Abstract
Background
This study aimed to predict metastasis risk and identify prognostic factors of gastrointestinal signet ring cell carcinoma (SRCC) using data from the SEER database, the largest cancer dataset in North America.Methods
Data were obtained from the SEER database, covering 17 cancer registries from 2004 to 2020. Demographic and clinical data included sex, age, race, tumor location, size, pathological grade, stage, overall survival time, and treatment modalities. Statistical analyses were conducted using SPSS and R software. Propensity Score Matching (PSM) ensured comparable baseline characteristics between gastric cancer (GC) and colorectal cancer (CRC) groups. LASSO regression analysis identified predictors of metastasis, leading to the construction of predictive models using the lrm function in R. Nomograms were visualized with the "rms" package and assessed via ROC curves, calibration curves, and decision curve analysis (DCA). Cox regression analyses identified prognostic indicators for overall survival (OS), and Kaplan-Meier curves compared OS between high-risk and low-risk groups.Results
From 2004 to 2020, 7680 SRCC patients were identified, including 4980 GC and 2700 CRC patients. CRC patients were older and had larger tumors, higher staging, and worse differentiation. Nomograms demonstrated good discriminative ability, with AUCs of 0.704 and 0.694 for GC, and 0.694 and 0.701 for CRC in training and validation cohorts, respectively. The DCA curve indicates that this predictive model has a high gain in predicting metastasis and OS.Conclusions
The nomograms effectively predicted metastasis risk and OS in metastatic SRCC patients, offering clinical utility in stratifying patients and guiding treatment decisions, thereby enhancing personalized treatment approaches.Free full text

A predictive and prognostic model for metastasis risk and prognostic factors in gastrointestinal signet ring cell carcinoma
Abstract
Background
This study aimed to predict metastasis risk and identify prognostic factors of gastrointestinal signet ring cell carcinoma (SRCC) using data from the SEER database, the largest cancer dataset in North America.
Methods
Data were obtained from the SEER database, covering 17 cancer registries from 2004 to 2020. Demographic and clinical data included sex, age, race, tumor location, size, pathological grade, stage, overall survival time, and treatment modalities. Statistical analyses were conducted using SPSS and R software. Propensity Score Matching (PSM) ensured comparable baseline characteristics between gastric cancer (GC) and colorectal cancer (CRC) groups. LASSO regression analysis identified predictors of metastasis, leading to the construction of predictive models using the lrm function in R. Nomograms were visualized with the “rms” package and assessed via ROC curves, calibration curves, and decision curve analysis (DCA). Cox regression analyses identified prognostic indicators for overall survival (OS), and Kaplan–Meier curves compared OS between high-risk and low-risk groups.
Results
From 2004 to 2020, 7680 SRCC patients were identified, including 4980 GC and 2700 CRC patients. CRC patients were older and had larger tumors, higher staging, and worse differentiation. Nomograms demonstrated good discriminative ability, with AUCs of 0.704 and 0.694 for GC, and 0.694 and 0.701 for CRC in training and validation cohorts, respectively. The DCA curve indicates that this predictive model has a high gain in predicting metastasis and OS.
Conclusions
The nomograms effectively predicted metastasis risk and OS in metastatic SRCC patients, offering clinical utility in stratifying patients and guiding treatment decisions, thereby enhancing personalized treatment approaches.
Introduction
Gastric cancer (GC) and colorectal cancer (CRC) are among the most frequently occurring tumors, ranking first and fifth in incidence among all malignancies, respectively [1]. They represent a significant global health burden, presenting considerable challenges in diagnosis and treatment. Although the mortality rates of gastric and colorectal cancers have declined annually due to the widespread use of Helicobacter pylori eradication therapy and advancements in early tumor resection techniques via electronic gastroscopy and colonoscopy, the incidence of signet ring cell carcinoma (SRCC) has increased annually [13]. SRCC of the stomach is more prevalent in females and tends to affect younger individuals [5], while colorectal SRCC occurs at a younger average age of 65 years compared to more common colorectal cancers, with similar incidence rates in males and females [14]. SRCC is most likely to occur in the stomach, accounting for ~ 15–28% of gastric cancers, whereas colorectal SRCC is relatively rare. SRCC also exhibits unique clinical characteristics and poorer prognosis compared to regular gastric and colorectal cancers patients [11].
Several studies indicates compare to traditional colorectal adenocarcinoma and gastric adenocarcinoma, SRCC originates from undifferentiated stem cells and often displays unique pathological features such as larger tumor size, poorer differentiation, greater invasiveness, and higher metastasis rates [10, 15, 28]. In gastrointestinal carcinoma, SRCC is commonly defined as more than 50% of tumor cells with intracytoplasmic mucin. The latest WHO Classification of Digestive Tumors categorizes more than 90% of SRCC as a poorly cohesive (PC) gastric cancer subtype [23]. In colorectal cancer, SRCC is more than half of the cells are signet ring cells, otherwise, it is characterized as having signet ring features [16]. Compared to non-SRCC, regardless of the tumor location in the stomach or colorectum, SRCC demonstrates higher aggressiveness and poorer patient prognosis [20, 37]. Additionally, as staging progresses, the prognosis of gastric SRCC becomes increasingly worse compared to well-differentiated or moderately differentiated gastric cancers [7]. Therefore, early prediction of metastasis in gastric and colorectal SRCC through clinical indicators is crucial.
In this study, we analyzed clinical and prognostic data from the Surveillance, Epidemiology, and End Results (SEER) database, the largest cancer dataset in North America [35], to predict metastasis risk and prognostic factors of gastrointestinal signet ring cell carcinoma.
Materials and methods
Patient selection
Based on the Surveillance, Epidemiology, and End Results (SEER) database released in November 2022, this study accessed the SEER database and collected data using SEER*Stat software 8.4.3. The data were obtained from 17 cancer registries between 2004 and 2020.
Demographic and clinical data were collected, including sex, age, race, tumor location, tumor size, pathological grade, pathological type, stage, survival time, survival status, surgery, radiotherapy, and chemotherapy.
The inclusion criteria included: 1. Diagnosis between 2004 and 2020; 2. Primary tumor site in the stomach (C16.0–C16.9), colon (C18.0–C18.9, C19.9), or rectum (C20.9) with malignant neoplasm, based on the International Classification of Diseases for Oncology, Third Edition (ICD-O-3) site codes; 3. Histologic code for signet ring cell carcinoma (8490).
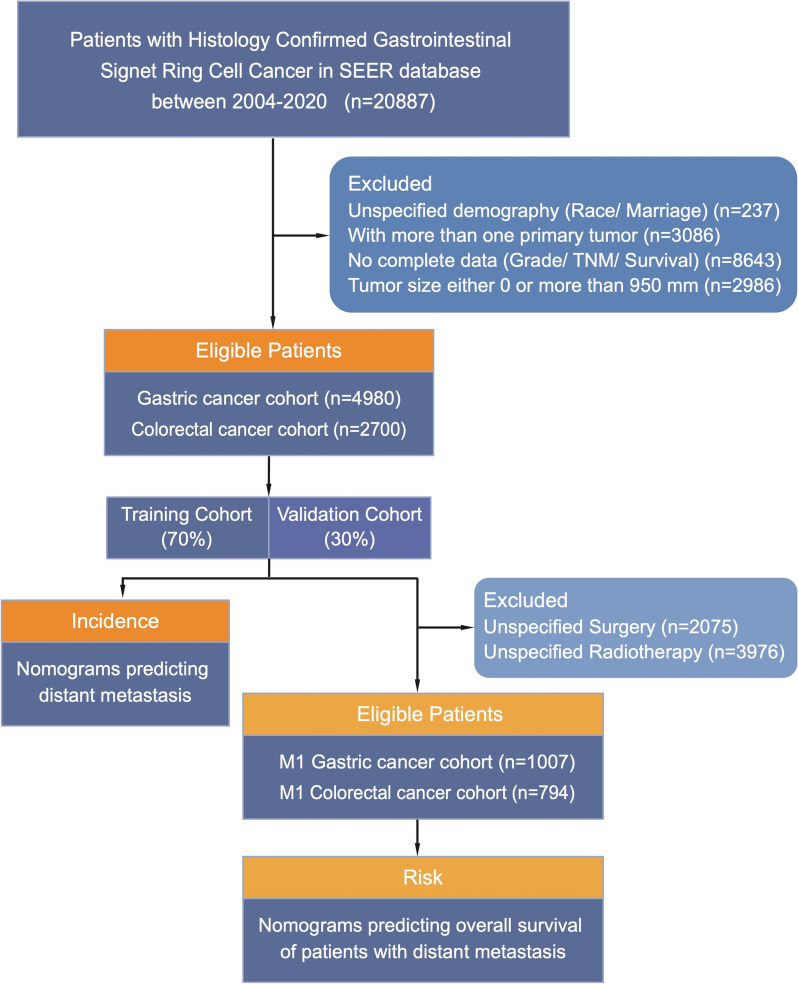
The study initially included 20,887 patients. Exclusion criteria comprised unspecified demographics (race/marital status), multiple primary tumors, incomplete data (grade/TNM stage/survival), and tumor size equal to 0 or exceeding 950 mm. Data from 4980 patients with gastric cancer and 2700 patients with colorectal cancer constituted separate cohorts to discuss risk factors and construct nomograms predicting tumor metastasis. Additionally, 1007 patients with gastric cancer and 794 patients with colorectal cancer who developed metastases were included for the analysis of epidemiological features and the construction of prognostic nomogram models. Figure 1 illustrates the specific screening process.
Nomogram construction
This study utilized SPSS 26.0 and R 4.3.1 for data analysis. Categorical variables were expressed as frequency and percentage, with the χ2 test or Fisher’s exact test used to compare them between the training and validation cohorts. Propensity Score Matching (PSM) was initially applied to identify differences in metastasis rates between patients with gastric and colorectal cancer.
The dataset was randomly split into training and validation sets in a 7:3 ratio. In the training set, Least Absolute Shrinkage and Selection Operator (LASSO) regression analysis was performed using the R package “glmnet.” Unlike traditional regression methods, LASSO reduces the coefficients of less significant variables to zero, excluding them from the model, which enhances interpretability, especially in high-dimensional data where predictors outnumber observations. Additionally, LASSO mitigates overfitting by constraining model complexity, improving generalizability to new data. After selecting predictor variables for the SRCC metastasis model, the model was constructed. The “nomogram” function in the “rms” package was used to visualize the model. Receiver operating characteristic (ROC) curves for the nomogram and independent variables were generated, with area under the curve (AUC) values calculated to assess discriminative power. Model performance was evaluated using calibration curves and decision curve analysis (DCA).
Univariate Cox regression analysis was performed on the training set to identify prognostic variables, and those with P <
< 0.05 were included in multivariate Cox regression analysis to determine independent prognostic indicators for metastatic SRCC. A prognostic nomogram, based on independent predictors, was established to predict overall survival (OS) in metastatic SRCC patients. Individual risk scores were calculated, classifying patients into “High risk” and “Low risk” groups. Time-dependent ROC curves and corresponding AUCs were generated for all independent variables at 6, 12, and 18 months. Calibration curves and DCA were plotted at the same time points to evaluate the nomograms. Kaplan–Meier survival curves and log-rank tests were applied to compare OS between the two risk groups.
0.05 were included in multivariate Cox regression analysis to determine independent prognostic indicators for metastatic SRCC. A prognostic nomogram, based on independent predictors, was established to predict overall survival (OS) in metastatic SRCC patients. Individual risk scores were calculated, classifying patients into “High risk” and “Low risk” groups. Time-dependent ROC curves and corresponding AUCs were generated for all independent variables at 6, 12, and 18 months. Calibration curves and DCA were plotted at the same time points to evaluate the nomograms. Kaplan–Meier survival curves and log-rank tests were applied to compare OS between the two risk groups.
Result
Baseline characteristics of the study population
From 2004 to 2020, a total of 7680 eligible patients with signet ring cell carcinoma (SRCC) were selected from the SEER database, including 4980 patients in the gastric cancer (GC) group and 2700 patients in the colorectal cancer (CRC) group. We compared patients in the GC and CRC groups and found that CRC patients were older, had larger tumor sizes, higher T and N staging, higher metastasis rates, and poorer differentiation. After propensity score matching, there were no statistically significant differences between the groups in terms of tumor diameter, metastasis rates, T stage, and N stage (Table 1). Subsequently, 70% of the patients in each group were randomly selected to form the training cohort, and 30% were assigned to the validation cohort. The baseline clinical characteristics of all patients are summarized in Tables 2, ,33.
Table 1
Clinical characteristics between GC and CRC cohort before and after PSM
| Variables | Before PSM | After PSM | ||||
|---|---|---|---|---|---|---|
| GC cohort | CRC cohort | P | GC cohort | CRC cohort | P | |
n = = 4980(%) 4980(%) | n = = 2700(%) 2700(%) | n = = 2604(%) 2604(%) | n = = 2604(%) 2604(%) | |||
| Age | 61.18 ± ± 14.11 14.11 | 64.24 ± ± 16.47 16.47 |  < < 0.001 0.001 | 61.76 ± ± 14.20 14.20 | 63.72 ± ± 16.49 16.49 |  < < 0.001 0.001 |
| Race |  < < 0.001 0.001 |  < < 0.001 0.001 | ||||
| Black | 537 (10.8) | 228 (8.4) | 258 (9.9) | 228 (8.8) | ||
| Other | 1095 (22.0) | 238 (8.8) | 521 (20.0) | 238 (9.1) | ||
| White | 3348 (67.2) | 2234 (82.7) | 1825 (70.1) | 2138 (82.1) | ||
| Sex | 1 | 0.488 | ||||
| Female | 2383 (47.9) | 1292 (47.9) | 1261 (48.4) | 1235 (47.4) | ||
| Male | 2597 (52.1) | 1408 (52.1) | 1343 (51.6) | 1369 (52.6) | ||
| Size | 53.05 ± ± 46.43 46.43 | 59.45 ± ± 33.08 33.08 |  < < 0.001 0.001 | 60.83 ± ± 44.67 44.67 | 59.63 ± ± 33.39 33.39 | 0.27 |
| Grade |  < < 0.001 0.001 |  < < 0.001 0.001 | ||||
| I | 10 (0.2) | 20 (0.7) | 2 (0.1) | 20 (0.8) | ||
| II | 126 (2.5) | 158 (5.9) | 51 (2.0) | 158 (6.1) | ||
| III | 4696 (94.3) | 2136 (79.1) | 2435 (93.5) | 2136 (82.0) | ||
| IV | 148 (3.0) | 386 (14.3) | 116 (4.5) | 290 (11.1) | ||
| T stage |  < < 0.001 0.001 | 0.279 | ||||
| T1–2 | 2817 (56.6) | 415 (15.4) | 445 (17.1) | 415 (15.9) | ||
| T3–4 | 2163 (43.4) | 2285 (84.6) | 2159 (82.9) | 2189 (84.1) | ||
| N stage |  < < 0.001 0.001 | 0.142 | ||||
N = = 0 0 | 1894 (38.0) | 688 (25.5) | 726 (27.9) | 678 (26.0) | ||
N > > 0 0 | 3086 (62.0) | 2012 (74.5) | 1878 (72.1) | 1926 (74.0) | ||
| Metastasis |  < < 0.001 0.001 | 0.784 | ||||
| No | 3973 (79.8) | 1906 (70.6) | 1850 (71.0) | 1840 (70.7) | ||
| Yes | 1007 (20.2) | 794 (29.4) | 754 (29.0) | 764 (29.3) | ||
Table 2
Baseline clinical characteristics of GC and CRC cohort
| Variables | GC cohort | CRC cohort | ||||
|---|---|---|---|---|---|---|
| Training group | Validation group | P | Training group | Validation group | P | |
n = = 3459(%) 3459(%) | n = = 1521(%) 1521(%) | n = = 1921(%) 1921(%) | n = = 779(%) 779(%) | |||
| Age | 61.24 ± ± 14.12 14.12 | 61.06 ± ± 14.10 14.10 | 0.693 | 64.55 ± ± 16.46 16.46 | 63.46 ± ± 16.47 16.47 | 0.118 |
| Race | 0.067 | 0.758 | ||||
| Black | 352 (10.2) | 185 (12.2) | 165 (8.6) | 63 (8.1) | ||
| Other | 2355 (68.1) | 993 (65.3) | 165 (8.6) | 73 (9.4) | ||
| White | 752 (21.7) | 343 (22.6) | 1591 (82.8) | 643 (82.5) | ||
| Sex | 0.743 | 0.95 | ||||
| Female | 1661 (48.0) | 722 (47.5) | 918 (47.8) | 374 (48.0) | ||
| Male | 1798 (52.0) | 799 (52.5) | 0.268 | 1003 (52.2) | 405 (52.0) | 0.064 |
| Size | 53.53 ± ± 45.96 45.96 | 51.95 ± ± 47.46 47.46 | 60.27 ± ± 34.73 34.73 | 57.44 ± ± 28.55 28.55 | ||
| Grade | 0.548 | 0.736 | ||||
| I | 5 (0.1) | 5 (0.3) | 13 (0.7) | 7 (0.9) | ||
| II | 90 (2.6) | 36 (2.4) | 113 (5.9) | 45 (5.8) | ||
| III | 3263 (94.3) | 1433 (94.2) | 1528 (79.5) | 608 (78.0) | ||
| IV | 101 (2.9) | 47 (3.1) | 267 (13.9) | 119 (15.3) | ||
| T stage | 0.944 | 0.186 | ||||
| T1–2 | 1955 (56.5) | 862 (56.7) | 307 (16.0) | 108 (13.9) | ||
| T3–4 | 1504 (43.5) | 659 (43.3) | 1614 (84.0) | 671 (86.1) | ||
| N stage | 0.484 | 1 | ||||
N = = 0 0 | 1304 (37.7) | 590 (38.8) | 489 (25.5) | 199 (25.5) | ||
N > > 0 0 | 2155 (62.3) | 931 (61.2) | 1432 (74.5) | 580 (74.5) | ||
| Metastasis | 0.589 | 0.247 | ||||
| No | 2752 (79.6) | 1221 (80.3) | 1369 (71.3) | 537 (68.9) | ||
| Yes | 707 (20.4) | 300 (19.7) | 552 (28.7) | 242 (31.1) | ||
Table 3
Baseline clinical characteristics of metastasis GC and CRC cohort
| Variables | Metastasis GC cohort | Metastasis CRC cohort | ||||
|---|---|---|---|---|---|---|
| Training group | Validation group | P | Training group | Validation group | P | |
n = = 702 (%) 702 (%) | n = = 305 (%) 305 (%) | n = = 552 (%) 552 (%) | n = = 242 (%) 242 (%) | |||
| Age | 58.58 ± ± 14.20 14.20 | 57.74 ± ± 15.39 15.39 | 0.398 | 61.20 ± ± 16.09 16.09 | 59.20 ± ± 16.41 16.41 | 0.11 |
| Race | 0.173 | 0.603 | ||||
| Black | 83 (11.8) | 24 (7.9) | 56 (10.1) | 23 (9.5) | ||
| Other | 138 (19.7) | 62 (20.3) | 41 (7.4) | 23 (9.5) | ||
| White | 481 (68.5) | 219 (71.8) | 455 (82.4) | 196 (81.0) | ||
| Sex | 0.133 | 0.637 | ||||
| Female | 319 (45.4) | 155 (50.8) | 274 (49.6) | 115 (47.5) | ||
| Male | 383 (54.6) | 150 (49.2) | 0.053 | 278 (50.4) | 127 (52.5) | 0.17 |
| Size | 64.62 ± ± 46.81 46.81 | 58.75 ± ± 37.47 37.47 | 65.17 ± ± 39.78 39.78 | 61.24 ± ± 30.14 30.14 | ||
| Grade | 0.62 | 0.881 | ||||
| I | 1 (0.1) | 1 (0.3) | 5 (0.9) | 2 (0.8) | ||
| II | 14 (2.0) | 3 (1.0) | 27 (4.9) | 9 (3.7) | ||
| III | 669 (95.3) | 292 (95.7) | 443 (80.3) | 199 (82.2) | ||
| IV | 18 (2.6) | 9 (3.0) | 77 (13.9) | 32 (13.2) | ||
| T stage | 0.195 | 0.411 | ||||
| T1–2 | 200 (28.5) | 100 (32.8) | 17 (3.1) | 11 (4.5) | ||
| T3–4 | 502 (71.5) | 205 (67.2) | 535 (96.9) | 231 (95.5) | ||
| N stage | 0.392 | 0.851 | ||||
N = = 0 0 | 232 (33.0) | 110 (36.1) | 53 (9.6) | 25 (10.3) | ||
N > > 0 0 | 470 (67.0) | 195 (63.9) | 499 (90.4) | 217 (89.7) | ||
| Surgery | 0.233 | 0.868 | ||||
| No | 331 (47.2) | 157 (51.5) | 60 (10.9) | 28 (11.6) | ||
| Yes | 371 (52.8) | 148 (48.5) | 492 (89.1) | 214 (88.4) | ||
| Radiotherapy | 1 | 1 | ||||
| No | 595 (84.8) | 258 (84.6) | 512 (92.8) | 225 (93.0) | ||
| Yes | 107 (15.2) | 47 (15.4) | 40 (7.2) | 17 (7.0) | ||
| Chemotherapy | 0.763 | 0.323 | ||||
| No | 241 (34.3) | 101 (33.1) | 177 (32.1) | 87 (36.0) | ||
| Yes | 461 (65.7) | 204 (66.9) | 375 (67.9) | 155 (64.0) | ||
In the gastric cancer cohort, the mean ages were 61.24 years and 61.06 years in the training and validation cohorts, respectively. The most common tumor differentiation grade was Grade III (94.3% in the training cohort and 94.2% in the validation cohort). The most common T and N stages were T1–2 (56.5% in the training cohort and 56.7% in the validation cohort) and N >
> 0 (62.3% in the training cohort and 61.2% in the validation cohort), with ~ 20% of patients having metastasized (20.4% in the training cohort and 19.7% in the validation cohort) (Fig. 2).
0 (62.3% in the training cohort and 61.2% in the validation cohort), with ~ 20% of patients having metastasized (20.4% in the training cohort and 19.7% in the validation cohort) (Fig. 2).
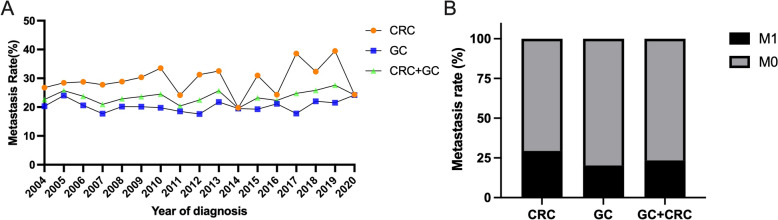
The metastasis rate of gastric and colorectal signet ring cell cancer. A Different years metastasis rate. B Overall metastasis rate. CRC colorectal signet ring cell cancer, GC gastric signet ring cell cancer
In the colorectal cancer cohort, the mean ages were 64.55 years and 63.46 years in the training and validation cohorts, respectively. The most common tumor differentiation grade was Grade III (79.5% in the training cohort and 78.0% in the validation cohort). The most common T and N stages were T3–4 (84.0% in the training cohort and 86.1% in the validation cohort) and N >
> 0 (74.5% in both cohorts), with approximately 30% of patients having metastasized (28.7% in the training cohort and 31.1% in the validation cohort) (Fig. 2).
0 (74.5% in both cohorts), with approximately 30% of patients having metastasized (28.7% in the training cohort and 31.1% in the validation cohort) (Fig. 2).
Clinical characteristics between GC and CRC cohort
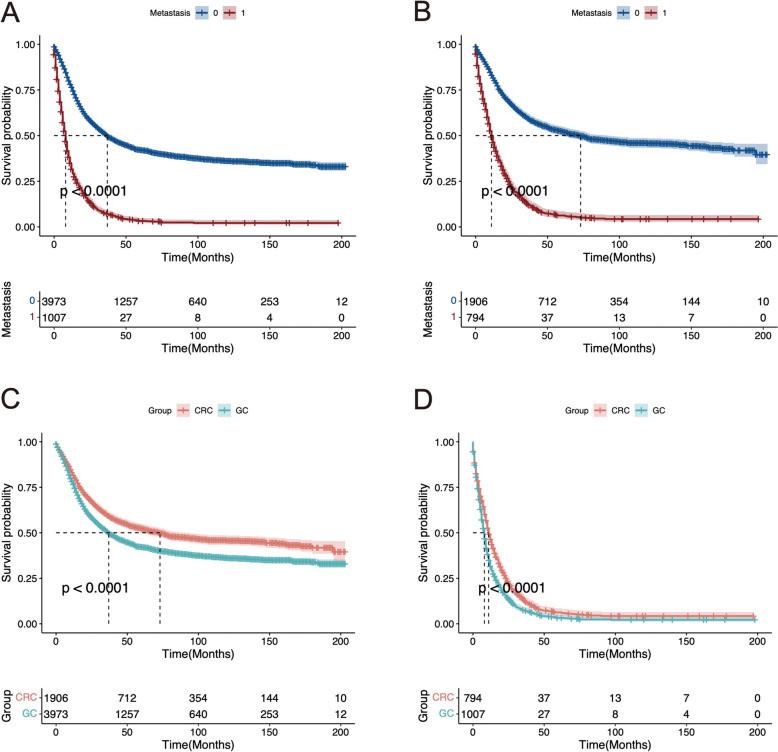
To investigate the impact of tumor metastasis on the prognosis of GC and CRC patients, we compared the survival rates of patients with and without metastasis using Kaplan–Meier (K–M) curves. The data indicated that the prognosis of patients with metastasis was significantly worse than that of patients without metastasis for both GC (P <
< 0.001) and CRC (P
0.001) and CRC (P <
< 0.001).
0.001).
To explore the differences in prognosis between GC and CRC patients, we compared the survival rates of GC and CRC patients. The results showed that the prognosis of GC patients was significantly worse than that of CRC patients in both the metastatic (P <
< 0.001) and non-metastatic groups (P
0.001) and non-metastatic groups (P <
< 0.001) (Fig. 3).
0.001) (Fig. 3).
Incidence and risk factors of distant metastasis in GC and CRC
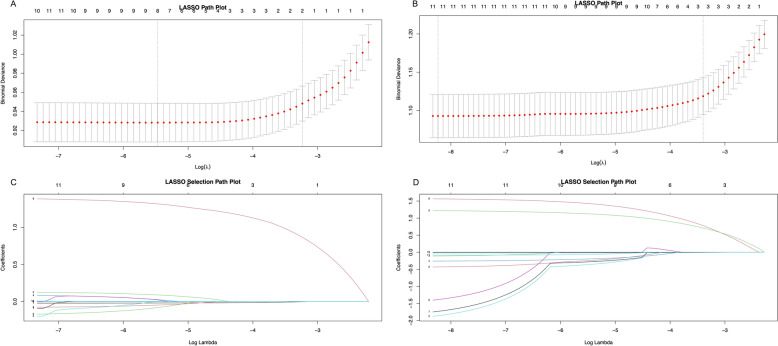
To identify variables associated with metastasis in GC and CRC patients, we utilized LASSO regression analysis to investigate the role of each variable. In the LASSO selection path diagram (Fig. 4A, B), two specific values of λ, lambda.min and lambda.1se, are shown. The LASSO path diagram (Fig. 4C, D) demonstrates that as the coefficients decrease, the predictors decrease accordingly. lambda.min is the regularization parameter that minimizes cross-validated error, providing the most accurate model. lambda.1se is a larger, more conservative value within one standard error of the minimum, offering a simpler model that may enhance generalizability. We established predictive models based on lambda.min and lambda.1se and conducted validation. Considering the overfitting risk and clinical usability of the models, we decided to select factors statistically significant from all 11 predictive variables based on lambda.1se. Ultimately, in GC patients, age and high T stage were identified as two independent risk predictors of metastasis. In CRC patients, age, high T stage, and high N stage were identified as independent risk predictors of metastasis.
Diagnostic nomogram development and validation
In the GC group, we developed a new nomogram prediction model based on two independent risk factors (Fig. 5A). The ROC curves for the nomogram in the training and validation cohorts showed AUCs of 0.704 and 0.694, respectively (Fig. 5B, E). More importantly, the calibration curves of the nomogram indicated good concordance between the observed and predicted outcomes (Fig. 5C, F) and the DCA demonstrated substantial positive net benefits across nearly all threshold probabilities at different time points, suggesting that the prediction model has good potential clinical efficacy (Fig. 5D, G).
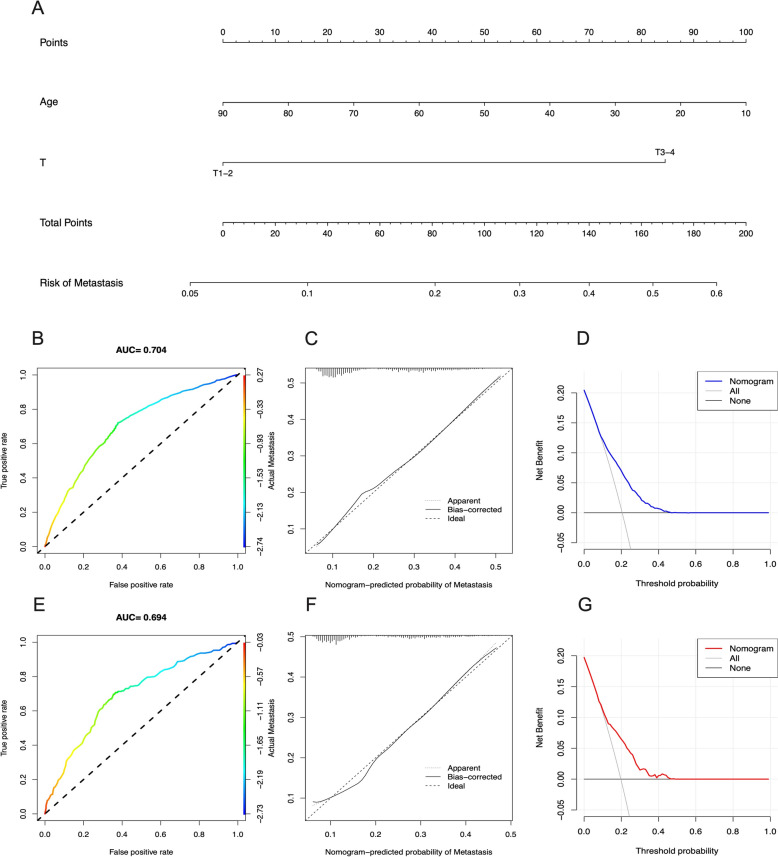
The nomogram of risk of metastasis of GC patients and validation. A Nomogram of risk of metastasis of GC patients in training cohort. ROC curves and AUC of (B) training cohort and (E) validation group. Calibration curves of (C) training cohort and (F) validation group. The DCA of the nomogram to (D) training cohort and (G) validation group
In the CRC group, we developed a new nomogram prediction model based on three independent risk factors (Fig. 6A). The ROC curves for the nomogram in the training and validation cohorts showed AUCs of 0.694 and 0.701, respectively (Fig. 6B, E). Additionally, in both the training and validation cohorts, the calibration curves displayed robust calibration of the nomogram (Fig. 6C, F). The DCA indicated that the nomogram could serve as an excellent diagnostic tool for predicting metastasis in CRC patients (Fig. 6D, G).
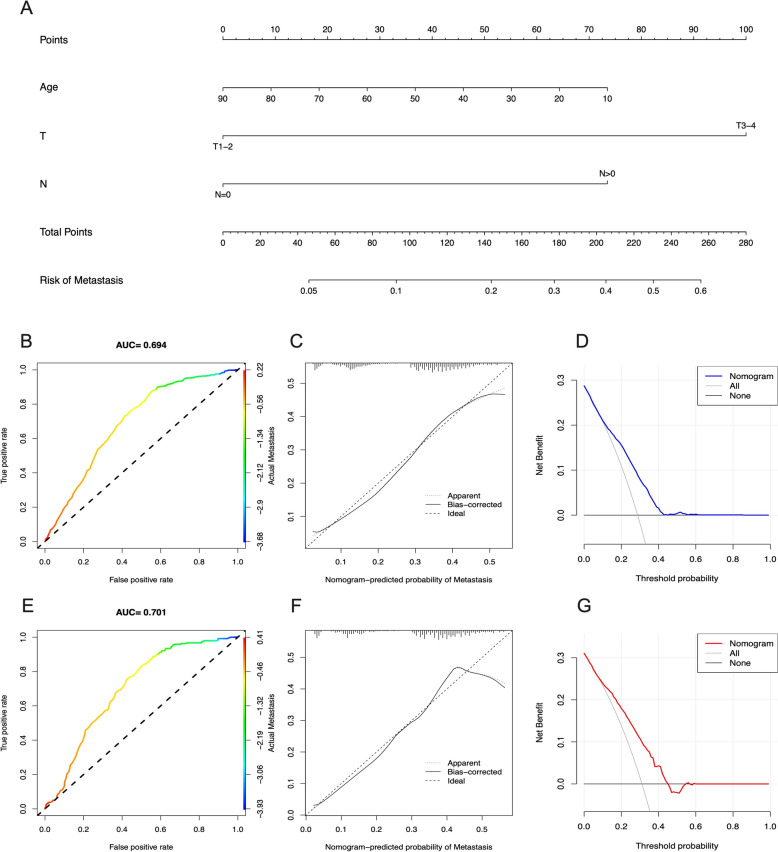
The nomogram of risk of metastasis of CRC patients and validation. A Nomogram of risk of metastasis of CRC patients in training cohort. ROC curves and AUC of (B) training cohort and (E) validation group. Calibration curves of (C) training cohort and (F) validation group. The DCA of the nomogram to (D) training cohort and (G) validation group
Prognostic factors for SRCC patients with metastasis
Based on whether patients received surgery and chemotherapy (Table 4), we constructed nomograms to predict the overall survival (OS) of metastatic GC and CRC patients (Fig. 7A). The calibration curves for the 12-, 24-, and 36-month OS probabilities demonstrated strong concordance between the actual outcomes and the nomogram predictions in both the training (Figs. 7B–D, 8B–D) and validation cohorts (Figs. 9A–C, 10A–C) for the GC and CRC groups. Additionally, the DCA curves confirmed that the nomograms performed well in clinical practice for both groups (Figs. 7E–G, 8E–G, 9D–F, 10D–F).
Table 4
Uni and multivariate cox analyses for the overall survival of metastasis GC and CRC cohort
| Variables | GC cohort | CRC cohort | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate Cox | Multivariate Cox | Univariate Cox | Multivariate Cox | |||||||||
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| Age | 1.007 | 1.002–1.012 | 0.004* | 1.000 | 0.995–1.005 | 0.962 | 1.012 | 1.007–1.017 |  < < 0.001* 0.001* | 1.007 | 1.003–1.012 | 0.003* |
| Race | ||||||||||||
| Black | Reference | Reference | Reference | |||||||||
| Other | 0.741 | 0.576–0.955 | 0.020* | 0.810 | 0.627–1.047 | 0.107 | 1.117 | 0.795–1.570 | 0.523 | |||
| White | 0.861 | 0.694–1.069 | 0.175 | 0.889 | 0.715–1.106 | 0.291 | 0.937 | 0.734–1.195 | 0.599 | |||
| Sex | ||||||||||||
| Female | Reference | Reference | ||||||||||
| Male | 1.008 | 0.883–1.151 | 0.903 | 1.009 | 0.872–1.168 | 0.900 | ||||||
| Size | 1.001 | 0.999–1.002 | 0.330 | 1.002 | 1.000–1.004 | 0.014* | 1.002 | 1.001–1.004 | 0.010* | |||
| Grade | ||||||||||||
| I | Reference | Reference | ||||||||||
| II | 0.760 | 0.174–3.326 | 0.716 | 0.696 | 0.290–1.674 | 0.419 | ||||||
| III | 0.736 | 0.184–2.950 | 0.665 | 1.049 | 0.469–2.345 | 0.908 | ||||||
| IV | 0.613 | 0.145–2.585 | 0.505 | 0.974 | 0.427–2.221 | 0.951 | ||||||
| T stage | ||||||||||||
| T1–2 | Reference | Reference | ||||||||||
| T3–4 | 1.035 | 0.897–1.195 | 0.636 | 1.015 | 0.686–1.501 | 0.941 | ||||||
| N stage | ||||||||||||
N = = 0 0 | Reference | Reference | Reference | Reference | ||||||||
N > > 0 0 | 0.738 | 0.642–0.849 |  < < 0.001* 0.001* | 0.967 | 0.828–1.130 | 0.675 | 0.923 | 0.721–1.182 | 0.526 | 1.280 | 0.979–1.674 | 0.071 |
| Surgery | ||||||||||||
| No | Reference | Reference | Reference | Reference | ||||||||
| Yes | 0.558 | 0.488–0.639 |  < < 0.001* 0.001* | 0.495 | 0.425–0.577 |  < < 0.001* 0.001* | 0.606 | 0.482–0.762 |  < < 0.001* 0.001* | 0.460 | 0.358–0.592 |  < < 0.001* 0.001* |
| Radiotherapy | ||||||||||||
| No | Reference | Reference | Reference | |||||||||
| Yes | 0.758 | 0.630–0.913 | 0.004* | 0.848 | 0.702–1.026 | 0.090 | 0.901 | 0.678–1.198 | 0.475 | |||
| Chemotherapy | ||||||||||||
| No | Reference | Reference | Reference | Reference | ||||||||
| Yes | 0.538 | 0.467–0.619 |  < < 0.001* 0.001* | 0.477 | 0.410–0.555 |  < < 0.001* 0.001* | 0.421 | 0.361–0.492 |  < < 0.001* 0.001* | 0.424 | 0.360–0.500 |  < < 0.001* 0.001* |
Cl Confidence interval, HR Hazard ratio, OS Overall survival, * means P<0.05
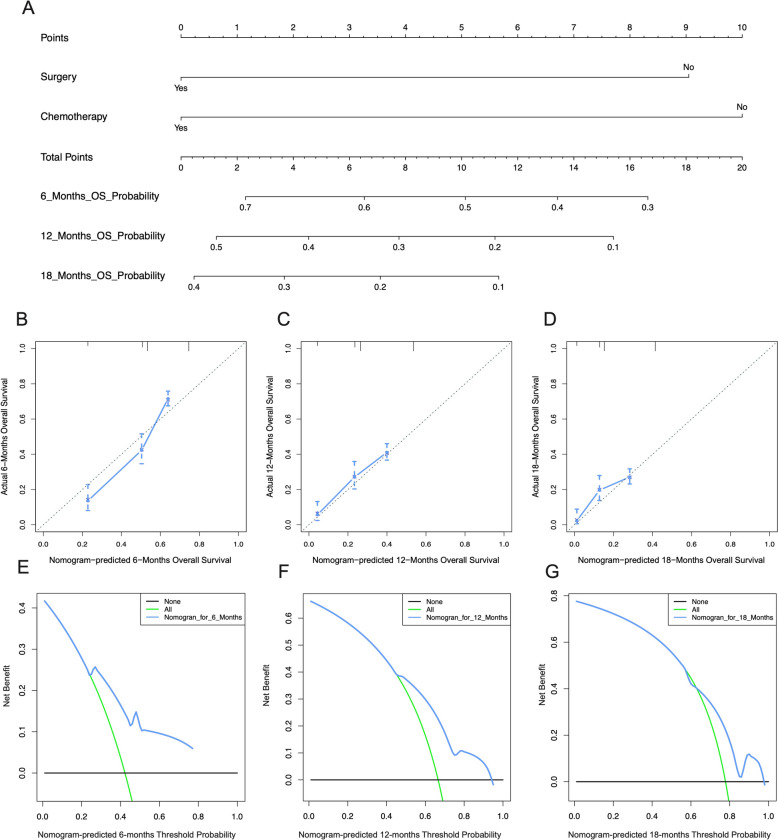
The predictive model of the 6-month, 12-month, and 18-month overall survival of metastasized GC patients in the training cohort. A Nomogram of the 6-month, 12-month, and 18-month overall survival of metastasized GC patients. Calibration curves at the 6-month (B), 12-month (C), and 18-month (D). The DCA of the nomogram at the 6-month (E), 12-month (F), and 18-month (G)
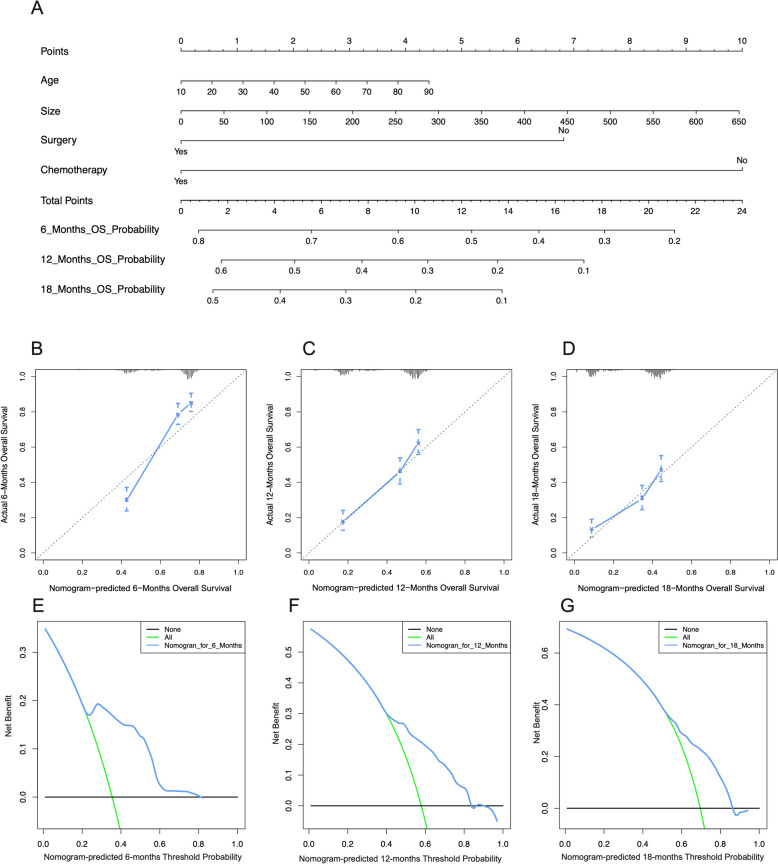
The predictive model of the 6-month, 12-month, and 18-month overall survival of metastasized CRC patients in the training cohort. (A) Nomogram of the 6-month, 12-month, and 18-month overall survival of metastasized CRC patients. Calibration curves at the 6-month (B), 12-month (C), and 18-month (D). The DCA of the nomogram at the 6-month (E), 12-month (F), and 18-month (G)
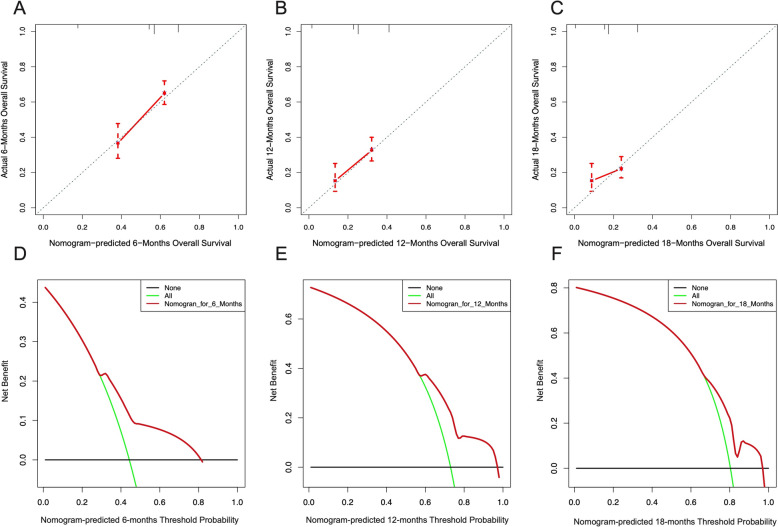
The validation of predictive model of the 6-month, 12-month, and 18-month overall survival of metastasized GC patients. Calibration curves at the 6-month (A), 12-month (B), and 18-month (C) in validation group. The DCA of the nomogram at the 6-month (D), 12-month (E), and 18-month (F) in validation group
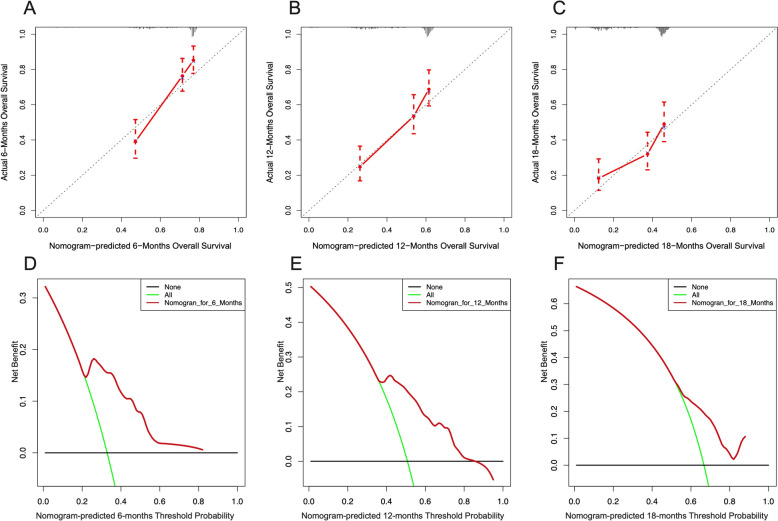
The validation of predictive model of the 6-month, 12-month, and 18-month overall survival of metastasized CRC patients. Calibration curves at the 6-month (A), 12-month (B), and 18-month (C) in validation group. The DCA of the nomogram at the 6-month (D), 12-month (E), and 18-month (F) in validation group
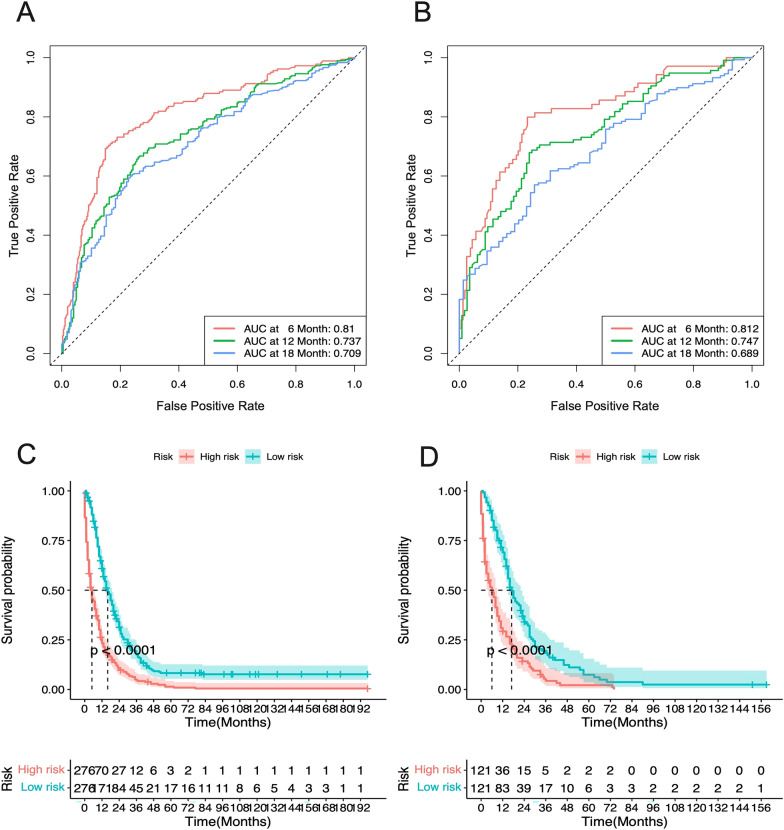
Furthermore, ROC analysis for the GC group indicated that the AUCs of the nomograms for the 12-, 24-, and 36-month OS in the training cohort were 0.771, 0.701, and 0.67, respectively (Fig. 9A), and in the validation cohort, they were 0.723, 0.702, and 0.655, respectively (Fig. 9B). For the CRC group, the ROC analysis revealed that the AUCs for the 12-, 24-, and 36-month OS in the training cohort were 0.81, 0.737, and 0.709, respectively (Fig. 10A), and in the validation cohort, they were 0.812, 0.747, and 0.689, respectively (Fig. 10B). These findings indicate that the model exhibits good discriminatory power in predicting the OS of metastatic GC and CRC patients.
The Kaplan–Meier (K–M) curves demonstrated that the OS of high-risk patients was significantly lower than that of low-risk patients in both the GC and CRC groups (Median Survival Time: GC 3 vs. 10 months; CRC 5 vs. 16 months) (Figs. (Figs.11C,11C, D, D,12C,12C, D). Moreover, the one-year survival rate of the high-risk group was significantly lower than that of the low-risk group (GC 24.1% vs. 46.0%; CRC 28.9% vs. 67.1%).
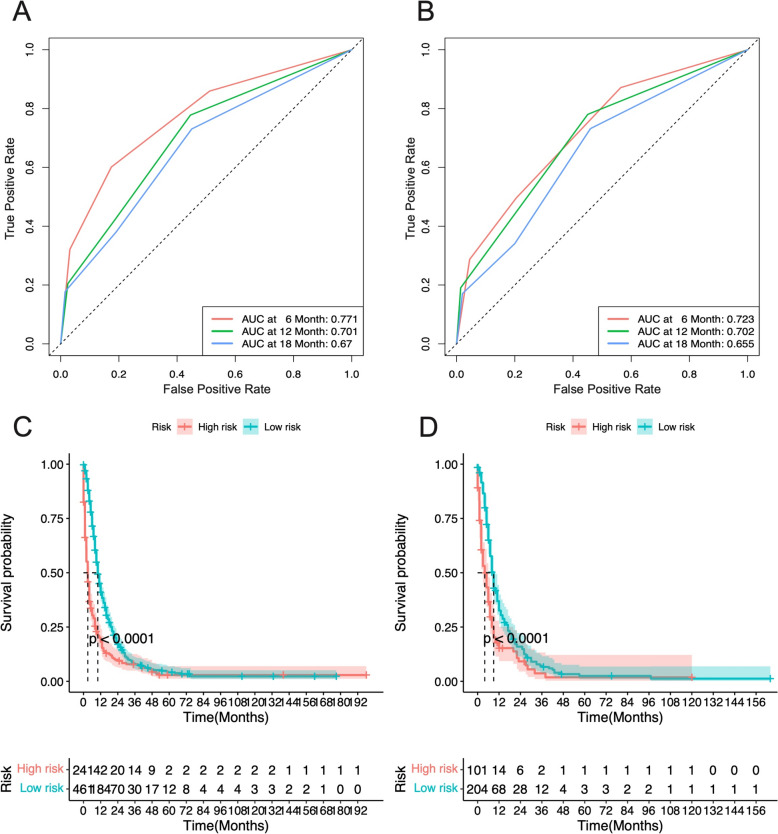
The ROC curves and AUC at 6-month, 12-month, and 18-month of metastasized GC patients in (A) Training cohort and (B) Validation group. Kaplan–Meier survival curves for (C) high-risk and (D) low-risk patients
Discussion
In signet ring cells, a large amount of mucin accumulates in the cytoplasm, pushing the nucleus to one side, thus giving the cell its characteristic ring shape. Generally, when more than half of the tumor cells are signet ring cells, the tumor is classified as signet ring cell carcinoma (SRCC) [2]. According to WHO standards [23], SRCC is a subtype of adenocarcinoma but differs significantly from conventional gastrointestinal adenocarcinoma in terms of clinical characteristics and prognosis [11]. In this study, we collected data from the SEER database on patients diagnosed with gastric or colorectal SRCC from 2004 to 2020. After propensity score matching between the GC and CRC groups, the differences between the two groups were not statistically significant. We then conducted LASSO regression analysis on both groups to identify independent predictive factors for SRCC metastasis. Using these independent predictors, we constructed nomogram-based prediction models, which were validated with internal cohort. Additionally, univariate and multivariate Cox regression analyses were used to identify independent risk factors for the prognosis of SRCC patients with metastasis, and stratified risk prediction models were developed and validated using the internal cohort. The discriminative ability of the models was assessed by the area under the ROC curve, and the performance of the models was evaluated using calibration curves and decision curves. The results indicated that the models' predictions were largely consistent with the actual outcomes, demonstrating good discriminative ability.
SRCC can occur in various malignancies, including breast cancer [36], bladder cancer [19], gallbladder cancer [24], and esophageal cancer [4], but its incidence is relatively low [2]. Compared to other histological types of gastric cancer, gastric SRCC is more common in females and affects younger individuals [5]. When confined to the mucosa or submucosa, its prognosis is relatively better than that of conventional adenocarcinoma. However, as it invades the gastric wall, its aggressiveness increases, along with the rates of lymph node and peritoneal metastasis. Additionally, once gastric SRCC metastasizes, the prognosis is poorer than that of other histological types of gastric cancer, regardless of the treatment method used [8]. Colorectal SRCC is less common than gastric SRCC, accounting for ~ 1% of all pathological types of colorectal cancer [2]. Also, studies indicate that married individuals tend to have better health outcomes compared to their unmarried counterparts. Specifically, in colorectal cancer cases, married individuals often exhibit better survival rates than those who are single, divorced, or widowed [9, 27]. The reason may be that married individuals may have better social support, and more likely to engage in preventive health measures. Besides, race and ethnicity significantly impact the incidence and outcomes of colorectal and gastric cancers. A study indicated that the black individuals have higher incidence rates of CRC compared to white individuals [17]. Similar differences are also observed in gastric cancer, where certain ethnic groups are at higher risk. For instance, Asian populations have been reported to have higher rates of gastric cancer compared to other racial groups in some regions [32]. Colorectal SRCC tends to be diagnosed at an earlier age compared to other pathological types of colorectal cancer [14], with an equal incidence in males and females. Tumors are often larger and more advanced at the time of diagnosis [2]. This finding is consistent with the results of our study. After the PSM of two cohorts, our research finds that the colorectal SRCC often has more advanced grade, but has older age compare to gastric SRCC. However, there was no significance for gender, marital status, tumor size, T-stage, N-stage。
The clinical characteristics and prognosis of signet ring cell carcinoma (SRCC) differ significantly from those of non-signet ring cell carcinoma. Hugen et al. [15] reported that in the colorectum, SRCC is more prone to distant metastasis to multiple organs compared to adenocarcinoma. Additionally, SRCC is more likely to metastasize to lymph nodes than mucinous adenocarcinoma and conventional adenocarcinoma. As our study found, the AJCC N stage is one of the independent predictive factors for metastasis in colorectal cancer. The mechanism underlying the increased propensity for distant metastasis in SRCC is not yet clear, but some studies Sung et al. [29] have observed that SRCC often appears as isolated cells or loose clusters under the microscope, possibly due to the loose intercellular connections that contribute to its metastatic characteristics. Signet ring cell carcinoma may be associated with genetic mutations in the CDH1 gene, which plays a crucial role in maintaining the integrity of E-cadherin [25]. Mutations in this gene lead to a loss of cohesion in the epithelial lining of transformed cells, thereby increasing the likelihood of early metastasis. At present, three biomarkers with therapeutic relevance are routinely assessed in adenocarcinoma: HER2 expression, PD-L1 expression, and deficient mismatch repair [6]. Other molecular signatures of SRCC include a higher frequency of BRAF mutations and the CpG island methylator phenotype (CIMP), reduced expression of p16 and p53, and a lower prevalence of KRAS and NRAS mutations [10]. After metastasis, SRCC progresses rapidly, necessitating adjustments in treatment plans, such as changing chemotherapy drugs or implementing neoadjuvant therapy. Therefore, early detection of metastasis is crucial. To date, no predictive model for metastasis in colorectal SRCC has been established. For gastric SRCC, only models predicting lymph node metastasis in early gastric cancer are available. You et al. [34] identified tumor size and lymphatic invasion as independent risk factors for lymph node metastasis in early gastric SRCC (AUC =
= 0.757, 95% CI 0.687–0.828). Other factors associated with lymph node metastasis in early gastric SRCC include tumor size, depth of invasion, lymphatic invasion, E-cadherin expression, positive mismatch repair function deficit, CA242, neutrophil to lymphocyte ratio, and macroscopic pathological type. Yang et al. [33] reported that gender, tumor size, depth of invasion, lymphatic invasion, ulceration, and immunological subtype are independent risk factors for lymph node metastasis (AUC
0.757, 95% CI 0.687–0.828). Other factors associated with lymph node metastasis in early gastric SRCC include tumor size, depth of invasion, lymphatic invasion, E-cadherin expression, positive mismatch repair function deficit, CA242, neutrophil to lymphocyte ratio, and macroscopic pathological type. Yang et al. [33] reported that gender, tumor size, depth of invasion, lymphatic invasion, ulceration, and immunological subtype are independent risk factors for lymph node metastasis (AUC =
= 0.734, 95%CI 0.643–0.826). In our study, we integrated comprehensive clinical information from a large sample in the SEER database, comparing patients with gastric and colorectal SRCC. We found that most SRCC patients had already developed metastasis at the time of tumor detection. We identified two independent risk predictors of metastasis in GC patients: age and high T stage, and three independent risk predictors in CRC patients: age, high T stage, and high N stage. These findings are consistent with previous reports. Using these independent risk predictors, we constructed predictive models via nomograms, evaluated by ROC curves, calibration curves, and DCA, demonstrating the models' good performance. This provides a valuable reference for clinicians to identify the risk of metastasis early.
0.734, 95%CI 0.643–0.826). In our study, we integrated comprehensive clinical information from a large sample in the SEER database, comparing patients with gastric and colorectal SRCC. We found that most SRCC patients had already developed metastasis at the time of tumor detection. We identified two independent risk predictors of metastasis in GC patients: age and high T stage, and three independent risk predictors in CRC patients: age, high T stage, and high N stage. These findings are consistent with previous reports. Using these independent risk predictors, we constructed predictive models via nomograms, evaluated by ROC curves, calibration curves, and DCA, demonstrating the models' good performance. This provides a valuable reference for clinicians to identify the risk of metastasis early.
Currently, there is no unified consensus on the treatment of signet ring cell carcinoma (SRCC). Most gastric SRCCs exhibit a double-layer structure (DLS), where the microscopic structure consists of two layers: the upper layer contains abundant mucin and an eccentric nucleus, while the lower layer contains small amounts of mucin and eosinophilic substances. This double-layer structure acts as a protective factor, preventing tumor invasion into lymph nodes [30]. For tumors staged as T1a, with a size ≤
≤ 2 cm and low risk of lymph node metastasis, endoscopic submucosal dissection (ESD) can be performed [18]. Moreover, even if the above conditions are not met, if the double-layer structure is observed in ESD specimens, further surgical treatment may not be necessary [22]. However, Wang et al. [31] reported that approximately 10.3% of gastric SRCCs had lymph node metastasis, which determines whether endoscopic treatment or surgical treatment is appropriate. Surgical options include extended margin resection with D2 lymph node dissection. Despite extended margin resection, the rate of positive margins remains high. Consequently, the overall survival of gastric SRCC patients after surgical resection is lower than that of non-SRCC patients, primarily due to the higher propensity for peritoneal and lymph node metastasis and earlier recurrence in SRCC [26]. Nonetheless, surgery remains the mainstay treatment for gastric SRCC. Colorectal SRCC is relatively less common than gastric SRCC, and there are currently no large-scale clinical trials specifically targeting colorectal SRCC. However, the NCCN guidelines indicate that for stage I, II, and III colorectal cancer, surgery is the primary treatment [3]. Our study also found that surgery is associated with the prognosis of SRCC patients, and surgical treatment can improve patient survival expectations.
2 cm and low risk of lymph node metastasis, endoscopic submucosal dissection (ESD) can be performed [18]. Moreover, even if the above conditions are not met, if the double-layer structure is observed in ESD specimens, further surgical treatment may not be necessary [22]. However, Wang et al. [31] reported that approximately 10.3% of gastric SRCCs had lymph node metastasis, which determines whether endoscopic treatment or surgical treatment is appropriate. Surgical options include extended margin resection with D2 lymph node dissection. Despite extended margin resection, the rate of positive margins remains high. Consequently, the overall survival of gastric SRCC patients after surgical resection is lower than that of non-SRCC patients, primarily due to the higher propensity for peritoneal and lymph node metastasis and earlier recurrence in SRCC [26]. Nonetheless, surgery remains the mainstay treatment for gastric SRCC. Colorectal SRCC is relatively less common than gastric SRCC, and there are currently no large-scale clinical trials specifically targeting colorectal SRCC. However, the NCCN guidelines indicate that for stage I, II, and III colorectal cancer, surgery is the primary treatment [3]. Our study also found that surgery is associated with the prognosis of SRCC patients, and surgical treatment can improve patient survival expectations.
For advanced gastric or colorectal signet ring cell carcinoma (SRCC), the prevailing view is that these tumors are generally resistant to chemotherapy and associated with poor prognosis. Some studies have suggested that neoadjuvant chemotherapy may worsen tumor progression and prognosis due to the cytotoxicity of the chemotherapy drugs [21]. However, other studies [12] have indicated that neoadjuvant chemotherapy can benefit overall survival. Our study found that perioperative chemotherapy is beneficial for patient survival, although the indications for perioperative chemotherapy require further clarification through additional clinical trials. For patients with unresectable gastric SRCC, the docetaxel-5FU-oxaliplatin (TEFOX) regimen can be used as first-line treatment. Our study also showed that chemotherapy is an independent prognostic factor for metastatic gastric SRCC patients. Therefore, we believe that effective chemotherapy regimens could significantly improve survival rates for SRCC patients.
Our study has several strengths. We utilized a large patient population from a nationwide database in the United States, avoiding biases associated with single-institution records or limited sample sizes, thereby ensuring the validity of our models. Additionally, we employed the propensity score matching (PSM) method to eliminate the interference of disease differences in the results. However, this study also has some potential limitations. First, as a retrospective study, it is susceptible to inaccuracies. Second, the SEER database lacks precise details on treatment methods, including specific surgical procedures and chemotherapy or radiotherapy regimens. Third, the limited number of patients included in this study might introduce some bias in the results.
Conclusion
This study demonstrates the utility of the SEER database in identifying significant predictors of metastasis and overall survival in patients with gastrointestinal SRCC. Through rigorous statistical analyses, including Propensity Score Matching and LASSO regression, we developed robust nomograms for GC and CRC cohorts. These models, validated by ROC curves, calibration curves, and decision curve analysis, exhibit substantial discriminative power and clinical efficacy. The identified independent risk factors, particularly age, high T stage, and high N stage, are critical in stratifying patients and guiding personalized treatment decisions. The nomograms offer a valuable tool for clinicians to predict metastasis risk and prognosis, ultimately improving patient management and outcomes in SRCC. Future research should focus on further validation of these models in diverse populations and integrating additional biomarkers to enhance predictive accuracy.
Author contributions
All authors contributed to the study conception and design. J.Y. and Y.L. proformed material preparation, data collection, and analysis. J.Y. write the first draft of the manuscript, and all authors commented on previous versions of the manuscript. Q.Z. and T.L. reviewed, edited and revisited the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by grants from the Natural Science Foundation of Shandong Province (ZR2020QH033).
Declarations
Since the SEER database is publicly accessible and all records are de-identified, no further ethical approval or informed consent was necessary once the SEER Research Data Agreement was executed to access the data. The study was conducted in compliance with the principles of the Helsinki Declaration.
Not applicable.
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Articles from European Journal of Medical Research are provided here courtesy of BMC
Funding
Funders who supported this work.
Natural Science Foundation of Shandong Province (1)
Grant ID: ZR2020QH033

 1 and
1 and