |
 8–15 8–15 | 34 | 5.36 |
 15–28 15–28 | 25 | 3.94 |
| Sex of the newborn | ||
 Male Male | 392 | 61.83 |
 Female Female | 242 | 38.17 |
| Place of delivery | ||
 Health institution Health institution | 567 | 89.43 |
 Home delivery Home delivery | 67 | 10.57 |
Obstetrical history of mother’s, and Obstetric complications of the mother
A total of 599 mothers (94.48%) received antenatal care (ANC) follow-up. Of these, 414 (65.30%) had four more ANC visits, while the remaining mothers had fewer than the minimum required four visits. The majority of mothers in the sample were multipara totaling 450 (70.95%). Among the 634 mothers, 530 (92.01%) were singletons, which accounted for more than half of the 422 mothers (66.56%) mothers who gave birth spontaneously vaginally. In terms of maternal complications, more than one-third of mothers experienced obstetric complications during the index pregnancy. The maternal obstetric complications in the current pregnancy include premature rupture of membranes (PROM) at 223 cases (35.17%), eclampsia at 28 cases (4.42%), placental hemorrhage at 49 cases (7.73%), gestational diabetes at 117 cases (18.45%), and polyhydramnios at 80 cases (12.62%) (Table 2).
Table 2
Obstetric history and obstetric complication of mothers whose neonates were admitted to NICU in Amhara region, Ethiopia, 2023 (n =
= 634)
634)
| Variables | Frequency(n) | Percentage (%) |
|---|---|---|
| ANC visit | ||
 Yes Yes | 599 | 94.48 |
 No No | 35 | 5.52 |
Number of ANC visit (n = = 506) 506) | ||
 1–3 1–3 | 185 | 29.18 |
 ≥ ≥ 4 4 | 414 | 65.30 |
| Parity | ||
 Primi para Primi para | 184 | 29.02 |
 Multi-para Multi-para | 450 | 70.98 |
| Mode of delivery | ||
 SVD SVD | 422 | 66.56 |
 C/S C/S | 86 | 13.56 |
 ISD ISD | 126 | 19.87 |
| Current pregnancy | ||
 Multiple Multiple | 46 | 7.99 |
 Single Single | 530 | 92.01 |
| PIH | ||
 Yes Yes | 121 | 19.09 |
 No No | 513 | 80.91 |
| Pre-eclampsia | ||
 Yes Yes | 91 | 14.35 |
 No No | 543 | 85.65 |
| Eclampsia | ||
 Yes Yes | 28 | 4.42 |
 No No | 606 | 95.58 |
| GDM | ||
 Yes Yes | 117 | 18.45 |
 No No | 517 | 81.55 |
| APH | ||
 Yes Yes | 49 | 7.73 |
 NO NO | 585 | 92.27 |
| Polyhydramnios | ||
 Yes Yes | 80 | 12.62 |
 NO NO | 554 | 87.38 |
| Oligohydramnios | ||
 Yes Yes | 87 | 13.72 |
 NO NO | 447 | 86.28 |
| PROM | ||
 Yes Yes | 223 | 35.17 |
 NO NO | 411 | 64.83 |
| Prenatal steroid | ||
 Yes Yes | 85 | 13.41 |
 NO NO | 549 | 86.59 |
| Prenatal antibiotics | ||
 Yes Yes | 121 | 19.09 |
 NO NO | 513 | 80.91 |
aPIH pregnancy induced hypertension APH ante partum hemorrhage PROM prolonged rupture of membrane ANC =
= Antenatal Care SVD
Antenatal Care SVD =
= spontaneous Vaginal Delivery
spontaneous Vaginal Delivery
Neonatal-related factors and neonatal clinical comorbidities
More than half of the 634 neonatal records reviewed showed that the 373 neonates (58.83%) had birth weights greater than 2500 g. The mean birth weight was 2813 g (SD ±
± 720) grams. In terms of gestational age, more half of the babies (324, 51.10%) were born after 37 completed weeks of gestation, and over three-fourths of the neonates (502, 79.18%) had an appropriate weight for gestational age. Regarding co-morbidities, more than three-fourths of the neonates admitted to the NICU had neonatal sepsis (259, 40.85%), while 170 (26.81%) had meningitis, and another 170 (26.81%) had perinatal asphyxia, according to a review of 634 charts (Table 3).
720) grams. In terms of gestational age, more half of the babies (324, 51.10%) were born after 37 completed weeks of gestation, and over three-fourths of the neonates (502, 79.18%) had an appropriate weight for gestational age. Regarding co-morbidities, more than three-fourths of the neonates admitted to the NICU had neonatal sepsis (259, 40.85%), while 170 (26.81%) had meningitis, and another 170 (26.81%) had perinatal asphyxia, according to a review of 634 charts (Table 3).
Table 3
Characteristics of neonates and clinical co-morbidities who were admitted at NICU in Northwest Amhara, Ethiopia, 2023 (n =
= 634)
634)
| Variable | Frequency | Percentage |
|---|---|---|
| Birth weight | ||
 Low birth weight Low birth weight | 235 | 37.07 |
 Normal birth weight Normal birth weight | 373 | 58.83 |
 Macrosomic Macrosomic | 26 | 4.10 |
| Gestational age | ||
 Preterm Preterm | 275 | 43.38 |
 Term Term | 324 | 51.10 |
 Post term Post term | 35 | 5.52 |
| Weight for gestation | ||
 AGA AGA | 502 | 79.18 |
 SGA SGA | 90 | 14.20 |
 LGA LGA | 42 | 6.62 |
| APGAR score first minute | ||
 Low Low | 109 | 17.19 |
 Mild Mild | 72 | 11.36 |
 Normal Normal | 453 | 71.45 |
| APGAR score five minute | ||
 Sever Sever | 92 | 14.51 |
 Mild Mild | 89 | 14.04 |
 Normal Normal | 453 | 71.45 |
| Sepsis | ||
 Yes Yes | 259 | 40.85 |
 No No | 375 | 59.15 |
| NEC | ||
 Yes Yes | 120 | 18.93 |
 No No | 514 | 81.07 |
| Shock | ||
 Yes Yes | 99 | 15.62 |
 No No | 535 | 84.38 |
| Chromosomal abnormality | ||
 Yes Yes | 49 | 7.73 |
 No No | 585 | 92.27 |
| Pneumonia | ||
 Yes Yes | 44 | 6.94 |
 No No | 590 | 93.06 |
| Surgical disorder | ||
 Yes Yes | 39 | 6.15 |
 No No | 595 | 93.85 |
| MAS | ||
 Yes Yes | 145 | 22.87 |
 No No | 489 | 77.13 |
| CHD | ||
 Yes Yes | 44 | 6.94 |
 No No | 590 | 93.06 |
| RDS | ||
 Yes Yes | 148 | 23.34 |
 No No | 486 | 76.66 |
| IVH | ||
 Yes Yes | 40 | 6.31 |
 No No | 594 | 93.69 |
| PNA | ||
 Yes Yes | 189 | 29.81 |
 No No | 445 | 70.19 |
| HIE | ||
 Stage1 Stage1 | 8 | 1.26 |
 Stage 2 Stage 2 | 66 | 10.41 |
 Stage 3 Stage 3 | 74 | 11.67 |
| Meningitis | ||
 Yes Yes | 170 | 26.81 |
 No No | 464 | 73.19 |
| Sodium | ||
 Hyponatremia Hyponatremia | 180 | 28.39 |
 Normal sodium level Normal sodium level | 331 | 52.21 |
 Hypernatremia Hypernatremia | 123 | 19.40 |
| Potassium | ||
 Hypokalemia Hypokalemia | 71 | 11.20 |
 Normo- potassium level Normo- potassium level | 374 | 58.99 |
 Hyperkalemia Hyperkalemia | 189 | 29.81 |
| Gentamicin | ||
 Yes Yes | 206 | 32.49 |
 No No | 428 | 67.51 |
| Gentamicin | ||
 Yes Yes | 206 | 32.49 |
 No No | 428 | 67.51 |
aAGA appropriate for gestational age, SGA Small for gestational age, LGA large for gestational age, APGAR activity pulse grimace appearance and respiration, HIE hypoxic ischemic encephalopathy, PNA perinatal asphyxia, IVH intraventricular hemorrhage, RDS Respiratory distress syndrome, MAS Meconium Aspiration syndrome, CHD congenital Heart disease, NEC necrotizing enterocolites
Overall neonatal out-com
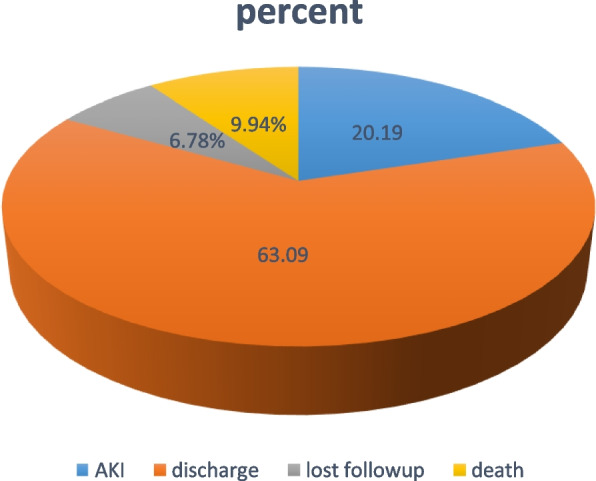
From the total follow-up outcome, 6.78% were lost to follow-up or left the hospitals without health professionals' permission or against medical advice (Fig. 1).
Proportional hazard assumption
Based on the proportional hazard assumption test using Schoenfeld residual, all of the covariates fulfilled the assumption and the overall global model satisfies the proportional hazard assumption (0.6638) Fig. 2.
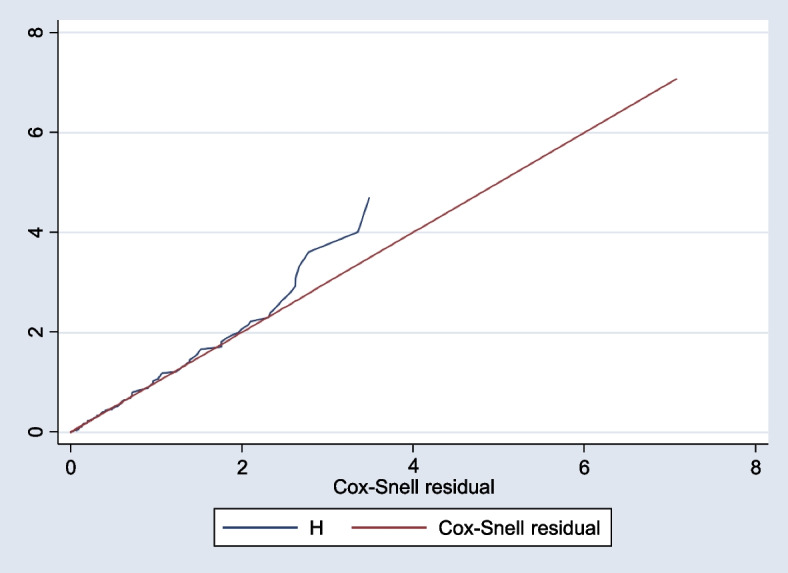
The Cox proportional hazard model, non-parametric Cox- Regression models using Snell residual graph, 2023. Semi-parametric Cox-proportional hazard (AIC =
= 1135.787) models
1135.787) models
Overall proportion and incidence rate of AKI in neonates
The overall proportion of neonates that develop the event of interest (AKI) among admitted 634 was found to be neonates, 128 (20.19%) (95% CI: 17.23–23.50). The overall incidence rate of AKI was found to be neonate-days observation for the entire follow-up time was 8573 person-days when 634 from the three hospitals with minimum and maximum follow-up times were 1 and 28 days respectively. The median follow-up survival time was 21 days (95% CI: 22–27). The overall incidence of AKI was 14.9 per 1000 neonate-day observations (95%CI: 12.5–17.7) during the entire follow-up time.
Overall failure Function (survivorship function)
The overall Kaplan–Meier failure function showed that the probability of acute kidney injury among neonates was increased during the follow-up period. During the 5–10 days of admission, a 7% probability of AKI was observed (Fig. 3).
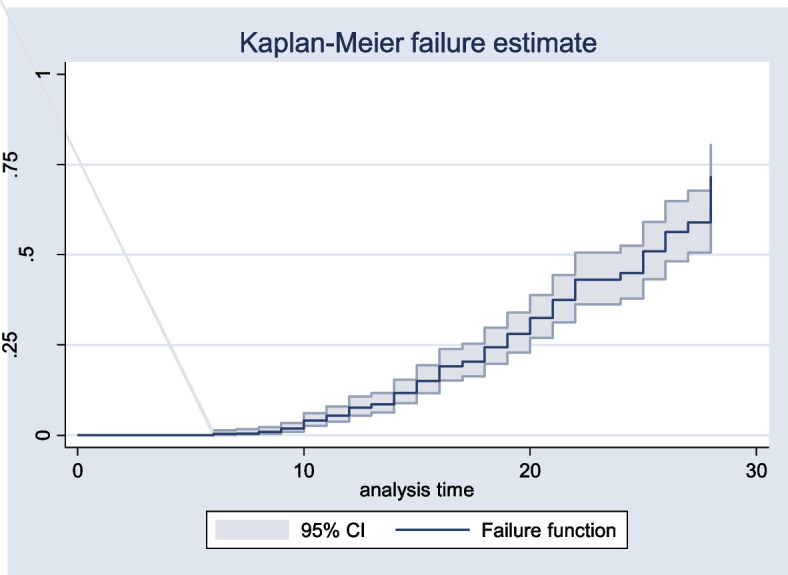
Kaplan Meier failure function of AKI among neonates from 2020 to 2022, Amhara régions Ethiopia, 2023 (N =
= 634)
634)
Predictors of AKI among neonates
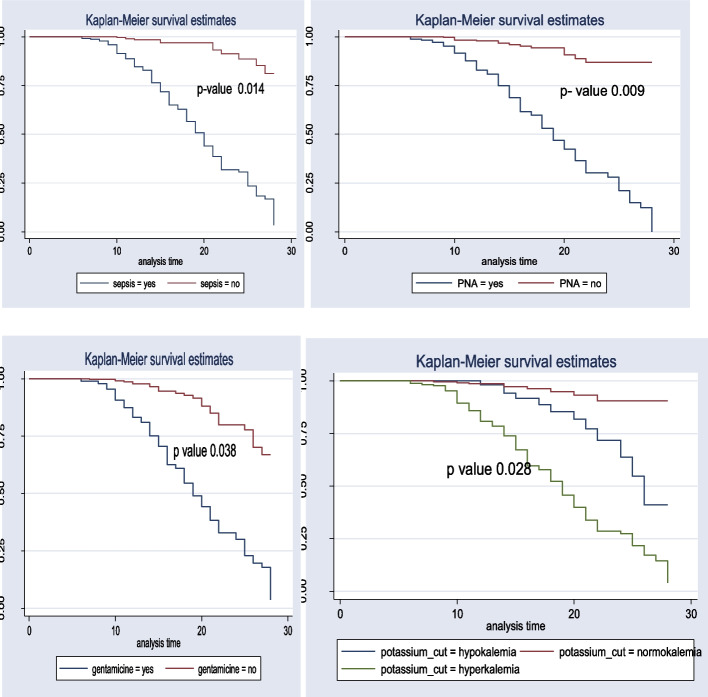
The test of equality for survival distribution for different categories of variables was performed with Kaplan–Meier and the log-rank test. In general, the pattern that one group's survivorship function lying above another group showed that the upper curve had a better probability of survival compared to the lower curve. On the other hand, the probability of AKI was high among the lower groups described by the Kaplan–Meier survival curve. Furthermore, the log-rank test confirmed whether the observed difference was seen on the KM graph statistical difference or not.
In the current study, newborns born with preterm have lower survival times compared with normal-term and, post-term neonates. Moreover, neonates without sepsis and perinatal asphyxia (PNA) have a more favorable survival probability than those neonates with sepsis and PNA respectively. This study also revealed that mothers who did not take prenatal steroids during antenatal care (ANC) follow-up periods had a better probability of survival compared with those mothers who took prenatal steroids. Furthermore, neonates born from mothers who had taken antenatal corticosteroids had a better probability of survival compared with newborns born from mothers who did not take antenatal corticosteroids during pregnancy while neonates born single had a better survival probability than those born multiple. These differences were statistically significant with a p-value <
< 0.001 in a log-rank test (Fig. 4).
0.001 in a log-rank test (Fig. 4).
Bivariable and multivariable Cox-regression analysis
In the bi-variable Cox regression analysis, place of delivery, neonatal age at admission, gestational DM, chronic hypertension, steroid drug used, polyhydramnios, PROM, prolonged labor, sepsis, types of pregnancy, birth weight, gestational age, NEC, PNA, meningitis, MAS, APGAR score first minute, APGAR score fifth minute, gentamicin, sodium level, and potassium level mode of delivery were significant predictors of AKI with P- value less 0.25. However, in the multivariable Cox regression analysis, sepsis, PNA, gentamicin, gestational age, potassium level, and sodium level were significant predictors of AKI among neonates admitted to NICU.
Keeping out other variables constant, the hazard of AKI among neonates born with sepsis was more than two times (AHR: 2.59; 95%CI: 1.21–5.56) more likely than neonates born without sepsis. Keeping The hazard of AKI among neonates born with perinatal asphyxia is almost three times more likely than neonates born without perinatal asphyxia (AHR: 2.70; 95%CI: 1.29–5.65). Neonates who received gentamicin drugs increased the hazard of developing AKI by 1.74 times (AHR =
= 1.74; 95%CI: 1.03–2.94) compared to newborns who did not receive gentamicin drugs. Moreover, being preterm was an independent predictor of AKI, which increased the hazard of AKI among new-borns two times (AHR; 1.77: 95%CI: 1.05 -2.98) than new-borns being term. The sodium level of the neonates develop Hyponatremia the hazard of AKI by almost two times than neonates have with normal sodium level (AHR: 2.14; 95%CI: (1.00 -4.9). by keeping other variables out constant, hyperkalemia was an independent predictor of AKI for neonates which increased the hazard of AKI almost by three times more than neonates with normal potassium levels (AHR: 2.64; 95 CI: (1.11- 6.2) (Table 4).
1.74; 95%CI: 1.03–2.94) compared to newborns who did not receive gentamicin drugs. Moreover, being preterm was an independent predictor of AKI, which increased the hazard of AKI among new-borns two times (AHR; 1.77: 95%CI: 1.05 -2.98) than new-borns being term. The sodium level of the neonates develop Hyponatremia the hazard of AKI by almost two times than neonates have with normal sodium level (AHR: 2.14; 95%CI: (1.00 -4.9). by keeping other variables out constant, hyperkalemia was an independent predictor of AKI for neonates which increased the hazard of AKI almost by three times more than neonates with normal potassium levels (AHR: 2.64; 95 CI: (1.11- 6.2) (Table 4).
Table 4
Bi-variable and multivariable Cox-regression analysis Cox regression analysis for predictors of acute kidney injury among neonates Northwest Ethiopia Comprehensive Specialized Hospitals from January 1, 2020, to December 30, 2022
| Variables | Category | Status | CHR(95%CI) | AHR (95%CI) | |
|---|---|---|---|---|---|
| Event | Censored | ||||
| Place of delivery | Homedelivy Institutional delivery | 24 104 | 43 463 | 0.65(0.4- 1.01) 1 | 1.25(0.71 2.21) 1 |
| GDM | Yes no | 43 85 | 96 410 | 0.67(0.46–0.47) 1 | 0.68(0.39 -1.18) |
| Multiple pregnancy | Yes no | 10 118 | 42 464 | 1.76(0.92–3.38) 1 | 1.2(0.65–2.63) 1 |
| Chronic HTN | Yes no | 5 123 | 14 492 | 0.48(0.19–1.18) 1 | 0.98(0.22 4.36) |
| polyhydramnios | Yes No | 12 166 | 68 438 | 2.22(1.22–4.02) 1 | 0.61(0.32–1.16) |
| PROM | Yes No | 39 89 | 184 322 | 1.26(0.86–1.83) 1 | 0.82(0.53–1.25) 1 |
| steroid | Yes No | 22 106 | 63 443 | 4.23(3–5.9) 1 | 0.60(0.33 1.10) |
| sepsis | yes No | 116 12 | 257 249 | 6.9(3.85–12.4)* 1 | 2.59(1.21–5.56)* 1 |
| NEC | Yes No | 22 106 | 91 415 | 0.52(0.33–0.84) 1 | 0.72(0.46 -1.15) 1 |
| MAS | Yes No | 26 102 | 122 384 | 0.53(0.34–0.82) 1 | 1.27(0.71–2.28) 1 |
| Meningitis | Yes No | 73 91 | 128 378 | 1.23(0.84–1.81) 1 | 1.54(0.89—2.66) 1 |
| PNA | Yes No | 104 24 | 84 422 | 7.28(4.6–11.36)* 1 | 2.70(1.29–5.65)* 1 |
| gentamicin | Yes No | 78 50 | 402 104 | 0.64(0.45–0.91)* 1 | 1.74(1.03–2.94)* 1 |
| Admission age |
8–15 15–28 | 112 7 9 | 437 12 57 | 1.82(0.67–4.91) 0.89(0.45–1.75) | 1.52(0.73 3.13) 1.16(0.50 2.67) |
| APGAR 5th | sever mild normal | 31 28 69 | 61 61 384 | 1.95(1.27–2.99) 1.97(1.27–3.07) 1 | 0.93(0.22 3.96) 0.87(0.25 3.03) 1 |
| APGAR 1st | sever mild normal | 36 23 69 | 73 49 384 | 1.98(1.32–2.98) 2.43(1.51–392) 1 | 1.01(0.24 4.28) 1.26(0.35 4.49) |
| Birth weight | NBW LBW Macrosomic | 93 30 5 | 324 169 13 | 0.82(0.54 1.26) 0.85(0.40 1.77) | 0.80(0.52 1.23) 0.85(0.40 1.78) |
| Gestational age | Term Preterm Post term | 67 61 | 260 246 | 1 1.77(1.05 2.97)* 0.83(0.36–2.95) | 1.77(1.05 2.98)* 1.06(0.36 3.04) |
| Sodium level | Normal Hyponatremia hypernatremia | 20 106 2 | 404 45 57 | 1 2.19(1.02 4.68)* 0.21(0.75 4.86) | 2.14(1.00 4.9)* 2.02 (0.79 5.14) |
| Potassium level | Normal Hypokalemia hyperkalemia | 10 2 116 | 358 39 109 | 1 1.19(0.694 5.26) 2.64(1.11 -6.28)* | 1.79(0.65 -4.93) 2.64(1.11 6.2)* |
aANC Antenatal Care, PROM Premature rupture of membrane, NEC Necrotizing enterocolitis, PNA Perinatal asphyxia, CS caesarean section, SVD Spontaneous vaginal delivery, NBW normal birth weight, LBW low birth weight, GDM gestational diabetic mellitus, HTN hypertension
Discussion
In a follow-up of 634 newborns among neonates, 128 (20.19%) (95% CI: 17.23–23.50) of them experienced the event of interest (AKI). Throughout the full follow-up period, the overall incidence of AKI was 14.9 per 1000 neonate-day observations (95%CI: 12.5–17.7).
Relatively, the proportion of acute kidney injury is comparable to or in line with studies conducted in the United States (21%) and Turkey (21%). However, it is lower than in multinational studies;30% [25] in Cairo, in Egypt 41% [26] in 56% in Saudi Arabia [11] This marked difference might be attributed to some factors,. The study was conducted in Saudi Arabia as a single-center, prospective design, utilizing urine output and creatinine measurements. Being single-center, it may reflect specific institutional protocols, expertise, and regional treatment variations. Prospective studies can be observational, monitoring participants without intervention, or interventional, involving specific treatments. In contrast, retrospective studies collect data from existing records or databases prior to the study's initiation. In addition the study in Egypt was conducted with a small sample size; this random variation can have a greater impact on the results which could be due to a difference. Multinational studies often involve participants from different countries or regions with diverse cultural, social, and genetic backgrounds, and heterogeneity can introduce variations in the study population, including differences in disease prevalence, lifestyle factors, healthcare systems, or genetic predispositions.
On the contrary, the proportion in this study was higher than the prospective study was Egypt 10.8% [27] in Turkey 8.4 [28] in India 12% [29] in Iran 1.54% [30] in USA 9% [31]. This variation might be due to differences in the source population, study design, and small sample size as well as study population as the study conducted in India followed preterm neonates, while this study population was all neonates. When compared to term infants, preterm neonates' kidneys are not fully mature and functional.
In this study, the hazards of developing acute kidney injury increased more than threefold in neonates admitted with sepsis compared with their control group; This finding was supported by a study conducted in Kenya and South Africa [8, 32] South Africa [33] Sri Lnka [34] Egypt [27]. The potential rationale Neonatal sepsis systemic inflammation can lead to impaired renal blood flow and oxygen delivery to the kidneys, causing renal ischemia and injury [29]. Neonatal sepsis can cause hypotension and hemodynamic instability, leading to reduced blood flow to the kidneys. Inadequate perfusion and oxygenation of the renal tissue can result in ischemic injury and contribute to the development of AKI [35, 36]. The gene expression profile of septic AKI showed increased upregulation of several genes related to inflammation, metabolism, and apoptosis [37]. therefore prevention of neonatal sepsis and/or early diagnosis and treatment of neonates suspected of sepsis can minimize the occurrence of acute kidney injury.
The risk of AKI was 2.70 higher in neonates born with perinatal asphyxia than in neonates born without perinatal asphyxia. This finding is supported by the study conducted in Congo [38] India [39] Kenyan [40] Sudan [41]. It might be due to Perinatal asphyxia leads to inadequate oxygenation, and perfusion can result in hypoxic-ischemic injury to the renal tissue, leading to AKI. There is a redistribution of blood flow to vital organs, such as the kidneys. This altered blood flow can cause renal hypo perfusion and ischemia, contributing to the development of AKI.
Jha and Tamrakar [42] in addition, reperfusion injury leads to the buildup of reactive oxygen species and the release of inflammatory mediators, both of which can cause further damage to the renal tissue and exacerbate AKI. The inflammatory response can lead to the release of inflammatory cytokines and mediators that can directly injure the kidneys and contribute to the development of AKI [43–45]. eventually, severe asphyxia in neonates results in widespread tubular injury and deformity due to sodium depletion or continued water reabsorption, leading to decreased glomerular filtration rate, high renin activity, and high renal vascular resistance in neonates with acute tubular necrosis after asphyxia, after developing tubular necrosis or cortical necrosis the neonates decrease glomerular filtration as well as develop acute kidney injury [26, 46, 47]. Asphyxiated neonates need strict follow-up to prevent secondary complications.
The neonates treated with gentamicin had 1.74 times the risk of developing compared with neonates not taking gentamicin medication. This study is supported by studies in the USA [9], Alabama [48] other study in, the USA [49, 50]. The possible reasons might be age-related immaturity, pharmacogenomics, severity of illness, the dosage of a drug, duration, and concomitant medication, all of which may be nephrotoxic, as well as the epidemiology impact of AKI. Whenever patients with AKI had an increased length of stay in the hospital, it was noted that the majority of nephrotoxic medications to which patients were exposed were antimicrobial agents [49, 50]. It is valuable if the neonates are provided with the gentamycin antibiotic under all important precautions, if needed.
In this study, being preterm neonates was an independent predictor of AKI that increased the hazard of AKI two times in neonates compared with term infants, This finding is confirmed by the studies conducted in Italy [51] France [52] University of Alabama [2], and University of Wisconsin-Madison [53]. may be due to the following Preterm infants are at high risk for acute kidney injury (AKI) due to vasomotor nephropathy; their glomerular filtration rate (GFR) is low and unstable; they have a lower adaptability to stressful situations, hemodynamic instability, and nephrotoxic drugs commonly used in neonatal intensive care units (NICU) [52, 54, 55]. Since preterm neonates’ organ function is inadequate because of immaturity, advance care and follow-up are important to decrease kidney injury.
Hyponatremia increases the risk of AKI almost twofold compared with neonates with normal sodium levels. This finding is supported by studies in Sudan [7], and India [56]. A possible reason might be that the capacity of sodium reabsorption is limited, and when the amount of sodium reaching the distal tubules increases significantly, reabsorption does not occur proportionally and the amount of sodium is excreted in the urine. Other Contributing Factors to Hyponatremia may lead to contraction of intravascular volume, further limiting renal function. Asphyxia-induced reduction in the number of functional nephrons leading to ARF elicits compensatory hypertrophy of the remaining nephrons, resulting in improved renal function in the first months of life and decreased creatinine clearance, renal tubular acidosis, or concentration defect. AKI was predicted by abnormal renal electrolyte imbalance and hyponatremia [56–58].
Neonates diagnosed with hyperkalemia had three times the risk of developing AKI compared with neonates with normal potassium levels. This study finding is supported by the studies conducted at Maimonides Medical Center [59], and USA [60]. The possible cause may be because severe hyperkalemia requires the administration of calcium to stabilize the myocardium, followed by drugs that shift extracellular potassium to the intracellular compartment, such as bicarbonate, glucose-insulin drip, and inhaled beta-agonists. Following this hyperkalemia, a neonates is at risk for acute renal injury. which results in decreased glomerular filtration rate, decreased tubular potassium secretion, tissue breakdown with intracellular potassium release, and metabolic acidosis with a transcellular potassium shift per 0.1 unit decrease in arterial PH. Increase serum potassium by 0.3 mEq/L [60]. Electrolyte balance can maintain the kidney function of neonates.
Limitations
Because this was a secondary design and/or retrospective data, the study could not exhaustively examine all predictor variables that might have an impact on AKI. the next researcher will be studying prospective studies including measuring urine or a put maternal paternal, and economic status.
Conclusions
This study found that the incidence of acute kidney injury in neonates was high. Besides, the overall incidence rate of AKI was 14.9 per 1000 neonates. Premature infants and neonates with sepsis who suffered perinatal asphyxia and had taken gentamicin drugs, whose sodium levels decreased and whose potassium levels increased, were at higher risk of developing acute kidney injury. Therefore, premature neonates and neonates with neonatal sepsis should get appropriate follow-up, and neonates diagnosed with asphyxia should not be treated with gentamycin antibiotics. Also, neonates’ electrolytes should be kept at a normal level, especially potassium and sodium, to decrease the incidence of AKI. Moreover, future researchers should conduct an interventional study to address further significant predictors of AKI in neonates.
Acknowledgements
The authors would like to express our gratitude to the respective administrations of each comprehensive hospital, card class, and data collectors.
All methods were done following BMC Pediatrics guidelines and regulations.
Abbreviations
| AHR | Adjusted hazard ratio |
| AKI | Acute kidney injury |
| ANC | Antenatal care |
| APGAR | Activity Pulse Grimace Appearance Respiration |
| CHD | Congenital Heart Disease |
| HIE | Hypoxic Ischemic Encephalopathy |
| IVH | Intra-Ventricular Hemorrhage |
| MAS | Meconium Aspiration Syndrome |
| NICU | Neonatal Intensive Care Unit |
| RDS | Respiratory distress syndrome |
| VIF | Variance Inflation Factor |
Authors’ contributions
Authors’ contributions GDG: conceptualization, methodology, software, formal analysis, writing original draft. BTL, YSE, HSM, AGA, &ATA validation, data curation, writing reviewing & editing. TMS&GBM: methodology, writing-review & editing. FBG, DTD & MTT: conceptualization, methodology, writing-review & editing. WTW&ATG: methodology, writing-review & editing, data curation. RAS, TGA & ZAG: methodology, writing-review & editing, data curation, validation. Finally, all authors approved the manuscript.
Funding
This study was funded by the University of Gondar. However, the funder had no role in data collection, preparation of the manuscript, and decision to publish.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to confidentiality but are available from the corresponding author on reasonable request.
Declarations
Before conducting this study ethical clearance letter was obtained from the ethical review committee of the school of Nursing on behalf of the institutional review board of the University of Gondar, after approval of the proposal [26/01/2015 E.C or 03/09/2022 G.C] and (Ref. No:—S/N/036/2015). A written permission letter was obtained from Amhara Public Health Institute for each Hospital
 . A waiver permission letter was obtained from hospital administrators before the data collection and since the neonates were not directly involved in this study (the data were obtained from charts review) informed consent was not required, but extracted data from medical records were kept confidentially. All methods were carried out under the declarations of Helsinki and relevant guidelines and regulations. So, anonymity was maintained by using the identified number instead of the patient’s name. Besides, all data extracted were kept confidential and not used for any other purpose than the stated objective.
. A waiver permission letter was obtained from hospital administrators before the data collection and since the neonates were not directly involved in this study (the data were obtained from charts review) informed consent was not required, but extracted data from medical records were kept confidentially. All methods were carried out under the declarations of Helsinki and relevant guidelines and regulations. So, anonymity was maintained by using the identified number instead of the patient’s name. Besides, all data extracted were kept confidential and not used for any other purpose than the stated objective.
Not Applicable.
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Gezahagn Demsu Gedefaw, Email: moc.liamg@86usmedngahazeg.
Bruck Tesfaye Legesse, Email: moc.liamg@341eyafsetkcurb.
References
Articles from BMC Pediatrics are provided here courtesy of BMC

 1
1